ABSTRACT
Glycerophosphoinositol (GPI) is a compatible solute present in a few hyperthermophiles. Interestingly, different GPI stereoisomers accumulate in Bacteria and Archaea, and the basis for this domain-dependent specificity was investigated herein. The archaeon Archaeoglobus fulgidus and the bacterium Aquifex aeolicus were used as model organisms. The synthesis of GPI involves glycerol phosphate cytidylyltransferase (GCT), which catalyzes the production of CDP-glycerol from CTP and glycerol phosphate, and di-myo-inositol phosphate-phosphate synthase (DIPPS), catalyzing the formation of phosphorylated GPI from CDP-glycerol and l-myo-inositol 1-phosphate. DIPPS of A. fulgidus recognized the two CDP-glycerol stereoisomers similarly. This feature and the ability of 31P nuclear magnetic resonance (NMR) to distinguish the GPI diastereomers provided a means to study the stereospecificity of GCTs. The AF1418 gene and genes aq_185 and aq_1368 are annotated as putative GCT genes in the genomes of A. fulgidus and Aq. aeolicus, respectively. The functions of these genes were determined by assaying the activity of the respective recombinant proteins: AQ1368 and AQ185 are GCTs, while AF1418 has flavin adenine dinucleotide (FAD) synthetase activity. AQ185 is absolutely specific for sn-glycerol 3-phosphate, while AQ1368 recognizes the two enantiomers but has a 2:1 preference for sn-glycerol 3-phosphate. In contrast, the partially purified A. fulgidus GCT uses sn-glycerol 1-phosphate preferentially (4:1). Significantly, the predominant GPI stereoforms found in the bacterium and the archaeon reflect the distinct stereospecificities of the respective GCTs: i.e., A. fulgidus accumulates predominantly sn-glycero-1-phospho-3-l-myo-inositol, while Aq. aeolicus accumulates sn-glycero-3-phospho-3-l-myo-inositol.
IMPORTANCE Compatible solutes of hyperthermophiles show high efficacy in thermal protection of proteins in comparison with solutes typical of mesophiles; therefore, they are potentially useful in several biotechnological applications. Glycerophosphoinositol (GPI) is synthesized from CDP-glycerol and l-myo-inositol 1-phosphate in a few hyperthermophiles. In this study, the molecular configuration of the GPI stereoisomers accumulated by members of the Bacteria and Archaea was established. The stereospecificity of glycerol phosphate cytidylyltransferase (GCT), the enzyme catalyzing the synthesis of CDP-glycerol, is crucial to the stereochemistry of GPI. However, the stereospecific properties of GCTs have not been investigated thus far. We devised a method to characterize GCT stereospecificity which does not require sn-glycerol 1-phosphate, a commercially unavailable substrate. This led us to understand the biochemical basis for the distinct GPI stereoisomer composition observed in archaea and bacteria.
KEYWORDS: Glycerol phosphate cytidylyltransferase, glycerophosphoinositol, molecular configuration, stereospecificity
INTRODUCTION
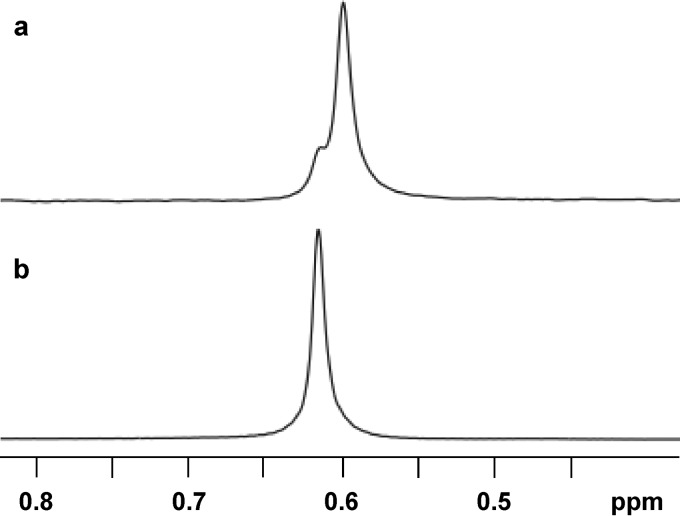
Glycerophosphoinositol (GPI) is an ionic compatible solute only found thus far in hyperthermophilic bacteria and archaea of the genera Aquifex and Archaeoglobus, respectively (1). 31P and 1H nuclear magnetic resonance (NMR) analysis of GPI preparations derived from biomass of the natural producers indicated that different stereoisomers accumulate in archaea and bacteria (2) (Fig. 1). The two compounds generate similar 1H NMR spectra but give rise to distinct 31P NMR resonances: hence, they are diastereomers. However, the full characterization of the stereochemistry of the two GPI forms remained elusive.
FIG 1.
31P NMR of GPI accumulated by Archaeoglobus fulgidus (a) and Aquifex aeolicus (b). The GPI preparations were obtained from cell biomass through ethanolic extraction (adapted from reference 2).
A large number of in vitro studies demonstrated that negatively charged compatible solutes, highly restricted to (hyper)thermophilic marine organisms, are better protectors of proteins against heat denaturation in comparison with compatible solutes typical of mesophilic organisms, such as trehalose or glycerol (3, 4). More recently, the efficacy of one such solute, mannosylglycerate, in inhibiting protein aggregation in the cytosol of a yeast model of Parkinson′s disease was also reported (5). However, the ambition for widespread utilization of these solutes in biotechnological applications is frustrated by the lack of efficient production methods. Large-scale production can be envisaged either by chemical synthesis or by introduction of the relevant biosynthetic pathways in suitable industrial hosts (synthetic biology approach). In either case, knowledge of the precise molecular structure of the natural compounds is required.
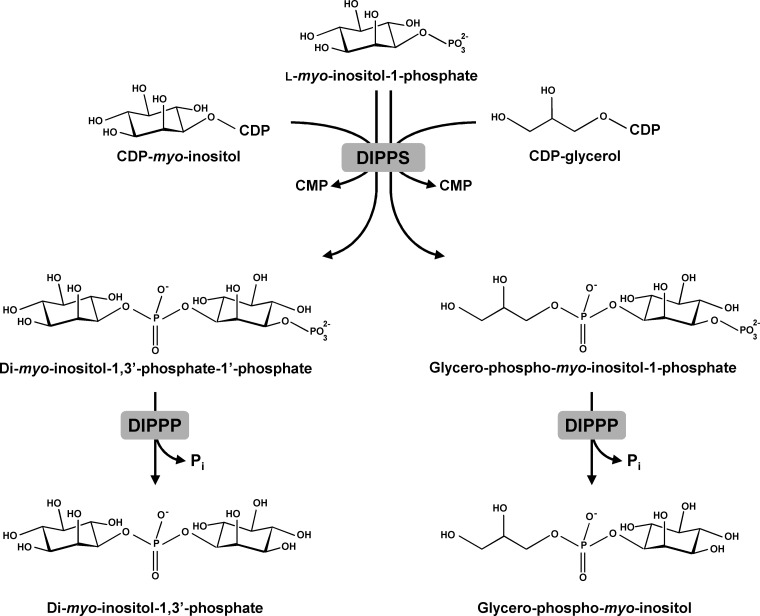
As with other polyol- or sugar-derived compatible solutes, the biosynthesis of GPI proceeds via a phosphorylated intermediate, GPI-phosphate (GPIP), which is further converted into GPI by a yet unknown phosphatase. GPIP is synthesized from CDP-glycerol and l-myo-inositol 1-phosphate in a reaction catalyzed by the membrane protein involved in the synthesis of di-myo-inositol phosphate (DIP), a canonical solute of marine hyperthermophiles. In some organisms, DIP-phosphate synthase (DIPPS), is part of a bifunctional enzyme that converts CTP and l-myo-inositol 1-phosphate into the phosphorylated form of di-myo-inositol phosphate (Fig. 2). Recently, the 3-dimensional structure of this bifunctional protein, comprising both cytosolic and transmembrane domains, has been determined (6). The soluble domain, inositol phosphate cytidylyltransferase (IPCT), catalyzes the formation of CDP-inositol from CTP and l-myo-inositol 1-phosphate, while the membrane domain, DIPPS, catalyzes the transfer of inositol phosphate from CDP-inositol to l-myo-inositol l-phosphate to yield the phosphorylated form of DIP. The latter enzyme uses exclusively l-myo-inositol 1-phosphate as an alcohol acceptor but is less specific for the alcohol donor as it recognizes CDP-l-myo-inositol, CDP-d-myo-inositol, and CDP-glycerol (7, 8). Consequently, phosphorylated GPI is a product of the reaction when CDP-glycerol and l-myo-inositol 1-phosphate are provided as the substrates (Fig. 2). All of the recombinant DIPPS enzymes characterized thus far are able to synthesize DIP and GPI (8). However, the solute GPI has been found only in species of the genera Archaeoglobus and Aquifex (1).
FIG 2.
Pathways for the synthesis of di-myo-inositol phosphate (DIP) and glycerophosphoinositol (GPI). The DIP-phosphate synthase (DIPPS) catalyzes the condensation of l-myo-inositol 1-phosphate with CDP-inositol or with CDP-glycerol, producing DIP-phosphate or GPI-phosphate, respectively. Subsequently, phosphorylated intermediates are converted into DIP or GPI by unknown DIPP phosphatase(s) (DIPPP).
We determined the stereochemistry of the l-myo-inositol 1-phosphate moiety in GPI by 13C isotopic labeling and NMR analysis to prove that the phosphate group is linked at position 3 of myo-inositol (the numbering of the inositol atoms is based on the l-configuration according to the “relaxation of lowest-locant rule” as recommended by the Nomenclature Committee of the International Union of Biochemistry), regardless of the bacterial or archaeal origin of the enzyme (8). However, the stereochemistry of the glycerol moiety has not been determined.
CDP-glycerol is synthesized by the action of glycerol phosphate cytidylyltransferase (GCT) from CTP and glycerol phosphate. All GCTs characterized thus far have a bacterial origin and can use sn-glycerol 3-phosphate to form CDP-3-glycerol, a precursor for the synthesis of poly(glycerol phosphate), which is a major component of the cell wall teichoic acids of Gram-positive bacteria (9). The observation that cell wall teichoic acids of Bacillus subtilis are essential for cell viability drew attention to this promising antimicrobial drug target. Prompted by this medical interest, several GCTs from Bacillus subtilis, Staphylococcus aureus, Listeria monocytogenes, and Enterococcus faecalis have been biochemically and structurally characterized (10–17). Oddly, in contrast with the great research effort directed to the determination of 3-dimensional structures and catalytic mechanisms, there is no information on the specificity of these enzymes for the enantiomers of glycerol phosphate.
In this work, the configuration of the GPI stereoisomers synthesized in members of the Bacteria and Archaea was identified and the biochemical basis for the domain-dependent specificity was investigated. To this end, we devised a method to study the stereospecificity of the GCTs, which does not require sn-glycerol 1-phosphate, a commercially unavailable substrate. Moreover, genes aq_1368, aq_185 and AF1418 were functionally characterized after heterologous expression in Escherichia coli.
RESULTS
Functional studies.
The archaeon Archaeoglobus fulgidus and the bacterium Aquifex aeolicus accumulate different diastereomers of GPI (Fig. 1). As previously established by 13C-specific isotopic labeling and NMR analysis, the inositol moiety has the same stereochemical configuration in the two natural diastereomers (2, 8); hence, the glycerol moieties must have distinct configurations.
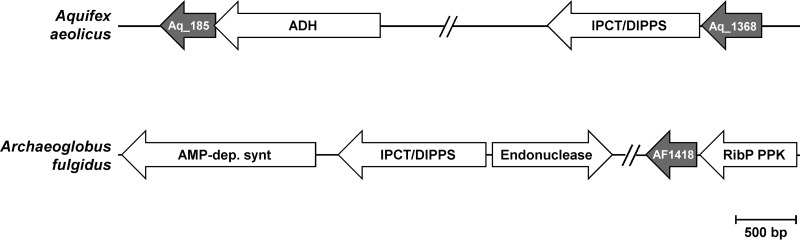
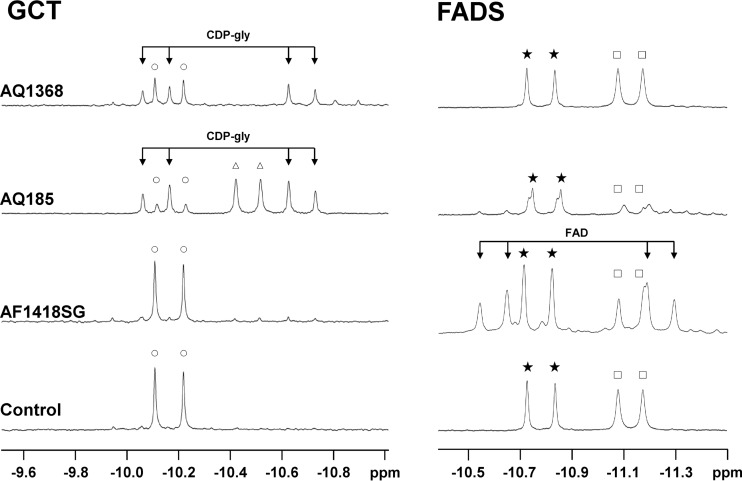
Glycerol phosphate cytidylyltransferases (GCTs) are the key enzymes in the synthesis of CDP-glycerol, one of the precursors of GPI (7) (Fig. 2). Genomic analysis revealed the presence of one gene in A. fulgidus (AF1418) and two genes in Aq. aeolicus (aq_185 and aq_1368) coding for putative GCTs (Fig. 3). These genes were cloned and expressed in Escherichia coli, and the activity of the encoded proteins was investigated in cell extracts. GCT activity was detected in cells producing either AQ185 or AQ1368 (Fig. 4), but no such activity was detected for the gene product of A. fulgidus (AF1418). Recently, the annotation of the A. fulgidus genome was revised, and the AF1418 gene was assigned to putative flavin adenine dinucleotide synthetase (FADS). We confirmed that cell extracts of E. coli harboring the synthetic AF1418 gene did activate flavin mononucleotide (FMN) with ATP, yielding FAD (Fig. 4). Pure recombinant AQ1368 and AQ185 were also assayed for FADS activity: very weak FAD signals were detected only for the latter enzyme. In summary, we found that AQ1368 and AQ185 are GCTs, while AF1418 is an FADS. BLAST searches were carried out in the genome of A. fulgidus, using the sequences of Aq. aeolicus GCTs (AQ1368 and AQ185) as the query, but no good candidate to encode a GCT was found. However, we confirmed the presence of GCT activity in cell extracts of A. fulgidus and decided to perform subsequent characterization studies with a partially purified preparation of the native enzyme, herein designated “partially purified A. fulgidus GCT.”
FIG 3.
Schematic organization of the genes encoding the enzymes implicated in the synthesis of glycerophosphoinositol (GPI), CDP-glycerol, or FAD in Aquifex aeolicus and Archaeoglobus fulgidus. Aq_185 and Aq_1368, genes encoding glycerol phosphate cytidylyltransferases; AF1418, gene encoding an FAD synthetase; ADH, aldehyde dehydrogenase; IPCT, inositol phosphate cytidylyltransferase; DIPPS, di-myo-inositol phosphate-phosphate synthase, which also catalyzes the synthesis of GPI-phosphate; AMP-dep. synt, AMP-dependent synthetase; and RibP PPK, ribose phosphate pyrophosphokinase.
FIG 4.
Assays for glycerol phosphate cytidylyltransferase (left panel) and FAD synthetase (right panel) activities. The traces are sections of 31P NMR spectra of reaction mixtures after incubation at 80°C of the relevant substrates and cofactors with cell extracts of E. coli harboring pET19b (control), pET19b:AF_1418SG, pET19b:aq_185, and pET19b:aq_1368. The reaction mixture for detection of GCT activity contained the following: 4 mM CTP, 4 mM rac-glycerol phosphate, and 10 mM MgCl2 in 50 mM Tris-HCl buffer (pH 8.6). The reaction mixture for detection of FADS activity contained the following: 10 mM ATP, 5 mM FMN, 15 mM DTT, and 10 mM MgCl2 in 50 mM Tris-HCl buffer (pH 8.6). FAD, flavin adenine dinucleotide; CDP-gly, CDP-glycerol. Resonance symbols: ○, CTP; △, CDP; ★, ATP; □, ADP.
Approach to study the stereospecificity of GCTs.
We designed a procedure to determine the stereospecificity of CGTs for sn-glycerol 3-phosphate and sn-glycerol 1-phosphate, which does not require pure sn-glycerol 1-phosphate, a compound not available commercially. The protocol comprises two steps (Fig. 5). In the first one, CDP-glycerol is produced by the target GCT from the substrates CTP and rac-glycerol phosphate. NMR cannot distinguish the stereoisomers of CDP-glycerol; hence, a second step is needed to produce diastereomeric products, which can be quantified by 31P NMR. We knew from previous work that archaea and bacteria that accumulate di-myo-inositol phosphate are able to synthesize GPI from CDP-glycerol and l-myo-inositol 1-phosphate (8). This results from a side reaction catalyzed by DIPPS, an enzyme that recognizes CDP-glycerol in addition to CDP-inositol, the preferred substrate. Therefore, cell extracts of A. fulgidus were selected to perform the conversion of CDP-glycerol into GPI. Additionally, we probed whether the relevant synthase, the transmembrane domain of IPCT/DIPPS, used the two stereoisomers of CDP-glycerol to a similar extent. For this purpose, cell extracts of A. fulgidus were incubated with a racemic mixture of CDP-glycerol (purchased from Sigma) and l-myo-inositol 1-phosphate. After 1 h of incubation, the proportion of the two forms of GPI was evaluated by 31P NMR. The average ratio from four independent experiments was 1:1.2, denoting only a slight preference for sn-glycerol 1-phosphate (see Fig. S1 in the supplemental material).
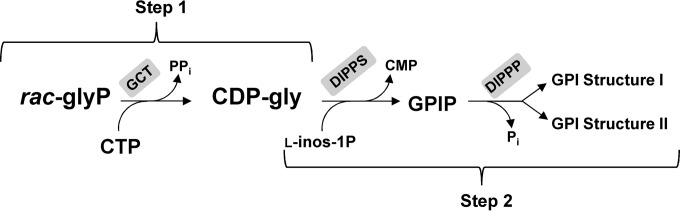
FIG 5.
Scheme representing the method used to characterize the stereospecificity of GCTs. Step 1 represents production of CDP-glycerol from rac-glycerol phosphate by the target glycerol phosphate cytidylyltransferase. Step 2 represents conversion of CDP-glycerol produced in step 1 into GPI via de action of the DIPP synthase and phosphatase activities present in the A. fulgidus cell extract provided. Finally, 31P NMR analysis of the reaction products enables the determination of the stereoforms of GPI produced and, indirectly, the proportions of sn-glycerol 3-phosphate and sn-glycerol 1-phosphate used by the GCT enzyme. GCT, glycerol phosphate cytidylyltransferase; DIPPS, di-myo-inositol phosphate-phosphate synthase; DIPPP, DIPP phosphatase; rac-glyP, rac-glycerol phosphate; l-inos-1P, l-myo-inositol 1-phosphate.
Identification of the stereochemical configuration of GPI in Aq. aeolicus and A. fulgidus.
To determine the configuration of the glycerol moiety in GPIs, CDP-3-glycerol was produced by using Aq. aeolicus GCT (AQ1368) and the substrates sn-glycerol 3-phosphate and CTP. Then CDP-3-glycerol was incubated with l-myo-inositol 1-phosphate and a cell extract of A. fulgidus, producing sn-glycero-3-phospho-3-l-myo-inositol (Fig. 6a). The phosphodiester group in this compound resonates at 0.62 ppm and coincides with the GPI detected in the bacterial species (Fig. 1). When the reaction was carried out with rac-glycerol phosphate instead of sn-glycerol 3-phosphate, two resonances (at 0.62 and 0.60 ppm) were observed in the 31P NMR spectrum of the reaction products (Fig. 6b). To exclude the possibility that these signals are due to the phosphorylated form of GPI, the reaction mixture was incubated with alkaline phosphatase, and no alteration of these resonances was observed (data not shown). Therefore the resonance at 0.62 ppm is due to sn-glycerol 3-phospho-3-l-myo-inositol, and the signal at 0.60 ppm is assigned to sn-glycero-1-phospho-3-l-myo-inositol. We conclude that the glycerol moiety of GPI encountered in the solute pool of Aq. aeolicus derives from sn-glycerol 3-phosphate, while the major GPI form in A. fulgidus derives from sn-glycerol 1-phosphate. The molecular structures of GPIs accumulated by A. fulgidus and Aq. aeolicus are shown in Fig. 7.
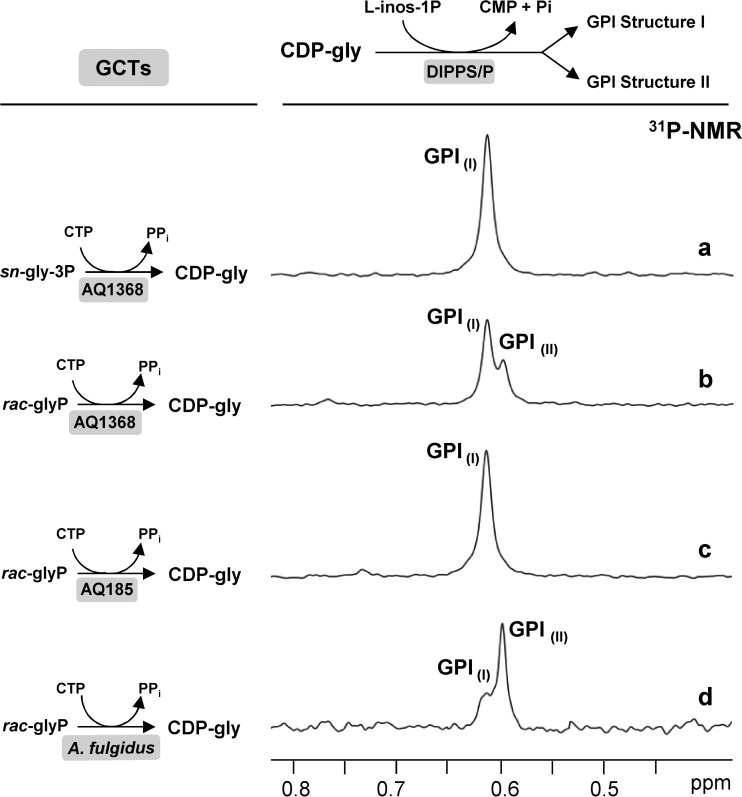
FIG 6.
Final step in the characterization of GCT stereospecific properties. Traces show the 31P NMR resonances due to the GPI formed by the auxiliary enzymes DIPP synthase/phosphatase in cell extracts of Archaeoglobus fulgidus using CDP-glycerol produced by the GCTs examined in this study (indicated on the left-hand side). (a and b) recombinant AQ1368, (c) recombinant AQ185, and (d) GCT activity partially purified from a cell extract of Archaeoglobus fulgidus. The stereospecificity of each GCT for sn-glycerol 3-phosphate and sn-glycerol 1-phosphate was determined from the areas of the resonances attributed to GPI structure I and GPI structure II in the 31P NMR spectra of the final reaction mixtures.
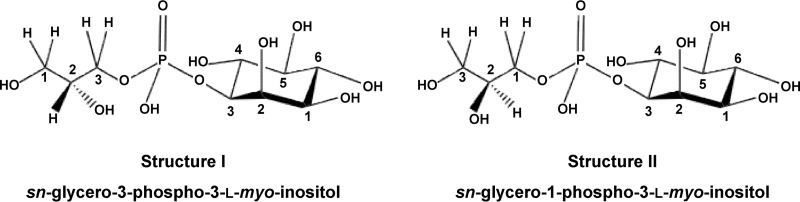
FIG 7.
The structures of two stereoisomers of GPI. Structure I is the sole form present in Aquifex spp. and a minor component in the archaeon Archaeoglobus fulgidus, which accumulates primarily the form represented by structure II. The carbon atoms of glycerol are numbered stereospecifically: i.e., the carbon atom that appears on the top of the Fischer projection that has the hydroxyl group at carbon 2 pointing to the left is designated C-1 (26).
Stereospecificity of the GCTs from Aq. aeolicus and A. fulgidus.
The strategy described above, which used a racemic mixture of glycerol phosphate, was followed to characterize the stereospecificity of recombinant Aq. aeolicus GCT (AQ185) and Aq. aeolicus GCT (AQ1368), as well as the partially purified A. fulgidus GCT. CDP-glycerol produced with Aq. aeolicus GCT (AQ1368) generated two forms of GPI (resonances at 0.60 and 0.62 ppm) in the subsequent step catalyzed by the auxiliary DIPPS present in A. fulgidus cell extract (Fig. 6b). Quantification of the respective signals shows that the Aq. aeolicus GCT (AQ1368) produces 66% CDP-3-glycerol and 34% CDP-1-glycerol. Therefore, the preference of GCT Aq. aeolicus (AQ1368) for sn-glycerol 3-phosphate over sn-glycerol 1-phosphate is approximately 2-fold. In contrast, GCT Aq. aeolicus (AQ185) has absolute specificity for sn-glycerol 3-phosphate since a single GPI product was observed (resonance at 0.62 ppm) (Fig. 6c).
The GCT activity of A. fulgidus recognized both enantiomers of glycerol phosphate, with a clear preference for sn-glycerol 1-phosphate. The proportion of sn-glycerol 1-phosphate incorporated is approximately 4 times higher than that of sn-glycerol 3-phosphate (Fig. 6d).
DISCUSSION
The genomes of A. fulgidus and Aq. aeolicus comprise one (AF1418) and two (aq_185 and aq_1368) genes encoding putative GCTs, respectively. Expression of these genes in E. coli confirmed that the gene products of Aq. aeolicus (AQ185 and AQ1368) catalyze the synthesis of CDP-glycerol from CTP and glycerol phosphate. AQ1368 is located immediately upstream of the IPCT/DIPPS gene, while the gene coding for AQ185 is located elsewhere in the genome. Therefore, we propose that AQ1368 is the GCT implicated in the synthesis of CDP-glycerol, a precursor for the synthesis of GPI by the DIPPS enzyme of Aq. aeolicus. AQ185 is also able to catalyze CDP-glycerol formation, but its physiological role is most likely unrelated to GPI synthesis. Interestingly, the AF1418 protein, earlier annotated as a putative GCT, showed no such activity; instead it catalyzed the synthesis of FAD from ATP and FMN. Moreover, BLAST searches with known GCTs revealed no hit in the A. fulgidus genome, despite the observation of GCT activity in cell extracts of this archaeon. This activity must be encoded by a gene with poor sequence similarity to known GCT genes; hence the identification of the gene encoding this novel archaeal GCT looks like an interesting challenge. To date, no GCT has been assigned in the domain Archaea.
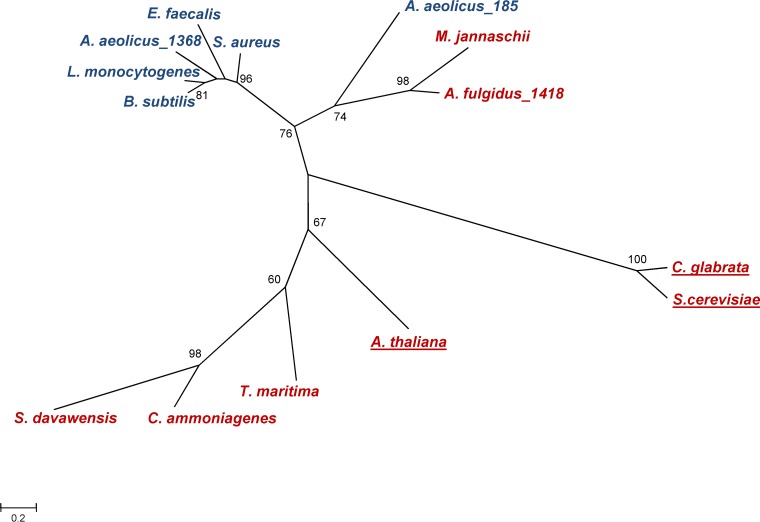
The enzymes GCTs and FADSs are nucleotidyltransferases that catalyze related reactions: formation of CDP-glycerol with release of PPi from the cytidylation of glycerol phosphate with CTP or formation of FAD with release of PPi from the adenylation of FMN with ATP. In bacteria, FADS is the N-terminal domain of a bifunctional protein in which the C-terminal domain is responsible for the phosphorylation of riboflavin, while in eukaryotes, the two proteins are encoded by separate genes. Bacterial and eukaryal FADSs show very little sequence similarity (18, 19); accordingly, they are classified into distinct protein families: the FADS family (PF06574), and the phosphoadenosine phosphosulfate reductase family (PF01507), respectively (http://pfam.xfam.org/). On the other hand, the two known archaeal FADS proteins from A. fulgidus and Methanocaldococcus jannaschii belong to yet a different protein family, the cytidylyltransferase family (PF01467), which also comprises all GCTs characterized thus far.
Three conserved motifs have been assigned in GCTs: the HXGH sequence, comprising two histidine residues involved in catalysis, is present in the GCTs of Bacillus subtilis, Staphylococcus aureus, Listeria monocytogenes, Enterococcus faecalis, and Aq. aeolicus (AQ1368 and AQ185) (Fig. 8). The two other conserved motifs of GCTs are RYVDEVI and, most importantly, RTXGISTT, which is considered to be a signature of this group of proteins (10, 11, 15). It is curious that the two GCTs of Aq. aeolicus are part of different clusters (Fig. 9): AQ1368 falls in the cluster comprising known bacterial GCTs, while the other is found in the archaeal FADS cluster, despite its poor activity for the synthesis of FAD. In brief, AQ185 shows a major GCT activity without possessing the so-called signature motif of GCTs.
FIG 8.
Multiple-sequence alignment of the amino acid sequences of the glycerol phosphate cytidylyltransferases of Aquifex aeolicus (AQ185 and AQ1368), Bacillus subtilis, Staphylococcus aureus, Listeria monocytogenes, and Enterococcus faecalis and of the FAD synthetase of Archaeoglobus fulgidus (AF1418), and Methanocaldococcus jannaschii (27). The alignment was generated with ClustalW2 (https://www.ebi.ac.uk/Tools/msa/clustalw2/). Gray boxes highlight conserved motifs.
FIG 9.
Phylogenetic tree based on amino acid sequences of glycerol phosphate cytidylyltransferases (GCTs), bifunctional riboflavin kinase/FMN adenylyltransferases (FADS/RibFKs), and flavin adenine dinucleotide synthetases (FADSs), whose functions have been proven. Only the FADS domain of the bifunctional FADS/RibFKs was used for this analysis. MEGA6 software (28) used for sequence alignment and to draw the tree. Bootstrap values were calculated from 1,000 replicates. Only bootstrap values greater than or equal to 60 are shown. The scale bar represents the branch lengths measured in the number of substitutions per site. Organisms harboring the GCT and FADS proteins are indicated in blue and red, respectively. Underlined names refer to eukaryotic organisms. GenPept accession numbers for the FADS and GCT proteins are provided in parentheses for the following organisms: for bifunctional RibFK/FADS, Corynebacterium ammoniagenes (Q59263.1), Thermotoga maritima (1T6Y), and Streptomyces davawensis (ABN55909.1); for FADS, Arabidopsis thaliana (Q9FMW8.1), Methanocaldococcus jannaschii (WP_010870692), and Archaeoglobus fulgidus AF1418 (WP_048064389.1); and for GCT, Aquifex aeolicus AQ1368 (NP_213944.1), Candida glabrata (3G5A), Saccharomyces cerevisiae (AAA65730.1), Bacillus subtilis (1COZ), Aquifex aeolicus AQ185 (NP_213132.1), Staphylococcus aureus (AAB51063.1), Listeria monocytogenes (WP_003727003), and Enterococcus faecalis (EEI58902).
To our knowledge, only four bacterial GCTs have been characterized thus far (10, 12, 13, 16, 17, 20, 21), but their stereospecific properties have not been reported. The strategy designed here to study the GCT stereospecific properties (Fig. 5) led to the conclusion that the recombinant Aq. aeolicus GCT (AQ1368) and Aq. aeolicus GCT (AQ185) showed a high preference (around 66%) and absolute preference for sn-glycerol 3-phosphate, respectively. In contrast, the A. fulgidus GCT exhibited a strong preference for sn-glycerol 1-phosphate (around 80%). Significantly, the bacterium Aq. aeolicus accumulates exclusively sn-glycero-3-phospho-3-l-myo-inositol, whereas the archaeon A. fulgidus accumulates mainly sn-glycero-1-phospho-3-l-myo-inositol, with small amounts of sn-glycero-1-phospho-3-l-myo-inositol (Fig. 1). Therefore, the ratio of the two GPI stereoforms in the solute pool of A. fulgidus appears to reflect perfectly the preference of its GCT for sn-glycerol 1-phosphate over sn-glycerol 3-phosphate. However, the issue of substrate availability should not be ignored in this discussion.
A. fulgidus possesses two enzymes involved in the synthesis of sn-glycerol 3-phosphate (WP_010878372.1 and WP_010878825.1) and one enzyme for the synthesis of sn-glycerol 1-phosphate (WP_010879170.1). Unfortunately, the lack of data on the relative intracellular concentrations of sn-glycerol 1-phosphate and sn-glycerol 3-phosphate in A. fulgidus, or any other archaeon, precludes further elaboration on the relevance of substrate availability. On the other hand, it is clear that the exclusive accumulation of one of the stereoforms of GPI (structure I) in Aq. aeolicus is determined ultimately by substrate availability. In fact, this bacterium does not possess sn-glycerol 1-phosphate dehydrogenase or any other enzyme involved in the synthesis of sn-glycerol 1-phosphate; hence, sn-glycerol 3-phosphate is the sole enantiomer available to be processed by GCTs in Aq. aeolicus.
Different enantiomers of glycerol phosphate are used in the glycerophosphate backbones of membrane phospholipids of bacteria and archaea. While sn-glycerol 1-phosphate is found in the phospholipids of members of the domain Archaea, sn-glycerol 3-phosphate is found in phospholipids of Bacteria and Eukarya (22). In fact, the presence of different enantiomers of glycerol phosphate in the membrane phospholipids of Bacteria and Archaea is the most distinctive feature of the two domains, and until now, no exceptions to this rule had been found (23). This feature seems to match up the occurrence of different stereoforms of GPI in archaea and bacteria together with the dissimilar stereospecific properties of the archaeal and bacterial GCTs studied in this work.
In conclusion, the molecular configuration of the GPI forms accumulated in the archaeon A. fulgidus and in the bacterium Aq. aeolicus were established herein. Moreover, we proved that the bacterial GCTs use primarily sn-glycerol 3-phosphate, while the GCT from A. fulgidus has a strong preference for sn-glycerol 1-phosphate. Therefore, the predominance of sn-glycero-1-phospho-3-l-myo-inositol in the archaeon and of sn-glycero-3-phospho-3-l-myo-inositol in the bacterium fits with the stereoisomer preference of the respective GCTs. This is the first report on the stereospecific properties of any GCT.
MATERIALS AND METHODS
Materials.
Glycerol phosphate (racemic mixture of sn-glycerol 3-phosphate and sn-glycerol 1-phosphate), sn-glycerol 3-phosphate, CTP, CDP-glycerol (racemic mixture of CDP-1-glycerol and CDP-3-glycerol), ATP, NAD+, flavin mononucleotide (FMN), flavin adenine dinucleotide (FAD), and 1,4-dithiothreitol (DTT), were purchased from Sigma-Aldrich (St. Louis, MO).
Growth of A. fulgidus and preparation of cell extracts.
A. fulgidus strain 7324 (Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany) was grown at 83°C in 2-liter static vessels with a gas phase composed of N2 in the medium described previously (7). Cell growth was assessed by measuring the optical density at 600 nm (OD600). Cells were harvested by centrifugation (8,671 × g, 20°C, 10 min) during the late exponential growth phase (maximal OD600 of 0.27) and washed with a solution of 3% (wt/vol) NaCl. The cell pellet was suspended in 10 mM Tris-HCl (pH 7.6) containing 5 mM MgCl2, and the cells were disrupted in a French press. The cell debris was removed by centrifugation (15,557 × g, 4°C, 45 min). For the enzymatic assays, the supernatant was applied to a PD-10 column (GE Healthcare) previously equilibrated with 50 mM Tris-HCl (pH 7.6) to remove low-molecular-mass compounds. For the partial purification of the native A. fulgidus GCT, the supernatant was dialyzed against 50 mM Tris-HCl (pH 7.6) using a membrane with a 3.5-kDa-molecular-mass cutoff. The protein content was estimated by the Bradford method (24).
Partial purification of the native A. fulgidus GCT.
The cell extracts (approximately 132 mg of total protein) were applied to a Resource Q column (GE Healthcare) equilibrated with 50 mM Tris-HCl (pH 7.6) and eluted with a linear gradient of NaCl (from 0 to 1 M) of the same buffer. GCT activity was detected in the flowthrough and in the fractions eluted between 0.25 and 0.5 M NaCl. The eluted fractions were pooled, dialyzed against 50 mM Tris-HCl (pH 7.6), and concentrated using a Centricon filter (Millipore).
Cloning, expression, and purification of putative GCTs.
The genes aq_185 (NP_213132.1) and aq_1368 (NP_213944.1) from Aq. aeolicus and the AF1418 gene (NP_070247.1) from A. fulgidus are annotated in their respective genomes as GCT-encoding genes. Chromosomal DNAs from Aq. aeolicus and A. fulgidus were isolated as described by Rodrigues et al. (8). The two genes from Aq. aeolicus were amplified by PCR using Taq DNA polymerase (Bioline) and cloned into pET19b following standard protocols (25). The correct sequences of the cloned genes were confirmed (Stab-Vida, Portugal). E. coli BL21(DE3) cells, containing the constructs, were grown at 37°C in LB medium with ampicillin (100 μg/μl) until an OD600 of 0.6 to 0.7 and induced with 0.5 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) for 4 h. The cells were harvested, suspended in 10 mM Tris-HCl (pH 7.6) containing 5 mM MgCl2, and disrupted in a French press; cell debris was removed as described above. The histidine-tagged recombinant proteins were purified from cell extracts (approximately 112 and 102 mg of total protein for AQ1368 and AQ185, respectively) by using a HisTrap HP column (GE Healthcare). Elution was carried out with 0.5 M imidazole. The enzymes were judged as pure by SDS-PAGE (data not shown). The gene AF1418 from A. fulgidus was cloned into pET19b, pET52b, and pTRC99a. The gene sequences of the constructs were confirmed by DNA sequencing (Stab-Vida, Portugal). These constructs were transformed into E. coli BL21(DE3) and Rosetta(DE3), and these cells were grown at different temperatures (18, 30, and 37°C) and IPTG concentrations (0, 0.5, and 1 mM). All recombinant proteins of AF1418 were expressed in inclusion bodies. The synthetic E. coli optimized gene was purchased from Geneart (Life Technologies). The synthetic gene (AF1418SG) was cloned into pET19b and transformed into E. coli BL21(DE3). Cells were grown at 37°C in LB medium with ampicillin (100 μg/μl) until an OD600 of 0.7 to 0.8, and protein expression was induced with 0.5 mM IPTG for 5 h. Several attempts to purify AF1418SG were performed, but the purification yield was very poor. Therefore, the characterization of the enzyme activity was performed in cell extracts of E. coli BL21(DE).
Production and purification of l-myo-inositol 1-phosphate.
l-myo-Inositol 1-phosphate was produced and purified as previously described (7, 8). Briefly, the recombinant l-myo-inositol 1-phosphate synthase from A. fulgidus was incubated with 20 mM Tris-HCl (pH 7.6), 10 mM MgCl2, 10 mM glucose-6-phosphate, and 5 mM NAD+ for 1 h at 85°C. The synthesized l-myo-inositol 1-phosphate was applied to a QAE-Sephadex A-25 column, equilibrated with 5 mM NaHCO3 (pH 9.8). The elution was carried out with a linear gradient of 5 mM to 1 M NaHCO3 (pH 9.8). The eluted fractions were analyzed by 1H NMR. The fractions containing l-myo-inositol 1-phosphate were pooled and desalted in the HCl-activated Dowex 50W-X8 resin. The elution was carried out with distilled H2O. Subsequently the fractions were pooled, and the pH was adjusted to 5.3. The sample was lyophilized, and the l-myo-inositol 1-phosphate was quantified by 1H NMR.
Production of CDP-glycerol using the GCTs from A. fulgidus and Aq. aeolicus.
Partially purified GCT from A. fulgidus (approximately 2 mg of total protein) was incubated at 80°C in 50 mM Tris-HCl (pH 8.6) containing 10 mM MgCl2 with 4 mM CTP and 4 mM rac-glycerol phosphate (or 4 mM sn-glycerol 3-phosphate) (total volume of 400 μl). The reactions were stopped after 45 min by the addition of 15 mM EDTA (pH 8.0). After centrifugation (16,100 × g, 15 min, 4°C), the supernatants were lyophilized. The residues were suspended in water and treated with alkaline phosphatase for 45 min at 37°C to dephosphorylate residual substrates. The alkaline phosphatase was inactivated at 80°C for 15 min. The CDP-glycerol was quantified by 31P NMR. For the production of CDP-glycerol with the Aq. aeolicus enzyme, the reaction mixtures were incubated at 85°C in 50 mM Tris-HCl (pH 8.6) containing 10 mM MgCl2 in the presence of 10 mM CTP and 10 mM rac-glycerol phosphate or 10 mM sn-glycerol 3-phosphate. Reactions were started by the addition of 150 μg of AQ185 or 50 μg of AQ1368. After 1 h of incubation, the mixtures were centrifuged (16,100 × g, 15 min, 4°C), and the supernatant was lyophilized and treated with alkaline phosphatase as described above. The CDP-glycerol produced was quantified by 31P NMR.
Analysis of the stereoform composition of CDP-glycerol.
CDP-glycerol produced with GCT of A. fulgidus or Aq. aeolicus was incubated at 80°C for 1 h with cell extracts of A. fulgidus. The reaction mixture (total volume of 800 μl), contained cell extract (around 18 mg of total protein), 50 mM Tris-HCl (pH 8.6), 10 mM MgCl2, 3.5 mM l-myo-inositol 1-phosphate, and 0.4 mM CDP-glycerol. CDP-glycerol was converted into GPI due to the consecutive actions of DIPPS and DIPP phosphatase present in A. fulgidus cell extract (Fig. 2). After lyophilizing, the residue was suspended in 500 μl of 2H2O with 30 mM EDTA (pH 8), and reaction products were analyzed by 31P NMR. The GPI diastereomers' resonances are partially overlapped, hampering direct integration. Therefore, deconvolution of the relevant spectral region was performed by fitting the sum of Lorentzian and Gaussian functions using Matlab V7.1 (Math Works, Natick, MA).
Enzyme assays.
The GCT and FAD synthetase activities were determined in the AF1418SG, AQ185, and AQ1368 preparations. For the detection of GCT activity, these enzymes were incubated with 50 mM Tris-HCl (pH 8.6), 10 mM MgCl2, 4 mM CTP, and 4 mM glycerol phosphate for 45 min at 80°C. The FAD synthetase activity was evaluated in reaction mixtures containing 50 mM Tris-HCl (pH 8.6), 10 mM MgCl2, 10 mM ATP, 5 mM FMN, and 15 mM DTT for 45 min at 80°C. The reaction products (CDP-glycerol or FAD) were analyzed by 31P NMR. Protein content was estimated by the Bradford method (24).
NMR spectroscopy.
31P NMR spectra were recorded at 202.45 MHz on a Bruker AVANCEII spectrometer using a 5-mm selective probe head at 25°C. Spectra were acquired with a 60° pulse and a repetition delay of 1.5 s. Proton broadband decoupling was applied during the acquisition time only to avoid heating. Quantification of CDP-glycerol was done using a repetition delay of 15 s, and di-myo-inositol phosphate was used as internal standard. Phosphoric acid (85% [vol/vol]) contained in a capillary tube designated at 0 ppm was used as chemical shift reference. l-myo-Inositol 1-phosphate, rac-glycerol phosphate, and sn-glycerol 3-phosphate were quantified by 1H NMR. Spectra were acquired with the same spectrometer operating at 500.13 MHz using a 5-mm inverse detection probe head, with presaturation of the water signal with a repetition delay of 60 s. Formate was used as an internal concentration standard.
Supplementary Material
ACKNOWLEDGMENTS
We thank Sara Rebelo for technical support and Luís Gonçalves for useful discussions.
This work was supported by project LISBOA-01-0145-FEDER-007660 (Microbiologia Molecular, Estrutural e Celular) funded by FEDER through COMPETE2020—Programa Operacional Competitividade e Internacionalização (POCI) and by national funds through Fundação para a Ciência e a Tecnologia (FCT). M.V.R. was awarded a fellowship from FCT (SFRH/BPD/80219/2011). The NMR spectrometers are part of The National NMR Facility, supported by FCT (RECI/BBB-BQB/0230/2012).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02462-16.
REFERENCES
- 1.Lamosa P, Gonçalves LG, Rodrigues MV, Martins LO, Raven ND, Santos H. 2006. Occurrence of 1-glyceryl-1-myo-inosityl phosphate in hyperthermophiles. Appl Environ Microbiol 72:6169–6173. doi: 10.1128/AEM.00852-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodrigues MV. 2011. Heat stress adaptation in hyperthermophiles: biosynthesis of inositol-containing compatible solutes. PhD thesis Instituto de Tecnologia Química e Biológica, Oeiras, Portugal. [Google Scholar]
- 3.Faria TQ, Mingote A, Siopa F, Ventura R, Maycock C, Santos H. 2008. Design of new enzyme stabilizers inspired by glycosides of hyperthermophilic microorganisms. Carbohydr Res 343:3025–3033. doi: 10.1016/j.carres.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 4.Santos H, Lamosa P, Borges N, Gonçalves LG, Pais T, Rodrigues MV. 2010. Organic compatible solutes of prokaryotes that thrive in hot environments: the importance of ionic compounds for thermostablization, p 497–520. In Horikoshi K. (ed), Extremophiles handbook. Springer Japan, Tokyo, Japan. [Google Scholar]
- 5.Faria C, Jorge CD, Borges N, Tenreiro S, Outeiro TF, Santos H. 2013. Inhibition of formation of α-synuclein inclusions by mannosylglycerate in a yeast model of Parkinson's disease. Biochim Biophys Acta 1830:4065–4072. doi: 10.1016/j.bbagen.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 6.Nogly P, Gushchin I, Remeeva A, Esteves AM, Borges N, Ma P, Ishchenko A, Grudinin S, Round E, Moraes I, Borshchevskiy V, Santos H, Gordeliy V, Archer M. 2014. X-ray structure of a CDP-alcohol phosphatidyltransferase membrane enzyme and insights into its catalytic mechanism. Nat Commun 5:4169. doi: 10.1038/ncomms5169. [DOI] [PubMed] [Google Scholar]
- 7.Borges N, Gonçalves LG, Rodrigues MV, Siopa F, Ventura R, Maycock C, Lamosa P, Santos H. 2006. Biosynthetic pathways of inositol and glycerol phosphodiesters used by the hyperthermophile Archaeoglobus fulgidus in stress adaptation. J Bacteriol 188:8128–8135. doi: 10.1128/JB.01129-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodrigues MV, Borges N, Henriques M, Lamosa P, Ventura R, Fernandes C, Empadinhas N, Maycock C, da Costa MS, Santos H. 2007. Bifunctional CTP:inositol-1-phosphate cytidylyltransferase/CDP-inositol:inositol-1-phosphate transferase, the key enzyme for di-myo-inositol-phosphate synthesis in several (hyper)thermophiles. J Bacteriol 189:5405–5412. doi: 10.1128/JB.00465-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown S, Santa Maria JP Jr, Walker S. 2013. Wall teichoic acids of Gram-positive bacteria. Annu Rev Microbiol 67:313–336. doi: 10.1146/annurev-micro-092412-155620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mericl AN, Friesen JA. 2012. Comparative kinetic analysis of glycerol 3-phosphate cytidylyltransferase from Enterococcus faecalis and Listeria monocytogenes. Med Sci Monit 18:BR427–BR434. doi: 10.12659/MSM.883535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park YS, Gee P, Sanker S, Schurter EJ, Zuiderweg ER, Kent C. 1997. Identification of functional conserved residues of CTP:glycerol-3-phosphate cytidylyltransferase. Role of histidines in the conserved HXGH in catalysis. J Biol Chem 272:15161–15166. [DOI] [PubMed] [Google Scholar]
- 12.Yim VC, Zolli M, Badurina DS, Rossi L, Brown ED, Berghuis AM. 2001. Crystallization and preliminary X-ray diffraction studies of glycerol 3-phosphate cytidylyltransferase from Staphylococcus aureus. Acta Crystallogr D Biol Crystallogr 57:918–920. doi: 10.1107/S0907444901005212. [DOI] [PubMed] [Google Scholar]
- 13.Badurina DS, Zolli-Juran M, Brown ED. 2003. CTP:glycerol 3-phosphate cytidylyltransferase (TarD) from Staphylococcus aureus catalyzes the cytidylyl transfer via an ordered Bi-Bi reaction mechanism with micromolar K(m) values. Biochim Biophys Acta 1646:196–206. doi: 10.1016/S1570-9639(03)00019-0. [DOI] [PubMed] [Google Scholar]
- 14.Fong DH, Yim VC, D'Elia MA, Brown ED, Berghuis AM. 2006. Crystal structure of CTP:glycerol-3-phosphate cytidylyltransferase from Staphylococcus aureus: examination of structural basis for kinetic mechanism. Biochim Biophys Acta 1764:63–69. doi: 10.1016/j.bbapap.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 15.Weber CH, Park YS, Sanker S, Kent C, Ludwig ML. 1999. A prototypical cytidylyltransferase: CTP:glycerol-3-phosphate cytidylyltransferase from Bacillus subtilis. Structure 7:1113–1124. doi: 10.1016/S0969-2126(99)80178-6. [DOI] [PubMed] [Google Scholar]
- 16.Sanker S, Campbell HA, Kent C. 2001. Negative cooperativity of substrate binding but not enzyme activity in wild-type and mutant forms of CTP:glycerol-3-phosphate cytidylyltransferase. J Biol Chem 276:37922–37928. [DOI] [PubMed] [Google Scholar]
- 17.Pattridge KA, Weber CH, Friesen JA, Sanker S, Kent C, Ludwig ML. 2003. Glycerol-3-phosphate cytidylyltransferase. Structural changes induced by binding of CDP-glycerol and the role of lysine residues in catalysis. J Biol Chem 278:51863–51871. [DOI] [PubMed] [Google Scholar]
- 18.Huerta C, Borek D, Machius M, Grishin NV, Zhang H. 2009. Structure and mechanism of a eukaryotic FMN adenylyltransferase. J Mol Biol 389:388–400. doi: 10.1016/j.jmb.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krupa A, Sandhya K, Srinivasan N, Jonnalagadda S. 2003. A conserved domain in prokaryotic bifunctional FAD synthetases can potentially catalyze nucleotide transfer. Trends Biochem Sci 28:9–12. doi: 10.1016/S0968-0004(02)00009-9. [DOI] [PubMed] [Google Scholar]
- 20.Park YS, Sweitzer TD, Dixon JE, Kent C. 1993. Expression, purification, and characterization of CTP:glycerol-3-phosphate cytidylyltransferase from Bacillus subtilis. J Biol Chem 268:16648–16654. [PubMed] [Google Scholar]
- 21.Stevens SY, Sanker S, Kent C, Zuiderweg ER. 2001. Delineation of the allosteric mechanism of a cytidylyltransferase exhibiting negative cooperativity. Nat Struct Biol 8:947–952. doi: 10.1038/nsb1101-947. [DOI] [PubMed] [Google Scholar]
- 22.Daiyasu H, Hiroike T, Koga Y, Toh H. 2002. Analysis of membrane stereochemistry with homology modeling of sn-glycerol-1-phosphate dehydrogenase. Protein Eng 15:987–995. doi: 10.1093/protein/15.12.987. [DOI] [PubMed] [Google Scholar]
- 23.Koga Y. 2014. From promiscuity to the lipid divide: on the evolution of distinct membranes in Archaea and Bacteria. J Mol Evol 78:234–242. doi: 10.1007/s00239-014-9613-4. [DOI] [PubMed] [Google Scholar]
- 24.Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 26.IUPAC-IUB Commission on Biochemical Nomenclature. 1967. The nomenclature of lipids. J Lipid Res 8:523–528. [PubMed] [Google Scholar]
- 27.Mashhadi Z, Xu H, Grochowski LL, White RH. 2010. Archaeal RibL: a new FAD synthetase that is air sensitive. Biochemistry 49:8748–8755. doi: 10.1021/bi100817q. [DOI] [PubMed] [Google Scholar]
- 28.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.