Abstract
During the end-Permian ecological crisis, terrestrial ecosystems experienced preferential dieback of woody vegetation. Across the world, surviving herbaceous lycopsids played a pioneering role in repopulating deforested terrain. We document that the microspores of these lycopsids were regularly released in unseparated tetrads indicative of failure to complete the normal process of spore development. Although involvement of mutation has long been hinted at or proposed in theory, this finding provides concrete evidence for chronic environmental mutagenesis at the time of global ecological crisis. Prolonged exposure to enhanced UV radiation could account satisfactorily for a worldwide increase in land plant mutation. At the end of the Permian, a period of raised UV stress may have been the consequence of severe disruption of the stratospheric ozone balance by excessive emission of hydrothermal organohalogens in the vast area of Siberian Traps volcanism.
At the end of the Permian Period, worldwide collapse of terrestrial and marine ecosystems resulted in major perturbation of global biogeochemical cycles and unrivaled extinction (1). End-Permian extinctions did not occur at an instantaneous time horizon; particularly, floral extinction was delayed in time (2). Among chronic environmental stress factors that have been advocated as killing agents, only atmospheric contamination could have exerted widespread destruction of plant life on land (3). Contaminants of our present-day atmosphere include chemicals (organic and inorganic) and radiation (ionizing and UV). Each of these qualifies as potent environmental mutagens. The most prominent fraction of present mutation is induced by UV radiation in the 290- to 315-nm spectral region (UV-B) (4). Under long-term background conditions, the biological impact of the mutagenic agents is a combined function of error-prone DNA-damage repair and adaptation. Excessive levels increase the frequency of mutation and can alter the genome or its proper functioning (5, 6). Modeling studies predict that accumulation of harmful mutations can cause or aid population decline and eventual extinction (7, 8). Severe environmental mutagenesis should therefore be a central component of mass-extinction scenarios. Ideas that mutation has affected end-Permian life have arisen repeatedly since the 1950s, when the German paleontologist Otto Schindewolf hypothesized that mutation induced by cosmic radiation could account not only for extinction, but also for accelerated origination of new clades (9). However, distinctive mutational symptoms have never been identified in the fossil record of end-Permian terrestrial and aquatic biota (10).
Because of its sensitivity to chemical and radiation mutagens (5, 6, 11, 12), land vegetation is one of the most obvious biotal constituents to be explored for worldwide mutational responses to atmospheric stress factors. Among fossil plants, assemblages of dispersed spores and pollen provide the sample size and temporal spacing needed to recognize morphogenetic traits that reflect mutation. We hypothesize that environmental mutagenesis could have imprinted a detectable signature in the end-Permian spore/pollen record.
Apart from cytological aberration, mutational effects on spore/pollen development can be discerned morphologically by anomalous variation in overall shape, size and wall thickness (13–16), disorganized wall structure (17), number and arrangement of germinal apertures (18, 19), and the presence of permanent tetrads in which four individual spores or pollen grains fail to separate from one another during sporogenesis (20–23). Unusual levels of morphological variants or imperfections have long been evident among Late Permian gymnosperm pollen, both in situ in pollen-bearing organs (24) and in dispersed spore/pollen assemblages (25). Although not explicitly linked to mutation, this variability has been interpreted as evidence of microevolutionary processes. Here, we document the worldwide proliferation of tetrads of lycopsid microspores that occurs in conjunction with the end-Permian ecological crisis. We present evidence and arguments that this conspicuous bioevent reflects environmental mutagenesis in response to increased fluxes of UV-B radiation triggered by stratospheric ozone destruction.
Proliferation of Lycopsid Microspore Tetrads
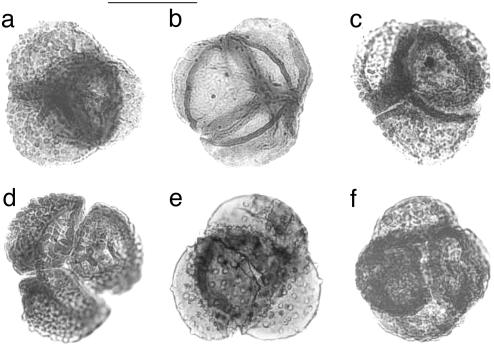
Superimposed on long-term effects of Late Permian–Early Triassic climatic trends, preferential dieback among dominant woody gymnosperms dramatically affected end-Permian terrestrial ecosystems (2, 3, 26, 27). Opportunistic herbs, capable of rapid population expansion into stressed environments, colonized the vacated ecospace. Palynological data confirm a pioneering role for herbaceous heterosporous lycopsids (2). Remarkably, in many Permian–Triassic (P-Tr) transition sequences, lycopsid microspores regularly occur in tetrahedral tetrads. These tetrads have been dispersed intact, with the four spores remaining firmly attached during transportation and burial (Fig. 1).
Fig. 1.
Selection of the latest Permian microspores of heterosporous lycopsids in tetrads from the Wordie Creek Formation, southern Jamesonland, East Greenland. Specimens can be assigned to various species of microspore form-genera Lundbladispora (a, c, e, and f), Densoisporites (b), and Uvaesporites (d). (Scale bar = 50 μm.)
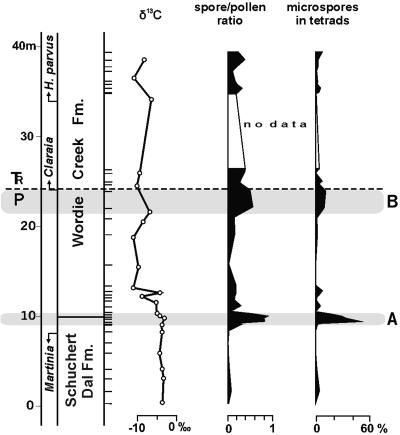
As a result of high sedimentation rates, fine-grained siliciclastic sediments in East Greenland provide an exceptionally detailed spore/pollen record of end-Permian vegetation succession (2, 28). From this section, we show the temporal distribution trend of lycopsid tetrads by calculating their proportion in successive spore/pollen assemblages (Fig. 2). A peak-occurrence of tetrads is apparent just above an interval with last-occurrences of Permian brachiopods, such as Martinia, and just below the onset of the characteristic negative shift in δ13C values for end-Permian carbonates. The peak corresponds to an initial rapid conversion from closed-canopied gymnosperm woodland to open lycopsid-dominant vegetation. After several hundred thousand years (28), a second increase in tetrad abundance occurs close to the P-Tr boundary, where renewed dieback of woody elements and renewed expansion of lycopsids marks the coup de grâce for lingering gymnosperms that were once dominant in the pre-crisis Permian vegetation (2). This time-delayed, selective extinction results in the establishment of low-diversity open-shrubland vegetation, in which lycopsid taxa continue to play a central role until full recovery of woodland ecosystems takes place ≈4 million years later at the transition between Early and Middle Triassic (29).
Fig. 2.
Carbon-isotope (δ13C) profile for carbonates, spore/pollen ratio, and distribution of lycopsid microspores preserved in tetrads in the P-Tr transition sequence of southern Jamesonland, East Greenland. Assuming uniform sedimentation rates, 1 m of lower Wordie Creek Formation represents between 20 and 60 kyr (28). Although the conodont element Hindeodus parvus is the first unquestionable indication of earliest Triassic age, first occurrences of the bivalve Claraia approximate the P-Tr boundary. Small horizontal lines represent position of palynological samples. The sample gap is due to lack of accessible exposures. Relative abundance of the microspores is expressed as a percentage of the total spore/pollen assemblage content. The spore/pollen ratio represents the counted number of spores of lycopsids, ferns, and bryophytes, divided by the total number of counted identifiable spores and pollen grains [n = 80–380 per sample (standard count 200), tetrads representing four spores]. Successive ratios depict two-step vegetation development from closed gymnosperm woodland to open shrubland dominated by herbaceous lycopsids. A, Collapse phase; B, delayed-extinction phase (for details, see ref. 2).
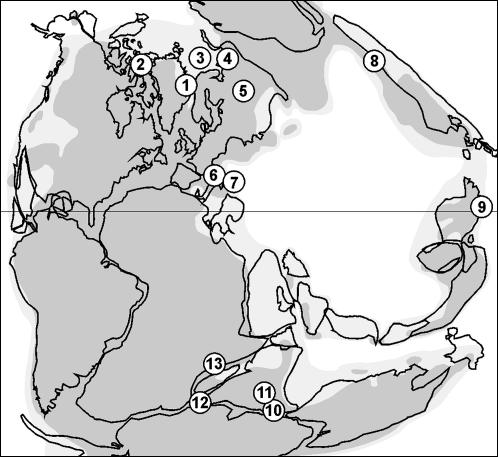
The tetrad abundances documented from Greenland can be traced across the world, irrespective of climatic zonation (Fig. 3). Identical or similar tetrads are known, often in relatively high frequencies, from time-equivalent sequences in North America (30), Europe (31–35), Asia (36–40), and Africa (41). The lycopsid tetrads are generally identified as species of form-genera for dispersed spores such as Decisporis, Densoisporites, Kraeuselisporites, Lundbladispora, and Uvaesporites. Alternatively, they are sometimes assigned to Lapposisporites, a separate form-genus for tetrahedral spore tetrads. Microspores corresponding to Densoisporites are known from spore-bearing organs of Early Triassic Pleuromeia, an extinct relative of modern Isoetales (43). Before and after their end-Permian proliferation, lycopsid tetrads can be found occasionally as subordinate elements in late Paleozoic, Mesozoic, and Cenozoic spore/pollen assemblages. Only at the Triassic–Jurassic transition is there evidence, at least locally, of another frequency increase. Facultative tetrads still occur in some extant species of Selaginella, such as S. selaginoides (44).
Fig. 3.
Known occurrences of lycopsid microspore tetrads in P-Tr transition sequences. 1, East Greenland; 2, Sverdrup Basin, Arctic Canada (30); 3, Barents Sea (31); 4, Pechora Basin/Urals, Russia (32); 5, Russian Platform (33); 6, Southern Alps, Italy (34); 7, Transdanubian Mountains, Hungary (35); 8, Jungar Basin, North China (36); 9, Meishan, South China (37); 10, Raniganj Basin, India (38); 11, Auranga Basin, India (39); 12, Sri Lanka (40); 13, Mombasa Basin, Kenya (41) (paleogeography after ref. 42).
Significance of Mutant Tetrads
In most extant plants, unseparated tetrads occur only in early spore and pollen ontogeny, immediately after male meiosis. Permanent tetrads as functional dispersal units are presently produced by a limited number of bryophyte taxa and, more particularly, by representatives of a wide variety of angiosperm families. With a few exceptions, tetrads of angiosperm pollen are associated with insect pollination (45). Retention of mature spores and pollen in a facultative tetrad configuration has a genetic basis. Experiments have identified two genes that are required for microspore separation during normal development (21). In the quartet (qrt1, qrt2) mutants of Arabidopsis thaliana, lesions in either of these genes lead to defects of pectin degradation in the primary wall of the pollen mother cell, preventing separation of juvenile pollen grains from one another (22). Other mutants produce tetrads in combination with unusually large and malformed pollen (23).
Coupled to male sterility, generation of cytologically and morphologically aberrant pollen may occur systematically in near-extinct plant species (15, 16, 19). Similarly, the wide variability in Late Permian conifer pollen involves genera that did not outlive the end-Permian crisis (25). In marked contrast, P-Tr lycopsid tetrads were produced by stress-tolerant survivors that profited by the ecological crisis. Nevertheless, the functional biology of microspore structure renders it unlikely that a tetrad condition among extinct and extant lycopsids has any adaptive significance. The contact area of the four attached spores covers their triradiate germinal apertures, preformed for release of internally produced antherozoids. As a consequence, although not necessarily sterile, mutant lycopsid tetrads may be less effective or even ineffective in sexual reproduction. This deficiency should not be a disadvantage for those plants. Because of the presence of unisexual gametophytes, free-sporing heterospory cannot be regarded as an efficient, long-range dispersal strategy in water-limited environments. However, under unfavorable environmental conditions, heterosporous lycopsids frequently employ asexual, apomictic reproduction strategies that complement or replace the sexual life cycle (46). Apomixis is genetically directed. Dispersed megaspores that have the capacity to produce sporophyte plants without fertilization provide an effective route to species survival. In populations of modern Selaginella that grow in stressful environments, generation of permanent tetrads or other mutant microspores can be coupled to apomixis (47). By analogy, it is entirely possible that opportunistic P-Tr lycopsids have been able to achieve their otherwise inexplicably wide dispersal into drought-prone habitats through apomixis.
Causation of Global Environmental Mutagenesis
Abundances of lycopsid tetrads recorded from the P-Tr transition in India have been interpreted as a developmental response to regional climate change (38). However, the cosmopolitan nature of the bioevent demands globally operating stress factors. A variety of astrophysical radiation models illustrate the far-reaching repercussions of excessive cosmic or solar radiation on the biosphere. Notably, nearby supernova explosions and gamma-ray bursts are considered to be powerful events, producing lethal or mutagenic radiation doses that could contribute to mass extinction (48–50). Predicted exposure periods and resultant effects of radiation stress on plant and animal life might last for years, decades, or even centuries. But, in the context of the end-Permian ecological crisis, these durations would be far too short. This short-term effect also applies to UV-B pulses caused by asteroid or comet impacts (51).
If extraterrestrial causation is ruled out, a scenario of multimillennial pulses of environmental mutagenesis would invoke a less cataclysmic terrestrial vector. Among chemical contaminants that could have disrupted end-Permian biota, volcanogenic SO2 gas is favored to explain extinction. This gas rapidly converts into H2SO4 aerosols that could produce acid precipitation. Large-scale atmospheric acidification is generally attributed to extensive P-Tr volcanism in Siberia (3, 52, 53). Flood basalts, pyroclastic deposits, and intrusives that characterize the vast Siberian Traps igneous province have been traced over an area of 6–7 × 106 km2 from the Siberian Platform to the Ural Mountains (54–56). 40Ar/39Ar dating suggests that the main phase of volcanic activity was contemporaneous with the end-Permian crisis (52, 56). However, crisis scenarios that rely on adverse effects of acid precipitation suffer an obvious difficulty. Intercalated sediments in the lower part of the volcanic rock sequence (57, 58), as well as crater-lake deposits (59), contain leaf remains and spore/pollen assemblages that witness the presence of remarkably diverse floras coincident with the area of Siberian Traps volcanism.
Apart from producing acid rain, massive gas release associated with explosive volcanic eruptions causes severe disruption of the stratospheric ozone balance, resulting in elevated UV-B radiation. Volcanic H2SO4 aerosols do not directly deplete ozone but accelerate activation of chlorine (Cl) compounds that catalytically destroy ozone molecules (60, 61). Effects are short-lived. The most common Cl species in volcanic gases is HCl, but the fraction that reaches the stratosphere is small, due to rapid washout in the ascending volcanic plume (62, 63). By contrast, volcanogenic bromine (Br), although less abundant than chlorine, contributes to ozone depletion (63). Notwithstanding the possibility that explosive gas emissions related to the Siberian Traps volcanism have been unusual in frequency and intensity, any UV-B increase triggered by volcanic gases would still remain a short-term event.
Within multimillennial time frames, effective stratospheric Cl and Br buildup could result potentially from excessive emission of hydrothermal organohalogens. A wide variety of chlorinated and brominated hydrocarbons have been detected in fumaroles, sometimes in concentrations that are two orders of magnitude greater than average atmospheric background levels (64–66). Most abundant is CH3Cl (methyl chloride, chloromethane). Although frequently termed “volcanogenic,” proposed mechanisms for the synthesis of these noneruptive volatile organohalogens are still controversial. Thermodynamic equilibrium calculations do not favor “deep” formation at magma-chamber conditions (67). Similar to CH4 (methane) and other common hydrocarbon gases in hydrothermal systems (68), it is likely that the compounds are generated in the relatively shallow subsurface by thermal degradation of organic precursors buried in fossil soil or sediments, in the presence of appropriate halide-ion concentrations. Pyrolysis experiments with chloride-impregnated lignocellulosic plant material show that peak production of CH3Cl may proceed under temperatures between 240°C and 270°C (69).
Fluxes of organohalogens from modern hydrothermal systems are still unquantified but are constrained primarily by the size of the system, in combination with the availability of suitable source materials. Due to a modest total size, any effect of this source on global organohalogen budgets should be limited in comparison to other natural sources of CH3Cl and CH3Br (70). However, a completely different situation existed at the time of the end-Permian crisis. Over large areas of Siberia, up to 50% of the volume of the Siberian Traps igneous rocks consists of dolerite sills and other intrusives in the Neoproterozoic–Paleozoic sedimentary succession (71), many of which are synmagmatic with the main eruptive lava flows. It is conceivable that these intrusives have driven a giant hydrothermal system in which wide-spread coal-bearing deposits (72), Neoproterozoic-sourced oil accumulations (71), and salt layers (73) in the Paleozoic formed an unlimited source of suitable ingredients for the formation of hydrothermal organohalogens.
Extensive borehole information from the Siberian Platform and modeling indicate that thermal maturation of Carboniferous–Permian sedimentary organic matter was related to non-uniform heating (>150°C) by contact metamorphism from dolerite sills, rather than to the heat effect of deep burial (71). Due to dissolution by hydrothermal water, Cambrian salt layers were reduced considerably (73). We postulate, therefore, that the size of the Siberian Traps province and the emplacement of voluminous intrusives in the organic-rich and halogen-rich Paleozoic sediments furnished appropriate preconditions for significant generation of hydrothermal organohalogens. These volatiles could have been responsible for end-Permian pulses of stratospheric ozone destruction and resulting UV-B stress that contributed to worldwide ecosystem destabilization, ecosystem collapse, and extinction.
Concluding Remarks
In comparison with all other major ecological crises in Earth history, recovery from the end-Permian crisis proceeded exceptionally slowly (74). Reestablishment of woodland ecosystems took place eventually after ≈4 million years, at the end of the Early Triassic (29). On the Siberian Platform, the principal outcrop area of the Siberian Traps, radiometric dating implies an eruption time of a million years or less for the exposed volcanic sequence (75). The probability of rapid eruption is strengthened by paleomagnetic evidence indicating that the volcanics were largely laid down during a single normal-polarity interval (76). However, detection of at least three successive normal intervals in volcanic rocks encountered in deep exploration wells in the West Siberian Basin (76, 77) suggests that lengthy phases of degassing at hydrothermal fields may have continued to exist during the Early Triassic. Hence, resulting UV-B stress could have retarded the process of biotic recovery.
A coherent scenario explaining the end-Permian ecological crisis still needs to be established. Yet, our findings strongly suggest that mutagenesis in an enhanced UV-B radiation environment has played a critical role. To corroborate the concept, we need to recover additional morphological and chemical evidence of mutagenesis that is still preserved in the fossil record. Complementary to further analysis of pollen variability in P-Tr gymnosperms,** variation in structure and composition of leaf cuticles is another potential source of botanical evidence on mutational effects of environmental stress factors (78) affecting both victims and survivors of the crisis.
Acknowledgments
We thank Paul B. Wignall (University of Leeds, Leeds, U.K.) and Richard J. Twitchett (University of Plymouth, Plymouth, U.K.) for making available sample material from Greenland. We appreciate helpful discussion on the topic by participants in the Symposium Past Ecological Crises: a Phytocentric Perspective (Utrecht, 2002). We thank two anonymous reviewers for constructive comments. The study was in part supported by the Council for Earth and Life Sciences of the Netherlands Organization for Scientific Research. This paper is Netherlands Research School of Sedimentary Geology publication no. 20040402.
Abbreviation: P-Tr, Permian–Triassic.
See Commentary on page 12779.
Footnotes
Foster, C. B., Oral Presentation, 15th International Congress on Carboniferous and Permian Stratigraphy, Aug. 10–16, 2003, Utrecht, The Netherlands.
References
- 1.Erwin, D. H. (1993) The Great Paleozoic Crisis (Columbia Univ. Press, New York).
- 2.Looy, C. V., Twitchett, R. J., Dilcher, D. L., Van Konijnenburg-Van Cittert, J. H. A. & Visscher, H. (2001) Proc. Natl. Acad. Sci. USA 98, 7879–7883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Visscher, H., Brinkhuis, H., Dilcher, D. L., Elsik, W. C., Eshet, Y., Looy, C. V., Rampino, M. R. & Traverse, A. (1996) Proc. Natl. Acad. Sci. USA 93, 2155–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alpen, E. L. (1998) Radiation Biophysics (Academic, New York), 2nd Ed.
- 5.Taylor, G. E., Jr., Pitelka, L. F. & Clegg, M. T., eds. (1991) Ecological Genetics and Air Pollution (Springer-Verlag, Berlin).
- 6.Ries, G., Heller, W., Puchta, H., Sandermann, H., Seidlitz, H. K. & Hohn, B. (2000) Nature 406, 98–101. [DOI] [PubMed] [Google Scholar]
- 7.Higgins, K. & Lynch, M. (2000) Proc. Natl. Acad. Sci. USA 98, 2928–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Droz, M. & Pekalski, A. (2002) Phys. Rev. E 65, 051911. [DOI] [PubMed] [Google Scholar]
- 9.Schindewolf, O. H. (1954) Z. Deutsch. Geol. Ges. 105, 153–182. [Google Scholar]
- 10.Cockel, C. S. (1999) Paleobiology 25, 212–225. [Google Scholar]
- 11.Rozema, J., Van de Staaij, J., Björn, L. O. & Caldwell, M. (1997) Trends Ecol. Evol. 12, 22–28. [DOI] [PubMed] [Google Scholar]
- 12.Grant, W. F. (1998) Ecosyst. Health 4, 210–229. [Google Scholar]
- 13.Chen, Y. S. & McCormick, S. (1996) Development (Cambridge, U.K.) 122, 3243–3253. [DOI] [PubMed] [Google Scholar]
- 14.Park, S. K., Howden, R. & Twell, D. (1998) Development (Cambridge, U.K.) 125, 3789–3799. [DOI] [PubMed] [Google Scholar]
- 15.Pandit, M. K. & Babu, C. R. (2000) Bot. J. Linn. Soc. 133, 525–533. [Google Scholar]
- 16.El Maâtaoui, M. & Pichot, C. (2001) Planta 213, 543–549. [DOI] [PubMed] [Google Scholar]
- 17.Jackson, R. C., Skvarla, J. J. & Chissoe, W. F. (2000) Am. J. Bot. 87, 1571–1577. [PubMed] [Google Scholar]
- 18.Dreyer, L. L. & Van Wyk, A. E. (1998) Grana 37, 337–342. [Google Scholar]
- 19.Kimpton, S. K., James, E. A. & Drinnan, A. N. (2002) Aust. Syst. Bot. 15, 485–492. [Google Scholar]
- 20.Izhar, S. & Frankel, R. (1971) Theor. Appl. Genet. 41, 104–108. [DOI] [PubMed] [Google Scholar]
- 21.Preuss, D., Rhee, S. Y. & Davis, R. W. (1994) Science 264, 1458–1460. [DOI] [PubMed] [Google Scholar]
- 22.Rhee, S. Y. & Sommerville, C. R. (1998) Plant J. 15, 79–88. [DOI] [PubMed] [Google Scholar]
- 23.Soyano, T., Nishihama, R., Morikiyo, K., Ishikawa, M. & Machida, Y. (2003) Genes Dev. 17, 1055–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Potonié, R. & Schweitzer, H. J. (1960) Paläontol. Z. 34, 27–39. [Google Scholar]
- 25.Visscher, H. (1971) Geol. Surv. Ireland Spec. Pap. 1, 1–114. [Google Scholar]
- 26.Ward, P. D., Montgomery, D. R. & Smith, R. (2000) Science 289, 1740–1743. [DOI] [PubMed] [Google Scholar]
- 27.Michaelsen, P. (2002) Palaeogeogr. Palaeoclimatol. Palaeoecol. 179, 173–188. [Google Scholar]
- 28.Twitchett, R. J., Looy, C. V., Morante, R., Visscher, H. & Wignall, P. B. (2001) Geology 29, 351–354. [Google Scholar]
- 29.Looy, C. V., Brugman, W. A., Dilcher, D. L. & Visscher, H. (1999) Proc. Natl. Acad. Sci. USA 96, 13857–13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Utting, J. (1994) Geol. Surv. Can. Bull. 478, 1–107. [Google Scholar]
- 31.Mangerud, G. (1994) Rev. Palaeobot. Palynol. 82, 317–349. [Google Scholar]
- 32.Tuzhikova, V. I. (1985) Miospores and Stratigraphy of Reference Sections in the Triassic of the Urals (Akad. Nauk SSSR, Ural'sk Nauchn. Tsentr, Sverdlovsk).
- 33.Afonin, S. A. (2000) Palaeontol. J. 34, S29–S34. [Google Scholar]
- 34.Van de Schootbrugge, B. (1997) M.Sc. thesis (Utrecht University, Utrecht, The Netherlands).
- 35.Haas, J., Góczán, F., Oravecz-Scheffer, A., Barabás-Stuhl, A., Majoros, G. & Bérczi-Makk, A. (1986) Mem. Soc. Geol. It. 34, 221–241. [Google Scholar]
- 36.Oujang, S. & Norris, G. (1999) Rev. Palaeobot. Palynol. 106, 65–103. [Google Scholar]
- 37.Oujang, S. & Utting, J. (1990) Rev. Palaeobot. Palynol. 66, 1–56. [Google Scholar]
- 38.Tiwari, R. S. & Meena, K. L. (1988) Palaeobotanist 37, 210–214. [Google Scholar]
- 39.Banerji, J. & Maheswari, H. K. (1975) Palaeobotanist 22, 158–170. [Google Scholar]
- 40.Dahanayake, K., Jayasena, H. A. H., Singh, B. K., Tiwari, H. K. & Tripathi, A. (1989) Rev. Palaeobot. Palynol. 58, 197–203. [Google Scholar]
- 41.Hankel, O. (1992) Rev. Palaeobot. Palynol. 72, 129–147. [Google Scholar]
- 42.Scotese, C. R. (1997) Continental Drift (PALEOMAP Project, Arlington, TX), 7th Ed.
- 43.Lugardon, B., Grauvogel-Stamm, L. & Dobruskina, I. (1999) Compt. Rend. Acad. Sci. Ser. IIA 329, 435–442. [Google Scholar]
- 44.Tryon, A. F. & Lugardon, B. (1991) Spores of the Pteridophyta (Springer, New York).
- 45.Pacini, E. & Franchi, G. G. (1996) Acta Soc. Bot. Pol. 65, 11–16. [Google Scholar]
- 46.DiMichele, W. A., Davis, J. I. & Olmstead, R. G. (1989) Taxonomy 38, 1–11. [Google Scholar]
- 47.Graustein, J. E. (1930) Bot. Gaz. 90, 46–74. [Google Scholar]
- 48.Dar, A., Laor, A. & Shaviv, N. J. (1998) Phys. Rev. Lett. 80, 5813–5816. [Google Scholar]
- 49.Scalo, J. & Wheeler, J. C. (2002) Astrophys. J. 566, 723–737. [Google Scholar]
- 50.Scalo, J., Wheeler, J. C. & Williams, P. (2003) in Frontiers of Life: Proceedings XIIth Rencontres de Blois, eds. Celnikier, L. M. & Tran Thanh Van, J. (The Gioi Publishers, Hanoi, Vietnam), pp. 221–228.
- 51.Cockell, C. S. & Blaustein, A. R. (2000) Ecol. Lett. 3, 77–81. [Google Scholar]
- 52.Renne, P. R., Zichao, Z., Richards, M. A., Black, M. T. & Basu, A. R. (1995) Science 269, 1413–1416. [DOI] [PubMed] [Google Scholar]
- 53.Maruoka, T., Koeberl, C., Hancox, P. J. & Reimold, W. U. (2003) Earth Planet. Sci. Lett. 206, 101–117. [Google Scholar]
- 54.Al'Mukhamedov, A. I., Medvedev, A. Ya. & Kirda, N. P. (1999) Russ. Geol. Geophys. 40, 1550–1561. [Google Scholar]
- 55.Nikishin, A. M., Ziegler, P. A., Abbott, D., Brunet, M. F. & Cloetingh, S. (2002) Tectonophysics 351, 3–39. [Google Scholar]
- 56.Reichow, M. K., Saunders, A. D., White, R. V., Pringle, M. S., Al'Mukhamedov, A. I., Medvedev, A. I. & Kirda, N. P. (2002) Science 296, 1846–1849. [DOI] [PubMed] [Google Scholar]
- 57.Dobruskina, I. A. (1994) Österr. Akad. Wiss. Schriftenr. Erdwiss. Kom. 10, 1–422. [Google Scholar]
- 58.Krugovykh, V. V. (2002) Atlas of Spores and Pollen from the Permo-Triassic Volcanogenic Formations of the Tunguska Syneclise (Krasnoyarskgeolsyomka, Krasnoyarsk, Russia).
- 59.Naumov, V. A. & Luzina, I. V. (1997) Stratigr. Geol. Correlation 5, 319–333. [Google Scholar]
- 60.Solomon, S., Sanders, R. W., Garcia, R. R. & Keys, J. G. (1993) Nature 363, 245–248. [Google Scholar]
- 61.Prather, M. (1992) J. Geophys. Res. 97, 10187–10191. [Google Scholar]
- 62.Tabazadeh, A. & Turco, R. P. (1993) Science 260, 1082–1086. [DOI] [PubMed] [Google Scholar]
- 63.Bureau, H., Keppler, H. & Metrich, N. (2000) Earth Planet. Sci. Lett. 183, 51–60. [Google Scholar]
- 64.Isidorov, V. A. (1990) Organic Chemistry of the Earth's Atmosphere (Springer-Verlag, Berlin).
- 65.Isidorov, V. A., Zenkevich, I. G. & Ioffe, B. V. (1990) J. Atmos. Chem. 10, 329–340. [Google Scholar]
- 66.Jordan, A., Harnisch, J., Borchers, R., Le Guern, F. & Shinohara, H. (2000) Environ. Sci. Technol. 34, 1122–1124. [Google Scholar]
- 67.Symonds, R. B., Rose, W. I. & Reed, M. H. (1988) Nature 334, 415–418. [Google Scholar]
- 68.Darling, W. G. (1998) Appl. Geochem. 13, 815–824. [Google Scholar]
- 69.Conesa, J. A., Marcilla, A. & Caballero, J. A. (1997) J. Anal. Appl. Pyrol. 43, 59–69. [Google Scholar]
- 70.Butler, J. H. (2000) Nature 403, 260–261. [DOI] [PubMed] [Google Scholar]
- 71.Kontorovich, A. E., Khomenko, A. V., Burshtein, L. M., Likhanov, I. I., Pavlov, A. L., Staroseltsev, V. S. & Ten, A. A. (1997) Petrol. Geosci. 3, 359–369. [Google Scholar]
- 72.Czamanske, G. K., Gurevitch, A. B., Fedorenko, V. & Simonov, O. (1998) Int. Geol. Rev. 40, 95–115. [Google Scholar]
- 73.Melnikov, N. V., Khomenko, A. V., Kuznetsova, E. N. & Zhidkova, L. V. (1997) Russ. Geol. Geophys. 38, 1378–1384. [Google Scholar]
- 74.Erwin, D. H. (1998) Trends Ecol. Evol. 13, 344–349. [DOI] [PubMed] [Google Scholar]
- 75.Kamo, S. L., Czamanske, G. K., Amelin, Yu., Fedorenko, V. A., Davis, D. W. & Trofimov, V. R. (2003) Earth Planet. Sci. Lett. 214, 75–91. [Google Scholar]
- 76.Gurevitch, E. L., Heunemann, C., Rad'ko, V., Westphal, M., Bachtadse, V., Pozzi, J. P. & Feinberg H. (2004) Tectonophysics 379, 211–226. [Google Scholar]
- 77.Westphal, M., Gurevitch, E. L., Samsonov, B. V., Feiberg, H. & Pozzi, J. P. (1998) Geophys. J. Int. 134, 254–266. [Google Scholar]
- 78.Bird, S. M. & Gray, J. E. (2003) New Phytol. 157, 9–23. [DOI] [PubMed] [Google Scholar]