Abstract
Study Design
Retrospective study.
Purpose
To analyze outcomes of posterior lumbar interbody fusion (PLIF) stand-alone cages.
Overview of Literature
PLIF for degenerative disk disease using stand-alone cages has lost its popularity owing to implant-related complications and pseudoarthrosis.
Methods
We analyzed the records of 45 patients (18 women, 27 men), operated between January 1994 and December 1996, with a mean follow-up of 18 years 3 months (20 years 3 months–22 years 3 months). Clinical outcomes were measured using visual analogue score (VAS), Oswestry disability index (ODI), Odom's criteria, and radiological measurements of fusion rate, Cobb angle, and implant-related complications conducted at the preoperative evaluation, hospital discharge, 12-month follow-up, and final follow-up.
Results
Preoperative mean VAS (back) was 6.9 and VAS (radicular) was 7.2, with mean improvements (p <0.05) of 2.9 and 3.1, respectively, at the final follow-up. Median preoperative ODI was 64.5, with a mean improvement to 34 and 42 at the 12-month and final follow-ups, respectively (p <0.05). Odom's criteria at the 12-month follow-up were excellent in 11.2% patients, good in 57.7%, fair in 31.1%, and poor in none of the patients; at the final follow-up, no patient was classified as excellent, 71.1% as good, 22.2% as fair, and 6.7% as poor (p <0.05). Pseudoarthrosis was observed in five patients (11.1%), of whom, three (6.6%) required re-operation. Preoperative disk height was 9.23 mm, which increased to 13.33 mm in the immediate postoperative evaluation and was maintained at 10.0 mm at the final follow-up (p <0.05). The preoperative mean L1–S1 Cobb angle was 34.7°, which changed to 44.7° in the immediate postoperative evaluation and dropped to 39.7° at the final follow-up (p <0.005).
Conclusions
PLIF stand-alone cages were associated with good clinical outcomes. Although the fusion rate was excellent, maintenance of disk heights and a lordotic alignment were not achieved in the long term.
Keywords: Spinal fusion, Stand-alone implants, Degenerative intervertebral disks, Treatment outcome
Introduction
Posterior lumbar interbody fusion (PLIF) creates intervertebral fusion using a posterior approach [1]. This technique is useful in managing degenerative disk disease, severe instability, spondylolisthesis, deformity, and pseudoarthrosis. Fusion is performed at the level of the intervertebral joint, and the anterior column is reconstructed as the load-bearing structures are restored. The resulting arthrodesis is biomechanically superior to posterolateral fusion and creates a biomechanically superior environment for bone healing [2]. The advantage of PLIF over anterior lumbar interbody fusion is the avoidance of direct anterior approach, reducing the risk of complications related to the vascular, abdominal, and reproductive systems [3,4] and with the added benefit of being less expensive [5]. PLIF is used to manage various spinal pathologies, including degenerative disk disease, severe instability, spondylolisthesis, deformity, and trauma. Acceptable indications for PLIF include degenerative scoliosis, wherein neural decompression and correction of the deformity or severe spinal instability are desired from an all-posterior approach, high-grade spondylolisthesis, and failure of posterolateral fusion (pseudoarthrosis) [6]. Relative indications for PLIF include treatment of debilitating low back pain caused by degenerative disk disease, a high degree of spinal instability, low-grade spondylolisthesis, and patients at a high risk of nonunion (e.g., obese individuals or smokers) [2,7,8,9]. Numerous interbody reconstruction options are available, including allograft (i.e., bone dowel or iliac crest), threaded cages (e.g., Bagby and Kuslich cage) [10,11], titanium mesh cages, polymeric rectangular cages (e.g., Brantigan cage), autograft (i.e., tricortical iliac crest), recombinant human bone morphogenetic protein-2, bioabsorbable materials, and ceramics. The primary goals of using interbody devices are to create immediate and permanent stability, circumvent bone graft resorption, maintain deformity correction, and decrease morbidity of autologous bone graft harvest. The surgical technique for PLIF is continuously being modified and refined [12]. Modifications include the addition of pedicle screw instrumentation, use of engineered interbody devices, and use of supplemental posterolateral bone grafting to add stability and increase fusion rates.
The objectives of this study were to analyze the outcomes and complications of stand-alone PLIF cages with the assessment of fusion rate, segmental lordosis, implant subsidence, adjacent disk degeneration, re-operations, and correlating these with the clinical course.
Materials and Methods
Retrospective analyses of a group of 79 patients with lumbosacral degenerative disk disease at one or two levels who were treated between January 1994 and December 1996 were performed. All the procedures were according to Declaration of Helsinki and were approved by the ethics committee, and written informed consent was obtained from all participants. Complete follow-up data were available for 45 patients (57%). Mean follow-up is 20 year and 3 months (range 18 years and 3 months to 22 years and 3 months). Overall, 27 patients (60%) underwent surgery during 1994, 6 (13.3%) during 1995, and 12 (26.7%) during 1996. Distribution according to sex was as follows: 18 women (40%) and 27 men (60%).
1. Surgical technique
The classic PLIF technique was performed through a wide laminotomy, with resection of the ligamentum flavum and partial or total removal of the cranial lamina. The lower one-third of the inferior facet and the medial two-thirds of the superior facets were resected to expose the pedicle of the vertebra as laterally as possible. This wide opening into the spinal canal minimizes the amount of neural retraction required. The traverse nerve root and dural sac were retracted medially. Particular attention was paid toward identifying and protecting the true axilla of the upper nerve root. Failure to identify it may result in undue tension, which can cause neurological damage. Retraction of the traversing nerve root and dural sac will aid in visualizing the widest possible field for disk resection. Subsequently, the disk and osteophytes were resected as anteriorly and laterally as possible; this aids in neural decompression as well as disk space visualization. Disk space augmentation was accomplished using disk distractors, which were designed so that the implant or bone graft could be inserted adjacent to these. The cartilaginous end plates were then removed with specially designed shavers and curettes. The bony end plates were preserved to help prevent implant subsidence.
In all cases, Ray Threaded Cage was selected as the implant for PLIF fusion (Fig. 1). This interbody device is a threaded hollow cylinder with fenestrations for bone-to-bone contact and promotes spinal fusion. All cages were filled with an autologous bone graft from the iliac crest, obtained through the same skin incision. The implant with bone graft was then posteriorly inserted, and supplemental bone graft could be packed medial to the spacer; no posterior screws were used. All patients underwent surgery according to the aforementioned surgical technique and kept in semirigid lumbosacral orthosis for 10 weeks.
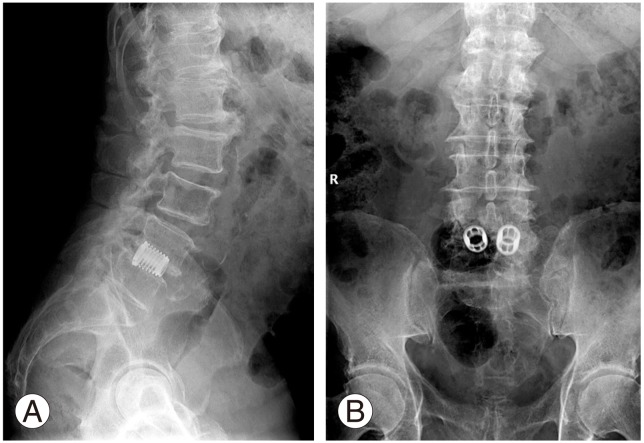
Fig. 1. Postoperative lateral (A) and anteroposterior (B) radiographs at 19-year follow-up. Follow-up of a 72-year-old female patient following L4–L5 posterior lumbar interbody fusion for degenerative lumbar spondylolisthesis. Postoperative standard radiographs show good lumbar lordosis as well as fusion without cage subsidence.
2. Clinical evaluation
Clinical outcomes were measured using visual analogue score (VAS) for both back and radicular pain at preoperative evaluation, hospital discharge, 12-month follow-up, and final follow-up. Oswestry disability index (ODI) and satisfaction questionnaire [13] were conducted at the final follow-up.
3. Radiological evaluation
Disk height, defined as the average of the anterior, middle, and posterior height on standing lateral radiographs. All measurements were performed on radiographs recorded on preoperative and postoperative evaluation as well as final follow-up. Fusion criteria were based on <1° of motion in dynamic (flexion-extension) radiographs, absence of halo around the implant, or bone bridge formation. At the final follow-up, degenerative signs (anterior–posterior osteophytes, subchondral sclerosis, and/or facet joint degeneration) were evaluated both in the cranial and caudal disks using plain radiographs.
4. Statistical analysis
Measurements among preoperative and postoperative evaluation and final follow-up were compared using Student's t-test for related samples. Correlations were made using Pearson's correlation test. All variables were analyzed using the SPSS ver. 20.0 (IBM Corp., Armonk, NY, USA). Statistical significance was defined as a p-value of <0.05.
Results
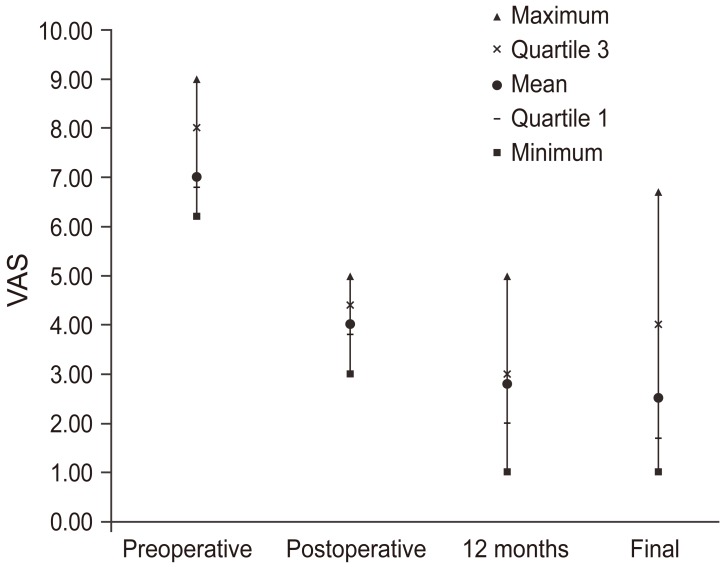
Preoperative mean VAS (back) was 7 (range, 6.2–9) and mean VAS (radicular) was 7.2 (range, 6.7–7.5). Postoperative (before discharge) values showed clinical improvements in VAS (back), with a mean value of 4 (range, 3–5), and VAS (radicular), with a mean value of 3.5 (range, 3.1–3.7). At the 12-month follow-up, mean VAS (back) was 2.8 (range, 1–5) and mean VAS (radicular) was 2.3 (range, 1.4–2.7), showing statistical significance (p<0.05). At the final follow-up, mean VAS (back) was 2.5 (range, 1–6.7) and mean VAS (radicular) was 3.1 (range, 1.9–3.5), being statistically significant compared with preoperative and immediate postoperative values (Fig. 2).
Fig. 2. Visual analog scale (VAS) (back) assessment at preoperative and postoperative follow-ups.
The mean operation time was 88 minutes (range, 73–121 minutes), with a mean blood loss of 285 mL. Postoperative hospital stay was 4.9 days (range, 3–9 days). Level distribution of disk disease was L3–L4 in 2 patients, L4–L5 in 24 patients, L5–S1 in 16 patients, and L4–L5–S1 in 3 patients.
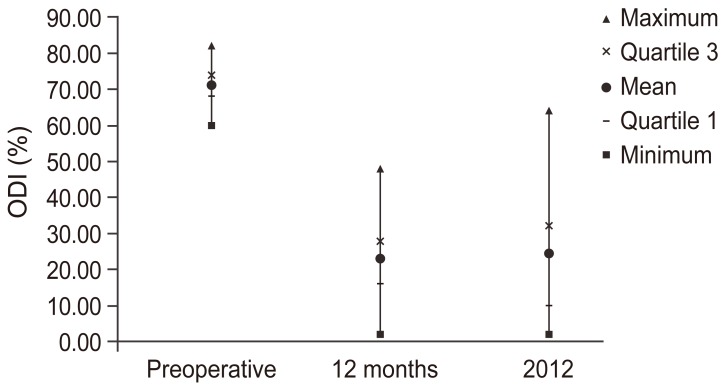
Median preoperative ODI was 70.8 (range, 60–82), with an improvement to a mean of 48.08 at the 12-month follow-up. ODI at the final follow-up was 24.16 (range, 2–64), with statistically significant differences among preoperative evaluation and 12-month and final follow-ups (p<0.05). No statistically significant differences were observed between the 12-month and final follow-ups (Fig. 3).
Fig. 3. Oswestry disability index (ODI) scores during preoperative and postoperative follow-ups.
According to Odom's criteria, in terms of outcomes at the 12-month follow-up, 5 (11.2%) patients were classified as excellent, 26 (57.7%) as good, 14 (31.1%) as fair, and none of the patients were classified as poor. Similarly, at the final follow-up, no patients were classified as excellent, 32 (71.1%) as good, 10 (22.2%) as fair, and 3 (6.7%) as poor. Differences between results of both follow-ups were statistically significant (p<0.05).
Satisfaction scale according to the final follow-up had a median value of 1.76 (1.10–4.40) and did not have a statistically significant correlation with the level of surgery (p>0.05), sex (p>0.05), age at the time of surgery (p>0.05), disk height (p=0.279), or sacral slope (p=0.06), although there was a positive correlation with lumbar lordosis at the final follow-up (p=0.03).
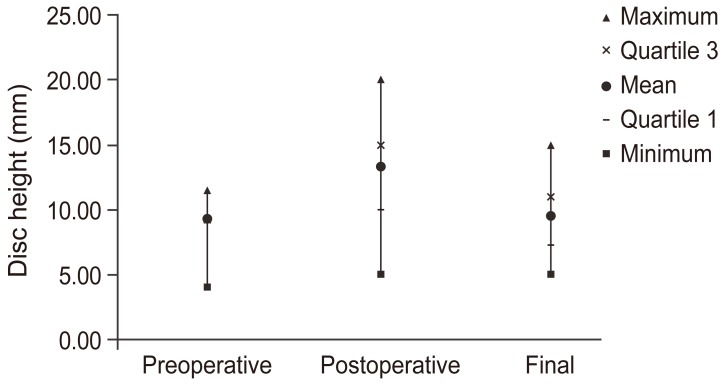
Fusion rates revealed that 5 (11.1%) patients presented with radiological pseudoarthrosis, although only 3 (6.6%) of them required another surgery to add posterior screw fixation. Preoperative disk height was 9.23 mm (range, 4–11.5 mm), which increased to 13.33 mm (range, 5–20 mm) in the immediate postoperative evaluation (Fig. 4). At the final follow-up, disk height was 9.5 mm (range, 5–15 mm). The height increase between preoperative and immediate postoperative evaluation was statistically significant (p<0.05) and so was the height loss between the immediate postoperative evaluation and final follow-up (p<0.05). The mean L1–S1 Cobb angle before surgery was 34.7° (range, 19.5°–55°), which increased to 44.67° (range, 21.3°–68°) in the immediate postoperative evaluation; however, at the final follow-up, the corrected lordosis was not maintained and was 39.73° (range, 8°–62°). The Cobb angle modification was statistically significant (p<0.005). Surgery-related complications of PLIF in our cohort were observed in 3 patients (6.6%): 1 (2.2%) case of dura tear occurred during the procedure, and 2 (4.4%) cases of postoperative radicular pain that was controlled within 6 weeks in both cases.
Fig. 4. Results of disk height measurements (mm) during preoperative and postoperative follow-ups.
Discussion
During the past decade, there has been a debate on the outcomes of stand-alone threaded cages for PLIF in the management of degenerative lumbar spine. The quality of surgical outcomes is usually provided by long-term studies, however, available literature is deficient of articles that provide evaluations from >5 years of monitoring. Certain clinical and radiological papers have reported favorable results with the use of stand-alone threaded cages for PLIF [10,14]. A multicenter clinical study of PLIF with stand-alone expandable cages (Tyche cage) for degenerative lumbar spinal disorders was conducted [11]. Four-year follow-up results of lumbar spine arthrodesis treated using the Bagby and Kuslich lumbar fusion cage were reported [15,16,17]. Nevertheless, several studies have reported a lack of efficacy and poor radiological outcomes with long-term follow-up [18,19].
Although the radiological fusion rate was 88.9% (with even lesser proportion of patients who underwent secondary surgery, 6.6%), within the range of series with posterior screws [20] or with series of stand-alone PLIF, the reported fusion rates were close to 100% [14,21]. In our study, only 5 patients (6.3%) exhibited radiological pseudoarthrosis. The overall functional improvement, according to ODI, decreased from the 12-month follow-up to the final follow-up, although it showed no correlation to the fusion rate.
Although this functional improvement appears in the range of stand-alone PLIF and 360° fusion procedures [5], there could be several reasons for this finding. The first reason is that the fusion criteria could be higher for this type of technique; therefore, all patients underwent 1-mm computed tomographic scan to check whether there was bone density inside the cage or any radiolucency between the implant and vertebral body. There were no changes in the fusion ratio [22,23]. The second reason could be that we found a correlation between the functional success and maintenance of the lumbar sagittal balance [24], which deteriorated along the follow-up by an average of 5°. This positive correlation is similar to that reported in certain recently published articles, which indicated the role of sagittal balance in clinical results.
In addition, patient satisfaction was evaluated at the final follow-up [1], and surprisingly, owing to VAS evolution, functional and radiological results showed excellent results, satisfaction scores were better in the group with lordotic control, and overall satisfaction results were positive. At the final follow-up, back and radicular pain scores were significantly lower compared with preoperative and immediate postoperative values.
In general, the success rates of PLIF and transforaminal lumbar interbody fusion (TLIF) procedures are high, with reported arthrodesis rates between 77% and 100% [25].
The most common complications associated with PLIF and TLIF are intraoperative neurological injury, interbody implant or bone graft migration, dural tear, infection, heterotopic ossification, postoperative radiculopathy, osteolysis, and subsidence. The overall reported complication rates of PLIF and TLIF range between 8% and 80% [26,27]. However, these percentages do not include potential pseudoarthrosis following attempted interbody fusion [19]. In addition, neural foraminal distraction may occur during PLIF, which can result in nerve root injury.
Moreover, consistent with a previous study, our study results suggested that the use of stand-alone cages is unsuccessful in controlling segmental lumbar lordosis [28]. Furthermore, because of this, functional results of segmental lumbar lordosis deteriorate with subsequent follow-ups. After a mean follow-up of 18 years 3 months, patient satisfaction was quite high, the number of new surgeries remained in the lower range (3 patients), and the most important conclusion was the need to restore and maintain the lordosis and sagittal balance to successfully maintain spine functionality.
Conclusions
Our study demonstrated that single, stand-alone PLIF with threaded cages is acceptable with good long-term outcomes, but it does not support maintenance of lumbar lordosis.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Park JH, Roh SW. Long-term clinical and radiological outcomes following stand-alone PLIF surgery using expandable cylindrical threaded cages in patients with degenerative lumbar spine disease. Acta Neurochir (Wien) 2011;153:1409–1416. doi: 10.1007/s00701-011-1044-z. [DOI] [PubMed] [Google Scholar]
- 2.Oxland TR, Grant JP, Dvorak MF, Fisher CG. Effects of endplate removal on the structural properties of the lower lumbar vertebral bodies. Spine (Phila Pa 1976) 2003;28:771–777. [PubMed] [Google Scholar]
- 3.DiPaola CP, Molinari RW. Posterior lumbar interbody fusion. J Am Acad Orthop Surg. 2008;16:130–139. doi: 10.5435/00124635-200803000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Faundez AA, Schwender JD, Safriel Y, et al. Clinical and radiological outcome of anterior-posterior fusion versus transforaminal lumbar interbody fusion for symptomatic disc degeneration: a retrospective comparative study of 133 patients. Eur Spine J. 2009;18:203–211. doi: 10.1007/s00586-008-0845-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitecloud TS, 3rd, Roesch WW, Ricciardi JE. Transforaminal interbody fusion versus anterior-posterior interbody fusion of the lumbar spine: a financial analysis. J Spinal Disord. 2001;14:100–103. doi: 10.1097/00002517-200104000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Christensen FB, Hansen ES, Eiskjaer SP, et al. Circumferential lumbar spinal fusion with Brantigan cage versus posterolateral fusion with titanium Cotrel-Dubousset instrumentation: a prospective, randomized clinical study of 146 patients. Spine (Phila Pa 1976) 2002;27:2674–2683. doi: 10.1097/00007632-200212010-00006. [DOI] [PubMed] [Google Scholar]
- 7.Dehoux E, Fourati E, Madi K, Reddy B, Segal P. Posterolateral versus interbody fusion in isthmic spondylolisthesis: functional results in 52 cases with a minimum follow-up of 6 years. Acta Orthop Belg. 2004;70:578–582. [PubMed] [Google Scholar]
- 8.McAfee PC, DeVine JG, Chaput CD, et al. The indications for interbody fusion cages in the treatment of spondylolisthesis: analysis of 120 cases. Spine (Phila Pa 1976) 2005;30(6 Suppl):S60–S65. doi: 10.1097/01.brs.0000155578.62680.dd. [DOI] [PubMed] [Google Scholar]
- 9.Molinari RW, Sloboda JF, Arrington EC. Low-grade isthmic spondylolisthesis treated with instrumented posterior lumbar interbody fusion in U.S. servicemen. J Spinal Disord Tech. 2005;18(Suppl):S24–S29. doi: 10.1097/01.bsd.0000140197.07619.8b. [DOI] [PubMed] [Google Scholar]
- 10.Bagby GW. Arthrodesis by the distraction-compression method using a stainless steel implant. Orthopedics. 1988;11:931–934. doi: 10.3928/0147-7447-19880601-13. [DOI] [PubMed] [Google Scholar]
- 11.Kuslich SD, Danielson G, Dowdle JD, et al. Four-year follow-up results of lumbar spine arthrodesis using the Bagby and Kuslich lumbar fusion cage. Spine (Phila Pa 1976) 2000;25:2656–2662. doi: 10.1097/00007632-200010150-00018. [DOI] [PubMed] [Google Scholar]
- 12.Lazennec JY, del Vecchio R, Ramare S, Mora N, Pouzet B, Saillant G. Revision and simplification of the minimal anterior approach of the lumbar spine. Rev Esp Cir Ortop Traumatol. 2001;45:195–205. [Google Scholar]
- 13.Ayerbe-Gracia J, Sousa-Casasnovas P. Outcome assessment in lumbar spine surgery: the patient's perspective. Neurocirugia (Astur) 2004;15:447–457. [PubMed] [Google Scholar]
- 14.Kim KH, Park JY, Chin DK. Fusion criteria for posterior lumbar interbody fusion with intervertebral cages : the significance of traction spur. J Korean Neurosurg Soc. 2009;46:328–332. doi: 10.3340/jkns.2009.46.4.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ray CD. Threaded titanium cages for lumbar interbody fusions. Spine (Phila Pa 1976) 1997;22:667–679. doi: 10.1097/00007632-199703150-00019. [DOI] [PubMed] [Google Scholar]
- 16.McAfee PC. Interbody fusion cages in reconstructive operations on the spine. J Bone Joint Surg Am. 1999;81:859–880. doi: 10.2106/00004623-199906000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Matge G, Leclercq TA. Rationale for interbody fusion with threaded titanium cages at cervical and lumbar levels. Results on 357 cases. Acta Neurochir (Wien) 2000;142:425–423. doi: 10.1007/s007010050453. [DOI] [PubMed] [Google Scholar]
- 18.Newman MH, Grinstead GL. Anterior lumbar interbody fusion for internal disc disruption. Spine (Phila Pa 1976) 1992;17:831–833. doi: 10.1097/00007632-199207000-00017. [DOI] [PubMed] [Google Scholar]
- 19.Oxland TR, Lund T. Biomechanics of stand-alone cages and cages in combination with posterior fixation: a literature review. Eur Spine J. 2000;9(Suppl 1):S95–S101. doi: 10.1007/PL00010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chrastil J, Patel AA. Complications associated with posterior and transforaminal lumbar interbody fusion. J Am Acad Orthop Surg. 2012;20:283–291. doi: 10.5435/JAAOS-20-05-283. [DOI] [PubMed] [Google Scholar]
- 21.Fuji T, Oda T, Kato Y, Fujita S, Tanaka M. Posterior lumbar interbody fusion using titanium cylindrical threaded cages: is optimal interbody fusion possible without other instrumentation? J Orthop Sci. 2003;8:142–147. doi: 10.1007/s007760300024. [DOI] [PubMed] [Google Scholar]
- 22.Kant AP, Daum WJ, Dean SM, Uchida T. Evaluation of lumbar spine fusion: plain radiographs versus direct surgical exploration and observation. Spine (Phila Pa 1976) 1995;20:2313–2317. [PubMed] [Google Scholar]
- 23.Cassinelli EH, Wallach C, Hanscom B, Vogt M, Kang JD. Prospective clinical outcomes of revision fusion surgery in patients with pseudarthrosis after posterior lumbar interbody fusions using stand-alone metallic cages. Spine J. 2006;6:428–434. doi: 10.1016/j.spinee.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Sears W. Posterior lumbar interbody fusion for degenerative spondylolisthesis: restoration of sagittal balance using insert-and-rotate interbody spacers. Spine J. 2005;5:170–179. doi: 10.1016/j.spinee.2004.05.257. [DOI] [PubMed] [Google Scholar]
- 25.Rihn JA, Patel R, Makda J, et al. Complications associated with single-level transforaminal lumbar interbody fusion. Spine J. 2009;9:623–629. doi: 10.1016/j.spinee.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Chen Z, Zhao J, Liu A, Yuan J, Li Z. Surgical treatment of recurrent lumbar disc herniation by transforaminal lumbar interbody fusion. Int Orthop. 2009;33:197–201. doi: 10.1007/s00264-008-0531-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng CW, Yue WM, Poh SY, Yeo W, Tan SB. Clinical and radiological outcomes of minimally invasive versus open transforaminal lumbar interbody fusion. Spine (Phila Pa 1976) 2009;34:1385–1389. doi: 10.1097/BRS.0b013e3181a4e3be. [DOI] [PubMed] [Google Scholar]
- 28.Klemme WR, Owens BD, Dhawan A, Zeidman S, Polly DW., Jr Lumbar sagittal contour after posterior interbody fusion: threaded devices alone versus vertical cages plus posterior instrumentation. Spine (Phila Pa 1976) 2001;26:534–537. doi: 10.1097/00007632-200103010-00017. [DOI] [PubMed] [Google Scholar]