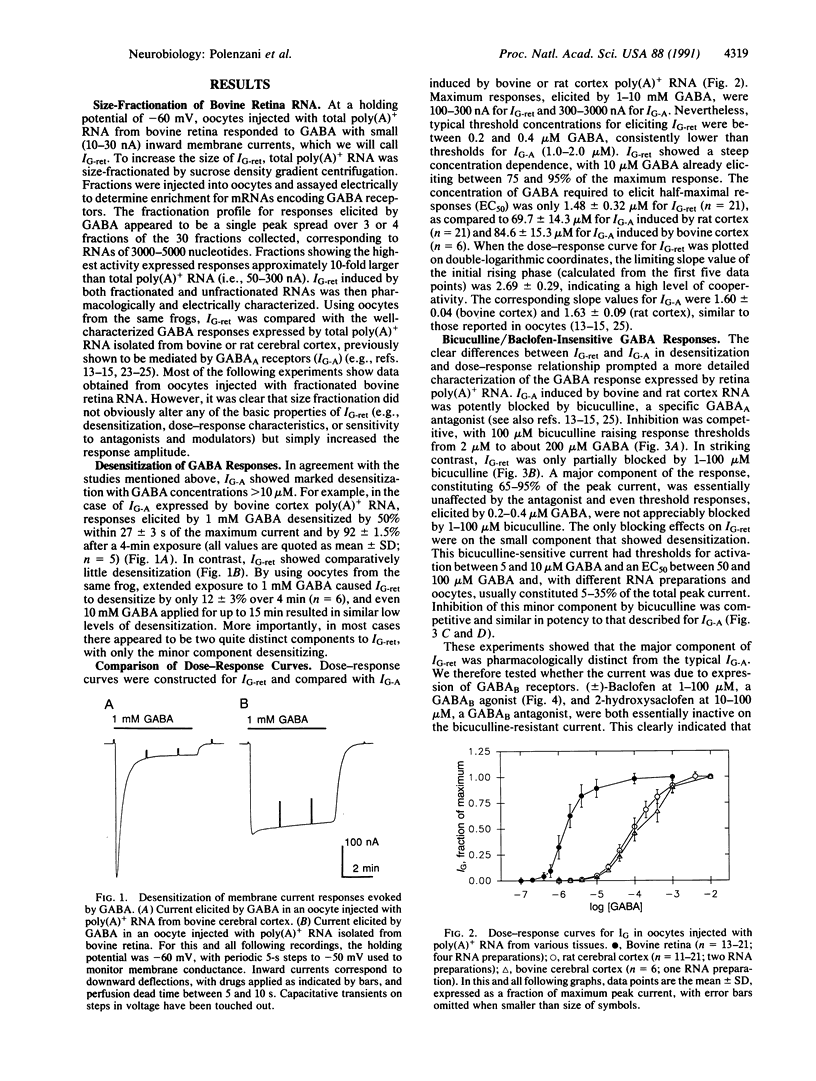
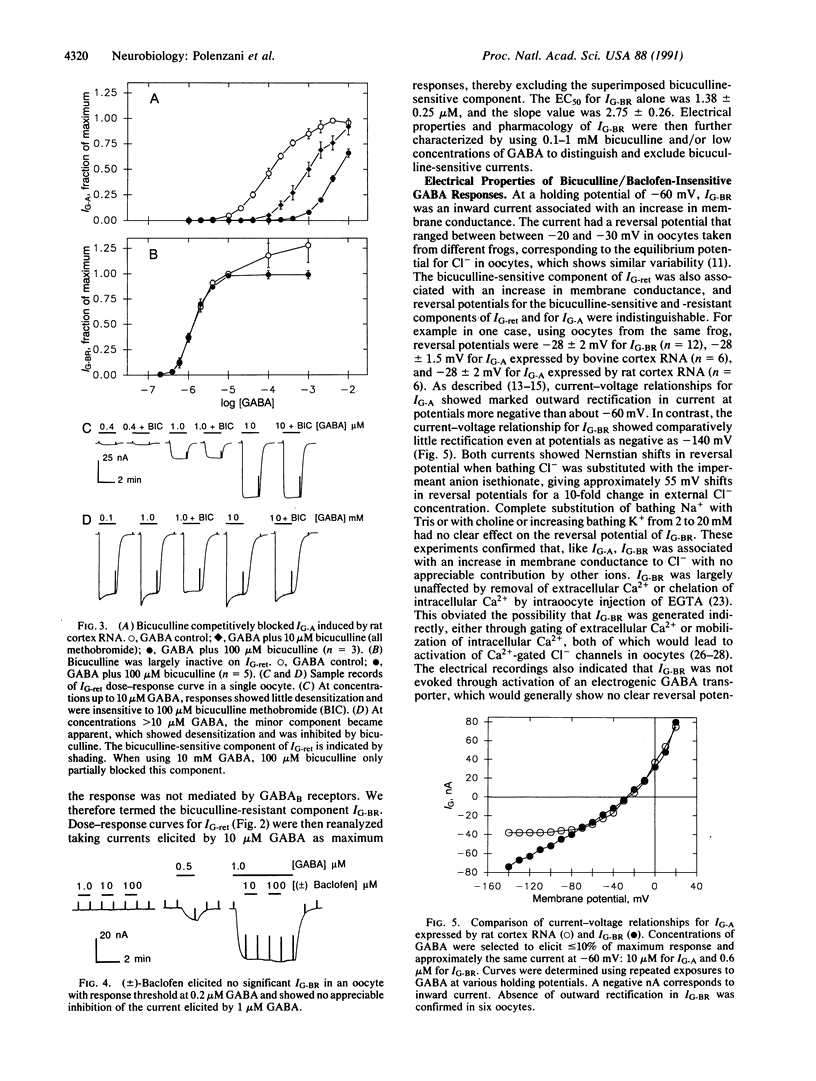
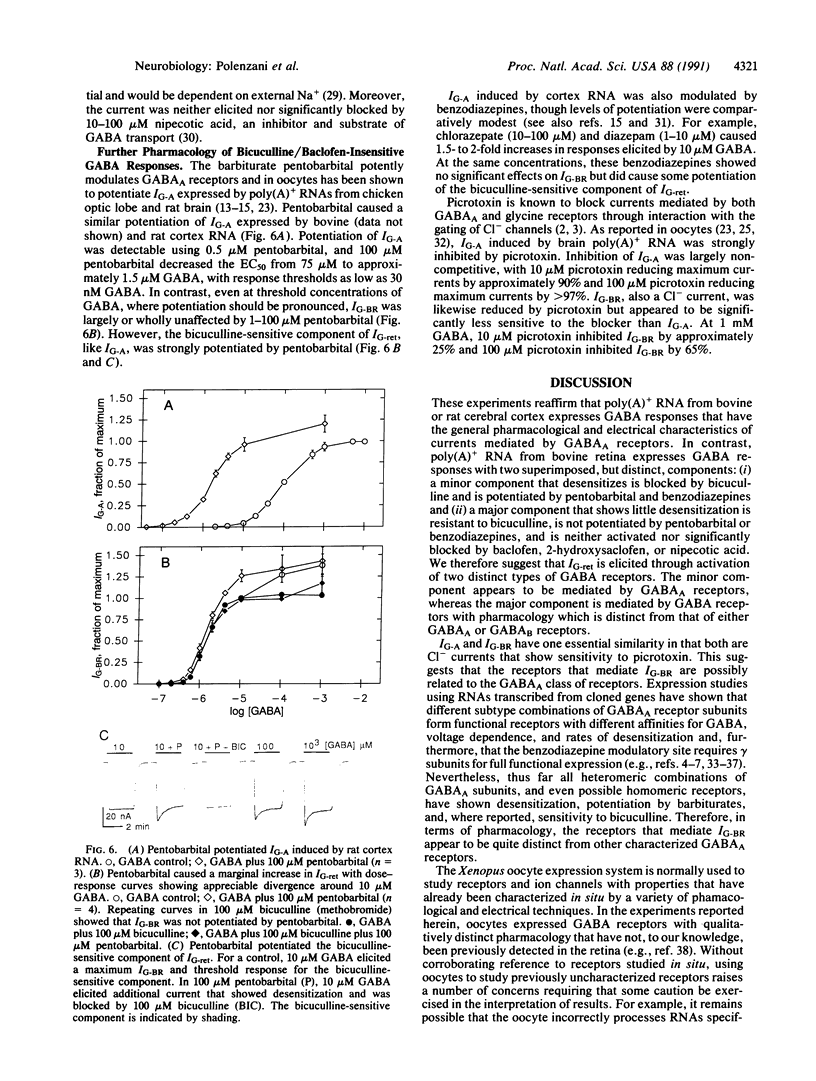
Abstract
Gamma-Aminobutyric acid (GABA), the major inhibitory neurotransmitter in mammalian brain, is known to interact with two classes of GABA receptors denoted GABAA and GABAB. Using Xenopus oocytes, we compared the electrical and pharmacological properties of GABA receptors expressed by poly(A)+ RNA isolated from mammalian brain and retina. RNA from cerebral cortex expressed GABA responses with features characteristic of currents mediated by GABAA receptors. In contrast, RNA from retina expressed responses mediated by GABAA receptors and, in addition, GABA responses that were insensitive to the GABAA antagonist bicuculline and the GABAB agonist baclofen and showed no modulation by barbiturates or benzodiazepines. The bicuculline/baclofen-insensitive GABA response was a Cl- current that was blocked by picrotoxin but showed little desensitization or outward rectification. Our results suggest that mammalian retina contains RNAs encoding GABA receptors with distinct pharmacology.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoshima H., Anan M., Ishii H., Iio H., Kobayashi S. Minimal model to account for the membrane conductance increase and desensitization of gamma-aminobutyric acid receptors synthesized in the Xenopus oocytes injected with rat brain mRNA. Biochemistry. 1987 Jul 28;26(15):4811–4816. doi: 10.1021/bi00389a031. [DOI] [PubMed] [Google Scholar]
- Barnard E. A., Bilbe G., Houamed K., Moss S. J., Van Renterghem C., Smart T. G. Functional expression in the Xenopus oocyte of messenger ribonucleic acids encoding brain neurotransmitter receptors: further characterisation of the implanted GABA receptor. Neuropharmacology. 1987 Jul;26(7B):837–844. doi: 10.1016/0028-3908(87)90060-8. [DOI] [PubMed] [Google Scholar]
- Blair L. A., Levitan E. S., Marshall J., Dionne V. E., Barnard E. A. Single subunits of the GABAA receptor form ion channels with properties of the native receptor. Science. 1988 Oct 28;242(4878):577–579. doi: 10.1126/science.2845583. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A. Bicuculline, an antagonist of GABA and synaptic inhibition in the spinal cord of the cat. Brain Res. 1971 Sep 10;32(1):69–96. doi: 10.1016/0006-8993(71)90156-9. [DOI] [PubMed] [Google Scholar]
- Dascal N. The use of Xenopus oocytes for the study of ion channels. CRC Crit Rev Biochem. 1987;22(4):317–387. doi: 10.3109/10409238709086960. [DOI] [PubMed] [Google Scholar]
- Hill D. R., Bowery N. G. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature. 1981 Mar 12;290(5802):149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- Houamed K. M., Bilbe G., Smart T. G., Constanti A., Brown D. A., Barnard E. A., Richards B. M. Expression of functional GABA, glycine and glutamate receptors in Xenopus oocytes injected with rat brain mRNA. 1984 Jul 26-Aug 1Nature. 310(5975):318–321. doi: 10.1038/310318a0. [DOI] [PubMed] [Google Scholar]
- Kerr D. I., Ong J., Johnston G. A., Abbenante J., Prager R. H. 2-Hydroxy-saclofen: an improved antagonist at central and peripheral GABAB receptors. Neurosci Lett. 1988 Sep 23;92(1):92–96. doi: 10.1016/0304-3940(88)90748-3. [DOI] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P. Inhibitors of the GABA uptake systems. Mol Cell Biochem. 1980 Jun 18;31(2):105–121. doi: 10.1007/BF00240816. [DOI] [PubMed] [Google Scholar]
- Larsson O. M., Hertz L., Schousboe A. Uptake of GABA and nipecotic acid in astrocytes and neurons in primary cultures: changes in the sodium coupling ratio during differentiation. J Neurosci Res. 1986;16(4):699–708. doi: 10.1002/jnr.490160410. [DOI] [PubMed] [Google Scholar]
- Levitan E. S., Blair L. A., Dionne V. E., Barnard E. A. Biophysical and pharmacological properties of cloned GABAA receptor subunits expressed in Xenopus oocytes. Neuron. 1988 Nov;1(9):773–781. doi: 10.1016/0896-6273(88)90125-0. [DOI] [PubMed] [Google Scholar]
- Levitan E. S., Schofield P. R., Burt D. R., Rhee L. M., Wisden W., Köhler M., Fujita N., Rodriguez H. F., Stephenson A., Darlison M. G. Structural and functional basis for GABAA receptor heterogeneity. Nature. 1988 Sep 1;335(6185):76–79. doi: 10.1038/335076a0. [DOI] [PubMed] [Google Scholar]
- Luthe D. S. A simple technique for the preparation and storage of sucrose gradients. Anal Biochem. 1983 Nov;135(1):230–232. doi: 10.1016/0003-2697(83)90755-8. [DOI] [PubMed] [Google Scholar]
- Massey S. C., Redburn D. A. Transmitter circuits in the vertebrate retina. Prog Neurobiol. 1987;28(1):55–96. doi: 10.1016/0301-0082(87)90005-0. [DOI] [PubMed] [Google Scholar]
- Miledi R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc R Soc Lond B Biol Sci. 1982 Jul 22;215(1201):491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I. Chloride current induced by injection of calcium into Xenopus oocytes. J Physiol. 1984 Dec;357:173–183. doi: 10.1113/jphysiol.1984.sp015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I., Sumikawa K. Synthesis of chick brain GABA receptors by frog oocytes. Proc R Soc Lond B Biol Sci. 1982 Nov 22;216(1205):509–515. doi: 10.1098/rspb.1982.0089. [DOI] [PubMed] [Google Scholar]
- Miledi R., Woodward R. M. Effects of defolliculation on membrane current responses of Xenopus oocytes. J Physiol. 1989 Sep;416:601–621. doi: 10.1113/jphysiol.1989.sp017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. W. Drug interactions at the GABA receptor-ionophore complex. Annu Rev Pharmacol Toxicol. 1982;22:245–277. doi: 10.1146/annurev.pa.22.040182.001333. [DOI] [PubMed] [Google Scholar]
- Oron Y., Dascal N., Nadler E., Lupu M. Inositol 1,4,5-trisphosphate mimics muscarinic response in Xenopus oocytes. Nature. 1985 Jan 10;313(5998):141–143. doi: 10.1038/313141a0. [DOI] [PubMed] [Google Scholar]
- Parker I., Gundersen C. B., Miledi R. Actions of pentobarbital on rat brain receptors expressed in Xenopus oocytes. J Neurosci. 1986 Aug;6(8):2290–2297. doi: 10.1523/JNEUROSCI.06-08-02290.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Miledi R. Changes in intracellular calcium and in membrane currents evoked by injection of inositol trisphosphate into Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1986 Aug 22;228(1252):307–315. doi: 10.1098/rspb.1986.0057. [DOI] [PubMed] [Google Scholar]
- Parker I., Sumikawa K., Miledi R. Messenger RNA from bovine retina induces kainate and glycine receptors in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1985 Jul 22;225(1238):99–106. doi: 10.1098/rspb.1985.0052. [DOI] [PubMed] [Google Scholar]
- Parker I., Sumikawa K., Miledi R. Responses to GABA, glycine and beta-alanine induced in Xenopus oocytes by messenger RNA from chick and rat brain. Proc R Soc Lond B Biol Sci. 1988 Mar 22;233(1271):201–216. doi: 10.1098/rspb.1988.0019. [DOI] [PubMed] [Google Scholar]
- Schofield P. R., Darlison M. G., Fujita N., Burt D. R., Stephenson F. A., Rodriguez H., Rhee L. M., Ramachandran J., Reale V., Glencorse T. A. Sequence and functional expression of the GABA A receptor shows a ligand-gated receptor super-family. Nature. 1987 Jul 16;328(6127):221–227. doi: 10.1038/328221a0. [DOI] [PubMed] [Google Scholar]
- Shivers B. D., Killisch I., Sprengel R., Sontheimer H., Köhler M., Schofield P. R., Seeburg P. H. Two novel GABAA receptor subunits exist in distinct neuronal subpopulations. Neuron. 1989 Sep;3(3):327–337. doi: 10.1016/0896-6273(89)90257-2. [DOI] [PubMed] [Google Scholar]
- Sigel E., Baur R. Allosteric modulation by benzodiazepine receptor ligands of the GABAA receptor channel expressed in Xenopus oocytes. J Neurosci. 1988 Jan;8(1):289–295. doi: 10.1523/JNEUROSCI.08-01-00289.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel E., Baur R., Trube G., Möhler H., Malherbe P. The effect of subunit composition of rat brain GABAA receptors on channel function. Neuron. 1990 Nov;5(5):703–711. doi: 10.1016/0896-6273(90)90224-4. [DOI] [PubMed] [Google Scholar]
- Smart T. G., Houamed K. M., Van Renterghem C., Constanti A. mRNA-directed synthesis and insertion of functional amino acid receptors in Xenopus laevis oocytes. Biochem Soc Trans. 1987 Feb;15(1):117–122. doi: 10.1042/bst0150117. [DOI] [PubMed] [Google Scholar]
- Verdoorn T. A., Draguhn A., Ymer S., Seeburg P. H., Sakmann B. Functional properties of recombinant rat GABAA receptors depend upon subunit composition. Neuron. 1990 Jun;4(6):919–928. doi: 10.1016/0896-6273(90)90145-6. [DOI] [PubMed] [Google Scholar]
- Ymer S., Draguhn A., Köhler M., Schofield P. R., Seeburg P. H. Sequence and expression of a novel GABAA receptor alpha subunit. FEBS Lett. 1989 Nov 20;258(1):119–122. doi: 10.1016/0014-5793(89)81630-8. [DOI] [PubMed] [Google Scholar]
- Ymer S., Schofield P. R., Draguhn A., Werner P., Köhler M., Seeburg P. H. GABAA receptor beta subunit heterogeneity: functional expression of cloned cDNAs. EMBO J. 1989 Jun;8(6):1665–1670. doi: 10.1002/j.1460-2075.1989.tb03557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]




