Abstract
The major surface glycoprotein of feline immunodeficiency virus (FIV) specifically binds to a 43-kDa glycoprotein expressed on the surface of a subset of T cells in peripheral blood mononuclear cells and IL-2-dependent T cell lines. Binding to this molecule, in conjunction with CXC chemokine receptor (CXCR) 4, is required for productive infection of these cells by primary isolates of FIV. Here, we demonstrate that the 43-kDa molecule is CD134, a receptor for FIV recently identified independently [Shimojima, M., et al. (2004) Science 303, 1192-1195]. Furthermore, we show that CD134 is specifically up-regulated on CD4+ T cells that have been activated by treatment with IL-2 and Con A. CD8+ T cells remained negative for CD134 expression regardless of the activation state. Binding of the FIV major surface glycoprotein on activated CD4+ T cells was observed through direct interaction with CD134 whereas, on activated CD8+ T cells, the binding was CD134-independent and mediated by CXCR4 and, to a lesser extent, heparan sulfate proteoglycans. However, this CD134-independent interaction was not sufficient to render CD8+ T cells permissive to FIV infection, as FIV replicated primarily in activated CD4+ T cells and not in cells negative for CD134 expression. Altogether, our results substantiate that CD134 acts as a primary binding receptor for FIV and explain the specific targeting and depletion of the CD4+ T cell population observed during the course of infection independent of the use of CD4 as a binding receptor/coreceptor.
Keywords: lentivirus, OX-40, CXC chemokine receptor 4, cytokine
Feline immunodeficiency virus (FIV) causes an AIDS-like syndrome in the domestic cat (1) and is thus a concern from an animal health care aspect but also offers an experimental model for the development of intervention therapies against HIV infection. As with HIV infection in humans, FIV causes depletion of CD4+ T cells (2-6), the primary target cell population early in infection (7, 8). CD8+ T cells and B cells also become infected later in the chronic phase of FIV infection (7, 8). Although FIV does not use CD4 as a binding receptor/coreceptor (9), the feline lentivirus does share with T-tropic HIV-1 isolates the use of the chemokine receptor CXC chemokine receptor (CXCR) 4 as an entry receptor for infection (10). FIV also uses cell-surface heparans (11) and DC-SIGN (12) as binding receptors to enhance infection, another characteristic shared with HIV (13, 14). In addition, the FIV major surface glycoprotein (SU) specifically binds to a 43-kDa glycoprotein on several IL-2-dependent T cell lines and peripheral blood mononuclear cells (PBMCs), and the presence of this receptor correlates with the ability of field strains to productively infect a particular target cell (11, 15). Recent studies by Shimojima et al. (16) have shown that the cell-surface marker CD134 facilitates CXCR4-dependent infection by FIV. In the present study, we demonstrate that FIV SU binds directly to CD134, and that its distribution and molecular size and properties precisely correspond to a 43-kDa receptor we identified in earlier studies (11, 15). We have also extended the analysis of receptor expression by subdividing PBMCs into CD4+ T cells, CD8+ T cells, and B cells and analyzing SU binding as well as FIV infectivity as a function of activation with Con A and IL-2. The results demonstrate the specific up-regulation of CD134 on activated CD4+ T cells, which explains the specific targeting of this critical cell population by FIV during in vivo infection.
Experimental Procedures
Cells. Cat PBMCs were prepared from heparinized whole feline blood by Ficoll/Paque gradient purification. PBMCs were left untreated and used immediately, or were activated in complete RPMI medium 1640 (11) in the presence of 100 units/ml recombinant human IL-2 (obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health) and 5 μg/ml Con A (Sigma). After 4-5 days of culture to allow full clonal expansion, the activated PBMCs were used for fluorescence-activated cell sorter (FACS) and infection analyses. The IL-2-dependent feline T cell line 104-C1 was isolated by limiting dilution cloning of PBMCs and was a gift from C. Grant (Custom Monoclonals, West Sacramento, CA). The IL-2-independent feline lymphoma cell line 3201 was obtained from W. Hardy (Sloan-Kettering Memorial Hospital, New York). Crandel feline kidney cells (CrFK) and 293T cells were obtained from the American Type Culture Collection. Propagation of the different cell lines was previously described (11).
CrFK CD134+ Cells. Human CD134 was cloned by PCR from activated PBMCs. Feline CD134 was cloned by RACE from a 104-C1 cDNA library constructed in the hygroMaRXII vector (17). Briefly, two CD134 regions of high homology at the amino acid level, QACK and PIQE, were identified by alignment of human, mouse, and rat CD134. Degenerative primers were synthesized; a sense primer corresponding to the relatively conserved amino acid sequence QACK had the sequence 5′-CARGCCTGCAAGCCCTGGACCAA-3′ and the other, antisense, corresponding to the conserved amino acid sequence PIQE, had the sequence 5′-GGCTAGATCTTGGCCAGAGTGGAGTKKGCGGTC-3′. After a first round of PCR using these two primers, a 350-bp amplicon was obtained and confirmed to be homologous to CD134 by sequencing. Next, the 5′ end and the 3′ end of feline CD134 were obtained by RACE. Finally, amplification of the entire cat CD134 was performed, and several clones were sequenced. CrFK cells were transduced with a murine stem cell retrovirus vector (MIGR1-CD134-GFP) (12) expressing either feline or human CD134 in tandem with GFP via an internal ribosome entry site linker. GFP positive cells (transduction efficiency typically 50%) were sorted by FACS and used for SU-binding studies.
Flow Cytometry Analyses. FIV-PPR SU-Fc adhesin (11) was incubated at 1 μg/ml in the presence or absence of AMD3100 (18-20) or heparin (Sigma), both at 1 μg/ml, with 2 × 105 cells for 1 h in 100 μl of Earl's balanced salt solution (Sigma) containing 0.1% BSA, washed once in Earl's balanced salt solution, and resuspended in 100 μl of Earl's balanced salt solution/0.1% BSA supplemented with a phycoerythrin-conjugated anti-human Fc antibody (ICN) at a dilution of 1:250. For two-color flow cytometry analyses, fluorescein isothiocyanate-conjugated CD4, CD8, and B220 antibodies (Southern Biotechnology Associates) were added for another 30 min, and samples were then washed and processed on a FACScan (Becton Dickinson). For double staining of CD4 and CD8, fluorescein isothiocyanate-conjugated CD4 and phycoerythri-nconjugated CD8 antibodies (Southern Biotechnology Associates) were used. Data were acquired on a FACScan and analyzed with cellquest (Becton Dickinson) and flowjo (Tree Star, San Carlos, CA) software, respectively. AMD3100 was obtained through the AIDS Research and Reference Reagent Program.
Receptor Overlay Assay. A modified virus overlay protein binding assay adapted from Wu et al. (21) was used. Cell lysates (in 20 mM Tris·HCl, pH7.5/150 mM NaCl/1% Nonidet P-40) were subjected to SDS/PAGE and Western blotting. Blots were incubated overnight with SU-Fc at 1 μg/ml in PBS at 4°C. The blots were then washed and further incubated for 1 h with horseradish peroxidase-conjugated anti-human Fc antibody (ICN-Cappel) at a 1:1,000 dilution. The blots were again washed, and SU-binding proteins were revealed by chemiluminescence.
Coimmunoprecipitation Assay. Cell lysates were incubated with FIV PPR SU-Fc and protein A-Sepharose beads overnight at 4°C. Washed precipitates were separated by SDS/PAGE and transferred to a nitrocellulose membrane. The membrane was then incubated with a rabbit polyclonal antibody raised against feline CD134 (see below). CD134 was detected by chemiluminescence with a horseradish peroxidase-conjugated anti-rabbit antibody.
Rabbit Anti-Feline CD134 Antibody. A construct containing the entire ectodomain of feline CD134 was amplified by PCR and cloned into pET28a in frame with an N-terminal 6-His tag. Clones were sequenced, and a single clone was transformed into competent Escherichia coli Rosetta(DE3)pLysS cells (Novagen). Isopropyl β-D-thiogalactoside-induced protein expression was scaled up, and purification was carried out by using three rounds of Ni-affinity chromatography. As a final purification step, the protein was transferred to an Immobilon membrane (Millipore) after SDS/PAGE and was eluted as described (22). The eluted protein was reanalyzed by SDS/PAGE and Western blotting, was quantitated, and was then injected into a rabbit to generate antibodies to CD134. Immunization was carried out every 2 weeks to boost antibody production. The prebleed from the same animal was used as a control to test for specificity of interaction in Westerns as well as immunoprecipitation assays.
Virus Entry Assay. Cytomegalovirus/FIV hybrid vectors (pCFIV) (23) pseudotyped with either a deleted envelope (Δenv) or an FIV-PPR envelope (PPR Env) genes were cotransfected with a β-galactosidase (β-gal) transgene, pTFIVCβ (23), in 293T cells. Three days later, viral supernatant was collected, and the reverse transcriptase activity was adjusted to 50,000 cpm/ml. One milliliter was used to transduced 2 × 105 cells in duplicate, and β-gal activity was measured 48 h later with the Tropix Galacto-Star chemiluminescent reporter gene assay (Applied Biosystems) according to the manufacturer's guidelines.
Virus Infectivity Assay. Cells (2 × 105) were infected with FIV at multiplicity of infection of 0.1 for 2 h at 37°C. Cells were then washed and cultured at 37°C in a 5% CO2 atmosphere. Reverse transcriptase activity was measured at 6 days postinfection from 50 μl of cell-free supernatant as previously described (11).
Results
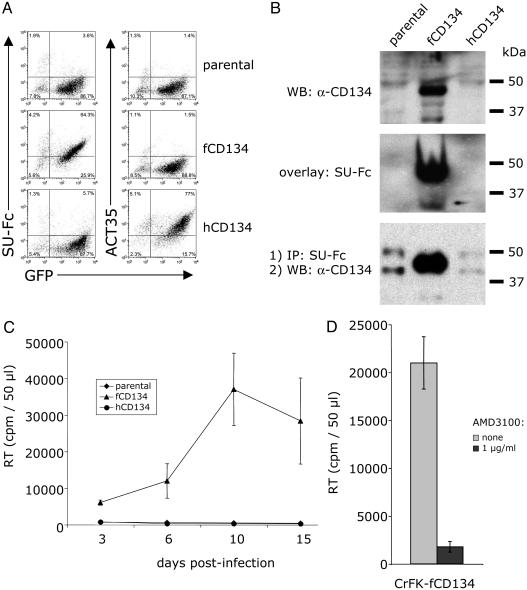
The SU of FIV envelope specifically binds to a 43-kDa glycoprotein expressed on PBMCs and IL-2-dependent T cell lines (11). It was suggested that this receptor might act as a primary binding receptor for FIV (11). The recent report that CD134 is involved in FIV infection (16) prompted comparisons to our earlier observations. We expressed feline and human CD134 in CrFK cells, a cell line refractory to infection by primary isolates (PIs) of FIV, and analyzed SU binding as well as infectivity by FIV as a function of CD134 expression. Our results showed that FIV SU specifically interacted with feline CD134 (Fig. 1). Binding of FIV SU by FACS analysis was observed on feline CD134+ cells but not on parental cells or human CD134+ cells (Fig. 1A). Both FIV SU-Fc and anti-CD134 polyclonal antibody recognized a similar 43-kDa protein species in feline CD134-transduced CrFK cells (Fig. 1B Top and Middle). Finally, coimmunoprecipitation assays revealed that CD134 specifically coprecipitated with FIV SU-Fc from feline CD134+ but not from CD134- cell lysates (Fig. 1B Bottom). CrFK cells are normally not permissive to infection by FIV-PPR, a PI of FIV (24). To investigate whether expression of fCD134 could render CrFK cells productively infected by FIV-PPR, parental and CD134-transduced cells were infected with FIV-PPR, and virus production was monitored by a reverse transcriptase assay for a period of 2 weeks. As shown in Fig. 1C, FIV specifically replicated in feline CD134+ cells but not in parental or human CD134+ cells. Because FIV infection is CXCR4-dependent (10), we determined whether infection of feline CD134+ cells required CXCR4. As shown on Fig. 1D, infection of feline CD134+ cells was efficiently inhibited by the CXCR4 antagonist, AMD3100 (19), indicating that both CD134 and CXCR4 are required for productive infection.
Fig. 1.
Specific interaction of FIV with CD134. (A Left) Plots represent a FACS analysis of FIV-PPR SU-Fc adhesin binding to CrFK cells transduced with MIGR1 GFP vector alone (parental) or MIGR1 GFP vector coexpressing either feline CD134 (fCD134) or human CD134 (hCD134). (Right) Plots represent a FACS analysis of the same transduced cells using mAb ACT35, specific for human CD134. (B) Direct evidence that FIV SU specifically interacts with feline CD134. (Top) Western blot using a rabbit polyclonal antiserum to fCD134 vs. cell lysates from CrFK cells transduced with MIGR1 GFP vector only (parental), vector expressing fCD134, or vector expressing hCD134. (Middle) Overlay assay performed as detailed in Experimental Procedures, using FIV SU-Fc vs. the extracts described under A to detect specific binding to fCD134. (Bottom) Drag-down assay using FIV SU-Fc and protein A-Sepharose vs. lysates detailed under A, followed by Western blotting of the resultant precipitates using polyclonal anti-fCD134. (C) CrFK cells transduced with vector expressing GFP only (parental), fCD134, or hCD134 were infected with FIV-PPR, and virus production was monitored by reverse transcriptase assay. Results are the means ± SD for triplicate determinations. (D) CrFK cells transduced with fCD134 were infected with FIV-PPR in the presence or absence of the CXCR4 antagonist, AMD3100 (1 μg/ml). Virus production was monitored by a reverse transcriptase assay 6 days postinfection. Results are the means ± SD for triplicate determinations.
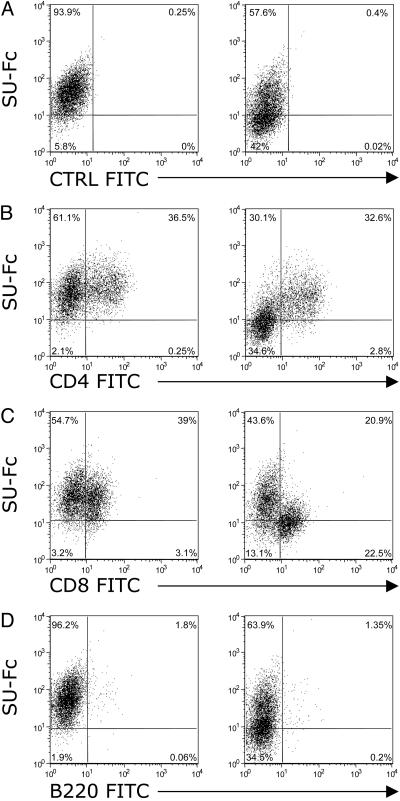
Because CD134 is specifically expressed on activated CD4+ T cells in human PBMCs (25), experiments were performed to assess the expression of feline CD134 as well as the extent and target specificity of FIV SU binding to subsets of PBMCs as a function of activation with IL-2 and Con A. FACS analyses using an SU-Fc adhesin (11) constructed from the SU protein of a PI, FIV-PPR (26), were carried out to assess relative binding of the FIV SU to naive PBMCs and to CD4, CD8, and B cell subsets (Fig. 2). Total PBMCs were Ficoll gradient-purified from whole blood and were then used immediately for analysis without stimulation. The majority of binding (77%) by the SU-Fc adhesin was inhibited by AMD3100 on total naive PBMCs, consistent with the interpretation that the majority of binding occurred through interaction with CXCR4 directly (Fig. 2A). Relative binding of FIV SU-Fc was then assessed on unstimulated CD4+ T cell, CD8+ T cell, and B220+ B cell subsets by two-color flow cytometry (Fig. 2 B-D, respectively). Binding to the CD4+ T cell subset reflected the pattern obtained with the unsorted PBMCs, with ≈70% of SU-Fc binding blocked by AMD3100 treatment (Fig. 2B). Similar results were observed on naive CD8+ T cells (81% inhibition, Fig. 2C). On the B220+ B cell subset, 50% inhibition was observed after AMD3100 treatment (Fig. 2D), and heparin treatment, which competes for heparan sulfate proteoglycans (HSPG) binding, had no effect on the binding of SU-Fc to B cells (data not shown).
Fig. 2.
Binding of FIV SU on naive PBMCs. FACS analysis showing the binding of FIV SU-Fc on freshly prepared, uncultured total PBMCs (A) and on CD4+, CD8+, and B cell subsets (B-D, respectively) in the absence (Left) or presence (Right) of the CXCR4 antagonist, AMD3100. Data are representative of two experiments.
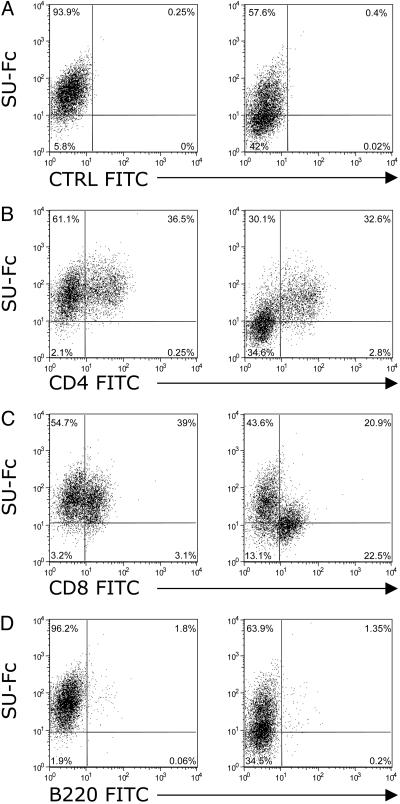
We next analyzed SU-Fc binding to total PBMCs that had been activated by 5 days of culture in the presence of Con A and IL-2 (Fig. 3). SU-Fc binding (≈40%) to activated total PBMCs was blocked in the presence of AMD3100 (Fig. 3A). Analysis of SU-Fc binding on activated CD4+ (Fig. 3B) and CD8+ (Fig. 3C) T cells revealed differential binding of SU by these two cell populations. Binding of SU-Fc to activated CD4+ T cells was inhibited only 10% by AMD3100 treatment (Fig. 3B Right). In contrast, binding to the CD8+ T cell population by SU-Fc was inhibited by ≈50% by AMD3100 treatment (Fig. 3C Right). B220-positive cells did not amplify and were lost after 5 days in culture, typical of cultured, nontransformed B cells (Fig. 3D). The findings indicate that the majority of binding to the activated CD4+ T cells is through an interaction other than with CXCR4. In contrast, binding to CD8+ T cells (Fig. 3C) and to the total CD4- cell population (Fig. 3B) remained sensitive to AMD3100 independent of cytokine activation.
Fig. 3.
Binding of FIV SU on activated PBMCs. FACS analysis showing the binding of FIV SU-Fc on total PBMCs (A) and on CD4+, CD8+, and B cell subsets (B-D, respectively) in the absence (Left) or presence (Right) of the CXCR4 antagonist, AMD3100. Total PBMCs were activated by 5 days of culture in the presence of IL-2 and Con A. Data are representative of two experiments.
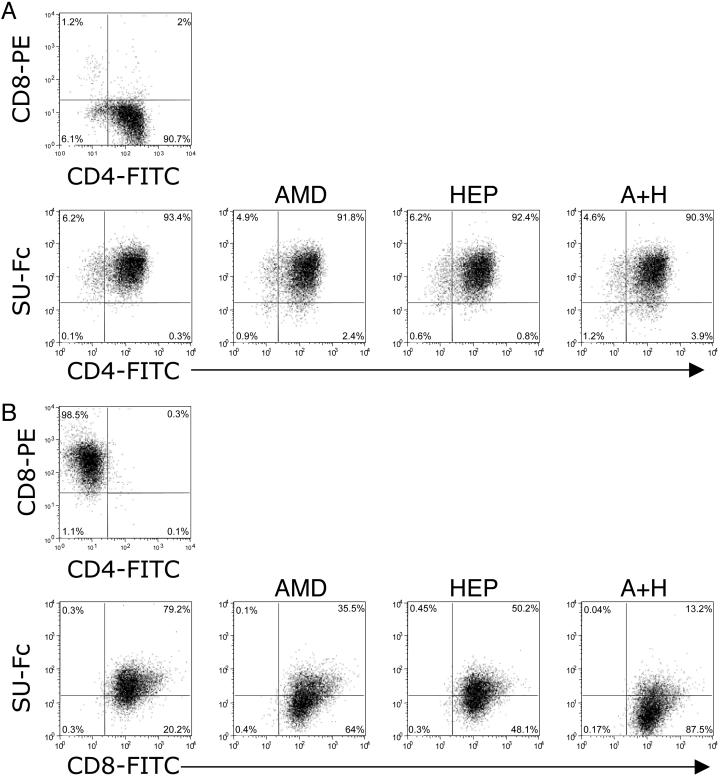
To address more precisely the relative binding of FIV SU to the activated CD4+ and CD8+ T cells, fresh PBMCs were sorted into CD4+/CD8- and CD4-/CD8+ cell populations and cultured in the presence of IL-2 and Con A for 5 days (Fig. 4). The phenotype of the resultant activated CD4+ sorted population was >96% positive for CD4 and negative for CD8, and the CD4- sorted cell population was >98% positive for CD8 and negative for CD4 (Fig. 4 A and B Upper, respectively). FACS analyses to assess SU binding were then carried out on these two cell populations in the presence and absence of AMD3100 and heparin. Heparin inhibits SU interactions with cell-surface heparans, binding to which occurs with some HIV (14) and FIV (11) isolates. The results reaffirm the findings with unsorted, activated PBMCs (Fig. 2B) relative to SU binding to CD4+ T cells in that virtually no inhibition was observed with AMD3100 (Fig. 4A Lower). Heparin also failed to inhibit the binding of SU-Fc to the CD4+ T cells, either alone or in combination with AMD3100. In contrast, SU binding to the CD4-CD8+ cell population was inhibited by ≈55% with AMD3100, 40% with heparin, and to nearly 90% with a combination of AMD3100 and heparin (Fig. 4B Lower). The results indicate that binding to the activated CD4+CD8- cell population is CXCR4- and HSPG-independent, whereas binding to the activated CD4-CD8+ cell population involved CXCR4 and HSPG.
Fig. 4.
Comparison of FIV SU-Fc binding to CD4+CD8- and CD4-CD8+ activated T cells and effect of AMD3100 and/or heparin treatment on SU-Fc binding. Fresh PBMCs were sorted into CD4+CD8- and CD4-CD8+ cell populations and activated by culture for 5 days in the presence of IL-2 and Con A. The activated CD4+CD8- sorted cell population was >90% positive for CD4 and essentially negative for CD8+ cells (A Upper). Conversely, the activated CD4-CD8+ sorted cells were >98.5% positive for CD8 and negative for CD4 expression (B Upper). The sorted cell populations were then used to analyze the degree of SU-Fc binding as a function of addition of the CXCR4 antagonist, AMD3100 (AMD), heparin (HEP), or both (A+H). No significant inhibition was noted with either antagonist on SU-Fc binding to the CD4+CD8- T cell population, indicating that the binding occurs through an interaction other than CXCR4 and HSPG (A Lower). However, both AMD3100 and heparin inhibited SU-Fc binding to the CD4-CD8+ cells by ≈55% and 40%, respectively, when added individually, and inhibited essentially 90% of binding when added in combination (B Lower). Data are representative of three experiments.
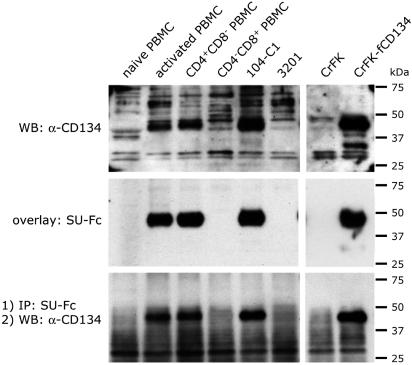
To determine whether the SU binding receptor on the activated CD4+CD8- T cell population is CD134, lysates were prepared from naive and activated total PBMCs and from the activated CD4+ and CD8+ sorted cell populations, and proteins were separated by SDS/PAGE, transferred to nitrocellulose, and subjected to Western blot analyses by using rabbit sera raised against feline CD134 (Fig. 5 Top). In agreement with the FACS analyses, the results revealed that CD134 was detected in extracts from Con A/IL-2 activated PBMCs (Fig. 5 Top, lane 2) but not in extracts from naive PBMCs (Fig. 5 Top, lane 1). Separation of PBMCs into activated CD4+ and CD8+ cell subsets, followed by Western blotting with anti-CD134, revealed that all of the reactivity colocalized with the activated CD4+ T cell population (Fig. 5 Top, compare lanes 3 and 4, respectively).
Fig. 5.
Specific up-regulation of CD134 on activated CD4+ T cells. (Top) The indicated cell lysates were subjected to SDS/PAGE and Western blotting with a rabbit anti-fCD134 polyclonal antibody. CD134 was detected in total activated PBMCs, activated CD4+ T cells, and 104-C1 cells but not on resting PBMCs, activated CD8+ T cells, and 3201 lymphoma cells. (Middle) An overlay assay with FIV SU-Fc was carried out with the indicated cell lysates. Results revealed a similar up-regulation of the 43-kDa SU binding receptor on activated CD4+ T cells. (Bottom) Coimmunoprecipitation of FIV SU-Fc and fCD134 was observed in lysates from total activated PBMCs, activated CD4+CD8- T cells, and 104-C1 T cells, but not from naive PBMCs, activated CD4-CD8+ T cells, and 3201 lymphoma cells. CrFK and CrFK-fCD134 cell lysates were used as controls.
Results of overlay assays with SU-Fc (Fig. 5 Middle) correlated with the expression of CD134 (Fig. 5 Top). Analysis by overlay blot of extracts from the sorted activated CD4+ and CD4- cell populations revealed that the CD4+CD8- cell population, but not the CD4-CD8+ cell population, was strongly positive for SU-Fc binding (Fig. 5 Middle, lanes 3 and 4, respectively). Consistent with our previous reports (11), 3201 cells were negative for SU-Fc binding, and 104-C1 cells were strongly positive (Fig. 5, lanes 5 and 6, respectively). Binding correlated with expression of CD134 in CD134-transduced CrFK cells (Fig. 5 Middle, lanes 7 and 8, respectively).
Coimmunoprecipitation experiments were next performed by using SU-Fc and protein A-Sepharose to demonstrate that the 43-kDa molecule bound by the SU-Fc adhesins corresponded to CD134 (Fig. 5 Bottom). Proteins precipitated from cell lysates by SU-Fc in combination with protein A-Sepharose were reacted in Western blots with the anti-CD134 antibody (Fig. 5 Bottom, lanes 1-6). The results demonstrated that SU-Fc coprecipitated with CD134 specifically from cells identified as positive for receptor activity, but not from receptor negative cells. Consistent with our previous report (11), CD134 was not coimmunoprecipitated with SU-Fc from the 3201 lymphoma cell line (Fig. 5 Bottom, lane 6) but was readily detected in SU-Fc coprecipitates from the primary T cell line, 104-C1 (Fig. 5 Bottom, lane 5).
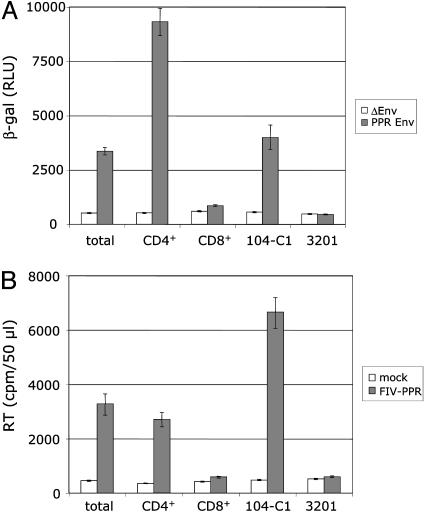
Infectivity assays were next performed to assess FIV replication in total activated PBMCs, activated CD4+ and CD8+ T cells, and 104-C1 and 3201 cells. First, a β-gal assay was used to assess the degree of viral entry after a single round of infection. FIV lentivectors (23) pseudotyped with either a deleted or an integral gene coding for the envelope of FIV-PPR, a PI FIV isolate, were used. The PI FIV strain replicates only in cells that express the 43-kDa receptor (11, 15). As shown in Fig. 6A, β-gal activity was observed only in total activated PBMCs, activated CD4+ T cells, and 104-C1 T cells, all cells that express CD134 (Fig. 5). The results are in agreement with a direct correlation between CD134 expression and susceptibility to FIV infection. Cells were also subjected to infection with the PI FIV-PPR strain, and virus production was monitored by a reverse transcriptase assay in cell-free supernatant after 6 days of culture. The results confirmed the entry assay in that total activated PBMCs, activated CD4+ T cells, and 104-C1 T cells, but not the CD134- cells (activated CD8+ T cells and 3201 cells), were productively infected by FIV.
Fig. 6.
FIV targets primarily activated CD4+ T cells but not CD8+ T cells. Susceptibility to FIV infection was analyzed on total activated PBMCs, CD4+CD8- activated T cells, CD4-CD8+ activated T cells, 104-C1 T cells, and 3201 lymphoma cells. (A) pCFIV vectors pseudotyped with the envelope of FIV-PPR (PPR Env) or a deleted envelope (ΔEnv) were used to assess the degree of viral entry. A β-gal assay performed 48 h posttransduction shows that pCFIV pseudotyped with the FIV-PPR envelope infected only total PBMCs, activated CD4+CD8- T cells, and 104-C1 T cells. RLU, relative luminescence units. (B) Productive infection by FIV was also assessed in parallel. Total activated PBMCs, activated CD4+CD8- and CD4-CD8+ T cells, 104-C1 T cells, and 3201 lymphoma cells were infected with FIV-PPR at a multiplicity of infection of 0.1, and virus production was analyzed by a reverse transcriptase (RT) assay at 6 days after the initiation of infection. Viral production was observed only in total activated PBMCs, activated CD4+CD8- T cells, and 104-C1 cells. Results are means and SD for duplicate determinations.
Discussion
The recent observation that CD134 can facilitate FIV infection (16) prompted us to examine the SU binding ability and expression of CD134 on cells that are potential targets for infection by FIV. The results show that a 43-kDa glycoprotein that we had previously proposed might act as a primary binding receptor for FIV (11) is, in fact, CD134. Furthermore, the findings demonstrated that FIV SU bound specifically to CrFK cells expressing CD134 but not to CD134- parental cells (Fig. 1A). Also, binding and infection of several primary cell subsets and cell lines correlated with CD134 expression. Coimmunoprecipitation studies clearly demonstrated specific interaction of FIV SU with feline CD134 (Fig. 1B). Of particular significance, CD134 expressed in conjunction with CXCR4 was strictly required for productive infection by PI FIV (Fig. 1 C and D). This observation is consistent with a binding/activation role for CD134 similar to the CD4/chemokine receptor interactions required for productive HIV infection.
The findings show that the CD4+ T cell subset, the same cell subset that is depleted during in vivo infection (2-5), also expresses CD134 after cytokine and mitogen activation and is the primary target for infection by the field isolate of FIV used in this study. Expression of CD134, which was not detected on naive PBMCs (Fig. 5 Top), was up-regulated specifically on the CD4+ T cell subset after stimulation with IL-2 and Con A (Fig. 5 Top). We observed a similar pattern in the CXCR4-independent binding of FIV SU (Figs. 2, 3, 4) that we demonstrated to be mediated by means of CD134 (Fig. 1B and 5). Furthermore, we showed that FIV infection is directly correlated to the expression of CD134 (Figs. 1C and 6). Indeed, only cells that expressed CD134 were found to be productively infected by PI FIV.
The results of SU binding to CD8+ T cells were distinct from the binding noted with the CD4+ T cell population. Activated CD8+ T cells remained CD134-negative (Fig. 5 Top) but expressed the entry receptor CXCR4. SU interaction with activated CD8+ T cells was essentially mediated by direct interactions with CXCR4 and HSPG (Fig. 4B). However, this interaction was not sufficient to allow productive infection of the activated CD8+ T cell population by FIV-PPR, a primary strain of FIV, suggesting that binding to CD134 is a prerequisite for efficient entry by field isolates of FIV. Whether distinct activation pathways up-regulate CD134 expression of other potential target cell populations including CD8+ T cells and B cells remains to be determined. The specific targeting of activated CD4+ T cells observed in our study correlated to the in vivo tropism previously reported for FIV (7, 8), where CD4+ T cells have been shown to be predominantly infected by FIV early in the infection (7, 8). However, later in the course of the infection, CD8+ T cells as well as B cells also become infected (7, 8). Given that CXCR4 expression is widely distributed and that tissue culture-adapted viruses can infect cells directly through CXCR4 (10, 24), the expansion of host cell tropism to include CD8+ T cells and B cells may result from an adaptation of FIV from a CD134-dependent to a CD134-independent phenotype. Alternatively, a low level of expression of CD134 on CD8+ T cells and B cells not seen with our analyses, but reported in humans and mice (27-29), could mask a slow and progressive infection of these two cell populations that is observed only later in the course of in vivo infection.
CD134 is expressed primarily on activated CD4+ T cells in humans (25) and on activated CD4+ T cells and, to a lesser extent, CD8+ T cells in mice (29). Activated B cells have also been reported to express CD134 at very low levels (27, 28). CD134 is a member of the tumor necrosis factor receptor superfamily and has been implicated in numerous immunological responses (for review see ref. 30). Engagement of CD134 by CD134 ligand after antigen-specific stimulation of T cells leads to enhanced effector and memory-effector T cell function and an increase in the survival rate of antigen-activated T cells by means of up-regulation of cytokine production and antiapoptotic gene expression. Thus, the direct interaction of FIV SU with CD134 may not only facilitate virus infection but may directly impact on signaling pathways activated by means of normal interactions between CD134 and its natural ligand, thus influencing the degree and specificity of the immune response in the host.
Our overall findings help to explain why FIV causes CD4+ T cell depletion as seen in HIV infection, despite the fact that the feline lentivirus does not use CD4 as a binding receptor. Up-regulation of CD134 as a consequence of activation may thus contribute to the demise of CD4+ T cells specifically elicited during immune responses to FIV infection, resulting in immune collapse and susceptibility to opportunistic infections.
Acknowledgments
We thank Ying-Chuan Lin and Sohela de Rozières for careful reading of the manuscript and valuable suggestions, Jackie Wold for manuscript preparation, and the AIDS Research and Reference Reagent Program for reagents used in these studies. This work was supported by National Institutes of Health (National Institute of Allergy and Infectious Diseases) Grant R01AI25825.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: FIV, feline immunodeficiency virus; SU, major surface glycoprotein; HSPG, heparan sulfate proteoglycans; PBMC, peripheral blood mononuclear cell; CrFK, Crandel feline kidney cells; β-gal, β-galactosidase; PI, primary isolate; CXCR4, CXC chemokine receptor 4; FACS, fluorescence-activated cell sorter.
References
- 1.Pedersen, N. C., Ho, E. W., Brown, M. L. & Yamamoto, J. K. (1987) Science 235, 790-793. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann-Fezer, G., Thum, J., Ackley, C., Herbold, M., Mysliwietz, J., Thefeld, S., Hartmann, K. & Kraft, W. (1992) J. Virol. 66, 1484-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ackley, C. D., Yamamoto, J. K., Levy, N., Pedersen, N. C. & Cooper, M. D. (1990) J. Virol. 64, 5652-5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Novotney, C., English, R. V., Housman, J., Davidson, M. G., Nasisse, M. P., Jeng, C. R., Davis, W. C. & Tompkins, M. B. (1990) AIDS 4, 1213-1218. [DOI] [PubMed] [Google Scholar]
- 5.Willett, B. J., Hosie, M. J., Dunsford, T. H., Neil, J. C. & Jarrett, O. (1991) AIDS 5, 1469-1475. [DOI] [PubMed] [Google Scholar]
- 6.Torten, M., Franchini, M., Barlough, J. E., George, J. W., Mozes, E., Lutz, H. & Pedersen, N. C. (1991) J. Virol. 65, 2225-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dean, G. A., Reubel, G. H., Moore, P. F. & Pedersen, N. C. (1996) J. Virol. 70, 5165-5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.English, R. V., Johnson, C. M., Gebhard, D. H. & Tompkins, M. B. (1993) J. Virol. 67, 5175-5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norimine, J., Miyazawa, T., Kawaguchi, Y., Tomonaga, K., Shin, Y. S., Toyosaki, T., Kohmoto, M., Niikura, M., Tohya, Y. & Mikami, T. (1993) Arch. Virol. 130, 171-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willett, B. J., Picard, L., Hosie, M. J., Turner, J. D., Adema, K. & Clapham, P. R. (1997) J. Virol. 71, 6407-6415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Parseval, A. & Elder, J. H. (2001) J. Virol. 75, 4528-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Parseval, A., Su, S. V., Elder, J. H. & Lee, B. (2004) J. Virol. 78, 2597-2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geijtenbeek, T. B., Kwon, D. S., Torensma, R., van Vliet, S. J., van Duijnhoven, G. C., Middel, J., Cornelissen, I. L., Nottet, H. S., KewalRamani, V. N., Littman, D. R., et al. (2000) Cell 100, 587-597. [DOI] [PubMed] [Google Scholar]
- 14.Saphire, A. C., Bobardt, M. D., Zhang, Z., David, G. & Gallay, P. A. (2001) J. Virol. 75, 9187-9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Parseval, A., Ngo, S. & Elder, J. H. (2004) J. Virol. 78, 9132-9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimojima, M., Miyazawa, T., Ikeda, Y., McMonagle, E. L., Haining, H., Akashi, H., Takeuchi, Y., Hosie, M. J. & Willett, B. J. (2004) Science 303, 1192-1195. [DOI] [PubMed] [Google Scholar]
- 17.Sun, P., Dong, P., Dai, K., Hannon, G. J. & Beach, D. (1998) Science 282, 2270-2272. [DOI] [PubMed] [Google Scholar]
- 18.Bridger, G. J., Skerlj, R. T., Thornton, D., Padmanabhan, S., Martellucci, S. A., Henson, G. W., Abrams, M. J., Yamamoto, N., De Vreese, K., Pauwels, R., et al. (1995) J. Med. Chem. 38, 366-378. [DOI] [PubMed] [Google Scholar]
- 19.De Clercq, E., Yamamoto, N., Pauwels, R., Balzarini, J., Witvrouw, M., De Vreese, K., Debyser, Z., Rosenwirth, B., Peichl, P., Datema, R., et al. (1994) Antimicrob. Agents Chemother. 38, 668-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hendrix, C. W., Flexner, C., MacFarland, R. T., Giandomenico, C., Fuchs, E. J., Redpath, E., Bridger, G. & Henson, G. W. (2000) Antimicrob. Agents Chemother. 44, 1667-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu, E., Fernandez, J., Fleck, S. K., Von Seggern, D. J., Huang, S. & Nemerow, G. R. (2001) Virology 279, 78-89. [DOI] [PubMed] [Google Scholar]
- 22.Chatterji, U., de Parseval, A. & Elder, J. H. (2002) J. Virol. 76, 9624-9634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnston, J. C., Gasmi, M., Lim, L. E., Elder, J. H., Yee, J. K., Jolly, D. J., Campbell, K. P., Davidson, B. L. & Sauter, S. L. (1999) J. Virol. 73, 4991-5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lerner, D. L. & Elder, J. H. (2000) J. Virol. 74, 1854-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Latza, U., Durkop, H., Schnittger, S., Ringeling, J., Eitelbach, F., Hummel, M., Fonatsch, C. & Stein, H. (1994) Eur. J. Immunol. 24, 677-683. [DOI] [PubMed] [Google Scholar]
- 26.Phillips, T. R., Talbott, R. L., Lamont, C., Muir, S., Lovelace, K. & Elder, J. H. (1990) J. Virol. 64, 4605-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durkop, H., Latza, U., Himmelreich, P. & Stein, H. (1995) Br. J. Haematol. 91, 927-931. [DOI] [PubMed] [Google Scholar]
- 28.Durkop, H., Anagnostopoulos, I., Bulfone-Paus, S. & Stein, H. (1997) Br. J. Haematol. 98, 863-868. [DOI] [PubMed] [Google Scholar]
- 29.Baum, P. R., Gayle, R. B., III, Ramsdell, F., Srinivasan, S., Sorensen, R. A., Watson, M. L., Seldin, M. F., Baker, E., Sutherland, G. R., Clifford, K. N., et al. (1994) EMBO J. 13, 3992-4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinberg, A. D., Evans, D. E., Thalhofer, C., Shi, T. & Prell, R. A. (2004) J. Leukocyte Biol. 75, 962-972. [DOI] [PubMed] [Google Scholar]