ABSTRACT
Staphylococcus aureus is an important human pathogen that relies on a large repertoire of secreted and cell wall-associated proteins for pathogenesis. Consequently, the ability of the organism to cause disease is absolutely dependent on its ability to synthesize and successfully secrete these proteins. In this study, we investigate the role of peptidyl-prolyl cis/trans isomerases (PPIases) on the activity of the S. aureus secreted virulence factor nuclease (Nuc). We identify a staphylococcal cyclophilin-type PPIase (PpiB) that is required for optimal activity of Nuc. Disruption of ppiB results in decreased nuclease activity in culture supernatants; however, the levels of Nuc protein are not altered, suggesting that the decrease in activity results from misfolding of Nuc in the absence of PpiB. We go on to demonstrate that PpiB exhibits PPIase activity in vitro, is localized to the bacterial cytosol, and directly interacts with Nuc in vitro to accelerate the rate of Nuc refolding. Finally, we demonstrate an additional role for PpiB in S. aureus hemolysis and demonstrate that the S. aureus parvulin-type PPIase PrsA also plays a role in the activity of secreted virulence factors. The deletion of prsA leads to a decrease in secreted protease and phospholipase activity, similar to that observed in other Gram-positive pathogens. Together, these results demonstrate, for the first time to our knowledge, that PPIases play an important role in the secretion of virulence factors in S. aureus.
IMPORTANCE Staphylococcus aureus is a highly dangerous bacterial pathogen capable of causing a variety of infections throughout the human body. The ability of S. aureus to cause disease is largely due to an extensive repertoire of secreted and cell wall-associated proteins, including adhesins, toxins, exoenzymes, and superantigens. These virulence factors, once produced, are typically transported across the cell membrane by the secretory (Sec) system in a denatured state. Consequently, once outside the cell, they must refold into their active form. This step often requires the assistance of bacterial folding proteins, such as PPIases. In this work, we investigate the role of PPIases in S. aureus and uncover a cyclophilin-type enzyme that assists in the folding/refolding of staphylococcal nuclease.
KEYWORDS: cyclophilin, Nuc, PI-PLC, PPIase, parvulin, PpiB, PrsA, Staphylococcus aureus, nuclease, protease
INTRODUCTION
The proline peptide bond is unique in nature in that both the cis and trans forms can occur in vivo, with the cis conformation existing approximately 6.5% of the time (1). In contrast, for all other naturally occurring amino acids, steric hindrance between side chains precludes the cis form and overwhelmingly favors the trans form (2). The presence of both the cis and trans forms of proline peptide bonds has important consequences for protein tertiary structure. In certain cases, the isomerization state of proline peptide bonds has been shown to be the rate-limiting step in protein folding (3). Consequently, the action of peptidyl-prolyl cis/trans isomerases (PPIases), enzymes that catalyze the isomerization of proline peptide bonds between the cis and trans forms, is required to facilitate timely folding and subsequent protein activity (4).
PPIases are found in both prokaryotes and eukaryotes and are divided into three functional classes: (i) the cyclophilins, (ii) the FK506 binding proteins (FKBPs), and (iii) the parvulins (5). While all three subgroups demonstrate PPIase activity, there is no sequence similarity between groups, and each group demonstrates functional independence. Known PPIase inhibitors are group specific and do not affect the activity of members of other groups (5). Examples of all three PPIase groups are found in bacteria. Perhaps the best-studied example of a bacterial PPIase is the trigger factor Tig (a member of the FKBPs), which is found to be associated with the ribosome and assists in folding nascent peptides immediately following translation (6). Another well-studied bacterial PPIase is PrsA, a member of the parvulin family (2, 5). PrsA is a membrane-anchored lipoprotein located at the interface between the bacterial cell membrane and the cell wall. It is thought to assist in the refolding of proteins as they are exported from the bacterial cell. PrsA has been well studied in two bacterial pathogens, Listeria monocytogenes and Streptococcus pyogenes, where alteration of prsA levels (either increased or decreased) leads to defects in protein secretion and/or virulence of the organism (7–10). The cyclophilins are by far the least-studied and least well understood group of PPIases in bacteria. Those that have been studied are typically lipoproteins (similar to PrsA) and are thought to function is a similar way (11, 12). However, in silico analysis reveals that cytoplasmic cyclophilins are predicted to exist in the genomes of a number of bacterial species, although no function has been demonstrated for these. Although PPIases have been studied in other bacteria, their role in S. aureus is not well understood. Recently, mutation of prsA was shown to affect antibiotic resistance in S. aureus (13, 14); however, its role in protein secretion is unknown, and no studies have been performed on additional S. aureus PPIases.
Staphylococcal nuclease (Nuc) is a secreted virulence factor that has recently been shown to play an important role in immune evasion by S. aureus (15–17). Specifically, Nuc degrades neutrophil extracellular traps (NETs), allowing bacteria to evade killing and exclude macrophages from abscesses (17). Nuc also plays an important role in biofilms, where it is thought to facilitate detachment and dispersal of the biofilm, allowing the bacteria to spread to additional sites (18–20). Although the role of Nuc in S. aureus virulence has only recently emerged, the enzyme itself has been the subject of intensive investigation for many years (3, 4, 21–25). It has served as a model system for studies on the kinetics of protein folding and the role of PPIases due to the low rate of cis-trans isomerization around the Lys116-Pro117 bond (3, 4, 24, 25). Studies have shown that the isomerization rate of this bond is the rate-limiting step in Nuc folding and can be greatly accelerated in the presence of a PPIase. These in vitro studies utilized purified human cyclophilin as the PPIase (4, 26, 27), and to date, it is unknown whether PPIase activity is required in vivo in S. aureus cells to assist Nuc folding, and if so, the identity of the PPIase involved.
Based on in vitro studies showing that Nuc requires PPIase activity for optimal folding, and the fact that PPIases are required for correct folding and subsequent activity of secreted virulence factors in a number of bacterial pathogens (7–10, 28–31), we hypothesized that a staphylococcal PPIase is required for activity of the S. aureus secreted virulence factor Nuc. In this study, we identify an S. aureus PPIase from the cyclophilin family (which we name PpiB) and demonstrate that it is required for optimal activity of Nuc. We purify PpiB and show that it exhibits PPIase activity and can directly increase the rate of Nuc refolding in vitro. Interestingly, our results indicate that PpiB is localized to the bacterial cytosol and affects the activity of additional secreted virulence factors, suggesting that this PPIase has a novel mechanism of action on S. aureus secretion. Finally, we also demonstrate a role for the S. aureus parvulin family PPIase, PrsA, in virulence factor secretion.
RESULTS
Identification of S. aureus PPIase-encoding genes.
Previous studies have demonstrated that Nuc requires PPIase activity for optimal protein folding (3, 4). Based on this observation and the fact that PPIase-mediated folding is required for subsequent activity of secreted virulence factors in a number of bacterial pathogens (8–11, 27–30), we hypothesized that a staphylococcal PPIase may be required for the activity of Nuc. To begin to investigate this, we examined the genome of the community-associated methicillin-resistant S. aureus (MRSA) isolate USA300 for genes that encode PPIase enzymes. Three potential PPIase-encoding genes were identified, one from each of the three PPIase subfamilies (i.e., the parvulins, FK506 binding proteins [FKBPs], and cyclophilins) (Fig. 1A and C). SAUSA300_1790 (prsA) encodes a parvulin-like protein with a secretion signal sequence and is predicted to be a cell wall-associated lipoprotein. A number of recent studies have implicated S. aureus PrsA in resistance to antibiotics (13, 14); however, it is unknown what role, if any, it has in protein secretion. SAUSA300_0857 (which we named ppiB due to its homology to ppiB of Bacillus subtilis) is an uncharacterized gene putatively encoding a PPIase with homology to the cyclophilin family. PpiB does not contain a secretion signal sequence and therefore is predicted to be cytoplasmic. SAUSA300_1622 (tig) encodes the S. aureus homologue of bacterial trigger factor (TF), a PPIase found in the cytoplasm of virtually all bacteria. The PPIase domain of TF has homology with the FKBP family. To investigate the role of PPIases in S. aureus, we generated strains with mutations in prsA, ppiB, and tig. The deletion of prsA, ppiB, and/or tig did not alter the growth characteristics of S. aureus (Fig. 1B). Furthermore, an analysis of previously published RNA sequencing (RNA-seq) data revealed that (i) each PPIase gene is expressed during growth in tryptic soy broth (TSB) and human serum, and (ii) each gene is on a monocistronic transcript; therefore, there are no polar affects resulting from the inactivation of each gene (32).
FIG 1.
(A) Genomic location and organization of PPIase-encoding genes in S. aureus strain USA300. Orientation of the prsA, ppiB, and tig genes (and the genes flanking them) is indicated by the arrows. The sizes of prsA, ppiB, and tig are indicated below the genes. (B) Growth of wild-type S. aureus strain USA300 and PPIase mutants. No difference in growth rate was observed between the wild-type (WT) strain and the prsA, ppiB, or tig mutant strain. Data shown are the averages from two independent biological replicate cultures. Error bars represent the standard deviation. (C) Schematic representation depicting the domain structure of the three PPIases of S. aureus. The PPIase domains of PrsA, PpiB, and Tig (rotamase, cyclophilin, and FKBP) are indicated. PrsA and Tig contain SurA domains, while Tig contains an N-terminal ribosome-binding domain. PrsA contains a predicted signal peptide (SP) domain that targets it to the bacterial membrane. Amino acid numbers are provided below, indicating the positions of each domain within the protein.
A number of recent S. aureus transposon-sequencing (TnSeq) studies have been carried out to identify genes that potentially contribute to fitness in vivo and/or during infection (33, 34). We hypothesized that if a PPIase contributes to the folding/activity of Nuc, it is likely that the corresponding mutant would have decreased virulence/fitness in vivo. To test this hypothesis, we examined the data from the two TnSeq studies to ascertain if any of the three PPIase genes potentially contribute to the virulence of S. aureus. Interestingly, mutants in each of the three PPIase genes demonstrated fitness defects under different conditions. A ppiB mutant had decreased fitness in an abscess model of infection (34), while a prsA mutant had decreased fitness in human blood and during osteomyelitis infection (33, 34). A tig mutant demonstrated decreased fitness in abscess and osteomyelitis infection models (33, 34). These results suggest that PPIases may play important roles during S. aureus infection, which we hypothesize is via an effect on protein secretion and activity.
A ppiB mutant demonstrates decreased secreted nuclease activity.
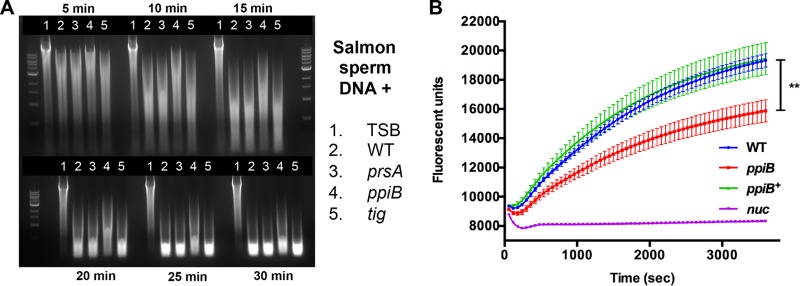
To determine if a PPIase is required for functional Nuc in S. aureus, we examined the activity of Nuc in culture supernatants from the wild-type and prsA, ppiB, and tig mutant strains. Nuc activity was initially measured by mixing culture supernatants with salmon sperm DNA, incubating at 37°C, and analyzing the resulting samples by agarose gel electrophoresis. Nuclease activity on the salmon sperm DNA was observed as a decrease in the apparent molecular weight of the DNA. The results demonstrated a comparable rate of digestion when DNA was incubated with culture supernatants from the wild-type and prsA and tig mutant strains (Fig. 2A, lanes 2, 3, and 5). However, salmon sperm DNA digestion was noticeably retarded when using culture supernatants from the ppiB mutant (Fig. 2A, lane 4). This result suggested that Nuc activity in culture supernatants from the ppiB mutant was reduced compared to that of the wild type. To confirm this result and investigate if the reduced Nuc activity is specifically due to PpiB, we performed an alternative nuclease activity assay using a single-stranded oligonucleotide DNA probe as the substrate for Nuc. The oligonucleotide probe contained a fluorophore and quencher at the 5′ and 3′ ends, respectively, and therefore, digestion of the probe results in an increase in fluorescence. The fluorescent nuclease activity assay was performed using culture supernatants from the wild-type strain as well as the ppiB mutant, nuc mutant (negative control), and a ppiB+ complement strain (where ppiB was provided in trans on a plasmid). The results confirm the decrease in Nuc activity in culture supernatants from the ppiB mutant (Fig. 2B). Nuc activity was restored to wild-type levels in the ppiB+ complement strain, confirming that the defect observed is due to PpiB. No nuclease activity was observed in the nuc mutant culture supernatants. Interestingly, the reduction in Nuc activity in the ppiB mutant strain was not as pronounced as that observed in a nuc mutant. This demonstrates that some functional Nuc is produced in the ppiB mutant strain. Consequently, we hypothesized that the reduction in activity observed is due to a defect in the folding of Nuc and not due to an absence/decreased levels of the protein.
FIG 2.
Culture supernatants from a ppiB mutant demonstrate decreased nuclease activity. (A) Sterile TSB or culture supernatants from the S. aureus wild-type (WT) or prsA, ppiB, or tig mutant strain were incubated at 37°C with salmon sperm DNA. At the time points indicated, samples from each reaction mixture were withdrawn and separated by agarose gel electrophoresis, and DNA was visualized with ethidium bromide. DNA degradation by nuclease present in S. aureus culture supernatants occurred in a time-dependent manner. At each time point, a higher-molecular-weight DNA product was observed in samples containing supernatants from the ppiB mutant, indicating decreased nuclease activity. (B) Culture supernatants from the S. aureus wild type and ppiB-lacking, ppiB+ (complement), and nuc-lacking mutants were incubated with a quenched fluorescent oligonucleotide probe. Cleavage of the DNA probe by secreted nuclease is measured by an increase in fluorescence over time. A significant decrease in nuclease activity was observed in the culture supernatant of the ppiB-lacking mutant. Nuclease activity was restored to wild-type levels in the complemented ppiB+ mutant strain. Data presented are the average of results from three independent biological replicates and two technical replicates. Error bars represent the standard error of the mean (SEM). Significance was determined by Student's t test. **, P < 0.01.
Secreted nuclease protein levels are not affected in a ppiB mutant.
To test the hypothesis that the decrease in Nuc activity observed in the ppiB mutant is not due to decreased levels of Nuc in the supernatants, we performed Western blotting to quantify Nuc in culture supernatants. Culture supernatants from the wild-type and prsA, ppiB, and nuc mutant strains were probed with a commercially available anti-Nuc antibody. The results show comparable levels of Nuc in the wild-type and prsA and ppiB mutant supernatants, while no Nuc was detected in the nuc mutant supernatant (Fig. 3). This result demonstrates that the decrease in Nuc activity in the ppiB culture supernatant does not result from decreased levels of the protein. Rather, it shows that in the absence of PpiB, there is reduced enzymatic activity from the Nuc protein produced, which we hypothesize is due to a decreased rate of folding and/or misfolding of the protein in the absence of PpiB.
FIG 3.
Western blot of secreted nuclease. Culture supernatants from wild-type and prsA, ppiB, and nuc mutant strains were separated by SDS-PAGE, and Western blotting was performed with an antinuclease antibody. No differences in nuclease levels were observed in the wild-type and prsA and ppiB mutant strains. No nuclease was detected in the nuc mutant strain.
PpiB demonstrates PPIase activity.
PpiB is predicted to be a PPIase from the cyclophilin family. Previous studies have shown that Nuc refolding is accelerated by PPIases from this family (4). These data and the results described above demonstrating that the activity of the Nuc protein is decreased in the absence of PpiB led us to hypothesize that the decrease in activity is due to misfolding of Nuc in the absence of PpiB PPIase activity. To test this hypothesis, we first investigated if PpiB is functional as a PPIase. Recombinant PpiB was purified and used in a chymotrypsin-coupled PPIase activity assay, as described by Fischer et al. (35). In the assay, a proline-containing oligopeptide is cleaved by chymotrypsin, resulting in an increase in absorbance at 420 nm. Chymotrypsin only cleaves the peptide when the prolyl peptide bond is in the trans conformation; therefore, the reaction initially proceeds rapidly and is then followed by a slow phase that is dependent on the rate of cis-to-trans isomerization of the remaining peptide. The addition of a PPIase results in a higher rate of isomerization and a higher rate of completion of the reaction. The results from the chymotrypsin cleavage assay demonstrated a higher rate of reaction when PpiB was included (Fig. 4). The rate of reaction was decreased when the cyclophilin inhibitor cyclosporine was included. These data clearly show that PpiB is functional as a cyclophilin family member PPIase.
FIG 4.

PpiB demonstrates PPIase activity. Purified recombinant PpiB (5 nM) was used in a chymotrypsin-coupled PPIase activity assay. In the absence of a PPIase (purple line), the initial high rate of oligopeptide cleavage decreases, and the reaction proceeds to completion based on the low rate of cis-to-trans isomerization of the peptide. Addition of PpiB to the reaction results in an increased rate of cleavage consistent with PpiB functioning as a PPIase (red line). The addition of cyclosporine, a cyclophilin family inhibitor, does not alter the rate of reaction (black line). When added to a reaction mixture containing PpiB (5 nM), cyclosporine completely inhibits the increased rate of cleavage observed upon addition of PpiB (blue line). Experiments were performed a minimum of three times. Representative data sets are shown.
PpiB is located in the bacterial cytoplasm.
The data presented above strongly suggest that PpiB is required for optimal folding of Nuc; however, PpiB does not contain a secretion signal sequence and is predicted to be located in the bacterial cytoplasm. PPIases that are known to assist in the refolding of secreted virulence factors are typically membrane associated. Therefore, to better understand how PpiB influences Nuc activity, we performed Western blotting to identify the cellular location of PpiB. The ppiB gene was cloned onto plasmid pMK4 with a hemagglutinin (HA) tag added to the C-terminal portion of the protein. Western blotting was then performed using an anti-HA antibody on protein samples from the bacterial cytoplasm, cell wall, and culture supernatant. A single band was detected in the cytoplasmic fraction that corresponded in size to HA-tagged PpiB (Fig. 5). Although this result cannot preclude the possibility that some PpiB exits the cell under certain conditions, it confirms the predicted cellular localization and demonstrates that under the conditions tested, PpiB is located in the bacterial cytoplasm.
FIG 5.
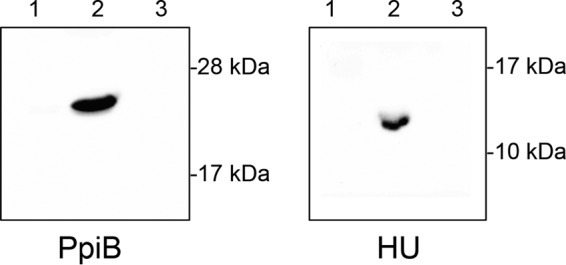
Cellular localization of PpiB. The ppiB gene was HA epitope tagged and expressed in S. aureus. Protein samples corresponding to the secreted fraction (lane 1), cytoplasm (lane 2), and cell envelope (lane 3) were taken. Western blotting was performed using an anti-HA antibody. Results show that PpiB is located in the bacterial cytoplasm. Cellular location of HA epitope-tagged HU (a nucleoid-associated protein) was determined as a control. Protein samples corresponding to the secreted fraction (lane 1), cytoplasm (lane 2), and cell envelope (lane 3) were prepared identically to those for PpiB. As expected, HA-tagged HU protein was located in the cytoplasmic fraction.
Nuc refolding is accelerated in the presence of PpiB.
The results described above show that PpiB is functional as a PPIase and strongly suggests that the PPIase activity of PpiB directly contributes to Nuc folding and activity. To test this hypothesis, we developed a Nuc PPIase assay whereby we measured the rate of Nuc refolding in the presence or absence of PpiB. Recombinant Nuc protein was purified and denatured in 8 M urea. Denatured Nuc was allowed to refold by diluting it into urea-free buffer in the presence or absence of PpiB. The activity of refolded Nuc in each sample was determined by adding a quenched single-stranded fluorescent oligonucleotide probe (as used as described above in Fig. 2). Nuc refolding/activation results in cleavage of the probe and fluorescence. An important benefit to this experimental approach (compared to others, such as tryptophan internalization) is that only the activity of correctly refolded Nuc will be measured. The results show that when PpiB is added to the reaction, the rate of Nuc refolding (and consequently fluorescence) is accelerated compared to reactions where PpiB is omitted (Fig. 6). Together, these data clearly demonstrate that PpiB is a cyclophilin family member PPIase that assists in folding the S. aureus secreted virulence factor Nuc.
FIG 6.

PpiB directly increases the rate of Nuc refolding. Denatured Nuc was diluted 1:40 into denaturant-free buffer containing a quenched fluorescent oligonucleotide probe. Refolding and subsequent activity of Nuc were visualized by fluorescence resulting from cleavage of the probe (blue line). The addition of PpiB, a PPIase, to the reaction resulted in a higher rate of refolding (red line). No activity was observed in the absence of Nuc protein (green line). Data presented are the average of results from three independent biological replicates and two technical replicates. Error bars represent SEM. Significance was determined by Student's t test. ****, P < 0.0001.
Disruption of PPIases does not alter the composition and abundance of secreted proteins in S. aureus.
Bacterial PPIases that are involved in protein secretion typically have more than one target in the cell. In L. monocytogenes, alterations in the abundance of secreted proteins were observed in a prsA mutant (7); however, the results for Nuc shown in Fig. 2 and 3 illustrate that the extracellular abundance of PPIase target proteins may not necessarily be altered in a PPIase mutant strain. Consequently, any attempt to identify additional targets of S. aureus PPIases cannot be solely based on an analysis of extracellular protein abundance alone, as the overall secreted protein profile may not be dramatically altered in composition and abundance. To test this hypothesis, we isolated the secreted protein fraction of S. aureus cultures and performed SDS-PAGE analysis, followed by silver staining, to visualize proteins in the supernatants. Although minor variations are evident, the overall secreted protein profiles of the wild-type and prsA, ppiB, and tig mutants are similar (Fig. 7). In contrast, the secreted protein profile of an agrB mutant (a strain known to have a dramatically altered protein secretion) shows widespread differences compared to all of the other strains. This result demonstrates that in S. aureus, the deletion of any of the three PPIases does not dramatically alter the composition and abundance of secreted proteins. It suggests that any additional PPIase targets will be present in equal amounts in culture supernatants (as is the case for Nuc); however, their activity may be decreased due to incorrect folding.
FIG 7.

SDS-PAGE and silver stain analysis of S. aureus culture supernatants. Cell-free 15-h-old culture supernatants (in duplicate) from the wild-type (WT) and prsA, ppiB, tig, and agrB mutant strains were concentrated 10-fold, separated by SDS-PAGE, and visualized by silver staining. The secreted protein profile of the agrB mutant exhibits widespread differences compared to the wild-type strain, reflecting the altered secretome of an agr mutant. In contrast, the secreted protein profiles of the wild-type and prsA, ppiB, and tig mutant strains are broadly similar, indicating that few differences exist in the extracellular composition and quantity of proteins in these strains.
Activity of S. aureus secreted virulence factors requires PPIase activity.
S. aureus secretes a large array of proteinaceous virulence factors, and as noted above, PPIases often have multiple targets. Therefore, we hypothesized that additional secreted protein targets may exist for S. aureus PPIases. As the abundance of secreted proteins is similar in the wild-type and PPIase mutants (Fig. 7), we elected to concentrate our search on proteins for which we could assay activity rather than abundance. To that end, we performed a targeted search for potential PPIase-dependent proteins by investigating secreted virulence factors for which functional assays were available and/or those that have known PPIase-dependent homologues in other bacterial species. Based on these criteria, we examined (i) protease activity, (ii) phospholipase activity, and (iii) hemolytic activity of S. aureus culture supernatants from wild-type and PPIase mutant strains.
In Streptococcus pyogenes, the activity of the secreted protease SpeB requires PPIase activity (9, 36). To determine if S. aureus secreted protease activity similarly requires a PPIase, we examined the ability of the prsA, ppiB, and tig mutant strains to degrade casein on milk agar plates (Fig. 8A). No decrease in protease activity was observed in the ppiB or tig mutant strain. However, a smaller zone of clearing was observed around the prsA mutant strain, indicating a reduction in protease activity in this strain. Protease activity was restored when prsA was provided in trans on a plasmid, demonstrating that PrsA is required for optimal protease activity.
FIG 8.

Decreased activity of S. aureus secreted virulence factors in PPIase mutant culture supernatants. (A) Protease activity assay. Deletion of the prsA gene leads to decreased protease activity on casein agar plates (prsA). Caseinolytic activity is restored to the prsA deletion mutant by providing the prsA gene in trans on a plasmid (prsA+). Protease activity is completely abolished in an aur mutant, while no decrease is observed in either the ppiB or the tig mutant strain. (B) Phospholipase activity assay. The activity of S. aureus secreted phospholipase C (PI-PLC) was determined by incubating cell-free supernatants with an artificial fluorogenic substrate. The fluorescence released is directly proportional to PI-PLC activity. A statistically significant decrease in activity is observed in culture supernatants from the prsA mutant but not the ppiB mutant. Data presented are the average of results from three independent biological replicates and two technical replicates. Error bars represent SEM. Significance was determined by Student's t test. ***, P < 0.005. (C) Human erythrocyte lysis assay. S. aureus culture supernatants were incubated with human red blood cells, and lysis was detected by pelleting intact erythrocytes and measuring the OD543 of the supernatant. A statistically significant decrease in hemolysis activity was observed between the ppiB mutant and wild type. No activity was observed in the agrB mutant or in cells incubated with TSB. Data shown are the average of results from four replicates. Error bars represent standard deviation. Significance was determined by Student's t test. **, P < 0.01.
In L. monocytogenes, the activity of the secreted phosphatidylinositol-specific phospholipase C (PI-PLC) is dependent on the PPIase PrsA2 (8). The S. aureus PI-PLC homologue has recently been shown to contribute to survival in human blood and neutrophils (37). To determine if S. aureus PI-PLC activity requires PPIase activity, we performed a PI-PLC assay using an artificial substrate and culture supernatants from the wild-type and prsA, ppiB, and plc mutant strains. No decrease in activity was observed between the wild-type and ppiB mutant strains; however, once again, a significant decrease in phospholipase activity was observed in the prsA mutant (Fig. 8B). No activity was observed in a phospholipase mutant strain (plc). These data demonstrate that similarly to L. monocytogenes, the S. aureus PI-PLC requires PrsA for optimal activity.
S. aureus produces a number of toxins capable of lysing human red blood cells (38). To determine if PPIases affect the activity of these toxins, an erythrocyte lysis assay was performed using culture supernatants from wild-type and prsA, ppiB, and tig mutant strains. An S. aureus strain containing a mutation in agrB (a regulator of S. aureus virulence gene expression) was included as a negative control. No decrease in hemolytic activity was observed using culture supernatants from the prsA or tig mutant strain; however, a significant decrease in hemolysis was observed with supernatants from the ppiB mutant (Fig. 8C). These results are similar to those observed in the nuclease assay (Fig. 2A). No hemolytic activity was observed in the agrB mutant or when using sterile TSB. Lysis of human red blood cells by S. aureus can be mediated by a number of toxins (38); therefore, the data in Fig. 8C strongly suggest that one or more of these factors require PpiB for optimal activity.
DISCUSSION
In this work, we investigated the role of three PPIases (PrsA, PpiB, and Tig) in the community-associated methicillin-resistant S. aureus (CA-MRSA) strain USA300. The study was initiated in an attempt to identify the staphylococcal PPIase responsible for assisting the folding of the secreted virulence factor Nuc. Previous studies had demonstrated that the isomerization state of the Lys116-Pro117 bond in Nuc is rate limiting for Nuc folding and can be accelerated by the action of a PPIase enzyme; however, the identity of the PPIase involved was not known. This led us to hypothesize that a PPIase, encoded on the S. aureus genome, is required for Nuc folding (and subsequent activity) in vivo. Here, we demonstrate that the S. aureus PPIase involved is an intracellular PPIase from the cyclophilin family, which we named PpiB. Although cyclophilins are known to exist in bacteria, their functions remain largely unexplored. Those that have been studied are typically cell wall-associated lipoproteins (11, 12), and there are very few reports on the role of cytoplasmic cyclophilins in bacteria. One known example of a cytoplasmic cyclophilin is the PpiB protein from B. subtilis (39). Deletion of the ppiB gene has no effect on bacterial growth/viability in rich medium; however, a growth defect was observed under starvation conditions (40). The reason for this growth defect is unknown, and no effect on protein secretion was demonstrated. Thus, our demonstration that PpiB affects the activity of secreted proteins is the first time, to our knowledge, that an intracellular cyclophilin has been implicated in bacterial protein secretion.
The general secretory (Sec) pathway is responsible for translocating the majority of extracellular proteins in S. aureus, including many virulence factors (41). Proteins exported via this system are in a denatured state during translocation; therefore, refolding of the proteins outside the cell is necessary for activity. Parvulin-type PPIases, e.g., PrsA, typically assist in this refolding after proteins have been translocated in the space between the cell membrane and cell wall (sometimes referred to as the Gram-positive periplasm). Our results, demonstrating that an intracellular PPIase affects the activity of secreted proteins (Nuc and one or more hemolytic toxins), raise some interesting and fundamental questions regarding the mechanism of action of PpiB and the location within the bacterial cell in which PpiB acts on its targets. Western blot analysis confirmed the in silico-predicted cytoplasmic location of PpiB (Fig. 5). This cellular localization is in agreement with previously published proteomic studies (42, 43). Therefore, it is reasonable to conclude that PpiB acts directly on its target proteins in the bacterial cytosol. Consequently, if the effect of PpiB on Nuc (and other secreted target proteins) is direct, as suggested by the results in Fig. 6, it most likely occurs in the cytoplasm, prior to Nuc secretion. If so, this suggests that PpiB can influence the isomerization state of prolyl bonds prior to protein secretion, and that this impact is maintained during translocation, allowing rapid refolding in the periplasmic space. A similar mechanism of action has been proposed for RopA in S. pyogenes (30). RopA is the S. pyogenes trigger factor homologue, an intracellular PPIase from the FKBP family that is required for activation of the cysteine protease SpeB after it has been secreted via the Sec pathway (30, 31, 36). Alternatively, it remains possible that PpiB exits the bacterial cell and exerts an influence on its targets posttranslocation. Previous studies have identified limited quantities of PpiB in secreted S. aureus protein fractions (43, 44); however, it is unknown if this is a result of unavoidable cellular lysis during growth or as a result of dedicated secretion of PpiB. If so, PpiB secretion likely occurs via some noncanonical mechanism, as it lacks a Sec-dependent signal sequence. Whether the activity of PpiB is confined to the cytoplasm or occurs outside the cell is the subject of ongoing investigations in our lab. Future work will also seek to identify the S. aureus hemolytic toxin(s) that requires PpiB for activity (Fig. 8C). Lysis of human red blood cells by S. aureus can be mediated by a number of different hemolytic toxins (38); therefore, the data in Fig. 8 strongly suggest that one or more of these factors require PpiB for optimal activity.
A number of recent reports have demonstrated a role for S. aureus PrsA in antibiotic resistance (13, 14). One proposed mechanism that contributes to methicillin resistance in MRSA strains is via export and/or folding of newly synthesized penicillin-binding protein 2A (PBP2A). Here, we also demonstrate that PrsA is required for the activity of S. aureus secreted virulence proteins, a role ascribed to PrsA homologues in other Gram-positive species but not previously demonstrated for S. aureus (7, 9, 29, 45). Due to the cellular location and demonstrated PPIase activity of S. aureus PrsA (46), it is highly likely that it functions in a manner similar to PrsA in other organisms. In support of this, it was recently demonstrated that S. aureus PrsA can functionally complement a L. monocytogenes prsA2 mutant for secreted phospholipase activity (47). Interestingly, in this work, we now demonstrate a similar role for PrsA in the activity of S. aureus PI-PLC (Fig. 8B).
A notable finding from this study is that PrsA and PpiB seem to act on distinct sets of secreted proteins, with no overlap in phenotypes evident. The prsA mutant exhibited decreased levels of protease and phospholipase activity but demonstrated wild-type levels of nuclease and hemolytic activity. The opposite was true for the ppiB mutant (i.e., wild-type levels of protease and phospholipase activity and reduced levels of nuclease and hemolytic activity). We expect that the four proteins/phenotypes identified in this study are not exhaustive, and additional secreted proteins exist that require PPIase activity (from either PrsA or PpiB). Determining what these proteins are and which PPIase they require for activity may provide novel insights into the mechanism of protein secretion in S. aureus and the role of PPIases in this process. Studies are under way in our lab to identify additional PPIase targets. We also plan to construct a prsA-ppiB double mutant (and possibly a prsA-ppiB-tig triple mutant). We will examine this strain to determine if the effects of deleting multiple PPIases are additive or if there is some functional overlap between PrsA and PpiB.
The continued emergence of antibiotic-resistant bacterial strains has led to an urgent need for new approaches to combat and treat infections caused by these organisms. Traditional antibiotic development approaches that target a limited number of bacterial components are increasingly ineffective, and therefore, novel bacterial targets must be investigated. PPIase enzymes represent one such promising novel target (2). The work presented here demonstrates that two functionally distinct PPIases are important for virulence factor production in S. aureus. Inhibition of PPIase activity is likely to lead to reduced activity from secreted virulence factors and decreased fitness/pathogenicity. Thus, inhibition of S. aureus PPIases may represent a novel antivirulence approach that would remove or reduce the ability of this dangerous pathogen to cause disease without killing the bacterial cell.
MATERIALS AND METHODS
Strains and strain construction.
All bacterial strains and plasmids used in this study are listed in Table 1. The oligonucleotides used are listed in Table 2 . S. aureus containing transposon insertions in the ppiB, tig, nuc, aur, agrB, and plc genes were obtained from the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) transposon mutant library (48) and transduced into S. aureus USA300 Houston (49) using bacteriophage ϕ11. A prsA mutant strain was constructed by allelic exchange using plasmid pJB38 (50). DNA sequences flanking the prsA gene were amplified using primer pairs OL2625/OL2626 and OL2627/OL2628 and cloned into pJB38 to generate plasmid pRKC0078. This plasmid was recombined onto the S. aureus chromosome and then excised to generate a prsA deletion strain, according to a published protocol (51). Complementation plasmids were constructed by cloning either the prsA or ppiB gene, along with its cognate promoter, into plasmid pMK4 (52). The prsA complementation plasmid, pRKC0126, was generated by amplifying and cloning the prsA gene using primers OL2064/OL2065. The primers used introduced a C-terminal His6 tag into the PrsA protein. The ppiB gene was amplified using primers OL2327/OL2328, which introduced a C-terminal hemagglutinin (HA) epitope tag. The resulting complementation plasmid, pRKC0131, was also used to identify the cellular location of PpiB (see below).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| S. aureus | ||
| USA300 HOU | Community-associated MRSA isolate cured of pUSA300-HOU-MRSA | 49 |
| RN4220 | Restriction-deficient transformation recipient | 56 |
| NE1769 | USA300 JE2 ppiB::Bursa; ppiB NARSA transposon mutant | NARSA |
| NE914 | USA300 JE2 tig::Bursa; tig NARSA transposon mutant | NARSA |
| NE710 | USA300 JE2 nuc::Bursa; nuc NARSA transposon mutant | NARSA |
| NE163 | USA300 JE2 aur::Bursa; aur NARSA transposon mutant | NARSA |
| NE95 | USA300 JE2 agrB::Bursa; agrB NARSA transposon mutant | NARSA |
| NE678 | USA300 JE2 plc::Bursa; plc NARSA transposon mutant | NARSA |
| RKC0085 | USA300 HOU ΔprsA | This work |
| RKC0113 | USA300 HOU ppiB::Bursa; ppiB mutant | This work |
| RKC0114 | USA300 HOU tig::Bursa; tig mutant | This work |
| RKC0165 | USA300 HOU agrB::Bursa; agrB mutant | This work |
| RKC0181 | USA300 HOU nuc::Bursa; nuc mutant | This work |
| RKC0182 | USA300 HOU aur::Bursa; aur mutant | This work |
| RCK0178 | USA300 HOU plc::Bursa; plc mutant | This work |
| RCK0063 | USA300 HOU hupA::pAZ106::hupA-HA | This work |
| RKC0129 | RKC0085 containing plasmid pRKC0126; prsA+ complement strain | This work |
| RKC0135 | RKC0113 containing plasmid pRKC0131; ppiB+ complement strain | This work |
| RKC0072 | USA300 HOU(pMK4) | This work |
| E. coli | ||
| DH5 | Cloning strain | Invitrogen |
| BL21(DE3)/pLysS | Protein expression strain | Promega |
| Plasmids | ||
| pMK4 | Gram-positive shuttle vector (Cmr) | 52 |
| pJB38 | Temperature-sensitive allelic exchange plasmid (Cmr) | 50 |
| pAZ106 | Suicide plasmid (Eryr) | |
| pET24d | C-terminal His6 tag expression vector | Novagen |
| pMALc5X | N-terminal MBP fusion expression vector | NEB |
| pRKC0078 | pJB38 containing DNA flanking prsA gene | This work |
| pRKC0126 | pMK4 prsA-His6 | This work |
| pRKC0131 | pMK4 ppiB-HA | This work |
| pRKC0061 | pAZ106 hupA-HA | This work |
| pRKC0212 | pMALc5X ppiB | This work |
| pRKC0276 | pET24d nuc | This work |
Cmr, chloramphenicol resistance; Eryr, erythromycin resistance.
TABLE 2.
Oligonucleotides used in this study
| Name | Sequencea |
|---|---|
| OL2625 | ATGGAATTCAGTGATGCTTTATTAGC |
| OL2626 | ATGACGCGTGTTGAAACTCCTTTGTA |
| OL2627 | ATGACGCGTGAGCTCATGTTTCAGTA |
| OL2628 | ATGGGTACCGGAAACGATGAGAAATATAG |
| OL2064 | CGCGGATCCGGCTTATATTCTATAATACCTACG |
| OL2065 | ATGCTGCAGTTAATGATGATGATGATGATGTTGGCTCATGCCGGATTGTCC |
| OL2372 | GGAATTCCAGTAGGGTTATGTAAATCACC |
| OL2373 | CGGGATCCTTAAGCGTAATCTGGAACATCGTATGGGTATTCTTCAACATCAATAGATTC |
| OL2161 | CGGAATTCGGAATTGGCTACGGTGATAATGC |
| OL2162 | CGGGATCCTTAAGCATAATCTGGAACATCATATGGATATTTTACAGCATCTTTTAATGC |
| #0127 | ATGGCTAACTATCCACAGTTAAAC |
| #0128 | GGAATTCTTATTCTTCAACATCAATAGATTC |
| #0199 | CATGCCATGGCAACTTCAACTAAAAAATTAC |
| #0200 | CCGCTCGAGTTGACCTGAATCAGCGTTGTC |
| FRET probe | Cy3-CCCCGGATCCACCCC-BHQ2 |
BHQ2, black hole quencher 2.
Bacterial growth conditions.
S. aureus cultures were routinely grown at 37°C with shaking in tryptic soy broth (TSB) and Escherichia coli cultures at 37°C with shaking in lysogeny broth (LB). Where appropriate, antibiotics were used at the following concentrations: 5 μg · ml−1 chloramphenicol, 100 μg · ml−1 ampicillin, and 50 μg · ml−1 kanamycin. For comparative analysis of supernatants, S. aureus cultures were synchronized as follows. Overnight starter cultures (5 ml) of each strain were diluted 1:100 in 100 ml of fresh prewarmed TSB and grown for 3 h to mid-exponential phase. The 3-h-old mid-exponential-phase cultures were subsequently diluted into 100 ml of fresh TSB at a starting optical density at 600 nm (OD600) of 0.05. The cultures were then grown for the time indicated, typically 15 h.
Nuclease activity assays.
The activity of secreted nuclease in S. aureus culture supernatants was determined in synchronized cultures that were grown for 15 h. Bacterial cells were pelleted by centrifugation and the resulting supernatants passed through a 0.22-μm-pore filter to remove any remaining cells. The cell-free supernatants were diluted 1:10 with fresh TSB and incubated with 500 μg · ml−1 salmon sperm DNA (Sigma), at 37°C in a thermocycler. At the time points indicated, samples from each reaction were withdrawn, separated on a 1% agarose gel, and visualized with ethidium bromide.
To further quantify Nuc activity in the ppiB mutant strain, we utilized a fluorescence resonance energy transfer (FRET)-based assay developed by Kiedrowski et al. (18), with minor modifications. Cell culture supernatants were diluted to 10−4 in 20 mM Tris (pH 8.0) with 10 mM CaCl2 and added to the FRET probe (described by Kiedrokski et al. [18]) (Table 2) diluted to 2 μM in the same buffer. Fluorescence of Cy3 released from the probe by nuclease activity was measured using a Synergy HTX plate reader (BioTek, Winooski, VT).
Western blotting.
Western blot analyses to quantify the amount of Nuc protein present in culture supernatants were performed as follows. Synchronized cultures were grown for 15 h, bacterial cells were removed, and the resulting cell-free supernatants were concentrated (10×) using Amicon Ultra-4 centrifugal filters with a 10,000 molecular weight cutoff (Millipore). Equal amounts of concentrated protein samples were separated by SDS-PAGE, transferred to a polyvinylidene difluoride (PVDF) membrane, and blocked with 5% skim milk, and Nuc detected using horseradish peroxidase (HRP)-linked anti-Staphylococcus DNase antibody (Abcam). Following three rounds of washing, Western blots were visualized with SuperSignal West Pico chemiluminescent substrate (Pierce) and exposure to X-ray film.
To determine the cellular location of PpiB, we constructed a plasmid (pRKC0131) in which the PpiB protein was N terminally epitope tagged with hemagglutinin (HA) and expressed from its native promoter. S. aureus containing this plasmid was grown to stationary phase and protein samples taken representing the intracellular (cytoplasmic), cell wall, and extracellular (secreted) fractions, as previously described (53). These samples were separated by SDS-PAGE, transferred to a PVDF membrane, and probed with an HA epitope tag antibody (Thermo Fisher). As a control, the cellular location of the nucleoid-associated protein HU was determined using a similar procedure. The hupA gene was amplified using the primer pair OL2161/OL2162. OL2162 included a 3′ extension corresponding to the HA epitope sequence. The resulting PCR product (hupA-HA) was cloned into suicide vector pAZ106 (to generate plasmid pRKC0061) and recombined into the S. aureus chromosome to generate strain RKC0063. Western blots using an HA epitope tag antibody were performed on this strain using protein samples (representing the cytoplasmic, cell wall, and secreted fractions) prepared identically to those outlined above for PpiB-HA.
Protein purification.
Recombinant forms of PpiB and Nuc were purified as follows. The ppiB gene was amplified using primer pair #0127/#0128 and cloned into vector pMALc5X (New England BioLabs [NEB]). The resulting plasmid, pRKC0212, expresses an N-terminal maltose binding protein (MBP) fusion to PpiB (MBP-PpiB). The presence of MBP fused to PpiB does not affect the PPIase activity of PpiB (this work). The MBP-PpiB fusion protein was expressed in E. coli as follows. A 100-ml flask of LB was inoculated with 1 ml of an overnight culture of NEB Express cells containing the pRKC0212 plasmid and grown to an OD600 of 0.6. Expression of the MBP-PpiB fusion was induced by the addition of isopropyl-β-d-thiogalactopyranoside (IPTG) (to a final concentration of 0.3 mM), and the culture was grown for an additional 3 h. The cells were harvested, sonicated in 5 ml of column buffer (20 mM Tris-HCl [pH 7.4], 200 mM NaCl, 1 mM EDTA), and centrifuged for 20 min at 20,000 × g. Cell lysates were loaded onto 0.5 ml of amylose resin that had been equilibrated with column buffer, washed two times with 10 ml of column buffer, and eluted in six 0.5-ml fractions with column buffer containing 10 mM maltose. Fractions containing MBP-PpiB were pooled and stored in 40% glycerol.
The nuc gene was amplified using primer pair #0199/#0200 and cloned into vector pET24d (Novagen). The resulting plasmid, pRKC0276, expresses the Nuc protein with a C-terminal 6-histidine tag (Nuc-His6). The presence of a C-terminal histidine tag does not affect the activity of Nuc (this work). The Nuc-His6 protein was expressed in E. coli BL21(DE3) pLysS and purified using nickel-nitrilotriacetic acid (Ni-NTA)–agarose resin (Qiagen), according to the manufacturer's protocol. Fractions containing Nuc were pooled and dialyzed against four changes of 1× phosphate-buffered saline (PBS) before storage in 40% glycerol (see Fig. S1 in the supplemental material for Coomassie blue-stained gels showing purified PpiB and Nuc).
Chymotrypsin-coupled PPIase activity assay.
The chymotrypsin-coupled PPIase activity assay originally described by Kofron et al. (54) was conducted as described by Zhang et al. (55), with minor modifications. Reaction mixtures containing assay buffer, chymotrypsin, and purified PpiB or water were prepared and stored briefly on ice. The reaction was initiated by adding the mixture to the oligopeptide Suc-AAFP-pNA (Sigma) in a cuvette and monitoring absorbance at 390 nm using a Genesys 5 spectrophotometer (Spectronic). Human cyclosporine (CsA; Sigma) was added as an inhibitor at a concentration of 5 μM.
Nuclease refolding assay.
A novel method for determining nuclease refolding and activity was developed based on the nuclease FRET assay described above. Recombinant histidine-tagged Nuc protein (Nuc-His6) was purified and denatured in 8 M urea. Denatured Nuc was diluted 1:40 into a reaction mixture (10 nM final concentration) containing buffer (20 mM Tris [pH 8.0], 10 mM CaCl2), the FRET probe (2 μM), and either purified recombinant PpiB (1 μM) or water. Dilution of the denatured Nuc (and the corresponding dilution of urea) facilitated refolding of the protein. The activity of refolded Nuc was observed by an increase in nuclease activity and cleavage of the oligonucleotide FRET probe. Variations in the rate of Nuc refolding are manifested as variations in the rate of nuclease activity against the FRET probe over time.
SDS-PAGE and silver staining.
The secreted protein profile of S. aureus was visualized by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and silver staining as follows. Samples of synchronized 15-h-old cell-free culture supernatants were concentrated (10×) using Amicon Ultra-4 centrifugal filters, mixed with 6× loading buffer, and separated on 12% SDS-PAGE gels. Proteins were visualized by staining with the Pierce color silver staining kit (Thermo Fisher Scientific), according to the manufacturer's protocol.
Protease activity assay.
Secreted protease activity of S. aureus strains was determined by plating onto casein agar plates (i.e., TSB agar plates containing 10% nonfat milk). Zones of clearing resulting from casein digestion were observed and photographed after 72 h of incubation at 37°C.
Lipase activity assay.
The activity of S. aureus secreted phosphatidylinositol-specific phospholipase C (PI-PLC) was determined using the fluorogenic substrate 4-methylumbelliferyl myo-inositol-1-phosphate, N-methyl-morpholine salt (Biosynth), as described previously (37). Synchronized cell-free S. aureus culture supernatants were prepared as outlined above and diluted 1:2 with sterile TSB. Diluted supernatants were incubated in 10 mM Tris (pH 6.8) containing 0.8 mM substrate. Reactions were performed in a 96-well plate and fluorescence (excitation/emission, 350 nm/450 nm) measured using a Synergy HTX plate reader (BioTek, Winooski, VT).
Hemolysis assay.
Synchronized cell-free S. aureus culture supernatants were diluted 1:2 in reaction buffer (40 mM CaCl2, 1.7% NaCl) and incubated at 37°C with 25 μl of whole human blood (BioreclamationIVT). Following a 10-min incubation, the samples were centrifuged at 5,500 × g, and the supernatant (100 μl) was transferred to a 96-well plate. The degree of erythrocyte lysis was determined by reading the OD543 of the samples.
Supplementary Material
ACKNOWLEDGMENTS
R.K.C. thanks members of the Shaw, Murphy, and Weyand labs for useful discussions. Special thanks also go to Stephen G. Smith for inspiration and insight.
This study was supported in part by a grant from the National Institute of Allergy and Infectious Diseases (grant AI080626 to L.N.S.).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JB.00453-16.
REFERENCES
- 1.Stewart DE, Sarkar A, Wampler JE. 1990. Occurrence and role of cis peptide bonds in protein structures. J Mol Biol 214:253–260. doi: 10.1016/0022-2836(90)90159-J. [DOI] [PubMed] [Google Scholar]
- 2.Ünal CM, Steinert M. 2014. Microbial peptidyl-prolyl cis/trans isomerases (PPIases): virulence factors and potential alternative drug targets. Microbiol Mol Biol Rev 78:544–571. doi: 10.1128/MMBR.00015-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tucker PW, Hazen EE Jr, Cotton FA. 1979. Staphylococcal nuclease reviewed: a prototypic study in contemporary enzymology. IV. The nuclease as a model for protein folding. Mol Cell Biochem 23:131–141. [DOI] [PubMed] [Google Scholar]
- 4.Veeraraghavan S, Nall BT, Fink AL. 1997. Effect of prolyl isomerase on the folding reactions of staphylococcal nuclease. Biochemistry 36:15134–15139. doi: 10.1021/bi971357r. [DOI] [PubMed] [Google Scholar]
- 5.Göthel SF, Marahiel MA. 1999. Peptidyl-prolyl cis-trans isomerases, a superfamily of ubiquitous folding catalysts. Cell Mol Life Sci 55:423–436. doi: 10.1007/s000180050299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann A, Bukau B, Kramer G. 2010. Structure and function of the molecular chaperone trigger factor. Biochim Biophys Acta 1803:650–661. doi: 10.1016/j.bbamcr.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Alonzo F III, Freitag NE. 2010. Listeria monocytogenes PrsA2 is required for virulence factor secretion and bacterial viability within the host cell cytosol. Infect Immun 78:4944–4957. doi: 10.1128/IAI.00532-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alonzo F III, Port GC, Cao M, Freitag NE. 2009. The posttranslocation chaperone PrsA2 contributes to multiple facets of Listeria monocytogenes pathogenesis. Infect Immun 77:2612–2623. doi: 10.1128/IAI.00280-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma Y, Bryant AE, Salmi DB, Hayes-Schroer SM, McIndoo E, Aldape MJ, Stevens DL. 2006. Identification and characterization of bicistronic speB and prsA gene expression in the group A streptococcus. J Bacteriol 188:7626–7634. doi: 10.1128/JB.01059-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olsen RJ, Sitkiewicz I, Ayeras AA, Gonulal VE, Cantu C, Beres SB, Green NM, Lei B, Humbird T, Greaver J, Chang E, Ragasa WP, Montgomery CA, Cartwright J Jr, McGeer A, Low DE, Whitney AR, Cagle PT, Blasdel TL, DeLeo FR, Musser JM. 2010. Decreased necrotizing fasciitis capacity caused by a single nucleotide mutation that alters a multiple gene virulence axis. Proc Natl Acad Sci U S A 107:888–893. doi: 10.1073/pnas.0911811107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hermans PW, Adrian PV, Albert C, Estevao S, Hoogenboezem T, Luijendijk IH, Kamphausen T, Hammerschmidt S. 2006. The streptococcal lipoprotein rotamase A (SlrA) is a functional peptidyl-prolyl isomerase involved in pneumococcal colonization. J Biol Chem 281:968–976. doi: 10.1074/jbc.M510014200. [DOI] [PubMed] [Google Scholar]
- 12.Cho K, Arimoto T, Igarashi T, Yamamoto M. 2013. Involvement of lipoprotein PpiA of Streptococcus gordonii in evasion of phagocytosis by macrophages. Mol Oral Microbiol 28:379–391. doi: 10.1111/omi.12031. [DOI] [PubMed] [Google Scholar]
- 13.Jousselin A, Manzano C, Biette A, Reed P, Pinho M, Rosato A, Kelley WL, Renzoni A. 2015. The Staphylococcus aureus chaperone PrsA is a new auxiliary factor of oxacillin resistance affecting penicillin-binding protein 2A. Antimicrob Agents Chemother 28:1656–1666. doi: 10.1128/AAC.02333-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jousselin A, Renzoni A, Andrey DO, Monod A, Lew DP, Kelley WL. 2012. The posttranslocational chaperone lipoprotein PrsA is involved in both glycopeptide and oxacillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother 56:3629–3640. doi: 10.1128/AAC.06264-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schilcher K, Andreoni F, Uchiyama S, Ogawa T, Schuepbach RA, Zinkernagel AS. 2014. Increased neutrophil extracellular trap-mediated Staphylococcus aureus clearance through inhibition of nuclease activity by clindamycin and immunoglobulin. J Infect Dis 210:473–482. doi: 10.1093/infdis/jiu091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olson ME, Nygaard TK, Ackermann L, Watkins RL, Zurek OW, Pallister KB, Griffith S, Kiedrowski MR, Flack CE, Kavanaugh JS, Kreiswirth BN, Horswill AR, Voyich JM. 2013. Staphylococcus aureus nuclease is an SaeRS-dependent virulence factor. Infect Immun 81:1316–1324. doi: 10.1128/IAI.01242-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thammavongsa V, Missiakas DM, Schneewind O. 2013. Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death. Science 342:863–866. doi: 10.1126/science.1242255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiedrowski MR, Kavanaugh JS, Malone CL, Mootz JM, Voyich JM, Smeltzer MS, Bayles KW, Horswill AR. 2011. Nuclease modulates biofilm formation in community-associated methicillin-resistant Staphylococcus aureus. PLoS One 6:e26714. doi: 10.1371/journal.pone.0026714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moormeier DE, Bose JL, Horswill AR, Bayles KW. 2014. Temporal and stochastic control of Staphylococcus aureus biofilm development. mBio 5:e01341-14. doi: 10.1128/mBio.01341-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mann EE, Rice KC, Boles BR, Endres JL, Ranjit D, Chandramohan L, Tsang LH, Smeltzer MS, Horswill AR, Bayles KW. 2009. Modulation of eDNA release and degradation affects Staphylococcus aureus biofilm maturation. PLoS One 4:e5822. doi: 10.1371/journal.pone.0005822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tucker PW, Hazen EE Jr, Cotton FA. 1978. Staphylococcal nuclease reviewed: a prototypic study in contemporary enzymology. I. Isolation; physical and enzymatic properties. Mol Cell Biochem 22:67–77. [DOI] [PubMed] [Google Scholar]
- 22.Tucker PW, Hazen EE Jr, Cotton FA. 1979. Staphylococcal nuclease reviewed: a prototypic study in contemporary enzymology. III. Correlation of the three-dimensional structure with the mechanisms of enzymatic action. Mol Cell Biochem 23:67–86. [DOI] [PubMed] [Google Scholar]
- 23.Tucker PW, Hazen EE Jr, Cotton FA. 1979. Staphylococcal nuclease reviewed: a prototypic study in contemporary enzymology. II. Solution studies of the nucleotide binding site and the effects of nucleotide binding. Mol Cell Biochem 23:3–16. [DOI] [PubMed] [Google Scholar]
- 24.Hodel A, Kautz RA, Jacobs MD, Fox RO. 1993. Stress and strain in staphylococcal nuclease. Protein Sci 2:838–850. doi: 10.1002/pro.5560020513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maki K, Ikura T, Hayano T, Takahashi N, Kuwajima K. 1999. Effects of proline mutations on the folding of staphylococcal nuclease. Biochemistry 38:2213–2223. doi: 10.1021/bi981962+. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Albers MW, Chen CM, Schreiber SL, Walsh CT. 1990. Cloning, expression, and purification of human cyclophilin in Escherichia coli and assessment of the catalytic role of cysteines by site-directed mutagenesis. Proc Natl Acad Sci U S A 87:2304–2308. doi: 10.1073/pnas.87.6.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer G, Wittmann-Liebold B, Lang K, Kiefhaber T, Schmid FX. 1989. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature 337:476–478. doi: 10.1038/337476a0. [DOI] [PubMed] [Google Scholar]
- 28.Alonzo F III, Xayarath B, Whisstock JC, Freitag NE. 2011. Functional analysis of the Listeria monocytogenes secretion chaperone PrsA2 and its multiple contributions to bacterial virulence. Mol Microbiol 80:1530–1548. doi: 10.1111/j.1365-2958.2011.07665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kontinen VP, Sarvas M. 1993. The PrsA lipoprotein is essential for protein secretion in Bacillus subtilis and sets a limit for high-level secretion. Mol Microbiol 8:727–737. doi: 10.1111/j.1365-2958.1993.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 30.Lyon WR, Caparon MG. 2003. Trigger factor-mediated prolyl isomerization influences maturation of the Streptococcus pyogenes cysteine protease. J Bacteriol 185:3661–3667. doi: 10.1128/JB.185.12.3661-3667.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyon WR, Gibson CM, Caparon MG. 1998. A role for trigger factor and an rgg-like regulator in the transcription, secretion and processing of the cysteine proteinase of Streptococcus pyogenes. EMBO J 17:6263–6275. doi: 10.1093/emboj/17.21.6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carroll RK, Weiss A, Broach WH, Wiemels RE, Mogen AB, Rice KC, Shaw LN. 2016. Genome-wide annotation, identification, and global transcriptomic analysis of regulatory or small RNA gene expression in Staphylococcus aureus. mBio 7:e01990-15. doi: 10.1128/mBio.01990-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilde AD, Snyder DJ, Putnam NE, Valentino MD, Hammer ND, Lonergan ZR, Hinger SA, Aysanoa EE, Blanchard C, Dunman PM, Wasserman GA, Chen J, Shopsin B, Gilmore MS, Skaar EP, Cassat JE. 2015. Bacterial hypoxic responses revealed as critical determinants of the host-pathogen outcome by TnSeq analysis of Staphylococcus aureus invasive infection. PLoS Pathog 11:e1005341. doi: 10.1371/journal.ppat.1005341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valentino MD, Foulston L, Sadaka A, Kos VN, Villet RA, Santa Maria J Jr, Lazinski DW, Camilli A, Walker S, Hooper DC, Gilmore MS. 2014. Genes contributing to Staphylococcus aureus fitness in abscess- and infection-related ecologies. mBio 5:e01729-14. doi: 10.1128/mBio.01729-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fischer G, Bang H, Mech C. 1984. Determination of enzymatic catalysis for the cis-trans-isomerization of peptide binding in proline-containing peptides. Biomed Biochim Acta 43:1101–1111. (In German.) [PubMed] [Google Scholar]
- 36.Carroll RK, Musser JM. 2011. From transcription to activation: how group A streptococcus, the flesh-eating pathogen, regulates SpeB cysteine protease production. Mol Microbiol 81:588–601. doi: 10.1111/j.1365-2958.2011.07709.x. [DOI] [PubMed] [Google Scholar]
- 37.White MJ, Boyd JM, Horswill AR, Nauseef WM. 2014. Phosphatidylinositol-specific phospholipase C contributes to survival of Staphylococcus aureus USA300 in human blood and neutrophils. Infect Immun 82:1559–1571. doi: 10.1128/IAI.01168-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spaan AN, Reyes-Robles T, Badiou C, Cochet S, Boguslawski KM, Yoong P, Day CJ, de Haas CJ, van Kessel KP, Vandenesch F, Jennings MP, Le Van Kim C, Colin Y, van Strijp JA, Henry T, Torres VJ. 2015. Staphylococcus aureus targets the Duffy antigen receptor for chemokines (DARC) to lyse erythrocytes. Cell Host Microbe 18:363–370. doi: 10.1016/j.chom.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herrler M, Bang H, Marahiel MA. 1994. Cloning and characterization of ppiB, a Bacillus subtilis gene which encodes a cyclosporin A-sensitive peptidyl-prolyl cis-trans isomerase. Mol Microbiol 11:1073–1083. doi: 10.1111/j.1365-2958.1994.tb00384.x. [DOI] [PubMed] [Google Scholar]
- 40.Göthel SF, Scholz C, Schmid FX, Marahiel MA. 1998. Cyclophilin and trigger factor from Bacillus subtilis catalyze in vitro protein folding and are necessary for viability under starvation conditions. Biochemistry 37:13392–13399. doi: 10.1021/bi981253w. [DOI] [PubMed] [Google Scholar]
- 41.Schallenberger MA, Niessen S, Shao C, Fowler BJ, Romesberg FE. 2012. Type I signal peptidase and protein secretion in Staphylococcus aureus. J Bacteriol 194:2677–2686. doi: 10.1128/JB.00064-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Becher D, Hempel K, Sievers S, Zuhlke D, Pane-Farre J, Otto A, Fuchs S, Albrecht D, Bernhardt J, Engelmann S, Volker U, van Dijl JM, Hecker M. 2009. A proteomic view of an important human pathogen–towards the quantification of the entire Staphylococcus aureus proteome. PLoS One 4:e8176. doi: 10.1371/journal.pone.0008176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hempel K, Herbst FA, Moche M, Hecker M, Becher D. 2011. Quantitative proteomic view on secreted, cell surface-associated, and cytoplasmic proteins of the methicillin-resistant human pathogen Staphylococcus aureus under iron-limited conditions. J Proteome Res 10:1657–1666. doi: 10.1021/pr1009838. [DOI] [PubMed] [Google Scholar]
- 44.Rivera FE, Miller HK, Kolar SL, Stevens SM Jr, Shaw LN. 2012. The impact of CodY on virulence determinant production in community-associated methicillin-resistant Staphylococcus aureus. Proteomics 12:263–268. doi: 10.1002/pmic.201100298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vitikainen M, Lappalainen I, Seppala R, Antelmann H, Boer H, Taira S, Savilahti H, Hecker M, Vihinen M, Sarvas M, Kontinen VP. 2004. Structure-function analysis of PrsA reveals roles for the parvulin-like and flanking N- and C-terminal domains in protein folding and secretion in Bacillus subtilis. J Biol Chem 279:19302–19314. doi: 10.1074/jbc.M400861200. [DOI] [PubMed] [Google Scholar]
- 46.Heikkinen O, Seppala R, Tossavainen H, Heikkinen S, Koskela H, Permi P, Kilpelainen I. 2009. Solution structure of the parvulin-type PPIase domain of Staphylococcus aureus PrsA–implications for the catalytic mechanism of parvulins. BMC Struct Biol 9:17. doi: 10.1186/1472-6807-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cahoon LA, Freitag NE. 2015. Identification of conserved and species-specific functions of the Listeria monocytogenes PrsA2 secretion chaperone. Infect Immun 83:4028–4041. doi: 10.1128/IAI.00504-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fey PD, Endres JL, Yajjala VK, Widhelm TJ, Boissy RJ, Bose JL, Bayles KW. 2013. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. mBio 4:e00537-12. doi: 10.1128/mBio.00537-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kolar SL, Nagarajan V, Oszmiana A, Rivera FE, Miller HK, Davenport JE, Riordan JT, Potempa J, Barber DS, Koziel J, Elasri MO, Shaw LN. 2011. NsaRS is a cell-envelope-stress-sensing two-component system of Staphylococcus aureus. Microbiology 157:2206–2219. doi: 10.1099/mic.0.049692-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bose JL, Fey PD, Bayles KW. 2013. Genetic tools to enhance the study of gene function and regulation in Staphylococcus aureus. Appl Environ Microbiol 79:2218–2224. doi: 10.1128/AEM.00136-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bose JL. 2014. Genetic manipulation of staphylococci. Methods Mol Biol 1106:101–111. doi: 10.1007/978-1-62703-736-5_8. [DOI] [PubMed] [Google Scholar]
- 52.Sullivan MA, Yasbin RE, Young FE. 1984. New shuttle vectors for Bacillus subtilis and Escherichia coli which allow rapid detection of inserted fragments. Gene 29:21–26. doi: 10.1016/0378-1119(84)90161-6. [DOI] [PubMed] [Google Scholar]
- 53.Carroll RK, Rivera FE, Cavaco CK, Johnson GM, Martin D, Shaw LN. 2014. The lone S41 family C-terminal processing protease in Staphylococcus aureus is localized to the cell wall and contributes to virulence. Microbiology 160:1737–1748. doi: 10.1099/mic.0.079798-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kofron JL, Kuzmic P, Kishore V, Colon-Bonilla E, Rich DH. 1991. Determination of kinetic constants for peptidyl prolyl cis-trans isomerases by an improved spectrophotometric assay. Biochemistry 30:6127–6134. doi: 10.1021/bi00239a007. [DOI] [PubMed] [Google Scholar]
- 55.Zhang XC, Wang WD, Wang JS, Pan JC. 2013. PPIase independent chaperone-like function of recombinant human cyclophilin A during arginine kinase refolding. FEBS Lett 587:666–672. doi: 10.1016/j.febslet.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 56.Kreiswirth BN, Lofdahl S, Betley MJ, O'Reilly M, Schlievert PM, Bergdoll MS, Novick RP. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305:709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.