Abstract
Objective
To evaluate the importance of Lynch syndrome associated risk screening in the patients aged less than 50 years affected from endometrial cancer.
Methods
From 2007 to 2014, 41 patients affected from endometrial cancer and aged less than 50 years underwent surgery at the Complex Operative Unit of Gynecology and Obstetrics, Cannizzaro Hospital of Catania, Italy. They were selected to undergo mismatch repair gene mutation analysis using immunohistochemistry (IHC; four markers: MLH1, MSH2, MSH6, PMS2) and microsatellite instability (MSI) test. For samples that resulted negative to IHC (abnormal finding), MSI test was performed to further study the suspected mutation. Samples were classified as MSI-high (MSI-H) if more than one marker was identified as unstable; MSI-low (MSI-L) if only one marker was identified as unstable; or MSI-stable (MSI-S) if no marker was identified as unstable. Samples were subdivided into two groups: MSI-H/L and MSI-S. Statistical analysis was performed to assess differences regarding survival, tumor staging, grading, and invasion of lymphovascular space between these two groups.
Results
IHC analysis showed that in 46% (19/41) of samples there was negative outcome. Forty-two percent (8/19) of these negative samples were unstable (either low or high). Of eight patients showing MSI, 75% were MSI-L, while 25% were MSI-H. Differences in survival, stage, grade, lymphovascular space invasion and Amsterdam criteria adherence were not statistically significant due to the small size of the cohort.
Conclusion
IHC and MSI test results of our cohort lead us to assess the relevance of performing Lynch syndrome genetic screening in endometrial cancer patients aged less than 50 years at the time of diagnosis.
Keywords: Endometrial Neoplasms, Genetic Testing, Immunohistochemistry, Lynch Syndrome, Young Patients
INTRODUCTION
Tumor development can be regarded as a series of individual changes, induced by oncogenes, tumor suppressors, and DNA repair genes [1] with multifactorial etiology [2]. Lynch syndrome (LS) is an autosomal dominant inherited disease characterized by DNA repair gene mutation that determines the predisposition to develop colorectal (type I) and/or other types of cancer (type II) [3].
LS is caused by mutations in mismatch repair (MMR) genes (MLH1, MSH2, MSH6, PMS2), which encode proteins involved in the identification and repair of DNA mismatch errors. MMR gene mutations determine an ‘error replication’ phenotype in the cell, called microsatellite instability (MSI) [4], that is more frequently observed following a germinal mutation with autosomal dominant inheritance, but may also result from a somatic mutation [5]. Women carrying MMR gene mutations have a lifetime risk of developing hereditary nonpolyposis colorectal cancer (HNPCC), endometrial cancer, and ovarian cancer of 80%, 60%, and 12%, respectively [6].
The American Society of Clinical Oncology has recently presented new evidence-based guidelines that emphasize the need to use immunohistochemistry (IHC) methods while searching for MMR gene mutations in patients with primary or metastatic colon cancer [7]. In several countries the genetic test with IHC analysis for the diagnosis of MSI is already performed in endometrial cancer patients aged less than 50 years old to identify potential LS patients [8]. In fact, for LS-related endometrial cancer patients, the 10- and 15-year risk of developing a second cancer is 25% and 50%, respectively. Hence, LS-related endometrial cancer can be regarded as a “sentinel tumor” for the patients themselves and their at-risk family members [9].
MSI, which occurs in 15% to 25% of cases of sporadic endometrial cancer [10], may derive from two molecular events: first, MLH1 hypermethylation (common event in sporadic endometrial cancer) with following gene inactivation through an epigenetic mechanism, or in the second event, a germinal mutation occurring in one or more MMR genes [11]. Clinically, LS-related endometrial cancer may be classified as either type I or type II [12] and occurs in younger patients than endometrial cancer with no MMR gene mutations. Indeed, the mean age of patients at diagnosis is 49 years among MMR mutation carriers and 60 years among the general population [13].
When there is clinical suspicion of the presence of MMR mutations, LS should be confirmed by searching for DNA germinal mutations, performing genetic counselling, and searching for tissue somatic mutations. IHC analysis specific for MMR proteins, MSI analysis and MLH1 methylation status have been used to identify individuals at risk of developing LS.
In 1991, an international group of researchers proposed the Amsterdam criteria I to define the population of patients that should be monitored for LS. These criteria have since been updated in 1999 to include extracolonic tumors [14]: One affected person is a first-degree relative of the other two, at least two successive generations are affected, at least one person was diagnosed before the age of 50 years, and familial adenomatous polyposis has been excluded. Unfortunately, around 50% of families that show positive Amsterdam criteria do not present tumor MSI, and are classified as MSI-stable (MSI-S) following MSI analysis. The LS definition applies only to MMR germinal mutation carriers [15]. Family history-based screening (Amsterdam and Bethesda criteria, predictive models) selects patients whose tissue should be screened to identify patients that need to undergo genetic testing (more difficult and costly to perform).
MMR predict is a predictive test with best performance (sensitivity 94% and specificity 91%). Indeed, it has been demonstrated that the use of MMR predict reduces the percentage of patients that would benefit from genetic analysis from 50% to 11%, in comparison with the assessment with Bethesda criteria, resulting in a notable reduction of cost and time during diagnosis.
Tissue molecular screening tests search for two characteristics of LS that result from MMR gene mutations using simple and low cost methods: MSI analysis and IHC assays specific for the protein that is encoded by the mutated gene and whose expression may have been lost [16]. Results from a recent study [17] demonstrate the utility of using IHC and MSI methods for LS diagnosis in patients at intermediate risk of developing colorectal cancer (CRC) as well. Of all CRC patients screened, 3.8% were MMR gene mutation carriers; all tumors of MMR gene mutation carriers were MSI-high (MSI-H; with MSI higher than 30%); and, in 94% of cases, IHC analysis was able to identify correctly the mutated gene. Only patients whose results from molecular analysis indicate LS (MSI-H and abnormal MMR protein expression on tissue) should undergo genetic testing, with considerable reduction of cost and time during diagnosis.
Gynecological standardized monitoring is nowadays recommended by international guidelines [18] and includes gynecological examination, transvaginal ultrasound, and endometrial biopsy, even if the efficacy of monitoring on the improvement of survival has not yet been demonstrated [19]. The development of a diagnostic-therapeutic-care program for endometrial cancer patients carrying MMR gene mutations derives from the need to standardize current diagnostic, therapeutic and care criteria available to clinicians using a multidisciplinary approach.
The aim of our study is to evaluate the importance of LS associated risk screening in the patients aged less than 50 years affected from endometrial cancer.
MATERIALS AND METHODS
From 2007 to 2014, 430 women with endometrial cancer were referred to the Complex Operative Unit of Gynecology and Obstetrics, Cannizzaro Hospital (Catania, Italy) where they underwent surgery. Patients aged less than 50 years were selected at diagnosis to undergo MMR gene mutation analysis using IHC and MSI test. According to the literature, the incidence of MMR gene mutation is higher in this age group.
Forty-one patients (9.5%) were enrolled to this study. Family history, clinical data, surgical procedure, adjuvant treatment, and follow-up were collected for each patient and Amsterdam criteria were applied. A patient was considered positive for the Amsterdam criteria if she matched all criteria. Patients were grouped by histotype, grade, and stage, using International Federation of Gynecology and Obstetrics (FIGO) 2014 staging as reference.
MMR protein qualitative analysis was performed by IHC using Novocastra (Leica Biosystem, Wetzlar, Germany) monoclonal murine liquid primary antibodies specific for MLH1, MSH2, MSH6, and PMS2 proteins (bond ready-to-use kit, Leica Biosystem). IHC allows to detect antigens by a three-step sequential process: antigen-specific antibody (primary antibody), secondary antibody that binds the primary antibody, and an enzyme complex with a chromogen substrate. The enzymatic activation of the chromogen produces a reaction visible at the optical microscope in correspondence of the antigenic site. In the presence of a normally expressed protein, the antibody-chromogen complex binds to the substrate and the test’s outcome is considered positive (MMR protein present, normal finding), while the non-visualization of the chromogen indicates no protein expression, and the test’s outcome is considered negative (MMR protein absent, abnormal finding). IHC was considered negative if there was negative outcome (no expression) for at least one of the four markers (MLH1, MSH2, MSH6, and PMS2).
DNA was extracted from formalin-fixed, paraffin-embedded tissue and amplified using polymerase chain reaction. DNA quantification was done with a Qubit 2.0 fluorometer (Thermofisher, Waltham, MA, USA). MSI analysis was performed on DNA from paraffin-embedded normal (control) and tumor tissues. Differences between tumor and normal tissue DNA electrophoretic profiles from the same patient indicate MSI. In the present study, we analyzed MSI by searching mononucleotide repetitions (BAT25, BAT26, NR27), using the QIAxcel system (Qiagen, Venlo, the Netherlands) to analyze MSI [20]. For samples that resulted negative to IHC, MSI test was performed to further study the suspected mutation.
Samples were classified as MSI-H, if more than one marker was identified as unstable; MSI-low (MSI-L), if only one marker was identified as unstable; or MSI-S, if no MSI marker was identified as unstable. Based on MSI test, samples were subdivided in two groups: MSI-H/L and MSI-S. Statistical analysis was performed to assess differences regarding tumor staging, grading, invasion of lymphovascular space, and presence of synchronous ovarian cancer.
All the patients signed informed consent after Institutional Review Board of Cannizzaro Hospital approval.
RESULTS
All patients enrolled in this study were treated for endometrial cancer and were aged less than 50 years at the time of diagnosis. Mean age was 44.4 years old (range, 32 to 50 years old). Tumors were classified as endometrioid (type I) in 96% of cases (39/41), papillary serous in 2% (1/41), and undifferentiated in 2% (1/41).
IHC analysis on the four markers (MLH1, MSH2, MSH6, PMS2) showed that in 46% of samples (19/41) there was negative outcome (no expression) for at least one marker, indicating a possible MMR gene mutation (Fig. 1). Eight percent of samples (3/41) showed dubious/weak protein expression, while for 46% (19/41) of samples, all proteins were expressed (positive outcome, normal expression).
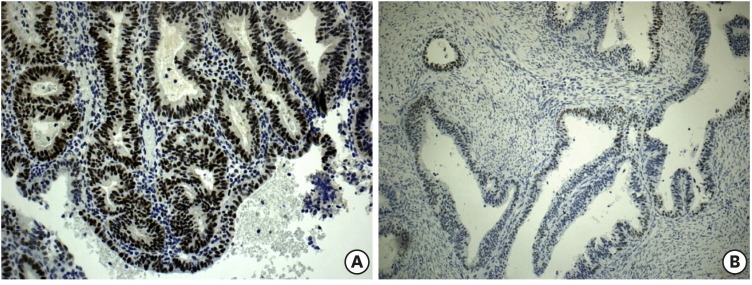
Fig. 1.
(A) Positivity of MLH1 protein and (B) weak positivity of MSH-2 protein. (×400)
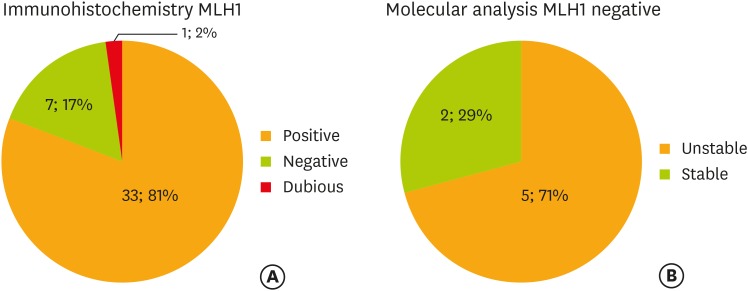
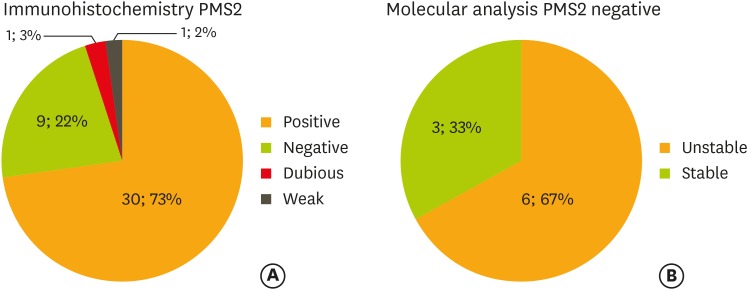
For samples that resulted negative to IHC (46%), DNA analysis was performed to further study the suspected mutation. Forty-two percent of the analyzed samples (8/19) showed MSI (either MSI-L or MSI-H), while the remaining 58% of samples (11/19) were stable. MLH1 and PMS2 were the most frequent markers with abnormal expression on IHC that were subsequently confirmed as unstable during MSI test (Figs. 2, 3).
Fig. 2.
(A) Immunohistochemistry and (B) molecular analysis on MLH1.
Fig. 3.
Immunohistochemistry (A) and molecular analysis (B) on PMS2.
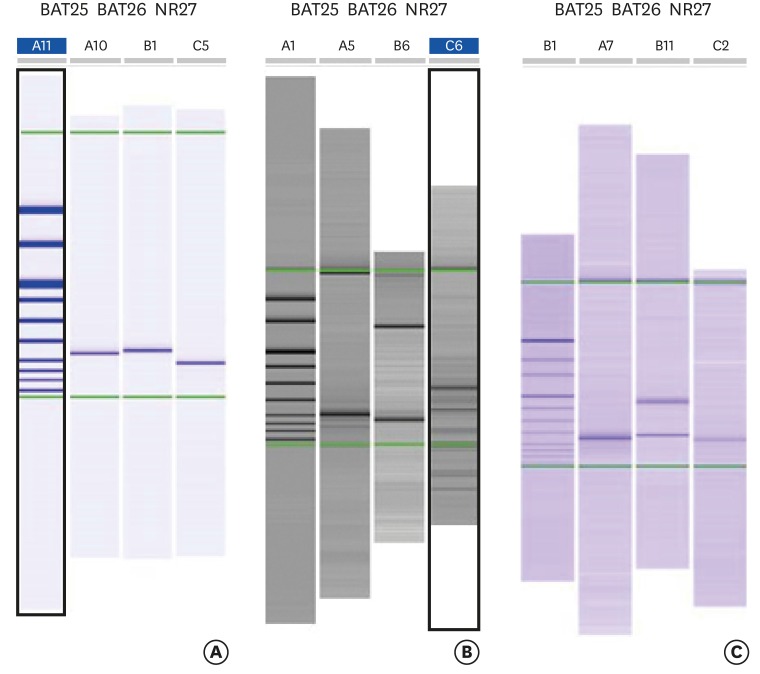
MSI test showed that 22% of endometrial cancer patients aged 50 years or less presented MSI with BAT25, BAT26, and NR27 repetitions (presumptive LS), in accordance with literature findings (Fig. 4).
Fig. 4.
(A) Single bands indicate no instability for the three markers analyzed. (B) The sample shows a single band for BAT25, and a microsatellite instability (MSI)-typical shift for BAT26 and NR27 markers; this sample is classified as MSI-high (instability identified in two out of three markers). (C) The sample shows MSI-typical shift for BAT26 and a single band for BAT25 and NR27; this sample is classified as MSI-low (instability identified in one out of three markers).
Of eight patients showing MSI, 75% presented only one suspected gene mutation (MSI-L), while 25% presented more than one suspected gene mutation (MSI-H).
Sample analysis, done before genetic testing, demonstrated that 68% of tumors were diagnosed at an early stage (stage I and II, disease confined to the uterus) and 32% of tumors were diagnosed at advanced stage (stage III to IV) (Supplementary Fig. 1). Supplementary Fig. 2 shows the number of patients with or without MSI subdivided according to FIGO stage. At diagnosis, tumors were graded as G2 in 33% and as G3 in 67% of MSI endometrial cancer patients; no patient with MSI was diagnosed with a G1 tumor (Supplementary Fig. 3). Thirty-three percent of MSI-H/L patients show early lymphovascular invasion, while in the MSI-S group this occurs in only 9% of patients.
In our cohort, only 33% (3/9) of MSI-H/L patients fulfill the Amsterdam criteria, while the remaining 67% (6/9) of patients do not have family cancer risk or previous cancers related to the syndrome; in the MSI-S group (sporadic cancer), 18% of patients have positive LS-related criteria (either due to family history or previous tumors).
Mean follow-up time was 42 months (range, 11 to 120 months). Eight patients were lost to follow-up. Only one death and three relapses were registered. There were no relapses or deaths in the group of patients that were positive for the molecular analysis (total of eight cases, of which two were lost to follow-up).
The number of patients with suspected mutations (MSI-H/L) was not enough to obtain a survival curve to compare with MSI-S patients. Similarly, differences related to stage, grade, lymphovascular space invasion and Amsterdam criteria adherence were not statistically significant due to the small size of the cohort.
Shown below are four cases that reflect the clinical importance of IHC/MSI tests for the diagnosis of endometrial cancer in young women.
1. Case 1
44 year-old Amsterdam positive patient (father died from stomach and colon cancer at 47) underwent totally laparoscopic hysterectomy, bilateral salpingo-oophorectomy and systematic pelvic lymphadenectomy with IB G3 (N0 V1) FIGO stage. Patient was treated with adjuvant antiblastic platinum-based combination chemotherapy with paclitaxel and continued follow-up. IHC analysis was negative for all proteins (MLH1, MSH2, MSH6 and PMS2); MSI test showed MSI for BAT26 (MSI-L).
2. Case 2
45 year-old Amsterdam negative patient underwent open total hysterectomy, systematic pelvic and para-aortic lymphadenectomy and total omentectomy with IIIA G3 FIGO stage (bilateral ovarian endometrioid adenocarcinoma, negative lymph nodes and omentum). Patient was treated with antiblastic adjuvant chemotherapy (paclitaxel + carboplatin for six cycles q 21 days) and continued follow-up. IHC analysis was positive only for MSH2; MSI test identified BAT25 and NR27 instability (MSI-H).
3. Case 3
50 year-old, diabetic, Amsterdam negative patient underwent laparotomy with total hysterectomy, bilateral salpingo-oophorectomy, and systematic pelvic lymphadenectomy with IIIC1 G3 (V1) FIGO stage (endometrioid adenocarcinoma metastasis in one hypogastric lymph node). Patient was treated with adjuvant antiblastic platinum- and taxane-based chemotherapy (four cycles), radiotherapy and continued to follow-up. IHC analysis showed absence of MLH1 and PMS2. MSI test showed MSI with BAT26 and NR27 repetitions (MSI-H).
4. Case 4
50 year-old Amsterdam positive patient (mother died from colon cancer at 45 and sister underwent hysterectomy due to endometrial cancer at 50) underwent open total hysterectomy and bilateral salpingo-oophorectomy with IA G2 FIGO stage. Intervention was adequate and the patient was addressed to follow-up. MSI test did not reveal MSI (MSI-S).
DISCUSSION
Identification and diagnosis of LS are very important not only to the patient but also to the entire at-risk family, since it is known that screening for and monitoring the disease reduce colorectal carcinoma mortality by more than 69% [21].
Amsterdam and Bethesda criteria were introduced to identify subjects with high risk of developing LS. Amsterdam criteria were reviewed in 1998 and Bethesda criteria were updated in 2002. However, although sensitivity and specificity levels have increased with these revisions, both criteria are still not able to select efficiently which cancer patients should be screened for the disease [22]. Indeed, in our cohort, only 33% of patients showing MSI events met the Amsterdam criteria, while most patients (67%) did not have LS-related personal or family history; this fact indicates that studying the patient’s clinical history is not enough to identify patients that would benefit from MMR genetic screening. The four clinical cases presented here also corroborate our conclusion: two Amsterdam positive cases, one MSI-S and one MSI-L; two Amsterdam negative cases, both MSI-H.
Most LS cases are due to germinal mutations in three MMR genes: MLH1, MSH2, and MSH6. Mutations in these genes, known to reduce the activity of MMR proteins that results in DNA MSI, may also derive from methylation of the MLH1 promoter, an event that occurs in somatic cells and, thus, is non-transmissible and does not require further screening for LS. A great limitation of our work is the lack of hypermethylation status test of MLH1. In fact, several studies support this method because it improves the selection of patients for genetic test. Therefore, in our study, this bias leads to an inaccurate selection of patients and consequent disputable results.
Since molecular analysis is costly, it is current clinical practice that patients that meet clinical criteria undergo IHC analysis first and, only when needed, genetic testing be performed afterwards. In our study, IHC for MMR proteins (MLH1, MSH2, MSH6, PMS2) showed that 46% of patients had a negative result. For patients with negative IHC result, MSI testing was performed and showed that in 42% of cases MSI (MSI-L or MSI-H) was observed. A negative outcome in IHC analysis may be due to errors during assessment or may also be explained, as previously stated, by protein MLH1 hypermethylation that leads to gene inactivation. In the latter case, negative protein expression, assessed by IHC, is considered an epigenetic event and is common in sporadic endometrial carcinoma. Of note, no patient with positive IHC (normal) was found to have MSI during molecular analysis; in other words, there were no IHC “false negatives.” This shows that IHC analysis is valid as a first screening step to identify patients that should undergo molecular analysis.
MSI-related endometrial cancer arises in younger patients, is frequently diagnosed in more advanced stages, and invades the lymphovascular space earlier than sporadic cancer; MSI-related endometrial cancer seems to be more biologically aggressive. In the subset of MSI-H/L patients, only 11% of cases was identified with stage IA tumors, while 53% of MSI-S patients was diagnosed with stage IA tumors. This fact confirms that the diagnosis of endometrial cancer in LS patients, if the tumor is confined to the uterus, occurs more frequently when there is more than half myometrial invasion. We also identified stage IIIA tumors in 33% of MSI-H/L patients and in only 9% of MSI-S patients. The increased frequency of stage IIIA tumors in MSI patients might be due to the involvement of the ovary in the disease at the time of diagnosis; in our cohort, two out of nine cases were diagnosed with synchronous or metastatic ovarian cancer histologically similar to the endometrial tumor diagnosed in that same patient.
In spite of these results, the prognostic importance of LS-related endometrial cancer is not considered on a routine basis, particularly in regard to subsequent personal follow-up and management of at-risk relatives. This information is particularly important for patients who wish to have children in the future and choose a fertility-sparing treatment; these patients should know if they are carriers of a genetic condition associated with higher cancer risk. However, no standard procedures for the correct diagnosis of LS-related endometrial cancer are currently available to physicians; it is not clear which genetic tests are necessary to confirm the diagnosis and which subset of patients should undergo genetic screening.
Endometrial cancer patients with MMR gene mutations have a 10- and 15-year risk of developing a second tumor of 25% and of 50%, respectively. For half of LS patients, endometrial cancer occurs before CRC; thus, uterine cancer may also be considered as a "sentinel tumor” for the patients themselves and potentially for the members of their families. Thus, it could be useful to advise endometrial cancer patients to perform CRC screening tests (fecal occult blood test, colonoscopy). For the general population, the incidence of LS-related endometrial cancer is 2.3%; in individuals younger than 50 years, this incidence rises to 9%. In our study, the incidence of LS-related endometrial cancer was 22%; such a high percentage may be due to the small size of our cohort.
IHC and MSI test results of our cohort are characterized by several abnormal findings suspected for gene mutations; this issue leads us to assess the relevance of performing LS-genetic screening in endometrial cancer patients aged less than 50 years at the time of diagnosis. Unfortunately, the lack of DNA sequencing for screening of LS is a limitation of our work, because there is not confirmation about IHC and MSI test results. Anyway current testing practices in the setting of LS include screening assays that use IHC and MSI analysis, which may suggest the presence of a germ line mutation. These assays are typically followed by Sanger sequencing of the MMR genes to identify specific mutations. MSI can be useful as a surrogate marker for defective MMR when the presence of MSI in the tumor suggests an MMR defect [23].
We believe such screening would have three advantages:
(1) Identification of mutation carriers in pre-operative phase. Genetic screening on the preoperative biopsy would allow to tailor the surgery and possible adjuvant treatments to the patient; the high prevalence of grade 3 tumors and of invasion of the lymphovascular space might lead us to consider these patients, with tumors apparently at stage I, as high-risk patients and, consequently, to perform additional surgical procedures (for example, lymphadenectomy). In addition, as for patients with MSI-positive CRC that do not benefit from postoperative 5-fluorouracil chemotherapy, LS-related endometrial cancer patients could have a personalized adjuvant treatment.; (2) Activation of LS-related cancer patient-specific screening algorithms for the early diagnosis of a possible second tumor.; (3) Improvement of genetic counseling for family members. The role of LS-related endometrial cancer as sentinel event would impose the genetic screening of family members.
Recent studies have proposed that IHC analysis should be done on all patients with endometrial cancer aged less than 60 years and on those older than 60 years who present suspicious factors possibly related to LS: endometrial cancer in absence of risk factors derived from estrogen hyperstimulation; synchronous ovarian cancer, particularly if presenting clear cell histology; LS family history or CRC personal history. According to our current knowledge, LS should be suspected when endometrial cancer arises in young patients without risk factors, with low body mass index, and with family history of LS-related tumors.
Our follow-up data and the small size of the cohort do not allow us, at this time, to have conclusive data regarding overall survival and disease-free survival statistically significant differences between MSI-H/L and MSI-S patients.
IHC analysis is a good cost-benefit tool to identify endometrial tumors that lack expression of MMR genes; however, the use of this tool requires professionals trained to develop and perform modern genetic tests when needed, to prevent disease and provide the best treatment to these cancer patients. Thus, a multidisciplinary approach designed by both clinicians and pathologists is needed. Ethical issues that inevitably derive from the knowledge of a genetic predisposition to cancer and that need to be adequately communicated should not be overlooked. In conclusion, the possibility to perform genetic screening in endometrial cancer patients requires, not only a correct clinical assessment by the physician, but also a multidisciplinary team composed of pathologists, oncologists, and geneticists, capable of integrating all information to reach a common clinical goal; this goal should not be limited to the assessment of the diagnostic and therapeutic strategy for each patient, but should also include a screening strategy for the patient’s family members.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
Supplementary Materials
The International Federation of Gynecology and Obstetrics (FIGO) stage of complete series.
The International Federation of Gynecology and Obstetrics (FIGO) stage in the patients with or without microsatellite instability; (A) unstable and (B) stable.
Grading in the patients with or without microsatellite instability (MSI); (A) MSI-stable (MSI-S) and (B) MSI-high/low (MSI-H/L).
References
- 1.Lynch HT, de la Chapelle A. Genetic susceptibility to non-polyposis colorectal cancer. J Med Genet. 1999;36:801–818. [PMC free article] [PubMed] [Google Scholar]
- 2.Weitz J, Koch M, Debus J, Höhler T, Galle PR, Büchler MW. Colorectal cancer. Lancet. 2005;365:153–165. doi: 10.1016/S0140-6736(05)17706-X. [DOI] [PubMed] [Google Scholar]
- 3.Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044–2058. doi: 10.1053/j.gastro.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lynch HT, Lynch PM, Lanspa SJ, Snyder CL, Lynch JF, Boland CR. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet. 2009;76:1–18. doi: 10.1111/j.1399-0004.2009.01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu KH, Schorge JO, Rodabaugh KJ, Daniels MS, Sun CC, Soliman PT, et al. Prospective determination of prevalence of lynch syndrome in young women with endometrial cancer. J Clin Oncol. 2007;25:5158–5164. doi: 10.1200/JCO.2007.10.8597. [DOI] [PubMed] [Google Scholar]
- 6.Barrow E, Robinson L, Alduaij W, Shenton A, Clancy T, Lalloo F, et al. Cumulative lifetime incidence of extracolonic cancers in Lynch syndrome: a report of 121 families with proven mutations. Clin Genet. 2009;75:141–149. doi: 10.1111/j.1399-0004.2008.01125.x. [DOI] [PubMed] [Google Scholar]
- 7.Nelson R. New guidelines on colorectal cancer molecular testing [Internet] New York, NY: Medscape; c1994. [cited 2016 Aug 10]. Available from: http://www.medscape.com/viewarticle/842496. [Google Scholar]
- 8.Wang Y, Wang Y, Li J, Cragun J, Hatch K, Chambers SK, et al. Lynch syndrome related endometrial cancer: clinical significance beyond the endometrium. J Hematol Oncol. 2013;6:22. doi: 10.1186/1756-8722-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato M, Takano M, Miyamoto M, Sasaki N, Goto T, Tsuda H, et al. DNA mismatch repair-related protein loss as a prognostic factor in endometrial cancers. J Gynecol Oncol. 2015;26:40–45. doi: 10.3802/jgo.2015.26.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peltomäki P. Role of DNA mismatch repair defects in the pathogenesis of human cancer. J Clin Oncol. 2003;21:1174–1179. doi: 10.1200/JCO.2003.04.060. [DOI] [PubMed] [Google Scholar]
- 11.Peltomäki P, Vasen H. Mutations associated with HNPCC predisposition -- Update of ICG-HNPCC/INSiGHT mutation database. Dis Markers. 2004;20:269–276. doi: 10.1155/2004/305058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carcangiu ML, Radice P, Casalini P, Bertario L, Merola M, Sala P. Lynch syndrome--related endometrial carcinomas show a high frequency of nonendometrioid types and of high FIGO grade endometrioid types. Int J Surg Pathol. 2010;18:21–26. doi: 10.1177/1066896909332117. [DOI] [PubMed] [Google Scholar]
- 13.Win AK, Lindor NM, Winship I, Tucker KM, Buchanan DD, Young JP, et al. Risks of colorectal and other cancers after endometrial cancer for women with Lynch syndrome. J Natl Cancer Inst. 2013;105:274–279. doi: 10.1093/jnci/djs525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116:1453–1456. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 15.Vasen HF, Möslein G, Alonso A, Bernstein I, Bertario L, Blanco I, et al. Guidelines for the clinical management of Lynch syndrome (hereditary non-polyposis cancer) J Med Genet. 2007;44:353–362. doi: 10.1136/jmg.2007.048991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138:2073–2087.e3. doi: 10.1053/j.gastro.2009.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26:5783–5788. doi: 10.1200/JCO.2008.17.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Comprehensive Cancer Network. NCCN practice guidelines in oncology: Lynch syndrome, version 1.2010 [Internet] Fort Washington, PA: National Comprehensive Cancer Network; c2016. [cited 2016 Aug 10]. Available from: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [DOI] [PubMed] [Google Scholar]
- 19.National Comprehensive Cancer Network. NCCN practice guidelines in oncology: uterine neoplasm, version 2012 [Internet] Fort Washington, PA: National Comprehensive Cancer Network; c2016. [cited 2016 Aug 10]. Available from: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [Google Scholar]
- 20.Dean DA, Wadl PA, Hadziabdic D, Wang X, Trigiano RN. Analyzing microsatellites using the QIAxcel system. Methods Mol Biol. 2013;1006:223–243. doi: 10.1007/978-1-62703-389-3_16. [DOI] [PubMed] [Google Scholar]
- 21.Järvinen HJ, Aarnio M, Mustonen H, Aktan-Collan K, Aaltonen LA, Peltomäki P, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118:829–834. doi: 10.1016/s0016-5085(00)70168-5. [DOI] [PubMed] [Google Scholar]
- 22.Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Rüschoff J, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tafe LJ, Riggs ER, Tsongalis GJ. Lynch syndrome presenting as endometrial cancer. Clin Chem. 2014;60:111–121. doi: 10.1373/clinchem.2013.206888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The International Federation of Gynecology and Obstetrics (FIGO) stage of complete series.
The International Federation of Gynecology and Obstetrics (FIGO) stage in the patients with or without microsatellite instability; (A) unstable and (B) stable.
Grading in the patients with or without microsatellite instability (MSI); (A) MSI-stable (MSI-S) and (B) MSI-high/low (MSI-H/L).