Abstract
STUDY QUESTION
What is the familial childhood mortality in first-degree (FDR) and second-degree relatives (SDR) of patients undergoing semen analysis (SA)?
SUMMARY ANSWER
The relationship between infertility and congenital malformations (CM) in offspring is complex, with an increased risk of death due to CM in FDR, but not SDR, of men with lower semen parameters.
WHAT IS KNOWN ALREADY
Semen quality is an established predictor of men's somatic health. We can gain a better understanding of possible genetic or environmental determinants of the infertility phenotype by exploring familial aggregation of childhood mortality in relatives of men with poor semen quality.
STUDY DESIGN, SIZE, DURATION
Retrospective cohort study from the Subfertility, Health and Assisted Reproduction study (cohort compiled 1996–2011) linked with patient/familial information from the Utah Population Database (UPDB). Index cases included a clinic-referred sample of 12 889 men who underwent SA and had adequate familial and follow-up data in the UPDB. Parameters of semen quality included: semen concentration, sperm count, motility, total motile count, sperm head morphology, sperm tail morphology and vitality.
PARTICIPANTS/MATERIALS, SETTING, METHODS
SA data were collected from two tertiary medical center andrology laboratories that have captured ~90% of all SA performed in Utah since 2004. Age- and sex-matched fertile controls were selected to create the comparison group for determining risk of childhood death (to age 20 years) in family members. A total of 79 750 siblings and 160 016 aunts/uncles were used to investigate the familial aggregation of childhood mortality. The main outcome was childhood mortality in FDR and SDR of men with SA and their matched controls. All-cause and cause-specific Cox proportional hazard models were used to test the association between semen quality and childhood mortality in family members. Cause-specific models were considered for cancer and CM.
MAIN RESULTS AND THE ROLE OF CHANCE
In the cohort of men with SA, there were 406 (1.0%) deaths in FDR and 772 (1.1%) deaths in SDR due to any cause. There was no significant difference in the risk of all-cause childhood mortality between the relatives of men with SA and the fertile control group [hazard ratio (HR)Female = 1.08, 95% CI = 0.88, 1.32; HRMale = 0.88, 95% CI = 0.75, 1.04]. We found no association between semen quality and risk for childhood cancer mortality in FDR or SDR (HRFDR = 0.98, 95% CI = 0.62, 1.54; HRSDR = 1.12, 95% CI = 0.83, 1.50). The FDR of men with SA and fertile controls were followed on average for 19.71 and 19.73 years, respectively. During this period of follow-up, FDR of men with SA had an unadjusted 40% relative risk of increased CM-related death. After stratifying by semen parameters and adjusting for birth year, we found FDR of men with worse semen quality, and notably azoospermic men (HR = 2.69, 95% CI = 1.24,5.84), were at higher risk of CM-related death.
LIMITATIONS REASONS FOR CAUTION
A large proportion of men with SA in the study had normal semen parameters. It is important to note that these men themselves may not be subfertile, but they were subfertile at the couple level (i.e. the female partner may be infertile). In addition, care is needed when interpreting our results, as we do not have semen measures on our sample of fertile men. Second, we were unable to include potential confounders such as medical comorbidities, smoking status, or environmental exposures. Third, men with SA were seen at the University of Utah or Intermountain Health Care clinics for a fertility evaluation thereby suggesting a more select population. Fourth, we chose to categorize morphology into equally distributed quartiles as a response to the fact that the World Health Organization threshold for normal motility changed multiple times during our study period. Lastly, we do not know the proportion of female partners with diagnosed infertility. We chose not to subcategorize each infertile male by infertile diagnosis because our goal was to understand how semen parameters influenced familial childhood mortality.
WIDER IMPLICATIONS OF THE FINDINGS
We are not the first study to show a relationship between fertility and CMs. Children conceived through ART may be at higher risk of birth defects, however it is not known if the relationship is causal or if there is some underlying factor linking infertility and birth outcomes. This study provides further evidence that the increased risk of congenital birth defects may not be due to the ART, but rather genetic or environmental factors that link the two outcomes. We encourage further research in order to confirm a relationship between semen quality and increased risk for CM.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by the National Institutes of Health – National Institute of Aging [Grant numbers 1R21AG036938-01, 2R01 AG022095 and 1K12HD085852-01]. Authors have no competing interests to disclose.
TRIAL REGISTRATION NUMBER
Not applicable.
Keywords: childhood mortality, semen, Utah Population Database, congenital malformations, familial risk, testicular dysgenesis syndrome, infertility, familial aggregation
Introduction
Semen quality and male fertility are indicators of somatic health. Male factor infertility is associated with an increased risk of mortality and cancer, suggesting that it is an important marker of men's general health throughout life (Giwercman et al., 1987; Moller and Skakkebaek, 1999; Jacobsen et al., 2000, 2009; Groos et al., 2006; Walsh et al., 2010; Eisenberg et al., 2013, 2014a; Hanson et al., 2015). Multiple studies have confirmed this finding for both short and long-term mortality in relation to the semen analysis (SA) (Groos et al., 2006; Jensen et al., 2009; Eisenberg et al., 2014b). Disorders of spermatogenesis involve a myriad of genetic and epigenetic variants that may be pleiotropic and/or lead to variable phenotypic expression among carriers (Carrell et al., 2015). In addition, male factor infertility may represent just one disease condition among a cluster of reproductive and somatic health disorders linked to the same underlying mechanisms. Mechanisms linking fertility to overall health include health behaviors, environmental exposures (particularly during gestation and childhood), and genetic or epigenetic factors with pleiotropic effects (Groos et al., 2006; Eisenberg et al., 2014a, 2015; Ventimiglia et al., 2015). However, little is known about the mechanisms underlying this association.
Familial studies offer a unique opportunity to better characterize the infertility phenotype. These have the potential to increase our understanding of the familial and environmental components associated with male factor infertility and expand the phenotypic definition to include multiple diseases. Within the last 10 years, the testicular dysgenesis syndrome (TDS) hypothesis, which proposes that disturbed testicular development in utero may result in male reproductive disorders, has been suggested as an explanation for a constellation of symptoms and diseases, which includes poor semen quality (Swan et al., 2000; Jouannet et al., 2001; Skakkebaek et al., 2001; McGlynn et al., 2003; Bray et al., 2006a, b; Centola et al., 2016).
To what degree the environment, genetics, or epigenetics during fetal growth are responsible for TDS remains unresolved, but consensus is building concerning an expanded role for environmental influences and exposures (Virtanen et al., 2005; Wohlfahrt-Veje et al., 2009; Nordkap et al., 2012; Skakkebaek et al., 2016). Therefore, investigating semen parameters in relation to familial early-life mortality represents an area of novel contribution to the TDS hypothesis. In men with poor semen quality, exploring excess disease and mortality in their relatives allows us to gain better understanding of possible genetic or shared environmental determinants of health.
Our study seeks to examine familial childhood mortality in first- (FDR) and second-degree relatives (SDR) of patients undergoing SA. We chose to examine childhood mortality for several reasons. First, young adult deaths are largely due to external causes, such as motor vehicle deaths and homicide, and there is not a plausible link between semen quality and external causes of death of relatives. Second, the biological mechanisms leading to childhood mortality are quite different from those in adulthood and therefore we argue that separate analyses were warranted. We hypothesize that FDR and SDR of men with poor semen quality have increased childhood mortality and that the magnitude of the effect will vary by the age and sex of the child. To our knowledge, there are no published studies that associate familial mortality with primary patient semen quality. Population-level genealogical data linked to over a century of death certificates and medical records spanning two decades, maintained in the Utah Population Database (UPDB) and linked to a SA database, make this innovative study feasible.
Materials and Methods
This study used data compiled by the Subfertility, Health and Assisted Reproduction (SHARE) study and the integration of demographic and follow-up information within the UPDB. SHARE data are based on a collection of men who underwent SA at the University of Utah Andrology Clinic (UU) from 1996 to 2011 and at Intermountain Healthcare (IHC) from 2002 to 2011. Together, these two tertiary medical centers’ andrology laboratories have captured ~90% of all SA performed in Utah since 2004. The UPDB has supported numerous bio-demographic, epidemiological and genetic studies in large part because of its comprehensive coverage of the population, pedigree complexity, and linkages across data sources, including statewide birth certificate records and cancer diagnoses (Kerber and O'Brien, 2005; DuVall et al., 2012; Hurdle et al., 2013; Samadder et al., 2014).
Study design
A retrospective cohort analysis of childhood mortality in FDR and SDR of men with measured semen parameters and fertile population controls was used to assess familial clustering between semen quality and childhood mortality. Semen quality was measured by seven semen parameters stored in the SHARE data: semen concentration, sperm count, motility, total motile count, sperm head morphology, sperm tail morphology and vitality. Family members were followed until time of childhood death, age 20 years, or until their last known date of residence in the state of Utah.
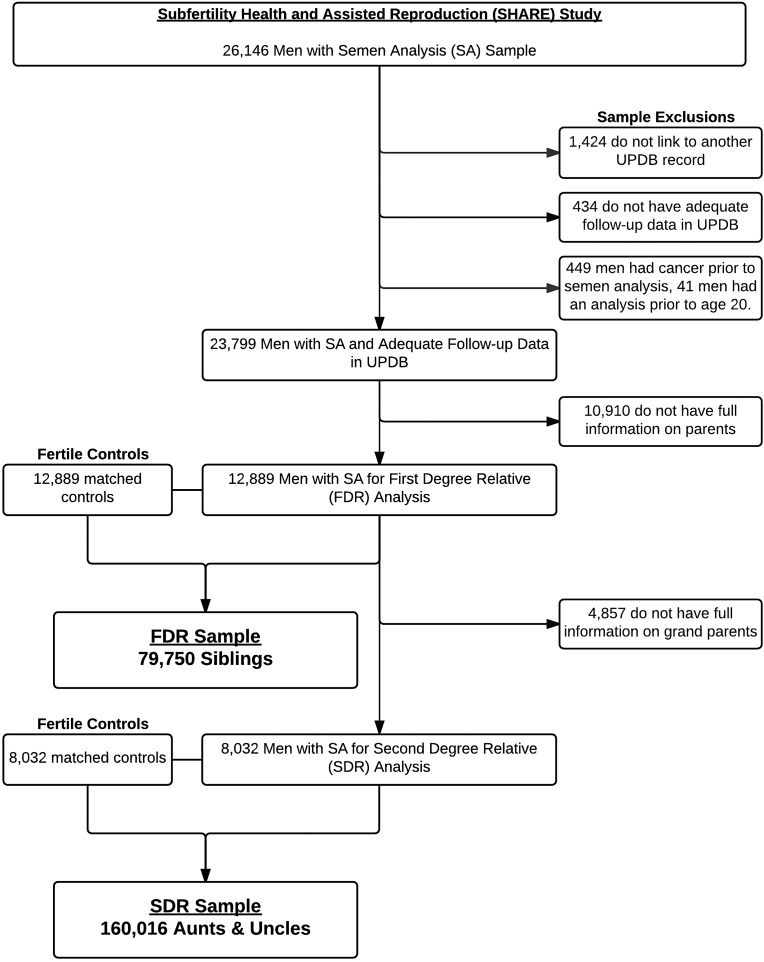
Figure 1 shows the inclusion/exclusion criteria and sample selection of men with SA. Our final sample included 12 889 men with FDR information and 8032 men with complete SDR information. Fertile population controls were selected randomly without replacement from the UPDB. Men seen at the IHC or UU clinics for fertility related issues were excluded from the pool of potential controls. Controls were required to be residents of the state of Utah, with adequate follow-up data in the UPDB. They were matched 1:1 by age and birth year. We used birth certificate data to define a man as being fertile by virtue of having at least one naturally conceived child. A total of 25 778 men were utilized for the basis of the FDR analyses and 16 064 men for the SDR.
Figure 1.
Sample selection criteria in a study of childhood mortality in family members of men with poor semen quality. We identified 26 147 men with semen analysis (SA) performed during our study period. We excluded 1424 men who did not link to another record in the Utah Population Database (UPDB), 434 with inadequate follow-up, 449 with cancer prior to SA, and 41 with an analysis prior to age 20. There were 10 910 men without complete information on their parents, and therefore a total of 12 889 men were available with first-degree relative (FDR) information. This cohort of men was used to study the childhood mortality in FDR. Another 4857 men did not have full information on their grandparents, which left 8032 men with complete second-degree relative (SDR) information available for analysis.
Following identification of men with SA and their matched population controls, we specifically selected siblings (FDR) and aunts and uncles (SDR) of these men from the UPDB for the familial analysis. Parents and grandparents were not included in the FDR and SDR analyses because, by definition, they survived long enough to have a child. Relatives were excluded from the analysis if they had incomplete birth and follow-up information in UPDB, were adopted, or were born prior to 1904 (lack of death certificate information). We identified a total of 79 750 siblings and 160 016 aunts/uncles.
Semen parameters
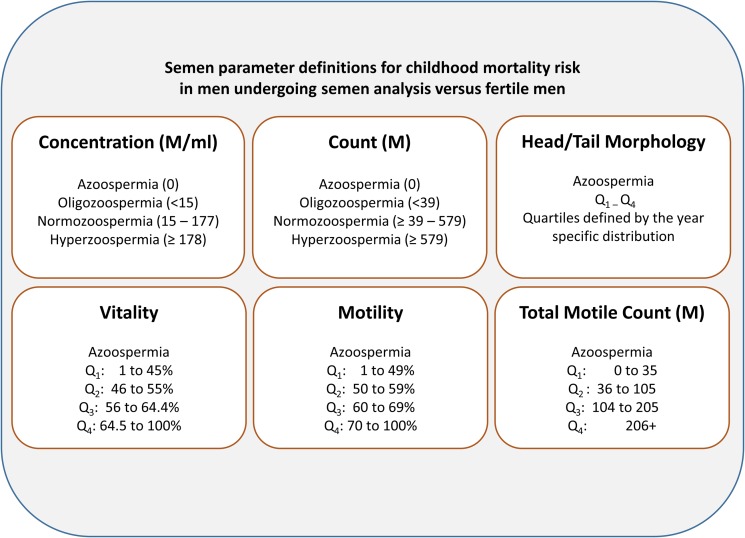
SA were processed based on the 2010 World Health Organization (WHO) guidelines (WHO, 2010). In cases where men had more than one semen sample (17% of patients), the average concentration, count, and motility across measures was used. Figure 2 shows the definitions for each semen parameter (Hanson et al., 2016). In general, each parameter is either divided into azoospermic, oligozoospermic, normozoospermic and hyperzoospermic categories or is divided into quartiles.
Figure 2.
Semen parameter definitions for childhood mortality risk in men undergoing SA versus fertile men. Morphology was categorized into equally distributed quartiles (Q) because the World Health Organization threshold for normal motility changed multiple times during the study period.
Cause of death data for family members
Death certificate information in Utah since 1904 combined with genealogical data provide the mortality data in family members of men with SA. Childhood mortality was defined as death prior to age 20 years. The underlying cause of death was coded using the International Classification of Diseases (ICD) cause-of-death coding (revisions 6–10). Causes of death were then categorized into the 17 disease and injury categories based on the ICD-9 categorization of disease (see Supplementary data, Table SI).
Ethical approval
This study was approved, with a waiver of informed consent, by the Institutional Review Boards of the UU and IHC by the Utah Resource for Genetic and Epidemiologic Research (www.research.utah.edu/rge/) #IRB_00069711.
Statistical methods
Power analyses were conducted using NCSS Statistical Software program PASS v14 (NCSS Statistical Software, Kaysville, UT, USA) (Schoenfeld, 1983; Hsieh and Lavori, 2000). All-cause childhood mortality and mortality due to congenital malformation (CM) are both rare events in this cohort, therefore large sample sizes are required to achieve sufficient power at a 0.05 significance level. Supplementary data, Fig. S1 displays the necessary sample sizes at 80% and 90% power by Hazard Rate Ratio (HR) for all-cause and CM-related childhood mortality. For all childhood mortality, the number of observations needed to achieve 90% power at a 0.05 significance level range from 5189 for a regression coefficient β = 0.7 (HR = 2.46) to 105 075 for regression coefficient β = 0.2 (HR = 1.22). When the outcome is CM, the range for the necessary number of observations increases to 25 945–525 372 for the same β values. A Bonferroni correction was used to correct for multiple testing. However, because the outcome is rare, correcting for multiple tests requires an inordinately large sample size to see an association between semen quality and childhood mortality (the probability of a type II error is increased). As such, results with and without the correction are presented in the Results section.
We first examined differences in childhood mortality in FDR and SDR of men who underwent SA (any man experiencing fertility problems at the couple level), relative to fertile controls. We then evaluated the association between familial mortality based on the previously specified seven semen parameters. All-cause and cause-specific models were used to assess the relationship between semen quality and childhood mortality in FDR and SDR. Cause-specific models were for death due to cancer and CMs.
Cox proportional hazard models were used to test the association between semen quality and childhood mortality in FDR and SDR of men with SA and their matched controls. The risk in relatives of men who underwent SA compared to relatives of controls was determined independently for each relation type (FDR and SDR). To determine the risk of childhood mortality in relatives of men with male factor infertility and men with normal semen parameters, case–case analyses (i.e. all subjects who had SA) were also completed in which relatives of men with abnormal semen parameters were compared to relatives of men with normal semen parameters.
All relatives of men who underwent SA and their fertile population controls were included in the analyses, even if that relative had been previously counted. For example, for families containing multiple men with SA, each man was included as a separate index case and risk among relatives of each case was handled as being distinct, an approach that has been shown to lead to unbiased estimates of risk (Kerber and O'Brien, 2005). Huber-White sandwich estimator of variance of regression parameters in the Cox models was used to correct for the non-independence of observations within families (Williams, 2000). Relatives of fertile men who were not patients at a fertility clinic were used as the reference group in all analyses (except as noted above).
If no death occurred, last known date residing in Utah was used as the date of right-censoring. Analyses were performed for all-cause mortality, mortality related to CMs, and cancer-related mortality. All models were stratified by birth year, allowing for a separate baseline hazard for each birth year. We tested for non-proportional effects based on differences in SA effects for three age groups among relatives: under age 1 year, ages 1–4 years, and ages 5–20 years.
Results
Table I displays the descriptive statistics for relatives of men with SA and their fertile controls. The FDR are members of a younger birth cohort than the SDR (average birth year 1975 and range 1917–2009 versus average 1948 and range 1905–1989, respectively) and both groups of relatives have slightly more males (53% and 52% respectively). Men with SA had slightly fewer siblings than men from the fertile control population (3.04 versus 3.15, respectively). Men with SA also have fewer SDR than men from the fertile control population (8.8 versus 11.1, respectively). The rate of childhood (under age 20 years) death is 10.7 and 11.7 per 1000 births in the FDR and SDR samples, respectively. FDR and SDR of men with SA have slightly lower rates of all-cause childhood mortality compared to relatives of fertile controls (10.4 and 11.1 per 1000, respectively for FDR; 10.9 and 12.4 per 1000, respectively for SDR). However, FDR of men with SA have a 40% increased rate of CM related deaths (2.2 versus 1.6 per 1000) relative to FDR of fertile controls.
Table I.
Characteristics of relatives of men undergoing semen analysis in a study of semen quality and childhood mortality.
| FDR: siblings (n = 79 750) | SDR: aunts/uncles (n = 160 016) | |||
|---|---|---|---|---|
| Fertile controls (n = 40 537) | Men with SA (n = 39 213) | Fertile controls (n = 89 145) | Men with SA (n = 70 871) | |
| Birth year (mean) | 1974 | 1975 | 1948 | 1948 |
| Years of follow-up (mean) | 19.73 | 19.71 | 19.53 | 19.56 |
| All-cause mortality, n (%) | 449 (1.1%) | 406 (1.0%) | 1107 (1.2%) | 772 (1.1%) |
| Congenital malformation-related mortality, n (%) | 65 (0.2%) | 88 (0.2%) | 89 (0.1%) | 61 (0.1%) |
| Cancer-related mortality n (%) | 38 (0.1%) | 34 (0.1%) | 94 (0.1%) | 83 (0.1%) |
| Gender | ||||
| Male, n (%) | 21 264 (52%) | 20 641 (53%) | 46 155 (52%) | 36 642 (52%) |
| Female, n (%) | 19 273 (48%) | 18 572 (47%) | 42 990 (48%) | 34 229 (48%) |
| Relation by semen quality, n (% of men with SA) | ||||
| Sibling of azoospermic | 1588 (4.0%) | 2918 (4.1%) | ||
| Sibling of oligozoospermic | 4131 (10.5%) | 7459 (10.5%) | ||
| Sibling of normozoospermic | 20 202 (51.5%) | 36 543 (51.6%) | ||
| Sibling of hyperzoospermic | 13 292 (33.9%) | 23 951 (33.8%) | ||
FDR: first-degree relative, SDR: second-degree relative, SA: semen analysis.
Table II displays the counts of childhood deaths by disease category for FDR (siblings) and SDR (aunts/uncles) relatives of men with SA. Deaths from external causes (0.4% of the total sample and 41% of all childhood deaths) and CMs (0.19% of the total sample and 16% of all childhood deaths) were the most common causes of death among FDR. For SDR, external causes (0.5% of the total sample and 43% of all childhood deaths) and cancer (0.11% of the total sample and 9% of all childhood deaths) were the most common. As expected, given their ages, a larger proportion of SDR relative to FDR died outside of the state of UT and therefore the cause of death is unknown. Table II displays information on the CMs as a cause of death. Cardiac/circulatory/respiratory and nervous system/musculoskeletal/skin anomalies were the most common CM deaths in FDR and SDR.
Table II.
Distribution of types of congenital malformation-related deaths.
| FDR | SDR | |||
|---|---|---|---|---|
| Malformation category | Frequency | Percentage | Frequency | Percentage |
| Cardiac/circulatory/respiratory | 29 | 42.65 | 20 | 39.22 |
| GI/GU | 10 | 14.70 | 11 | 21.57 |
| Nervous/musculoskeletal/skin | 17 | 25.00 | 13 | 25.49 |
| Other | 12 | 17.65 | 7 | 13.72 |
| Grand total | 68 | 100.00 | 51 | 100.00 |
GI/GU: Gastrointestinal system/Genito-urinary system.
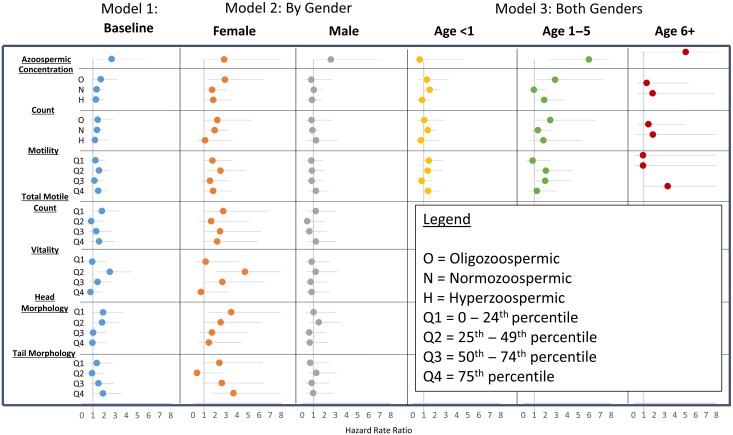
The Cox regression results are displayed in Fig. 3 (full FDR results can be found in Supplementary data, Fig. S1). Overall, we find that there is no difference in the risk of all-cause childhood mortality between the relatives of men with SA and the fertile control group. We further differentiated men with SA based on the seven semen parameters, and found no consistent significant differences in all-cause mortality in FDR or SDR relatives.
Figure 3.
Hazard Rate Ratios and 95% CI for the risk of congenital malformation-related mortality in siblings of subfertile men by semen quality category relative to siblings of fertile controls. Model 1 shows the baseline results for males and females combined. Model 2 shows the gender specific estimates. Model 3 shows the non-proportional effects for males and females combined by age group. Model 3 results for total motile count, vitality, head morphology, and tail morphology are not shown because these measures are only available for a subset of the sample and cell counts become too small when parsed by semen quality and three categories of age. Azoospermia was included as a separate category in each model. The results are stable across models because they constitute the same group of men, therefore only the azoospermic models from the concentration model are displayed in the figure.
Cause-specific models were estimated for CM and cancer-related death. Model 1 displays the results of the baseline model controlling for sex and birth year. In the adjusted models, we find that FDR of men with SA have a 41% increase in the risk of death due to CM (HR = 1.41; 95% CI = 1.02, 1.95) relative to fertile controls. Models that explore variation in risk by semen parameter show that FDR relatives of azoospermic men are 2.7 times (95% CI = 1.24, 5.84) more likely to have a CM related death. The risk of CM related death decreases linearly as semen concentration and count increases (ptrend = 0.007). We find no differences in risk by quartile of motility. We found that FDR of men with semen vitality in the second quartile (HR = 2.53, 95% CI = 1.42, 4.52) have an increased risk of CM related death. Likewise, the first and second quartiles of head morphology (HRQ1 = 1.92, 95% CI = 1.00–3.70; HRQ2 = 1.83, 95% CI = 0.99, 3.39) have increased risk of CM related death. The tail morphology findings differ, with those in Q4 at increased risk of CM related deaths (HR = 1.91, 95% CI = 1.02–3.59). When concentration is treated as a continuous variable, we find that increase in semen concentration is associated with a decreased risk of CM related death, however the trend is not significant at the P = 0.05 level. The results presented here have not been corrected for multiple testing. When a Bonferroni correction was applied, the increased risk of CM related deaths in siblings of azoospermic men remained significant and all other associations were not.
When we examined the differences in risk by sex of the FDR and SDR (Fig. 3, Model 2), we found that there was no sex difference in the risk of CM related death for the FDR of men with azoospermia. However, we found that females were at greater risk of CM related death for all other measures of semen quality. Female FDR of men with low values of semen concentration and sperm count had an increased risk of CM related death (HROligo = 2.90, 95% CI = 1.31, 6.39; HRNormo = 1.74, 95% CI = 1.00, 3.01). These findings were not significant after the Bonferroni correction. Similar findings were observed for female FDR of men with poor measures of total motile count, motility, vitality and head morphology, with increased risk in the lowest quality parameters. The pattern of risk for tail morphology is not consistent with the other parameters, with increased risk of CM mortality in the highest quartile of tail morphology. Other than male FDR of azoospermic men, CM related mortality did not vary between FDR of men with poor semen quality and FDR of fertile controls.
In addition to sex differences, the effect may vary by time-period due to changes in medical technology that have increased survival in children with a CM. To test for these differences, the sample was stratified by birth year (born pre- and post-1975). We found that the risk of mortality related to CM in siblings of men with poor semen quality is higher in the pre-1975 birth cohort, but the differences were not significant.
Non-proportional models showed that effects vary by age at death. We found that the largest effects were between ages 1 and 5 years, with FDR of azoospermic men (HR = 5.98, 95% CI = 2.28, 15.65) and oligozoospermic men (HR = 2.90, 95% CI = 1.16, 7.23) having an elevated rate of CM related death. There was not a significant difference in the rate of CM-related mortality between FDR of men with SA and fertile controls under the age of 1 year or after age 5 years.
There was no association between all-cause and CM related death for SDR. There was a pattern of decreased risk with increased semen quality, with significant protective effects for the highest categories of concentration, count, motility, total motile count, and morphology.
We found no association between semen quality and risk for childhood cancer mortality in FDR or SDR.
Discussion
This study utilized a unique Utah population resource to study the familial clustering of male factor infertility and childhood mortality. We found that FDR, and not SDR, of azoospermic men were at an increased risk of childhood death due to CMs and that there were no sex differences in the association. However, the largest differences in mortality were not for infant mortality, as expected, but after age 1 year. For men with SA, lower semen parameters were associated with an increased risk of childhood mortality due to CMs for FDR females and this effect was most prominent during the ages 1–5 years. Contrary to our hypothesis, there was no association between male factor infertility and childhood cancer mortality in FDR or SDR relatives of men with SA.
Some studies have shown a relationship between infertility and CMs (Zhu et al., 2006). Children conceived through ART are at higher risk of birth defects, however it is not known if the relationship is causal or if there is an underlying factor linking infertility and birth outcomes (Hansen et al., 2005; Tararbit et al., 2013; Liu et al., 2015). Prior to this study, there has been no evaluation of the relationship between semen quality and CMs in relatives. Our findings suggest that the relationship between ART and CMs is complex and that male factor infertility should be considered when investigating the relationship between infertility and CMs. This study suggests that the increased risk of birth defects in previous studies linking ART and CMs may not be due to the ART, but rather genetic or environmental factors that link the two outcomes. We encourage further research in order to confirm a relationship between semen quality and increased risk for CM.
A null association between childhood cancer mortality and semen quality was unexpected. However, it is important to consider the differences between cancer incidence and cancer mortality. If semen quality were associated with cancers with a very high survival rate, the association between semen quality and cancer may be different for incidence and survival. Future studies should consider the relationship between semen quality and cancer incidence in FDR and SDR of men with SA.
Our hypothesis that the associations may differ by semen parameter was based on the physiological underpinnings of the association and therefore the multiple tests were not exploratory in nature. While a strict methodological stance would require multiple testing correction, this increases type II error rates and is not supported by many researchers (Rothman, 1990; Perneger, 1998; Streiner, 2015). For example, with a rare event such as CMs, a sample size of over 600 000 individuals would be necessary to achieve 90% power to observe a HR of 1.35 at the Bonferroni corrected significance value. Our study was underpowered to detect significance after the Bonferroni correction for many of the observed effect sizes. The increased risk for siblings of azoospermic men between ages 1 and 5 years remains significant after the Bonferroni correction, but the other results do not. Therefore, any conclusions made using the uncorrected results should be reached with caution. More research on the relationship between male factor infertility and risk for CMs in offspring should be conducted.
Although we used fertile, age-matched men to compare the men evaluated in an assisted reproductive clinic, there are several limitations. First, a large proportion of men with SA in the study had normal semen parameters. It is important to note that these men themselves may not be subfertile, but they were subfertile at the couple level (i.e. the female partner may have infertility problems). In addition, care is needed when interpreting our results, as we do not have semen measures on our sample of fertile men. Second, we were unable to include potential confounders such as medical comorbidities, smoking status, or environmental exposures. Third, men with SA were seen at the UU or IHC clinics for a fertility evaluation. These men were therefore a select subsample of the population that experienced fertility problems at the couple level and have the socioeconomic resources to be evaluated by a physician. It is possible that a small fraction of these men are seen for reasons other than infertility at the couple level, however, these men would likely fall into the two categories for normozoospermic men and do not confound the results presented here. Fourth, we chose to categorize morphology into equally distributed quartiles due to the fact that the WHO threshold for normal motility changed multiple times during our study period. This study also involved men living in Utah, which has less racial and ethnic diversity compared to other parts of the country. Fifth, we do not know the proportion of female partners with diagnosed infertility. We chose not to subcategorize each infertile male by infertile diagnosis because our goal was to understand how semen parameters influenced familial childhood mortality.
There are a number of novel aspects of the study. The UPDB extensive familial linkages allowed reporting of childhood mortality risk not only for men with SA data, but also multiple generations of family members. We are the first to report specific HRs for the childhood mortality risk of relatives based on common semen parameters. Finally, we are the first to attempt to control for this by using age-matched, fertile population controls from the same state. Investigating the role of shared early-life environments and spatial aggregation of family members is a neglected and yet promising research direction to take toward understanding familial aggregation of poor semen quality and its implications for familial health.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Acknowledgments
The authors wish to thank the Huntsman Cancer Foundation for database support provided to the Pedigree and Population Resource of the HCI, University of Utah. We also thank Alison Fraser, Jennifer West and Diana Lane Reed for valuable assistance in managing the data.
Authors’ roles
H.A.H., PhD, MS: Substantial contributions to conception and design, data acquisition, and analysis and interpretation of data, primary author in drafting the article and editing it critically for important intellectual content, and final approval of the version to be published. E.N.M.: Substantial contributions to conception and design and analysis and interpretation of data, secondary author in drafting the article and editing it critically for important intellectual content, and final approval of the version to be published. R.E.A.: Substantial contributions to conception and design, contributing author in editing the manuscript critically for important intellectual content, and final approval of the version to be published. K.I.A., PhD: Substantial contributions to conception and design, contributing author in editing the manuscript critically for important intellectual content, and final approval of the version to be published. D.T.C., PhD, HCLD: Substantial contributions to conception and design, contributing author in editing the manuscript critically for important intellectual content, and final approval of the version to be published. J.B., BS: Substantial contributions to data acquisition contributing author in editing the manuscript critically for important intellectual content, and final approval of the version to be published. W.T.L., MD, MPH: Substantial contributions to conception and design contributing author in editing the manuscript critically for important intellectual content, and final approval of the version to be published. K.R.S., PhD: Substantial contributions to conception and design, contributing author in editing the manuscript critically for important intellectual content, and final approval of the version to be published. J.M.H. MD, MS: Substantial contributions to conception and design and data acquisition, contributing author in drafting the article and editing it critically for important intellectual content, and final approval of the version to be published.
Funding
National Institutes of Health – National Institute of Aging (Grant Numbers 1R21AG036938-01, 2R01 AG022095, 1K12HD085852-01). Partial support for all datasets within the UPDB was provided by the HCI Cancer Center Support Grant (P30 CA42014) from National Cancer Institute.
Conflict of interest
None declared.
References
- Bray F, Ferlay J, Devesa SS, McGlynn KA, Moller H. Interpreting the international trends in testicular seminoma and nonseminoma incidence. Nat Clin Pract Urol 2006. a;3:532–543. [DOI] [PubMed] [Google Scholar]
- Bray F, Richiardi L, Ekbom A, Pukkala E, Cuninkova M, Moller H. Trends in testicular cancer incidence and mortality in 22 European countries: continuing increases in incidence and declines in mortality. Int J Cancer 2006. b;118:3099–3111. [DOI] [PubMed] [Google Scholar]
- Carrell DT, Aston KI, Oliva R, Emery BR, Jonge CJ. The ‘omics’ of human male infertility: integrating big data in a systems biology approach. Cell Tissue Res 2015;363:295–312. [DOI] [PubMed] [Google Scholar]
- Centola GM, Blanchard A, Demick J, Li S, Eisenberg ML. Decline in sperm count and motility in young adult men from 2003 to 2013: observations from a U.S. Sperm Bank. Andrology 2016. [DOI] [PubMed] [Google Scholar]
- DuVall SL, Fraser AM, Rowe K, Thomas A, Mineau GP. Evaluation of record linkage between a large healthcare provider and the Utah Population Database. J Am Med Inform Assoc 2012;19:e54–e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg ML, Betts P, Herder D, Lamb DJ, Lipshultz LI. Increased risk of cancer among azoospermic men. Fertil Steril 2013;100:681–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg ML, Kim S, Chen Z, Sundaram R, Schisterman EF, Buck Louis GM. The relationship between male BMI and waist circumference on semen quality: data from the LIFE study. Hum Reprod 2014. a;29:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg ML, Li S, Behr B, Cullen MR, Galusha D, Lamb DJ, Lipshultz LI. Semen quality, infertility and mortality in the USA. Hum Reprod 2014. b;29:1567–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg ML, Li S, Behr B, Pera RR, Cullen MR. Relationship between semen production and medical comorbidity. Fertil Steril 2015;103:66–71. [DOI] [PubMed] [Google Scholar]
- Giwercman A, Grindsted J, Hansen B, Jensen OM, Skakkebaek NE. Testicular cancer risk in boys with maldescended testis: a cohort study. J Urol 1987;138:1214–1216. [DOI] [PubMed] [Google Scholar]
- Groos S, Krause W, Mueller UO. Men with subnormal sperm counts live shorter lives. Soc Biol 2006;53:46–60. [DOI] [PubMed] [Google Scholar]
- Hansen M, Bower C, Milne E, de Klerk N, Kurinczuk J, Assisted J. Reproductive technologies and the risk of birth defects—a systematic review. Hum Reprod 2005;20:328–338. [DOI] [PubMed] [Google Scholar]
- Hanson HA, Anderson RE, Aston KI, Carrell DT, Smith KR, Hotaling JM. Subfertility increases risk of testicular cancer: evidence from population-based semen samples. Fertil Steril 2016;105:322–328 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh FY, Lavori PW. Sample-size calculations for the Cox Proportional Hazards Regression Model with nonbinary covariates. Control Clin Trials 2000;21:552–560. [DOI] [PubMed] [Google Scholar]
- Hurdle JF, Smith KR, Mineau GP. Mining electronic health records: an additional perspective. Nat Rev Genet 2013;14:75–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen R, Bostofte E, Engholm G, Hansen J, Olsen JH, Skakkebaek NE, Moller H. Risk of testicular cancer in men with abnormal semen characteristics: cohort study. BMJ 2000;321:789–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TK, Jacobsen R, Christensen K, Nielsen NC, Bostofte E. Good semen quality and life expectancy: a cohort study of 43 277 men. Am J Epidemiol 2009;170:559–565. [DOI] [PubMed] [Google Scholar]
- Jouannet P, Wang C, Eustache F, Kold-Jensen T, Auger J. Semen quality and male reproductive health: the controversy about human sperm concentration decline. APMIS 2001;109:333–344. [DOI] [PubMed] [Google Scholar]
- Kerber RA, O'Brien E. A cohort study of cancer risk in relation to family histories of cancer in the Utah population database. Cancer 2005;103:1906–1915. [DOI] [PubMed] [Google Scholar]
- Liu H, Zhang Y, Gu H, Feng QL, Liu JY, Zhou J, Yan F. Association between assisted reproductive technology and cardiac alteration at age 5 years. JAMA Pediatr 2015;169:603–605. [DOI] [PubMed] [Google Scholar]
- McGlynn KA, Devesa SS, Sigurdson AJ, Brown LM, Tsao L, Tarone RE. Trends in the incidence of testicular germ cell tumors in the United States. Cancer 2003;97:63–70. [DOI] [PubMed] [Google Scholar]
- Moller H, Skakkebaek NE. Risk of testicular cancer in subfertile men: case-control study. BMJ 1999;318:559–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordkap L, Joensen UN, Blomberg Jensen M, Jorgensen N. Regional differences and temporal trends in male reproductive health disorders: semen quality may be a sensitive marker of environmental exposures. Mol Cell Endocrinol 2012;355:221–230. [DOI] [PubMed] [Google Scholar]
- WHO WHO Laboratory Manual for the Examination and Processing of Human Semen. Switzerland: World Health Organization, 2010. [Google Scholar]
- Perneger TV. What's wrong with Bonferroni adjustments. BMJ 1998;316:1236–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990;1:43–46. [PubMed] [Google Scholar]
- Samadder NJ, Curtin K, Tuohy TMF, Rowe KG, Mineau GP, Smith KR, Pimentel R, Wong J, Boucher K, Burt RW. Increased risk of colorectal neoplasia among family members of patients with colorectal cancer: a population-based study in Utah. Gastroenterology 2014;147:814–821.e815. [DOI] [PubMed] [Google Scholar]
- Schoenfeld DA. Sample-size formula for the proportional-hazards regression model. Biometrics 1983;39:499–503. [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Buck Louis GM, Toppari J, Andersson AM, Eisenberg ML, Jensen TK, Jorgensen N, Swan SH, Sapra KJ et al. . Male Reproductive disorders and fertility trends: influences of environment and genetic susceptibility. Physiol Rev 2016;96:55–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod 2001;16:972–978. [DOI] [PubMed] [Google Scholar]
- Streiner DL. Best (but oft-forgotten) practices: the multiple problems of multiplicity-whether and how to correct for many statistical tests. Am J Clin Nutr 2015;102:721–728. [DOI] [PubMed] [Google Scholar]
- Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934–1996. Environ Health Perspect 2000;108:961–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tararbit K, Lelong N, Thieulin AC, Houyel L, Bonnet D, Goffinet F, Khoshnood B. The risk for four specific congenital heart defects associated with assisted reproductive techniques: a population-based evaluation. Hum Reprod 2013;28:367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventimiglia E, Capogrosso P, Boeri L, Serino A, Colicchia M, Ippolito S, Scano R, Papaleo E, Damiano R, Montorsi F et al. . Infertility as a proxy of general male health: results of a cross-sectional survey. Fertil Steril 2015;104:48–55. [DOI] [PubMed] [Google Scholar]
- Virtanen HE, Rajpert-De Meyts E, Main KM, Skakkebaek NE, Toppari J. Testicular dysgenesis syndrome and the development and occurrence of male reproductive disorders. Toxicol Appl Pharmacol 2005;207:501–505. [DOI] [PubMed] [Google Scholar]
- Walsh TJ, Schembri M, Turek PJ, Chan JM, Carroll PR, Smith JF, Eisenberg ML, Van Den Eeden SK, Croughan MS. Increased risk of high-grade prostate cancer among infertile men. Cancer 2010;116:2140–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics 2000;56:645–646. [DOI] [PubMed] [Google Scholar]
- Wohlfahrt-Veje C, Main KM, Skakkebaek NE. Testicular dysgenesis syndrome: foetal origin of adult reproductive problems. Clin Endocrinol (Oxf) 2009;71:459–465. [DOI] [PubMed] [Google Scholar]
- Zhu JL, Basso O, Obel C, Bille C, Olsen J. Infertility, infertility treatment, and congenital malformations: Danish national birth cohort. BMJ 2006;333:679. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.