Abstract
The study of the hepatitis C virus (HCV) has been hindered by the lack of in vitro model systems. The recent development of HCV subgenomic RNA replicons has permitted the study of viral RNA replication in cell culture; however, the requirements for efficient replication of replicons in this system are poorly understood. Many viral isolates do not function as replicons and most require conserved changes, termed adaptive mutations, to replicate efficiently. In this report, we focus on the HCV nonstructural protein 5A (NS5A), a frequent locus for adaptive mutation. We found the interaction between NS5A and human vesicle-associated membrane protein-associated protein A (hVAP-A), a cellular target N-ethylmaleimide-sensitive factor attachment protein receptor, to be required for efficient RNA replication: NS5A mutations that blocked interaction with hVAP-A strongly reduced HCV RNA replication. Further analyses revealed an inverse correlation between NS5A phosphorylation and hVAP-A interaction. A subset of the previously identified adaptive mutations suppressed NS5A hyperphosphorylation and promoted hVAP-A binding. Our results support a model in which NS5A hyperphosphorylation disrupts interaction with hVAP-A and negatively regulates viral RNA replication, suggesting that replicon-adaptive mutations act by preventing the phosphorylation-dependent dissociation of the RNA replication complex.
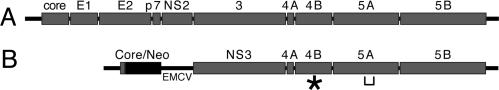
Approximately 3% of the worldwide population is infected by the hepatitis C virus (HCV) (1), a positive-strand RNA virus of the family Flaviviridae. Infections frequently become chronic, persisting for the life of the individual, often leading to liver cirrhosis or cancer. The HCV genome is a single-stranded, positive-strand (coding), uncapped RNA ≈9,600 nt in length, which encodes a single ≈3,000-aa polypeptide, cleaved by cellular and viral proteases into at least 10 mature viral peptides (Fig. 1A) (reviewed in refs. 2 and 3). This genome is replicated through a minus-strand RNA intermediate in the cytoplasm of an infected cell.
Fig. 1.
Organization of the HCV genome/replicon. (A) Diagram of the HCV genomic RNA. (B) Structure of the HCV subgenomic replicon. The majority of the structural region of the polyprotein has been replaced with the neomycin phosphotransferase gene (Neo) such that translation is driven from the HCV internal ribosome entry site. Translation of the remaining NS proteins is conducted by the encephalomyocarditis virus internal ribosome entry site (EMCV). Position of the NS4B adaptive mutation K1846T is indicated by an asterisk. A bracket represents the location of the cluster of NS5A adaptive mutations (P2196S, S2197P, S2197C, A2199S, A2199T, and S2204I) and the hVAP-A binding determinants.
Study of HCV has been hindered by the lack of convenient model systems capable of robust viral replication. The development of HCV subgenomic RNA replicons has enabled study of viral RNA replication in cell culture (4). In this system, hepatocarcinoma Huh7 cells are transfected with a modified HCV RNA encoding the neomycin phosphotransferase gene in place of the structural region of the viral polyprotein (Fig. 1B). Replication of this RNA through the action of the remaining nonstructural (NS) proteins confers G418-resistance to the host cell. The replication potential of particular replicons can be scored as the frequency of G418-resistant colonies derived from each RNA.
The determinants for replicon efficiency are poorly understood. Not all HCV isolates make functional subgenomic replicons. Six closely related viral genotypes, classified by sequence homology, comprise the HCV genus (5). Genotype 1, including subtypes 1a and 1b, is the most abundant worldwide (6). Of the six known functional replicons, four have been derived from 1b HCV isolates (7-11). Replicons of a single subtype 1a isolate, H77, have been produced, but replicate less efficiently than subtype 1b replicons.
The determinants for this apparent genotype-specific replication capacity are unknown.
Most HCV isolates require at least some mutations to “adapt” to the cell culture environment. Early experiments with a replicon derived from the Con1 strain, subtype 1b, showed only inefficient replication (4). Isolation and sequencing of replicon RNAs present in the infrequent G418-resistant colonies revealed mutations that, when engineered into the original Con1 replicon, yielded dramatic increases in replication efficiency (8, 12-14). Highly adaptive mutations have been found throughout the NS4B/5A/5B region, with the majority clustered in the central region of NS5A. Two of the most highly adaptive mutations, K1846T in NS4B (15) and S2204I in NS5A (12), each result in a >10,000-fold increase in G418-resistant colony formation over the parental Con1 replicon.
The mechanisms by which adaptive mutations increase RNA replication efficiency are not understood. One possibility is that adaptive mutations influence protein interactions critical for HCV replicase function. As a hot spot for highly adaptive mutations, NS5A is an attractive target for initial examination. Although the role of NS5A in the HCV life cycle is unknown, many NS5A interactors have been reported, including RNA-regulated protein kinase (16, 17), apolipoproteins (18), p53 (19, 20), Grb2 (21, 22), and amphiphysin II (23), but none have been shown to have a defined role in the viral life cycle.
One previously reported NS5A-interacting protein is human vesicle-associated membrane protein-associated protein A (hVAPA), a widely expressed, endoplasmic reticulum/Golgi-localized protein involved in intracellular vesicle trafficking. This interaction was originally identified in a yeast two-hybrid screen with NS5A as bait and further validated in biochemical and immunofluorescence colocalization experiments (24). hVAP-A has been hypothesized to function as a docking site for assembly or localization of the HCV replicase (24). In support of this hypothesis, inhibition of hVAP-A, either through RNA interference or expression of truncated, dominant negative fragments of this protein, has been shown to have negative effects on HCV RNA replication (25, 26).
In this study, we found a striking correlation between the ability of NS5A to interact with hVAP-A and to participate in HCV RNA replication in cell culture. In addition, a subset of replicon adaptive mutations suppressed the negative effects of hVAP-A noninteracting NS5A mutations on RNA replication. Further investigation indicated that the ability to bind hVAP-A was determined by the phosphorylation state of NS5A. We propose a model in which hyperphosphorylated NS5A represents a “closed” conformation that is unable to associate with hVAP-A or other proteins required for viral RNA replication. In this manner, NS5A phosphorylation is hypothesized to profoundly regulate the HCV life cycle.
Experimental Procedures
Plasmid Construction. For yeast two-hybrid expression, the LexA DNA-binding domain fusion vector pSH2-1 (27) and the Gal4 activation domain fusion vector pACTII (28) were used. I.M. A.G.E. (Integrated Molecular Analysis of Genomes and their Expression) consortium EST clone ID 724209 (ATCC catalog no. 292618) was used as template to amplify the full-length hVAP-A coding sequence. The HCV clone H77 (29) served as template for subtype 1a NS5A PCR amplification. Con1 subtype 1b sequences were amplified from subgenomic HCV Con1 replicon clones kindly provided by Keril Blight (Washington University, St. Louis) (12). Clones were generated as described in Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Yeast Two-Hybrid. Saccharomyces cerevisiae strain CTY10-5d (MATa ade2 trp1-901 leu2-3,112 his3-200 gal4- gal80-URA3::lexA-lacZ) (gift from Stanley Fields, University of Washington, Seattle), containing an integrated GAL1-lacZ gene with the lexA operator, was transformed with yeast two-hybrid baits and preys by the standard lithium acetate method (30). Colonies were qualitatively and quantitatively assayed for β-galactosidase activity by filter lift or o-nitrophenyl β-D-galactoside liquid assay, respectively, as described in ref. 31.
Replicon Assay. Huh7 cells were maintained in DMEM (Specialty Media, Lavellette, NJ) supplemented with 10% FBS, 1% glutamine, and 1% penicillin/streptomycin (Invitrogen) at 37°C in 5% CO2. For preparation of subgenomic replicon RNAs, 1 μg of ScaI linearized replicon plasmid was used in T7-MEGAscript kit (Ambion, Austin, TX) according to the manufacturer's instructions. Reactions were purified with an RNAeasy RNA clean-up kit (Qiagen, Valencia, CA) with on column DNase digestion. Eluted RNA was quantified by spectrophotometer and agarose gel analysis.
RNAs were electroporated into Huh7 cells as described in ref. 12. After transfection, 1 × 106 cells were plated per 10-cm tissue culture plate. At 24 h after transfection, media was changed to DMEM/10% FBS containing 1 mg/ml G418 (Geneticin, Invitrogen), which was changed every 3-5 days until colonies were clearly selected. For quantification, plates were methanol-fixed and Coomassie-stained. To quantify highly efficient replicons, dilutions were made with cells transfected with polymerase-defective RNAs, which were also plated alone as a negative control in each experiment.
Western Analysis of NS5A from Huh7 Cells. Individual G418-selected replicon colonies were expanded in DMEM/10% FBS with 500 μg/ml G418. For protein analysis, subconfluent, 35-mm dishes were lysed in 500 μl of SDS loading buffer. Twenty microliters of each lysate, denatured by boiling, was resolved on 7.5% SDS-polyacrylamide gels, and transferred to Hybond-N nitrocellulose membranes (Millipore). Membranes were probed with an anti-NS5A monoclonal antibody (Maine Biotechnology, Portland, ME). Detection was with enhanced chemiluminescence (ECL-Plus, Amersham Pharmacia).
Western Analysis of NS5A Preys from Yeast. Yeast expressing the NS5A prey of interest were used to seed selective media cultures and grown to log-phase (OD600 0.5-0.8) at 30°C with shaking. Cultures were collected by centrifugation, washed with TE buffer (10 mM Tris/1 mM EDTA, pH 8.0), and repelleted as 10 OD600 aliquots in 1.5-ml microcentrifuge tubes. Yeast lysates were prepared by glass bead disruption in 200 μl of SDS loading buffer by vortexing and boiling. For immunoblotting, lysates normalized for NS5A expression were resolved by SDS/PAGE, transferred, and probed as described above. For dephosphorylation, lysates were diluted with 1× restriction enzyme buffer 3 (New England Biolabs) and incubated at 37°C for 3 h with 5,000 units of calf intestinal alkaline phosphatase (CIP) (New England Biolabs).
Results
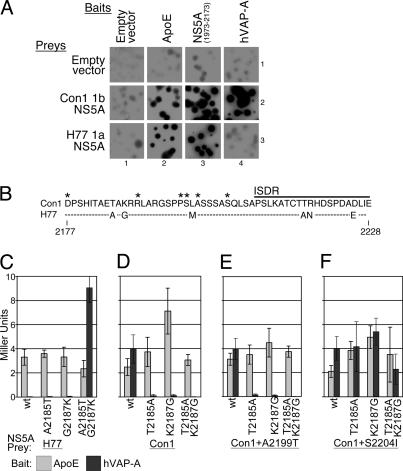
Interaction of NS5A with hVAP-A Depends on Genotype. To identify NS5A-binding proteins required for HCV RNA replication, the yeast two-hybrid system was used to compare the interaction profiles of NS5A proteins from two HCV isolates: Con1 subtype 1b or H77 subtype 1a. As discussed above, replicons derived from the Con1 1b isolate replicate much more efficiently those derived from the H77 1a genome. NS5A proteins from these two isolates, expressed as Gal4 activation domain fusion “preys,” displayed similar reporter activity against a wide range of cellular and viral NS5A-interacting protein “baits,” cloned as LexA DNA-binding domain fusions, including the NS5A interactors apolipoprotein E (ApoE) and an amino-terminal fragment of NS5A (Fig. 2A, columns 2 and 3, and unpublished data). This result indicated that the NS5A preys were comparably expressed and functional.
Fig. 2.
Identifying and mapping NS5A subtype-specific hVAP-A interaction determinants. (A) β-galactosidase filter lift assay of yeast transformed with the indicated yeast two-hybrid baits and preys. Interaction strength is proportional to colony color intensity. Empty DBD and AD fusion vectors control for reporter activation by protein fusions in the absence of a suitable partner. (B) Alignment of Con1 and H77 amino acids 2,177-2,228 encoding NS5A determinants for hVAP-A interaction. Differences between genotypes are indicated, and those unchanged are indicated by a dashed line. Positions of previously identified replicon adaptive mutations, shown as asterisks, and the beginning of the interferon sensitivity determining region (ISDR) are indicated. (C-F) Quantitative analysis of NS5A/hVAP-A interactions. Wild-type (wt) and hVAP-A-binding determinant mutant NS5A preys were assayed in yeast for interaction against baits containing either ApoE (light bars) or hVAP-A (dark bars). Reporter activation was quantified in Miller units by β-galactosidase liquid assay. (C) Assays with H77 1a NS5A preys. (D-F) Assays with Con1 1b NS5A preys, with either no adaptive mutation (D), the A2199T adaptive mutation (E), or the S2204I adaptive mutation (F).
In contrast to the results with most interacting partners, a striking difference in interaction ability was observed when the two NS5A preys were tested for interaction with hVAP-A. Although subtype 1b NS5A preys interacted strongly with hVAP-A, the subtype 1a NS5A exhibited no detectable hVAP-A interaction (Fig. 2A, column 4). It should be noted that the hVAP-A fusions performed well in this system per se, given that a strong dimerization was detected between an hVAP-A bait and prey (data not shown), as reported in refs. 32 and 33. The finding that the 1a subtype NS5A was specifically defective for hVAP-A binding raised the possibility that the NS5A/hVAP-A interaction might be a determinant of replicon efficiency.
Two NS5A Residues Determine hVAP-A Interaction Capacity. The determinants for the NS5A subtype-specific hVAP-A interaction were mapped by DNA fragment exchange (as described in Fig. 6, which is published as supporting information on the PNAS web site) to a 51-aa region within NS5A that overlaps with the NS5A adaptive mutation cluster (Fig. 2B). Within this region, two amino acid differences between these NS5A proteins at amino acid positions 2,185 and 2,187, relative to the polyprotein, were found to modulate the hVAP-A interaction. Changing either one of these residues in 1a NS5A to the corresponding 1b sequence (H77 A2185T or G2187K) significantly raised reporter activation, whereas the double substitution (H77 A2185T and G2187K) conferred strong hVAP-A binding (Fig. 2C). Conversely, changing either one of these residues in 1b NS5A to the 1a sequence (Con1 T2185A or K2187G) reduced interaction with hVAP-A ≈50-fold, whereas changing both (Con1 T2185A and K2187G) reduced reporter activity >100-fold to undetectable levels (Fig. 2D). The ApoE control bait showed no significant variation in its interaction with any of these preys (Fig. 2 C and D, gray bars). Thus, Con1 1b residues T2185 and K2187 were defined as key specific determinants for efficient hVAP-A binding, and substitution to the 1a residues at these two positions (T2185A and K2187G) yielded an hVAP-A noninteracting mutant.
hVAP-A Noninteracting NS5A Mutations Can Be Suppressed by the Highly Adaptive S2204I Adaptive Mutation. To examine the influence of replicon adaptive mutations on the hVAP-A interaction, the hVAP-A noninteracting substitutions (T2185A and K2187G) were engineered into Con1 NS5A preys containing different NS5A adaptive mutations (P2196S, S2197P, S2197C, A2199S, A2199T, or S2204I) (12). Most NS5A adaptive mutations had no measurable effect on the hVAP-A interaction (Fig. 2E and data not shown). A representative example is depicted for the A2199T adaptive mutation (Fig. 2, compare D with E). As shown, the ability to interact with the hVAP-A bait continued to be disrupted by the 1a substitutions even in the context of this adaptive mutation.
A dramatically different result was obtained in the presence of the strongest NS5A adaptive mutation, S2204I. In this background, the 1a substitutions (T2185A and K2187G), either alone or together, no longer disrupted the NS5A and hVAP-A interaction (Fig. 2F). Thus, the S2204I mutation suppressed the defect caused by the T2185A and K2187G substitutions. It is important to note that the S2204I change did not increase the basal strength of the NS5A and hVAP-A interaction but instead perpetuated the interaction in the presence of the otherwise disrupting mutations. This result suggests this particular adaptive mutation modulates in some capacity the interaction between NS5A and hVAP-A.
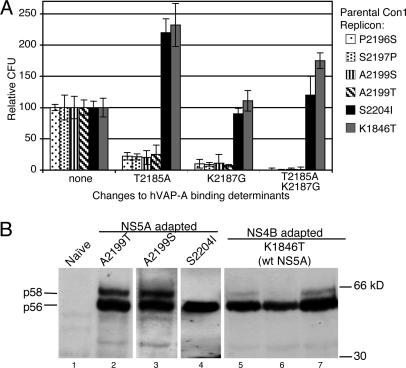
Replicon Fitness Correlates with NS5A/hVAP-A Interaction. To test the importance of the hVAP-A/NS5A interaction for replication, a variety of adapted Con1 replicons containing the hVAP-A noninteracting mutations were tested for ability to form G418-resistant colonies in Huh7 cells. Because each of the adaptive mutations tested confer dramatically different replication potentials, all data were normalized to the parental replicon with unmodified hVAP-A interaction determinants. Absolute replication efficiencies of each parental replicon (data not shown) correlated well with previously reported data (12, 15).
In the context of most adaptive mutations (tested P2196S, S2197P, A2199S, and A2199T), where the 1a substitutions T2185A and K2187G disrupted the NS5A/hVAP-A interaction in the yeast two-hybrid system (Fig. 2E and data not shown), these substitutions also dramatically reduced replicon efficiency (Fig. 3A). Introduction of either hVAP-A interaction-disrupting mutation alone, T2185A or K2187G, reduced G418 colony-forming activity of these replicons by ≈5- or 10-fold, respectively, whereas changing both these determinants together decreased replication efficiencies by ≈100-fold.
Fig. 3.
Analysis of effects of mutations in replicon system. (A) Various adapted Con1 replicons were constructed containing the 1a amino acids at positions 2,185 and 2,187. Huh7 cells electroporated with equivalent quantities of in vitro transcribed RNAs were selected with G418 for several weeks. Colony-forming units (CFU) have been normalized to that of the parental adapted replicon with wild-type hVAP-A-binding determinants. The adaptive mutation used in each data series is indicated by fill pattern. (B) Both the NS5A S2204I and NS4B K1846T adaptive mutations reduce NS5A hyperphosphorylation. G418 resistant clones transfected with the indicated adapted Con1 replicons were isolated and expanded. Total protein extracts from these cells were resolved by SDS/PAGE and Western blotted for NS5A. Molecular masses are labeled to the right, and the positions of the hypophosphorylated p56 and the hyperphosphorylated p58 NS5A species are indicated to the left. wt, wild-type.
In the highly adapted S2204I replicon background, neither 1a substitution alone or in combination decreased replicon fitness (Fig. 3A, black bars). Indeed the T2185A mutation even moderately increased replication efficiency. These results mirror those obtained in yeast, for which the S2204I adaptive change suppressed the effect of the 1a substitutions and perpetuated interaction with hVAP-A (Fig. 2F). Based on these data, the Con1 S2204I mutation can be seen as a positive effector of both hVAP-A binding and viral RNA replication.
In NS5A-adapted replicons there was a perfect correlation between the ability of NS5A to bind hVAP-A in the yeast-two hybrid assay and the efficiency of RNA replication in Huh7 cells. However, when tested in a replicon containing no NS5A adaptive mutations but instead in a replicon containing an adaptive mutation in NS4B (K1846T) (15), the hVAP-A noninteracting substitutions failed to disrupt replication (Fig. 3A, gray bars). The relative effects of the single or double mutations at positions 2,185 and 2,187 closely parallel those seen in the Con1 S2204I replicon (Fig. 4, black bars). Thus, in a manner similar to the S2204I mutation, the NS4B K1846T mutation acts as a second site suppressor of the replication defect caused by the hVAP-A noninteracting NS5A mutations.
Fig. 4.
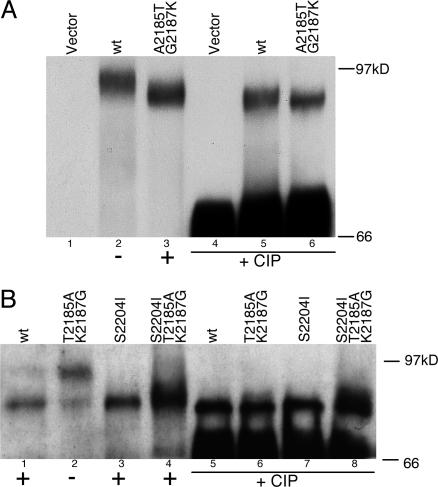
NS5A prey expression in yeast. NS5A immunoblots of total cell lysates from yeast expressing various NS5A preys. The capacity for each prey to interact with hVAP-A in the yeast two-hybrid system is indicated below each lane as a plus or minus sign. (A) Blot of H77 subtype 1a wild-type (wt) and mutant (interactor) NS5A preys, with or without CIP treatment. (B) Blot of Con1 subtype 1b NS5A preys with or without CIP treatment. Both wild-type and S2204I preys are shown without or with the hVAP-A noninteracting mutations.
The NS4B Adaptive Mutation K1846T Reduces NS5A Hyperphosphorylation. The ability of both the S2204I- and K1846T-adaptive changes to suppress the deleterious effect of the hVAP-A noninteracting NS5A mutations on RNA replication suggested that they might act by a common mechanism. It has been reported that the S2204I adaptive mutation disrupts NS5A hyperphosphorylation (12). In addition, several studies have shown that NS5A hyperphosphorylation is modulated by upstream sequences in the polyprotein (34-36). It was therefore possible that both the NS4B K1846T and NS5A S2204I adaptive mutations acted similarly by decreasing NS5A hyperphosphorylation.
To investigate this possibility, Huh-7 cells stably replicating various adapted replicon RNAs were examined for NS5A expression by immunoblotting. As expected, both Con1 A2199T and Con1 A2199S replicon clones exhibited significant levels of both hypoand hyperphosphorylated NS5A isoforms (Fig. 3B, lanes 2 and 3), whereas only the hypophosphorylated species was detectable in a Con1 S2204I clone (Fig. 3B, lane 4). All three Con1 K1846T clones examined exhibited dramatically lower levels of hyperphosphorylated NS5A (Fig. 3B, lanes 5-7). In fact, two of these clones appear to express almost no hyperphosphorylated NS5A. This finding provides the first insight into how NS4B adaptive mutations may mediate their enhancement of RNA replication.
The loss of NS5A hyperphosphorylation correlated with the ability of highly adaptive mutations to suppress the replication defect caused by the hVAP-A noninteracting mutations. This finding suggested a unifying model in which NS5A phosphorylation might modulate its capacity to interact with hVAP-A. The hVAP-A binding determinants may actually influence the phosphorylation state of NS5A, thus indirectly affecting its capacity to interact with hVAP-A. Both the S2204I and K1846T adaptive mutations, which dramatically reduce or eliminate NS5A hyperphosphorylation, would suppress the effects of the 1a substitutions by preserving both the hVAP-A interaction and replication capacity.
NS5A Phosphorylation in Yeast and Interaction with hVAP-A. The validity of the above model required that NS5A phosphorylation regulate its interaction with hVAP-A in the yeast two-hybrid system, because this was the setting for study of the NS5A/hVAP-A interaction. Although this finding would be remarkable because it would require an endogenous yeast kinase (perhaps a homolog of the mammalian NS5A kinase) to act to modify the interaction capacities of the NS5A prey, it was possible because a kinase capable of correctly phosphorylating NS5A has been found in yeast (37). To examine NS5A phosphorylation in yeast, total protein extracts from yeast transformed with various NS5A preys were resolved by immunoblotting for NS5A.
When 1a NS5A preys were visualized, the NS5A proteins migrated at different rates: Wild-type, noninteracting H77 prey migrated significantly slower than the same prey with the 1b substitutions (A2185T and G2187K) that promoted interaction with hVAP-A (Fig. 4A, lanes 2 and 3). These bands were confirmed to correspond to the expected NS5A fusion proteins by probing replica blots with antibodies against both the Gal4-activation domain and hemagglutinin epitope (data not shown), which were both present in the fusion protein. These bands collapsed to a single species when the lysates were treated with CIP, showing that the differences in mobility were indeed due to phosphorylation (Fig. 4A, lanes 5 and 6).
Con1 1b NS5A preys showed the reciprocal gel migration pattern to that of H77: The wild-type, interacting Con1 prey was predominantly in the faster-migrating form, whereas the same prey with the hVAP-A noninteracting mutations (T2185A and K2187G) exhibited a strong shift toward the slower migrating, more phosphorylated isoform (Fig. 4B, lanes 1 and 2). Introduction of the noninteracting mutations into the Con1 S2204I prey did not appreciably affect the phosphorylation state (Fig. 4B, lanes 3 and 4). Therefore, a serine residue at NS5A position 2,204 is required for detectable phosphorylation of NS5A in yeast, suggesting that this modification closely resembles NS5A hyperphosphorylation observed in mammalian cells (Fig. 3B, lane 4). All of the 1b NS5A bands collapsed to a single species when treated with CIP (Fig. 4B, lanes 5-8).
These data show that NS5A preys are substrates for an endogenous yeast kinase, and that the hVAP-A interaction determinants identified affect the capacity of this modification to occur. An inverse correlation between the phosphorylation state of NS5A in yeast and its ability to associate with hVAP-A was seen, suggesting that hyperphosphorylation impairs the ability of NS5A to interact with hVAP-A in yeast.
Discussion
Characterization of the NS5A and hVAP-A Interaction. In this report, we examine the importance of the interaction between HCV NS5A and hVAP-A. By using the yeast two-hybrid system, we found distinct differences in the hVAP-A interaction with NS5A proteins from different HCV subtypes: NS5A from the Con1 1b genome strongly associated with hVAP-A, whereas NS5A from the H77 1a subtype was unable to bind hVAP-A. The determinants of subtype-specific binding were mapped to amino acids 2,185 and 2,187. Interestingly, in a Con1 1b prey, the loss of interaction caused by substitution of the 1a amino acids at these positions was suppressed by the highly adaptive S2204I mutation in NS5A.
Mutations in NS5A that abrogated interaction with hVAP-A were introduced into Con1 1b-derived adapted subgenomic replicons. In most adapted replicons, the hVAP-A noninteracting NS5A mutations strongly impaired replication ability. This replication defect was suppressed by two particular adaptive mutations: K1846T in NS4B and S2204I in NS5A. Interestingly, both these adaptive mutations highly reduced or eliminated the presence of hyperphosphorylated p58 NS5A in replicon clones (Fig. 3B) (12), implying a connection between NS5A hypophosphorylation, the ability to associate with hVAP-A, and RNA replication capacity. It is possible that other mutations in this region will similarly modulate hyperphosphorylation, hVAP-A interaction, and efficiency of RNA replication.
NS5A Phosphorylation State and hVAP-A Binding. The inverse correlation between NS5A phosphorylation and hVAP-A binding raised the intriguing possibility that the NS5A phosphorylation state governs its ability to interact with hVAP-A. In support of this model, NS5A was differentially phosphorylated in yeast: NS5A preys that failed to interact with hVAP-A were expressed as a more phosphorylated isoform in yeast than preys that interacted strongly with hVAP-A. Based on these data, we propose that the identified hVAP-A noninteracting mutations act to enhance NS5A hyperphosphorylation. Furthermore, we propose that hyperphosphorylated p58 NS5A represents a closed conformation that cannot interact with hVAP-A and perhaps with other host and/or viral proteins, whereas hypophosphorylated p56 NS5A represents an “open” conformation capable of strong interactions with hVAP-A.
The phosphorylation of NS5A by a yeast kinase and subsequent modulation of its interaction capacities is remarkable. Although it has been demonstrated that yeast express a kinase capable of phosphorylating NS5A at a serine acceptor site used in mammalian cells (37), it was quite unexpected that phosphorylation would faithfully occur in the yeast two-hybrid system and modulate the activity of an NS5A prey. Requirements for NS5A hyperphosphorylation in mammalian cells are complex: Either NS5A expression in the context of the polyprotein (34, 35) or, in some studies, coexpression of NS4A (38), is necessary for production of this isoform. The presence of other HCV proteins may lead to particular protein conformations, subcellular localization, or host factor recruitments that promote NS5A phosphorylation. NS5A prey hyperphosphorylation in yeast implies that requirements for this modification are somewhat less strict in this setting.
We were unsuccessful in demonstrating an NS5A genotype-specific hVAP-A interaction in other assays. In fact, in our hands, NS5A derived from either subtype expressed in Huh7 cells interacted equally well with hVAP-A in vitro (Fig. 7A, which is published as supporting information on the PNAS web site). This result is not surprising, however, because NS5A produced in these expression systems, out of the context of the viral polyprotein, is not hyperphosphorylated and thus represents an open, hVAP-A-interacting conformation. Demonstration of the hVAP-A noninteracting NS5A phenotype in vitro may require a deeper understanding of the NS5A phospho-acceptor sites and responsible kinases, such that correctly phosphorylated recombinant NS5A can be produced. We did attempt to selectively purify hypophosphorylated NS5A from Huh7 replicon cell lysates with recombinant hVAP-A but were unsuccessful (Fig. 7B). However, selective purification of one NS5A isoform may be difficult if numerous protein contacts are made between both NS5A isoforms and the various proteins likely found within the replicase. For such an approach to be successful, the NS5A phospho-isoforms would first need to be partially purified, or ideal buffer conditions would need to be identified that dissociate complicating protein interactions while maintaining the hVAP-A/NS5A association.
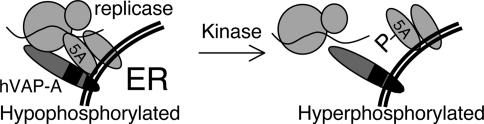
NS5A Phosphorylation, HCV Replication, and Regulation of the Viral Life Cycle. We propose that NS5A phosphorylation plays an important regulatory role in the life cycle of HCV by modulating the ability of NS5A to participate in critical protein-protein interactions. According to this model, hypophosphorylated NS5A (p56) is present in an open conformation capable of making contacts required for HCV replicase assembly and/or function, such as with hVAP-A. Hyperphosphorylation of NS5A could serve as a switch to disassemble or otherwise inactivate a particular replicase by inducing a closed conformation of NS5A. Dissociation of the complex would release viral proteins and RNA for later viral life cycle events, such as virion assembly or packaging (Fig. 5).
Fig. 5.
Model of NS5A phosphorylation modulating assembly of the HCV RNA replication complex. (Left) In the HCV RNA replicase, NS5A exists as a hypophosphorylated species capable of interacting with hVAP-A. (Right) NS5A hyperphosphorylation impairs hVAP-A interaction capacity, breaking apart this complex and allowing postRNA replication viral life cycle events to occur. ER, endoplasmic reticulum.
In support of the involvement of hVAP-A in HCV RNA replication, recent reports have demonstrated that inhibition of hVAP-A expression with small interfering RNAs or expression of hVAP-A fragments as dominant-negative proteins substantially inhibits HCV replicons in Huh7 cells (25, 26). Our data supports the importance of hVAP-A in HCV RNA replication and provides a direct mechanism to explain its role in this process.
Although the tight correlation we present between the ability of NS5A to interact with hVAP-A and the efficiency of RNA replication is compelling, it remains formally possible that the hVAP-A binding determinants we have identified may also affect the interaction between NS5A and other host or viral proteins critical for replicase assembly and/or function; thus, we may have inadvertently examined the consequences of disrupting interactions between NS5A and these other proteins. Our model requires that any such NS5A-interacting proteins closely mimic the interaction pattern that we have observed between NS5A and hVAP-A. Notably, other NS5A interactions, including with itself (dimerization), apolipoproteins (Fig. 2A), and other viral proteins (NS3 and NS4B, data not shown), were not affected by NS5A phosphorylation and are therefore excluded. The interaction between RNA-regulated protein kinase and NS5A was not observed in this study (data not shown).
Implications for the Replicon System. In the replicon system, modifications that make RNA replication more efficient are positively selected. Strengthening contacts with replicase components, possibly including hVAP-A, could certainly be one way to improve replication efficiency. Thus, we propose a model for a mechanism of action of adaptive mutations: The two most active replicon adaptive mutations, S2204I in NS5A (12) and K1846T in NS4B (15), impair NS5A hyperphosphorylation and prevent replicase disassociation. This model could explain why the replicon system has not led to the development of a cell culture system representing the complete HCV life cycle. Full-length HCV genomic RNAs containing adaptive mutations replicate well in Huh7 cells, yet no virions are produced (7, 11, 39). In addition, these adapted RNAs are no longer infectious in chimpanzees (40). Adaptive mutations may prevent disassociation of the replication complex, freezing the viral life cycle at the RNA replication stage, and thus inhibit virion production and viral spread.
It is not clear whether impaired NS5A hyperphosphorylation is the exclusive mechanism of replicon adaptation. Weaker NS5A adaptive mutations assayed did not suppress the effects of the hVAP-A noninteracting mutations in the yeast two-hybrid systems (Fig. 2E) or the replicon (Fig. 3A). In addition, some of these mutations do not appear to impair NS5A hyperphosphorylation in Huh7 cells. As another possible mechanism of RNA replication enhancement, perhaps these weaker adaptive mutations alter the NS5A response to phosphorylation such that in the hyperphosphorylated state NS5A remains capable of making the required interactions. In this way, weaker adaptive mutations may be ways of maintaining the closed NS5A confirmation without changing the overall phosphorylation state of NS5A. Alternatively, these adaptive mutations could influence the kinetics of hyperphosphorylation, perhaps by impairing NS5A recognition by the cognate kinase(s), which may allow NS5A to remain in the hypophosphorylated, open conformation longer and, thus, the RNA replication complex to have extended, and therefore greater, activity. Mutations that directly enhance hyperphosphorylation, such as the 1a substitutions identified in this study, may act in a dominant manner over such adaptive mutations.
The model that impaired NS5A hyperphosphorylation is a critical requirement for efficient RNA replication in Huh7 cells would predict that a wild-type, unadapted HCV genome could replicate in a cellular environment lacking the NS5A kinase. Recent findings by Neddermann et al. (P. Neddermann, M. Quintavalle, C. Di Pietro, A. Clementi, M. Cerretoni, S. Altamura, L. Bartholomew, and R. De Francesco, personal communication) confirms this hypothesis and substantiates our model. This group screened a panel of kinase inhibitors to identify chemicals that selectively impaired NS5A hyperphosphorylation. In the presence of these compounds, the Con1 HCV RNA no longer required adaptive mutations to replicate efficiently in Huh7 cells. Excluding any nonspecific effects of these compounds, these data demonstrate a causal relationship between impaired NS5A hyperphosphorylation and enhanced RNA replication.
The NS5A Kinase. These studies suggest that NS5A phosphorylation is a critical modification that controls its interactions with hVAP-A, perhaps to act as a switch between middle and late viral life cycle events. The identification of the kinase(s) that phosphorylates NS5A is an important aim that would promote further study of NS5A function and possibly provide an antiviral target. A recent report (41) described the utilization of a biochemical genomics approach to identify the NS5A kinase(s) expressed in yeast. Although this group found numerous yeast kinases and, ultimately, their mammalian homologs that were capable of phosphorylating NS5A, the identity of the biologically relevant NS5A kinase(s) remains elusive. The capacity of the yeast two-hybrid system presented here to provide a functional readout for the phosphorylation state of NS5A and thus for the activity of the yeast homolog(s) of this kinase(s) may yield an attractive approach for identifying the biologically relevant NS5A kinase(s).
Supplementary Material
Acknowledgments
We thank Daniel Shaye, Carina Storrs, and Brett Lindenbach for critical reading of the manuscript and for helpful comments and Keril J. Blight for reagents. C.M.R. is supported by Public Health Service Grants CA57973 and AI40034 and the Greenberg Medical Research Institute. S.P.G. is an Investigator of the Howard Hughes Medical Institute.
Abbreviations: HCV, hepatitis C virus; hVAP-A, human vesicle-associated membrane protein-associated protein A; CIP, calf intestinal alkaline phosphatase; NS, nonstructural; ApoE, apolipoprotein E.
References
- 1.World Health Organization (1999) J. Viral. Hepat. 6, 35-47.10847128 [Google Scholar]
- 2.Bartenschlager, R. & Lohmann, V. (2000) J. Gen. Virol. 81, 1631-1648. [DOI] [PubMed] [Google Scholar]
- 3.Lindenbach, B. D. & Rice, C. M. (2001) in Fields Virology, eds. Howley, P. M. & Knipe, D. M. (Lippincott-Raven, Philadelphia, PA), Vol. 1, pp. 991-1041. [Google Scholar]
- 4.Lohmann, V., Korner, F., Koch, J. O., Herian, U., Theilmann, L. & Bartenschlager, R. (1999) Science 285, 110-113. [DOI] [PubMed] [Google Scholar]
- 5.Robertson, B., Myers, G., Howard, C., Brettin, T., Bukh, J., Gaschen, B., Gojobori, T., Maertens, G., Mizokami, M., Nainan, O., et al. (1998) Arch. Virol. 143, 2493-2503. [DOI] [PubMed] [Google Scholar]
- 6.Davis, G. L. (1999) Am. J. Med. 107, 21S-26S. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda, M., Yi, M. K., Li, K. & Lemon, S. M. (2002) J. Virol. 76, 2997-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo, J. T., Bichko, V. V. & Seeger, C. (2001) J. Virol. 75, 8516-8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kishine, H., Sugiyama, K., Hijikata, M., Kato, N., Takahashi, H., Noshi, T., Nio, Y., Hosaka, M., Miyanari, Y. & Shimotohno, K. (2002) Biochem. Biophys. Res. Commun. 293, 993-999. [DOI] [PubMed] [Google Scholar]
- 10.Kato, N., Sugiyama, K., Namba, K., Dansako, H., Nakamura, T., Takami, M., Naka, K., Nozaki, A. & Shimotohno, K. (2003) Biochem. Biophys. Res. Commun. 306, 756-766. [DOI] [PubMed] [Google Scholar]
- 11.Blight, K. J., McKeating, J. A., Marcotrigiano, J. & Rice, C. M. (2003) J. Virol. 77, 3181-3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blight, K. J., Kolykhalov, A. A. & Rice, C. M. (2000) Science 290, 1972-1974. [DOI] [PubMed] [Google Scholar]
- 13.Krieger, N., Lohmann, V. & Bartenschlager, R. (2001) J. Virol. 75, 4614-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lohmann, V., Korner, F., Dobierzewska, A. & Bartenschlager, R. (2001) J. Virol. 75, 1437-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lohmann, V., Hoffmann, S., Herian, U., Penin, F. & Bartenschlager, R. (2003) J. Virol. 77, 3007-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gale, M. J., Jr., Korth, M. J., Tang, N. M., Tan, S. L., Hopkins, D. A., Dever, T. E., Polyak, S. J., Gretch, D. R. & Katze, M. G. (1997) Virology 230, 217-227. [DOI] [PubMed] [Google Scholar]
- 17.Gale, M., Jr., Blakely, C. M., Kwieciszewski, B., Tan, S. L., Dossett, M., Tang, N. M., Korth, M. J., Polyak, S. J., Gretch, D. R. & Katze, M. G. (1998) Mol. Cell. Biol. 18, 5208-5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi, S. T., Polyak, S. J., Tu, H., Taylor, D. R., Gretch, D. R. & Lai, M. M. (2002) Virology 292, 198-210. [DOI] [PubMed] [Google Scholar]
- 19.Majumder, M., Ghosh, A. K., Steele, R., Ray, R. & Ray, R. B. (2001) J. Virol. 75, 1401-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lan, K. H., Sheu, M. L., Hwang, S. J., Yen, S. H., Chen, S. Y., Wu, J. C., Wang, Y. J., Kato, N., Omata, M., Chang, F. Y. & Lee, S. D. (2002) Oncogene 21, 4801-4811. [DOI] [PubMed] [Google Scholar]
- 21.He, Y., Nakao, H., Tan, S. L., Polyak, S. J., Neddermann, P., Vijaysri, S., Jacobs, B. L. & Katze, M. G. (2002) J. Virol. 76, 9207-9217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan, S. L., Nakao, H., He, Y., Vijaysri, S., Neddermann, P., Jacobs, B. L., Mayer, B. J. & Katze, M. G. (1999) Proc. Natl. Acad. Sci. USA 96, 5533-5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zech, B., Kurtenbach, A., Krieger, N., Strand, D., Blencke, S., Morbitzer, M., Salassidis, K., Cotten, M., Wissing, J., Obert, S., et al. (2003) J. Gen. Virol. 84, 555-560. [DOI] [PubMed] [Google Scholar]
- 24.Tu, H., Gao, L., Shi, S. T., Taylor, D. R., Yang, T., Mircheff, A. K., Wen, Y., Gorbalenya, A. E., Hwang, S. B. & Lai, M. M. (1999) Virology 263, 30-41. [DOI] [PubMed] [Google Scholar]
- 25.Gao, L., Aizaki, H., He, J.-W. & Lai, M. M. (2004) J. Virol. 78, 3480-3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang, J., Yamada, O., Sakamoto, T., Yoshida, H., Iwai, T., Matsushita, Y., Shimamura, H., Araki, H. & Shimotohno, K. (2004) Virology 320, 135-143. [DOI] [PubMed] [Google Scholar]
- 27.Hanes, S. D., Shank, P. R. & Bostian, K. A. (1989) Yeast 5, 55-72. [DOI] [PubMed] [Google Scholar]
- 28.Legrain, P., Dokhelar, M. C. & Transy, C. (1994) Nucleic Acids Res. 22, 3241-3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolykhalov, A. A., Agapov, E. V., Blight, K. J., Mihalik, K., Feinstone, S. M. & Rice, C. M. (1997) Science 277, 570-574. [DOI] [PubMed] [Google Scholar]
- 30.Shi, Y., Alin, K. & Goff, S. P. (1995) Genes Dev. 9, 2583-2597. [DOI] [PubMed] [Google Scholar]
- 31.Tachedjian, G., Aronson, H. E., de los Santos, M., Seehra, J., McCoy, J. M. & Goff, S. P. (2003) J. Mol. Biol. 326, 381-396. [DOI] [PubMed] [Google Scholar]
- 32.Weir, M. L., Xie, H., Klip, A. & Trimble, W. S. (2001) Biochem. Biophys. Res. Commun. 286, 616-621. [DOI] [PubMed] [Google Scholar]
- 33.Wyles, J. P., McMaster, C. R. & Ridgway, N. D. (2002) J. Biol. Chem. 277, 29908-29918. [DOI] [PubMed] [Google Scholar]
- 34.Neddermann, P., Clementi, A. & De Francesco, R. (1999) J. Virol. 73, 9984-9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koch, J. O. & Bartenschlager, R. (1999) J. Virol. 73, 7138-7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanji, Y., Kaneko, T., Satoh, S. & Shimotohno, K. (1995) J. Virol. 69, 3980-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katze, M. G., Kwieciszewski, B., Goodlett, D. R., Blakely, C. M., Neddermann, P., Tan, S. L. & Aebersold, R. (2000) Virology 278, 501-513. [DOI] [PubMed] [Google Scholar]
- 38.Asabe, S. I., Tanji, Y., Satoh, S., Kaneko, T., Kimura, K. & Shimotohno, K. (1997) J. Virol. 71, 790-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pietschmann, T., Lohmann, V., Kaul, A., Krieger, N., Rinck, G., Rutter, G., Strand, D. & Bartenschlager, R. (2002) J. Virol. 76, 4008-4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bukh, J., Pietschmann, T., Lohmann, V., Krieger, N., Faulk, K., Engle, R. E., Govindarajan, S., Shapiro, M., St Claire, M. & Bartenschlager, R. (2002) Proc. Natl. Acad. Sci. USA 99, 14416-14421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coito, C., Diamond, D. L., Neddermann, P., Korth, M. J. & Katze, M. G. (2004) J. Virol. 78, 3502-3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.