Abstract
Background. Long-term outcomes (mortality and health-related quality of life) of sepsis have risen as important indicators for health care. Pulmonary infection and abdominal infection are the leading causes of sepsis. However, few researches about long-term outcomes focused on the origin of sepsis. Here we aim to study the clinical differences between pulmonary-sepsis and abdominal-sepsis and to investigate whether different infection foci were associated with long-term outcomes. Methods. Patients who survived after hospital discharge were followed up by telephone interview. Quality of life (QoL) was assessed using the EuroQol 5-dimension (EQ5D) questionnaire. Results. Four hundred and eighty-three sepsis patients were included, 272 (56.3%) had pulmonary-sepsis, and 180 (37.3%) had abdominal-sepsis. The overall ICU and one-year mortality rates of the cohort were 17.8% and 36.1%, respectively. Compared with abdominal-sepsis, pulmonary-sepsis patients had older age, higher APACHE II, higher ICU mortality (31.7% versus 12.6%), and one-year mortality (45.4% versus 24.4%), together with worse QoL. Age, septic shock, acute renal failure, fungus infection, anion gap, and pulmonary infection were predictors for one-year mortality and pulmonary infection was a risk factor for poor QoL. Conclusions. Pulmonary-sepsis showed worse outcome than abdominal-sepsis. Pulmonary infection is a risk factor for one-year mortality and QoL after sepsis.
1. Introduction
Despite advances in organ support and guidelines for sepsis management, the incidence of sepsis is still increasing [1–5]. Sepsis is the leading cause of death among hospitalized patients [6], mortality of which ranging from 20 to 80%. Sepsis survivors also suffered persistent reduction in long-term health-related quality of life (HR-QoL), such as depression, morbidity, and cognitive impairment [7–9]. This reduction can persist up to 5 years after hospital discharge [10]. For better evaluation of the long-term outcomes of sepsis, we should focus not only on its long-term mortality but also on HR-QoL.
More and more researches have showed that the EQ5D questionnaire can be used in critically ill patients to evaluate long-term HR-QoL [11–13]. The EQ5D questionnaire includes five dimensions, namely, mobility ability, self-care, usual activity, pain/discomfort, and anxiety/depression. Each dimension has three different levels, separately none, moderate, and severe problem. An EQ5D index can be obtained based on the EQ5D questionnaire via a Japanese version conversion table [14]. The visual analog scale (VAS), as a part of the questionnaire, is also used. The EQ-VAS, a score ranging from 0 to 100, can subjectively reflect the health state of patients, where 0 means the worst state and 100 the best [14].
Pneumonia is one of the most common reasons for admission to intensive care units (ICUs). Studies have revealed that pneumonia is the primary kind of sepsis [15–17]. Kim and his colleagues' study showed that pneumonia is associated with higher mortality when compared to other infection sources [18].
Abdominal infection is another common indication for admission to ICU, and abdomen is the second popular site of invasive infection among critically ill patients [19–21]. Poor control of abdominal infection frequently results in abdominal-sepsis [22].
Lung and abdomen are the most common sources of sepsis [4, 12]. Existing research on the outcome of sepsis according to the infection foci is sparse and information about difference between pulmonary-sepsis and abdominal-sepsis is still limited. Our study focuses on elucidating the clinical difference between pulmonary-sepsis and abdominal-sepsis, the variation in long-term mortality, and QoL of different sepsis origin and identifying the predictors of long-term mortality and QoL for sepsis survivors.
2. Materials and Methods
2.1. Study Population
This prospective cohort study was carried out among patients admitted to the combined surgical, respiratory, and medical intensive care units of West China hospital of Sichuan University (from December 2013 to December 2014). Patients diagnosed with sepsis as the primary cause for ICU administration were identified and enrolled within the first 24 hours. Patients younger than 18 years were excluded, and so were patients with a length of ICU stay less than 24 hours. If the patient was admitted to the ICU more than once, only the first sepsis episode was enrolled. HR-QoL was assessed using the EQ5D questionnaire. Permission to perform the follow-up study was granted by the Clinical Trials and Biomedical Ethics Committee of West China Hospital.
2.2. Definitions, Data Collection, and Outcome Measures
Sepsis was defined as at least two systemic inflammatory syndrome criteria together with infection evidence [23]. At least one of the following criteria was required for diagnosis of pneumonia: (1) clinical features including fever (>38°C) or hypothermia (≤35°C), new cough wherever with or without sputum, dyspnea, pleuritic chest pain, or changed respiratory sounds; (2) radiographic evidence of lung infection with a newly onset or changed infiltrate focus based on the guidelines of German College of Pulmonology [24]. Abdominal infection includes bacterial liver abscess, acute peritonitis, acute binary tract infection cholecystitis, and acute pancreatitis complicated with secondary bacterial infections.
Demographic characteristics, infection site, type of infection (G+/G−, fungus, or virus), laboratory results in the first 24 hours, comorbidities, length of ICU and hospital stay, ICU administration strategy such as mechanical ventilation, continuous renal replacement therapy (CRRT), and use of vasoactive agent were recorded. The Acute Physiology and Chronic Health Evaluation (APACHE) II score [25] and Sepsis-related Organ Failure Assessment (SOFA) score [26] in the first 24 hours of ICU admission, were also collected to assess the severity of illness. Primary outcome was one-year mortality, and secondary outcome was one-year QoL assessed via EQ5D. All clinical data were obtained from the Hospital Information System of West China Hospital and follow-up information was recorded by the telephone interviewer.
2.3. Statistical Analysis
Statistical analysis was conducted in SPSS software version 19.0 (SPSS Inc., Chicago, IL, USA). The Kolmogorov-Smirnov test was used to assess the data normality. Quantitative data exhibiting normal distributions were expressed as mean and standard deviation (SD) or, otherwise, presented as median with 25th and 75th percentile on rejection of the normality hypothesis. Students' t-test was used for the analysis of normally distributed continuous variables. The Mann–Whitney test was used to explore the difference between the independent groups when the data was not normally distributed. For categorical variables, the χ2 (for large sample) or Fisher's exact test (for small sample) was applied appropriately to calculate the difference between groups. Backward stepwise binary logistic regression was conducted to find predictors for one-year mortality and QoL after the sepsis episode. All the tests were two-tailed and a p value less than 0.05 was considered statistically significant. Missing data were handled via simple deletion method and patients lost to follow-up were excluded when analyzing one-year mortality and quality of life.
3. Results
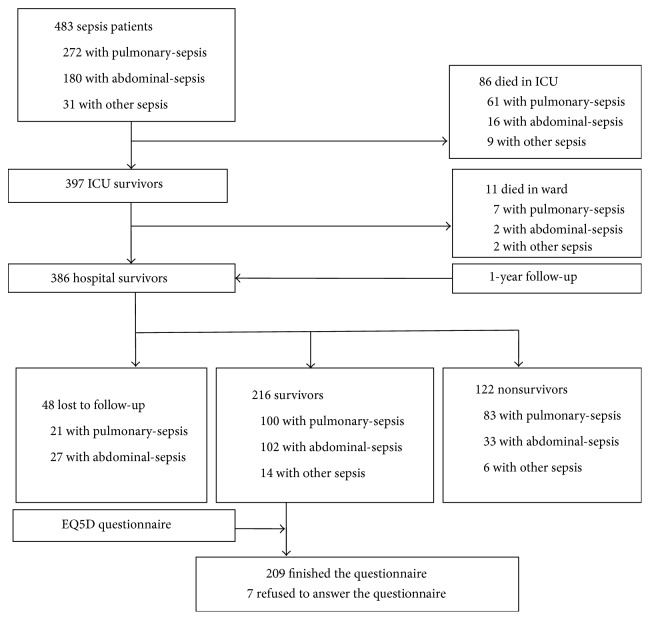
Study flow was presented in Figure 1. Of the 483 patients, 86 died in ICU and 11 died in ward. Three hundred and eighty-six hospital survivors were followed up by telephone one year after ICU discharge. Forty-eight patients were lost to follow-up. Of the others, 216 patients survived one year after ICU discharge, and then EQ5D questionnaire was used for the assessment of QoL for 1-year survivors and 209 of them finished the questionnaire (Figure 1).
Figure 1.
Flow chart of the follow-up study. EQ5D, EuroQol-5D.
3.1. Demographic Characteristics of the Sepsis Cohort
Among the 483 sepsis patients, pulmonary-sepsis (56.3%, n = 272) was the most common type of sepsis, followed by abdominal infection (37.3%, n = 180). The mean age of the sepsis cohort was 60.3 years, and the average APACHE II score was 21.5. For the whole study population, the incidence of sepsis, severe sepsis, and septic shock was 7.7%, 27.5%, and 64.8%, respectively. Pulmonary-sepsis patients were much older (63.7 years old) than abdominal-sepsis patients (56.7 years old) (p = 0.000) and had higher APACHE II score (23.0 versus 18.6, p = 0.000). The SOFA score of pulmonary-sepsis (median 9, IQR 7–12) was significantly worse than that of abdominal-sepsis (median 7, IQR 5–11). Pulmonary-sepsis had a higher Charlson Comorbidity Index. Fungal or viral infection was more likely to be identified in the pneumonia-induced sepsis population. Pulmonary-sepsis was more prone to develop acute renal failure (17.6%, p = 0.043) and had greater need for CRRT during the whole ICU stay period (20.2% and 11.1%, p = 0.014). Pulmonary-sepsis had longer MV days and length of ICU stay (p = 0.000). Demographic characteristics of the study cohort were presented in Table 1.
Table 1.
Demographic characteristics of the sepsis cohort.
| Variables | All sepsis | Pulmonary-sepsis | Abdominal-sepsis | p a | Nonsurvivors | Survivors | p b |
|---|---|---|---|---|---|---|---|
| n = 483 | n = 272 | n = 180 | n = 219 | n = 216 | |||
| Age, mean (SD) | 60.3 (16.2) | 63.7 (15.3) | 56.7 (16.1) | 0.000∗∗ | 64.2 (15.3) | 56.7 (16.6) | 0.000∗∗ |
| Male sex, n% | 319 (66.0) | 186 (67.6) | 114 (63.3) | 0.363 | 140 (64.2) | 147 (68.1) | 0.418 |
| APACHE II, mean (SD) | 21.5 (7.9) | 23.0 (6.9) | 18.6 (8.0) | 0.000∗∗ | 24.4 (7.5) | 19.3 (7.4) | 0.000∗∗ |
| SOFA | 8 (6–11) | 9 (7–12) | 7 (5–11) | 0.003∗∗ | 10 (7–12) | 7 (5–10) | 0.000∗∗ |
| SOFA, nonpulmonary | 6 (4–8) | 6 (4–8) | 5 (3–8) | 0.125 | 7 (5–9) | 5 (3–8) | 0.000∗∗ |
| Charlson Comorbidity Index | 3 (1–5) | 4 (2–5) | 2 (1–4) | 0.000∗∗ | 4 (2–5) | 2 (1–4) | 0.000∗∗ |
| Comorbidities | |||||||
| Cardiovascular disease | 182 (37.7) | 121 (44.5) | 51 (28.3) | 0.001∗∗ | 98 (45.0) | 76 (35.2) | 0.040∗ |
| Cerebrovascular disease | 47 (9.7) | 35 (12.9) | 9 (5.0) | 0.006∗∗ | 25 (11.5) | 19 (8.8) | 0.427 |
| Diabetes mellitus | 88 (18.2) | 55 (20.2) | 25 (13.9) | 0.102 | 47 (21.6) | 36 (16.7) | 0.223 |
| Peripheral vascular disease | 65 (13.5) | 46 (16.9) | 18 (10.0) | 0.040∗ | 29 (13.3) | 30 (13.9) | 0.889 |
| Digestive and liver disease | 140 (29.0) | 70 (25.7) | 66 (36.7) | 0.016∗ | 68 (31.2) | 61 (28.2) | 0.529 |
| Malignancy | 71 (14.7) | 41 (15.1) | 26 (14.4) | 0.893 | 41 (18.8) | 25 (11.6) | 0.045∗ |
| Chronic pulmonary disease | 110 (22.8) | 87 (32.0) | 21 (11.7) | 0.000∗∗ | 64 (29.4) | 36 (16.7) | 0.002∗∗ |
| Chronic kidney disease | 40 (8.3) | 31 (11.4) | 7 (3.9) | 0.005∗∗ | 22 (10.1) | 16 (7.4) | 0.396 |
| Other diseases | 110 (22.8) | 76 (27.9) | 30 (16.7) | 0.006∗∗ | 58 (26.6) | 40 (20.4) | 0.141 |
| Pathogen, n% | |||||||
| Gram positive | 40 (8.3) | 19 (7.0) | 16 (8.9) | 0.591 | 12 (5.6) | 16 (7.6) | 0.439 |
| Gram negative | 265 (54.9) | 152 (55.9) | 97 (53.9) | 0.495 | 121 (56.3) | 111 (52.6) | 0.496 |
| Fungus | 175 (36.2) | 122 (44.9) | 42 (23.3) | 0.000∗∗ | 98 (45.0) | 60 (27.8) | 0.000∗∗ |
| Virus | 20 (4.1) | 16 (5.9) | 3 (1.7) | 0.031∗ | 7 (3.2) | 9 (4.2) | 0.621 |
| Type of sepsis, n% | 0.084 | 0.000∗∗ | |||||
| Sepsis | 37 (7.7) | 23 (8.5) | 11 (6.1) | 0.467 | 5 (2.3) | 23 (10.6) | 0.000∗∗ |
| Severe sepsis | 133 (27.5) | 83 (30.5) | 44 (24.4) | 0.166 | 50 (22.9) | 72 (33.3) | 0.019∗ |
| Septic shock | 313 (64.8) | 166 (61.0) | 125 (69.4) | 0.072 | 163 (74.8) | 121 (56.0) | 0.000∗∗ |
| Origin of sepsis, n% | |||||||
| Pulmonary | 272 (56.3) | — | — | — | 152 (69.7) | 100 (46.3) | 0.000∗∗ |
| Abdominal | 180 (37.3) | — | — | — | 50 (22.9) | 102 (47.2) | 0.000∗∗ |
| Other | 31 (6.4) | — | — | — | 16 (7.3) | 15 (6.9) | 1.000 |
| Pulmonary infectionc, n% | 346 (71.6) | 272 (100) | 62 (34.4) | 0.000∗∗ | 172 (78.9) | 135 (62.5) | 0.000∗∗ |
| ICU treatment within 24 h hours, n% | |||||||
| IPPV | 420 (87.0) | 228 (83.8) | 164 (91.1) | 0.033∗ | 195 (90.3) | 180 (88.7) | 0.634 |
| NPPV | 34 (7.0) | 24 (8.8) | 8 (4.4) | 0.092 | 15 (6.9) | 16 (7.9) | 0.852 |
| Tracheal extubationd | 40 (8.3) | 8 (2.9) | 30 (16.9) | 0.000∗∗ | 12 (5.5) | 26 (12.0) | 0.017∗ |
| Vasopressor | 181 (37.5) | 91 (33.5) | 74 (41.7) | 0.090 | 103 (47.2) | 64 (29.6) | 0.000∗∗ |
| ARF, n% | 74 (15.3) | 48 (17.6) | 19 (10.6) | 0.043∗ | 54 (24.8) | 17 (7.9) | 0.000∗∗ |
| CRRT, n% | 83 (17.2) | 55 (20.2) | 20 (11.1) | 0.014∗ | 60 (27.5) | 21 (9.7) | 0.000∗∗ |
| MV, d | 8 (3–16) | 11 (5–20.8) | 5 (2–11) | 0.000∗∗ | 11 (4–21) | 6 (3–13) | 0.000∗∗ |
| ICU LOS, d | 13 (6–23) | 14 (7–3) | 8.5 (4–19) | 0.000∗∗ | 14 (5–24) | 12 (6–22) | 0.605 |
| Hospital LOS, d | 23 (13–39) | 24 (13–37) | 23 (13–44) | 0.649 | 18 (8.8–33) | 28 (17–47) | 0.000∗∗ |
| ICU mortality | 86 (17.8) | 61 (22.4) | 16 (8.9) | 0.000∗∗ | — | — | — |
| Hospital mortality | 97 (20.1) | 68 (25.0) | 18 (10.0) | 0.000∗∗ | — | — | — |
| 28-day mortalitye | 79 (23.4) | 58 (31.7) | 17 (12.6) | 0.000∗∗ | — | — | — |
| 1-year mortalityf | 122 (36.1) | 83 (45.4) | 33 (24.4) | 0.000∗∗ | — | — | — |
| Laboratory parameters on admission | |||||||
| PLT 109 | 140 (88–219) | 137 (85–200) | 158 (89–248) | 0.041∗ | 133 (72–203) | 152 (92–232) | 0.027∗ |
| Albumin g/L, mean (SD) | 26.9 (6.8) | 29.1 (6.1) | 23.4 (6.7) | 0.000∗∗ | 27.5 (23.3–31.6) | 26.6 (21.7–31.1) | 0.071 |
| Creatinine, μmol/L | 82 (57–142) | 84 (59–171) | 79 (54–125) | 0.072 | 98 (61–197) | 73 (54–119) | 0.001∗∗ |
| Cystatin c, mg/L | 1.1 (0.9–1.9) | 1.3 (0.9–2.2) | 0.9 (0.7–1.4) | 0.000∗∗ | 1.4 (1.0–2.3) | 1.0 (0.8–1.4) | 0.000∗∗ |
| LDH IU/L | 272 (198–435) | 304 (232–476) | 217 (164–328) | 0.000∗∗ | 305 (230–487) | 242 (180–375) | 0.000∗∗ |
| Anion gap, mmol/L | 17.4 (14.5–21.0) | 17.0 (14.2–21.3) | 18.2 (15.6–20.6) | 0.129 | 17.9 (14.5–22.1) | 17.3 (14.4–20.2) | 0.012∗ |
| Lactateg, mmol/L | 1.9 (1.3–3.1) | 1.8 (1.3–2.5) | 1.9 (1.4–3.6) | 0.033∗ | 1.9 (1.4–3.3) | 1.8 (1.3–3.1) | 0.239 |
| PaO2/FiO2, mmHg | 189.0 (121.8–263.3) | 170.5 (109.4–229.9) | 208.5 (146.6–294.0) | 0.000∗∗ | 169.2 (100.6–238.6) | 204 (150.0–277.3) | 0.000∗∗ |
| PaO2, mmHg | 85 (70–119) | 80 (68–109) | 97 (77–135) | 0.000∗∗ | 81 (67–115) | 87 (73–131) | 0.055 |
Quantitative data was presented as median (IQR), and qualitative data was presented as n (%) except otherwise indicated. SD, standard deviation; IPPV, invasive ventilation; NPPV, noninvasive ventilation; ARF, acute renal failure; CRRT, continuous renal replacement therapy; MV, mechanical ventilation; ICU LOS, length of ICU stay; hospital LOS, length of hospital stay; PLT, platelet; LDH, lactate dehydrogenase.
aComparison between pulmonary-sepsis and abdominal-sepsis.
bComparison between one-year survivors and nonsurvivors.
cPulmonary infection was defined as pulmonary infection identified during the whole ICU stay period.
dWhen analyzing the ratio of extubation in the first 24 h of ICU administration, patients without mechanical ventilation were excluded.
e, fPatients who died in ICU or lost to follow-up were excluded. There was a total of 338 sepsis patients, 183 of them had pulmonary-sepsis, and 135 had abdominal-sepsis when analyzing 28-day or one-year mortality.
gThere was a total of 290 sepsis patients with measurement of lactate within the first 24 h; of those 155 had pulmonary-sepsis and 114 had abdominal-sepsis.
∗ p < 0.05. ∗∗ p < 0.01.
3.2. Mortality of the Sepsis Cohort
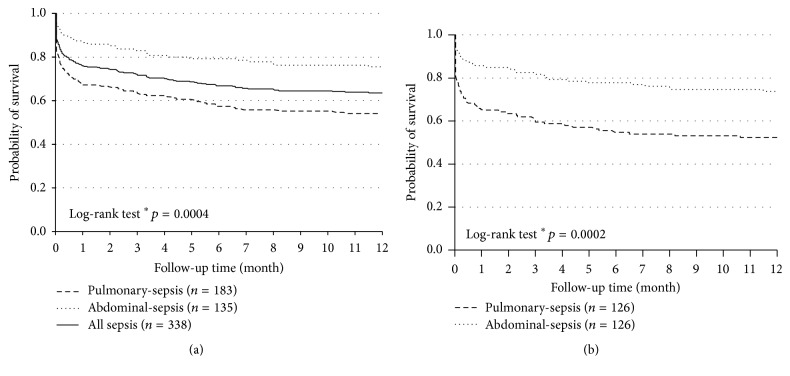
ICU mortality for all sepsis, pulmonary-sepsis, and abdominal-sepsis was 17.8% (n = 86), 22.4% (n = 61), and 8.9% (n = 16), respectively, and hospital mortality was 20.1% (n = 97), 25.0% (n = 68), and 10.0% (n = 18), respectively (Table 1). The overall 28-day mortality of the ICU survivors for all sepsis, pulmonary-sepsis, and abdominal-sepsis was 23.4% (n = 79), 31.7% (n = 58), and 12.6% (n = 17), respectively, and one-year mortality of ICU survivors was 36.1% (n = 122), 45.4% (n = 83), and 24.4% (n = 33), respectively (Table 1) (when analyzing one-year mortality, all sepsis n = 437, patients lost to follow-up were excluded). Kaplan-Meier curve also showed that patients with pulmonary-sepsis had higher one-year mortality than that of the patients with abdominal-sepsis (Figure 2(a)). Considering the older age and greater comorbidity burden on the pulmonary-sepsis cohort, we did an age-matched cohort study of ICU survivors to adjust the impact on long-term mortality. Similar results were obtained; that is, pulmonary-sepsis showed poor survival (Figure 2(b)). Background characteristics of the age-matched cohort were shown in Supplementary Table 1 in Supplementary Material available online at http://dx.doi.org/10.1155/2016/4213712.
Figure 2.
Probability of one-year survival for ICU survivors. (a) Probability of one-year survival for all ICU survivors of the unmatched cohorts. (b) Probability of one-year survival for ICU survivors of the age-matched cohorts. ∗ p value indicated for comparison between pulmonary-sepsis and abdominal-sepsis.
3.3. One-Year QoL (EQ5D) of Sepsis Survivors
The distribution of the five dimensions in the EQ5D questionnaire was described in Table 2. Of all the survivors who completed the EQ5D questionnaire, 18.7% had moderate to severe problem in mobility, 12.5% in self-care, 19.2% in pain/discomfort, 33.5% in anxiety/depression, and 19.1% in anxiety/depression. This showed that most patients had problems in the pain/discomfort dimension. The median EQ5D index was 0.848, and the median EQ-VAS was 80. Pulmonary-sepsis patients showed more problems than abdominal-sepsis patients in all the five dimensions (Figure 3, Table 2). Significant difference was found in both the EQ5D index and EQ-VAS (p = 0.001 for both). Pulmonary-sepsis patients showed worse one-year QoL (Table 2).
Table 2.
One-year HR-QoL (EQ-5D) of sepsis survivors and comparison between pulmonary-sepsis and abdominal-sepsis.
| Variable | Sepsis responders | Pulmonary-sepsis | Abdominal-sepsis | p |
|---|---|---|---|---|
| n = 209 | n = 97 | n = 98 | ||
| Mobility | ||||
| No problems | 170 (81.3) | 70 (72.2) | 91 (92.9) | 0.000∗∗ |
| Some problems | 25 (12.0) | 18 (18.6) | 5 (5.1) | 0.004∗∗ |
| Extreme problems | 14 (6.7) | 9 (9.3) | 2 (2.0) | 0.033∗ |
| Self-care | ||||
| No problems | 183 (87.6) | 78 (80.4) | 95 (96.9) | 0.000∗∗ |
| Some problems | 10 (4.8) | 8 (8.2) | 1 (1.0) | 0.018∗ |
| Extreme problems | 16 (7.7) | 11 (11.3) | 2 (2.0) | 0.010∗ |
| Usual activity | ||||
| No problems | 169 (80.9) | 67 (69.1) | 93 (94.9) | 0.000∗∗ |
| Some problems | 25 (12.0) | 20 (20.6) | 3 (3.1) | 0.000∗∗ |
| Extreme problems | 15 (7.2) | 10 (10.3) | 2 (2.0) | 0.018∗ |
| Pain/discomfort | ||||
| No problems | 139 (66.5) | 56 (57.7) | 72 (73.5) | 0.024∗ |
| Some problems | 64 (30.6) | 39 (40.2) | 23 (23.5) | 0.014∗ |
| Extreme problems | 6 (2.9) | 2 (2.1) | 3 (3.1) | 0.505 |
| Anxiety/depression | ||||
| No problems | 169 (80.9) | 72 (74.2) | 86 (87.8) | 0.018∗ |
| Some problems | 35 (16.7) | 23 (23.7) | 10 (10.2) | 0.013∗ |
| Extreme problems | 5 (2.4) | 2 (2.1) | 2 (2.0) | 1.000 |
| EQ5D index (IQR) | 0.848 (0.729–0.848) | 0.768 (0.668–0.848) | 0.848 (0.768–0.848) | 0.000∗∗ |
| EQ-VAS (IQR) | 80 (68.7–90) | 75 (60–85) | 80 (70–90) | 0.001∗∗ |
Data was presented as n (%). Patients who refused to finish the questionnaire were excluded. ∗ p < 0.05. ∗∗ p < 0.01.
Figure 3.
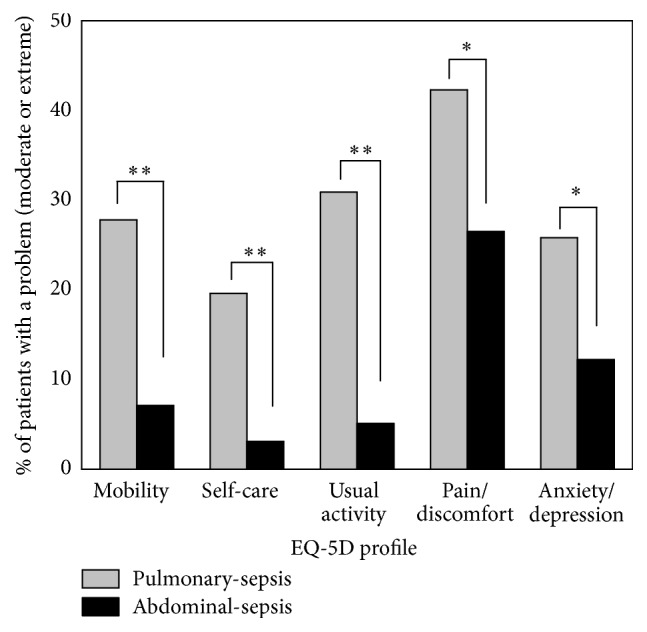
EQ5D profile in one-year survivors of pulmonary-sepsis and abdominal-sepsis. The EuroQol 5D profile is dichotomized into “no problems” and “moderate or extreme problems” 1 year after ICU discharge ∗ p < 0.05; ∗∗ p < 0.01.
3.4. Risk Factors for One-Year Mortality
To find risk factors for one-year mortality, a total of 435 sepsis patients were involved in the analysis. Of them, 216 (49.7%) survived one year after ICU discharge. Nonsurvivors tended to be much older and had apparently higher APACHE II, SOFA, and Charlson Comorbidity Index (all p = 0.000). Greater ratio of patients in nonsurvivors was identified with fungal infection (p = 0.000). The incidence of septic shock was obviously higher in the nonsurvivor group (74.8%) than that of the survivor group (56.0%) (p = 0.000). One hundred and fifty-two of the 219 (69.7%) nonsurvivors had pulmonary-sepsis, which was much higher than the survivor group (46.3%). However, abdominal-sepsis was more frequently found in the survivor group (47.2% versus 22.9%, p = 0.000). Within the first 24 hours after admission to the ICU, there was a greater need of vasopressor use for the nonsurvivors (47.2% versus 29.6%, p = 0.000). Similarly, nonsurvivors were more prone to develop acute renal failure than survivors and had more requirements for CRRT. Mechanical days and length of hospital stay (p = 0.000) were also longer in the nonsurvivors group, but there was no difference in ICU LOS (p = 0.605). Laboratory parameters such as creatinine, plates, cystatin c, LDH, and anion gag were also worse in the nonsurvivors (Table 1).
Univariate analysis of the mortality showed age, APACHE II, SOFA, Charlson Comorbidity Index, malignancy, acute renal failure, pulmonary infection, fungus infection, septic shock, cystatin c, and anion gap as potential predictors for one-year mortality. After multivariate adjustment, age (OR = 1.025; 95% CI, 1.011–1.039), septic shock (OR = 2.533; 95% CI, 1.591–4.032), fungus infection (OR = 1.846; 95% CI, 1.160–2.938), acute renal failure (OR = 2.914; 95% CI, 1.525–5.568), anion gap (OR = 1.070; 95% CI, 1.025–1.117), and pulmonary infection (OR = 2.547; 95% CI, 1.513–4.288) were risk factors for one-year mortality (Table 3).
Table 3.
Univariate and multivariate regression analysis for risk factors of one-year mortality and quality of life.
| Predictors | OR (95% CI) | p a | OR (95% CI) | p b |
|---|---|---|---|---|
| A: one-year mortality | ||||
| Age | 1.036 (1.017–1.043) | 0.000∗∗ | 1.025 (1.011–1.039) | 0.001∗∗ |
| APACHE II | 1.097 (1.067–1.129) | 0.000∗∗ | — | — |
| SOFA | 1.159 (1.102–1.219) | 0.000∗∗ | — | — |
| Charlson Comorbidity index | 1.068 (1.012–1.126) | 0.016∗ | — | — |
| Malignancy | 1.770 (1.034–3.030) | 0.045∗ | — | — |
| Septic shock | 2.327 (1.549–3.495) | 0.000∗∗ | 2.533 (1.525–5.568) | 0.000∗∗ |
| Fungus infection | 2.213 (1.424–3.167) | 0.000∗∗ | 1.846 (1.160–2.938) | 0.010∗ |
| Acute renal failure | 3.854 (2.152–6.904) | 0.000∗∗ | 2.914 (1.525–5.568) | 0.001∗∗ |
| Cystatin c | 1.453 (1.193–1.768) | 0.000∗∗ | — | — |
| Anion gap | 1.071 (1.031–1.113) | 0.000∗∗ | 1.070 (1.025–1.117) | 0.002∗∗ |
| Pulmonary infectionc | 2.243 (1.465–3.436) | 0.000∗∗ | 2.547 (1.513–4.288) | 0.000∗∗ |
|
| ||||
| B: one-year QoL | ||||
| APACHE II | 1.048 (1.008–1.088) | 0.017∗ | — | — |
| Chronic heart failure | 6.217 (1.343–28.786) | 0.019∗ | — | — |
| Pulmonary infectionc | 2.939 (1.621–5.329) | 0.000∗∗ | 2.846 (1.530–5.294) | 0.004∗∗ |
| Tracheal extubation in 24 hd | 0.231 (0.083–0.645) | 0.005∗∗ | 0.330 (0.110–0.989) | 0.048∗ |
| Mechanical ventilation days | 1.036 (1.009–1.064) | 0.008∗∗ | — | — |
A: n = 435. Variables eliminated from backward selection.
B: n = 209. Variables eliminated from backward selection.
aResults of univariate analysis.
bResults of multivariate analysis.
cPulmonary infection was defined as pulmonary infection identified during the whole ICU stay period.
d n = 188; patients without mechanical ventilation were excluded.
∗ p < 0.05. ∗∗ p < 0.01.
3.5. Predictors for One-Year Quality of Life
In order to find predictors for one-year QoL, QoL was evaluated by EQ5D index. EQ5D index less than 0.848 (median) was defined as poor QoL. Survivors were divided into poor and good QoL groups. Background characteristics were summarized in Table 4. Patients with poor QoL had higher APACHE II and Charlson Comorbidity Index, prolonged mechanical ventilation, longer ICU, and hospital LOS. Patients of the poor QoL group were more prone to suffer pulmonary infection (76.7% versus 47.2%), and 57.3% of them had pulmonary-sepsis, while only 25.8% of patients in the good QoL group had pulmonary-sepsis. Univariate analysis suggested that APACHE II, chronic heart failure, pulmonary infection, and tube extubation during the first 24 hours after admission to ICU were possible predictive factors of one-year QoL (Table 3). Multivariate logistic regression showed that pulmonary infection (OR = 2.846, 95% CI (1.530–5.294)) was a risk factor of one-year QoL, while tube extubation during the first 24 hours (OR = 0.330, 95% CI (0.110–0.989)) was a protective factor (Table 3).
Table 4.
Baseline characteristics of sepsis survivors with good/poor one-year QoL.
| Variables | Good QoL | Poor QoL | p |
|---|---|---|---|
| n = 106 | n = 103 | ||
| Age, mean (SD) | 54.6 (16.7) | 58.8 (16.4) | 0.066 |
| Male sex, n% | 80 (75.5) | 63 (61.2) | 0.037∗ |
| APACHE II, mean (SD) | 18.1 (7.0) | 20.6 (7.6) | 0.016∗ |
| SOFA, mean (SD) | 7.2 (3.7) | 7.9 (4.3) | 0.245 |
| Charlson Comorbidity Index | 2 (0,3) | 3 (1,4) | 0.021∗ |
| Septic shock, n% | 60 (56.6) | 57 (55.3) | 0.890 |
| Chronic heart failure, n% | 2 (1.9) | 11 (10.7) | 0.010∗ |
| Pulmonary infectiona, n% | 50 (47.2) | 79 (76.7) | 0.000∗∗ |
| Pulmonary-sepsis, n% | 38 (25.8) | 59 (57.3) | 0.002∗∗ |
| ICU treatment within 24 h, n% | |||
| IPPV | 88 (83.0) | 87 (84.5) | 0.852 |
| NPPV | 3 (2.8) | 11 (10.7) | 0.028∗ |
| Tube extubationb | 18 (20.0) | 5 (5.1) | 0.003∗∗ |
| Vasopressor | 28 (26.4) | 34 (33.0) | 0.364 |
| MV, d | 6 (22–12) | 8 (4–17) | 0.003∗∗ |
| ICU LOS, d | 11 (6–19) | 15 (7–27) | 0.016∗ |
| Hospital LOS, d | 26.5 (16.8–42.3) | 31 (20–58) | 0.034∗ |
Quantitative data was presented as median (IQR), and qualitative data was presented as n (%) except otherwise indicated. SD, standard deviation; IPPV, invasive ventilation; NPPV, noninvasive ventilation; MV, mechanical ventilation; ICU LOS, length of ICU stay; hospital LOS, length of hospital stay;
aPulmonary infection was defined as pulmonary infection identified during the whole ICU stay period.
b N = 188; patients without mechanical ventilation were excluded.
∗ p < 0.05. ∗∗ p < 0.01.
4. Discussion
This study showed that short- and long-term outcomes between patients with pulmonary-sepsis and abdominal-sepsis vary greatly. Our findings suggest that patients with pulmonary-sepsis were more prone to fungal infection, acute renal failure requiring CRRT, prolonged mechanical ventilation, longer ICU and hospital stays, and higher in-hospital and one-year mortality than the abdominal-sepsis group. In addition, the pulmonary-sepsis cohort had worse QoL indicators after hospital discharge. To our knowledge, our follow-up study was one of the few researches to investigate the clinical difference of the most frequently identified sepsis source, including short-term and long-term mortality, together with QoL.
Our study found that age, septic shock, acute renal failure, fungal infection, anion gap, and pulmonary infection were potential risk factors for increased one-year mortality. It is not surprising that older age positively correlates with higher long-term mortality. Septic shock is the most severe stage of sepsis and long-term outcome of septic shock was poor. Nesseler et al. [27] reported that 6-month mortality of septic shock was 45%. Harris et al. [28] found that critically ill patients with acute kidney injury had higher one-year mortality, and it is reasonable to speculate that there was higher one-year mortality in patients with acute renal failure. Fungal infection usually occurs in patients with immunosuppression and was associated with increased hospital mortality [29]. Previous researches have shown that anion gap increases in 72% of critically ill patients, and elevated AG has been found to be associated with mortality in serious diseases, including critical illness [30–35].
The research revealed that pulmonary infection was associated with increased short-term and long-term mortality which was in accordance with previous studies. Mansur et al.'s study [36] reported a higher 90-day mortality in pulmonary-sepsis than abdominal-sepsis. Kim et al. [18] reported significantly higher 28 d mortality of pneumonia (41%) than non-pulmonary-sepsis (30%), and pneumonia was demonstrated to be a risk factor for 28-day mortality. In our study cohort, we found that pulmonary-sepsis patients were much older and had higher APACHE II, SOFA score, and Charlson Comorbidity Index. Comorbidities and laboratory parameters on admission of the sepsis cohort were shown in Table 1. Consistent with our study, the PAO2/FiO2 and PaO2 of pulmonary-sepsis patients were worse than other sepsis source patients and previous research had already validated Pao2/FiO2 as a biomarker for prognosis of sepsis such as mortality [18]. What is more, patients in the pulmonary-sepsis cohort were significantly older and had a higher rate of renal failure, thus explaining their higher APCHE II scores. The SOFA score of pulmonary-sepsis was apparently higher than that of the abdominal group (p = 0.003); however, this difference disappeared when comparing the nonpulmonary SOFA scores (p = 0.125); that is, the difference of SOFA scores between groups was primarily caused by the pulmonary component which can be explained by pneumonia. Pneumonia patients had a greater probability to have chronic pulmonary disease (32% and 11.7%, p = 0.000). COPD was the most common chronic pulmonary disease and the quality of life for patients with COPD was apparently impaired [37]. Greater portion of patients with cardiovascular disease, cerebrovascular disease, chronic pulmonary disease, and chronic kidney disease in the pulmonary-sepsis group also contributed to high long-term mortality [38–41]. In order to eliminate the impact of older age and age-associated diseases on the pulmonary-sepsis cohort, an age-matched cohort analysis was conducted. Survival analysis of both the unmatched and the matched cohorts showed greater mortality in the pulmonary-sepsis group (Figures 2(a) and 2(b)).
Quality of life for sepsis was impaired [27, 42]. Patients with poor QoL were much older, had higher APACHE II, SOFA, and Charlson Comorbidity Index, and had prolonged mechanical ventilation days and ICU and hospital LOS (Table 4). Chronic heart failure was also found more commonly in the poor QoL group. A total of 57.3% of the 103 survivors in the poor QoL group were diagnosed with pulmonary-sepsis when admitted to the ICU and 76.7% of survivors with poor QoL suffered pulmonary infection in ICU (Table 4). In accordance with data shown in Table 1, pulmonary-sepsis cases had older age, higher APACHE II and SOFA score, and greater comorbidity burden (Table 1, Figure 2). Patients with tube weaning in the first 24 hours had better QoL, since these patients tended to be less serious, had less need for mechanical ventilation, and could soon recover from the sepsis attack. Pulmonary infection was already confirmed to be a risk factor for 28 d mortality [18]. Our study was the first to confirm its role in decreased QoL.
There were several limitations in our study. Firstly, this follow-up study was a single-center study conducted in a teaching hospital. This study design would result in lack of representativeness. Patients admitted to our hospital appeared to be much more serious, and they were much older and had more complications than patients admitted to ICUs of other hospitals, resulting in an overestimation of mortality. Moreover, a majority of patients were transferred from other hospitals and patients fulfilling the sepsis criteria at the onset of disease might fail to be diagnosed as having sepsis. These could all lead to selection bias. Secondly, the evaluation of GCS was inaccurate due to the use of sedation and approximately half of the cohort did not have a measurement of lactate during the 24 hours. Thirdly, Tibetan patients who could not speak Mandarin were excluded for language barrier, increasing the rate of patients lost to follow-up. Multicenter studies with larger samples were needed to confirm the study results.
5. Conclusions
Patients diagnosed with sepsis show ongoing mortality after the sepsis episode, with only 63.9% surviving one year after ICU discharge. Pulmonary-sepsis had worse short-term and long-term outcomes, including ICU/hospital mortality, one-year mortality, and one-year quality of life. Pulmonary infection is a risk factor for one-year mortality and is associated with decreased health-related quality of life.
Supplementary Material
Of the age-matched sepsis cohort, there was no difference in age, Charlson Comorbidity Index and SOFA score. However, pulmonary sepsis had apparently higher APACHE II(22.1 vs 18.6), prolonged mechanical ventilation days, longer ICU and hospital days. Abdominal-sepsis patients were more prone to develop septic shock.
Acknowledgments
The authors are grateful to Chao Jiang (Chongqing University, China) for designing the figures for the manuscript.
Competing Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Martin G. S., Mannino D. M., Eaton S., Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. The New England Journal of Medicine. 2003;348(16):1546–1554. doi: 10.1056/nejmoa022139. [DOI] [PubMed] [Google Scholar]
- 2.Dombrovskiy V. Y., Martin A. A., Sunderram J., Paz H. L. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Critical Care Medicine. 2007;35(5):1244–1250. doi: 10.1097/01.ccm.0000261890.41311.e9. [DOI] [PubMed] [Google Scholar]
- 3.Gaieski D. F., Edwards J. M., Kallan M. J., Carr B. G. Benchmarking the incidence and mortality of severe sepsis in the united states. Critical Care Medicine. 2013;41(5):1167–1174. doi: 10.1097/CCM.0b013e31827c09f8. [DOI] [PubMed] [Google Scholar]
- 4.Zhou J., Qian C., Zhao M., et al. Epidemiology and outcome of severe sepsis and septic shock in intensive care units in Mainland China. PLoS ONE. 2014;9(9) doi: 10.1371/journal.pone.0107181.e107181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy M. M., Dellinger R. P., Townsend S. R., et al. The surviving sepsis campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Medicine. 2010;36(2):222–231. doi: 10.1007/s00134-009-1738-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liesenfeld O., Lehman L., Hunfeld K., Kost G. Molecular diagnosis of sepsis: new aspects and recent developments. European Journal of Microbiology and Immunology. 2014;4(1):1–25. doi: 10.1556/eujmi.4.2014.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heyland D. K., Hopman W., Coo H., Tranmer J., McColl M. A. Long-term health-related quality of life in survivors of sepsis. Short Form 36: a valid and reliable measure of health-related quality of life. Critical Care Medicine. 2000;28(11):3599–3605. doi: 10.1097/00003246-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Reinhart K., Daniels R., Kissoon N., O'Brien J., Machado F. R., Jimenez E. The burden of sepsis-a call to action in support of World Sepsis Day 2013. Journal of Critical Care. 2013;28(4):526–528. doi: 10.1016/j.jcrc.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Götz T., Günther A., Witte O. W., Brunkhorst F. M., Seidel G., Hamzei F. Long-term sequelae of severe sepsis: cognitive impairment and structural brain alterations—an MRI study (LossCog MRI) BMC Neurology. 2014;14(1, article 145) doi: 10.1186/1471-2377-14-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alberti C., Brun-Buisson C., Goodman S. V., et al. Influence of systemic inflammatory response syndrome and sepsis on outcome of critically III infected patients. American Journal of Respiratory and Critical Care Medicine. 2003;168(1):77–84. doi: 10.1164/rccm.200208-785OC. [DOI] [PubMed] [Google Scholar]
- 11.The EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 12.Granja C., Dias C., Costa-Pereira A., Sarmento A. Quality of life of survivors from severe sepsis and septic shock may be similar to that of others who survive critical illness. Critical Care. 2004;8(2):R91–R98. doi: 10.1186/cc2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angus D. C., Carlet J. Surviving intensive care: a report from the 2002 Brussels Roundtable. Intensive Care Medicine. 2003;29(3):368–377. doi: 10.1007/s00134-002-1624-8. [DOI] [PubMed] [Google Scholar]
- 14.Tsuchiya A., Ikeda S., Ikegami N., et al. Estimating an EQ-5D population value set: the case of Japan. Health Economics. 2002;11(4):341–353. doi: 10.1002/hec.673. [DOI] [PubMed] [Google Scholar]
- 15.Puskarich M. A., Trzeciak S., Shapiro N. I., Heffner A. C., Kline J. A., Jones A. E. Outcomes of patients undergoing early sepsis resuscitation for cryptic shock compared with overt shock. Resuscitation. 2011;82(10):1289–1293. doi: 10.1016/j.resuscitation.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howell M. D., Donnino M., Clardy P., Talmor D., Shapiro N. I. Occult hypoperfusion and mortality in patients with suspected infection. Intensive Care Medicine. 2007;33(11):1892–1899. doi: 10.1007/s00134-007-0680-5. [DOI] [PubMed] [Google Scholar]
- 17.Trzeciak S., Dellinger R. P., Chansky M. E., et al. Serum lactate as a predictor of mortality in patients with infection. Intensive Care Medicine. 2007;33(6):970–977. doi: 10.1007/s00134-007-0563-9. [DOI] [PubMed] [Google Scholar]
- 18.Kim W.-Y., Lee Y.-J., Yeon Lim S., et al. Clinical characteristics and prognosis of pneumonia and sepsis: Multicenter Study. Minerva Anestesiologica. 2013;79(12):1356–1365. [PubMed] [Google Scholar]
- 19.Finfer S., Bellomo R., Lipman J., French C., Dobb G., Myburgh J. Adult-population incidence of severe sepsis in Australian and New Zealand intensive care units. Intensive Care Medicine. 2004;30(4):589–596. doi: 10.1007/s00134-004-2157-0. [DOI] [PubMed] [Google Scholar]
- 20.Vincent J.-L., Rello J., Marshall J., et al. International study of the prevalence and outcomes of infection in intensive care units. The Journal of the American Medical Association. 2009;302(21):2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 21.Ranieri V. M., Thompson B. T., Barie P. S., et al. Drotrecogin alfa (activated) in adults with septic shock. The New England Journal of Medicine. 2012;366(22):2055–2064. doi: 10.1056/nejmoa1202290. [DOI] [PubMed] [Google Scholar]
- 22.Russell J. A. Management of sepsis. The New England Journal of Medicine. 2006;355(16):1699–1713. doi: 10.1056/nejmra043632. [DOI] [PubMed] [Google Scholar]
- 23.Bone R. C., Balk R. A., Cerra F. B., et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 24.American Thoracic Society. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. American Journal of Respiratory and Critical Care Medicine. 2005;171(4):388–416. doi: 10.1164/rccm.200405-644st. [DOI] [PubMed] [Google Scholar]
- 25.Knaus W. A., Draper E. A., Wagner D. P., Zimmerman J. E. APACHE II: a severity of disease classification system. Critical Care Medicine. 1985;13(10):818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Vincent J.-L., de Mendonça A., Cantraine F., et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on ‘sepsis-related problems’ of the European Society of Intensive Care Medicine. Critical Care Medicine. 1998;26(11):1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 27.Nesseler N., Defontaine A., Launey Y., Morcet J., Mallédant Y., Seguin P. Long-term mortality and quality of life after septic shock: A Follow-Up Observational Study. Intensive Care Medicine. 2013;39(5):881–888. doi: 10.1007/s00134-013-2815-1. [DOI] [PubMed] [Google Scholar]
- 28.Harris D. G., McCrone M. P., Koo G., et al. Epidemiology and outcomes of acute kidney injury in critically ill surgical patients. Journal of Critical Care. 2015;30(1):102–106. doi: 10.1016/j.jcrc.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 29.Lichtenstern C., Herold C., Mieth M., et al. Relevance of Candida and other mycoses for morbidity and mortality in severe sepsis and septic shock due to peritonitis. Mycoses. 2015;58(7):399–407. doi: 10.1111/myc.12331. [DOI] [PubMed] [Google Scholar]
- 30.Domínguez-Cherit G., Ñamendys-Silva S. A. Changes in the anion gap: a novel marker of outcome in critically ill patients. Back to the basis. Critical Care Medicine. 2013;41(1):336–337. doi: 10.1097/ccm.0b013e318270e799. [DOI] [PubMed] [Google Scholar]
- 31.Abramowitz M. K., Hostetter T. H., Melamed M. L. The serum anion gap is altered in early kidney disease and associates with mortality. Kidney International. 2012;82(6):701–709. doi: 10.1038/ki.2012.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leskovan J. J., Justiniano C. F., Bach J. A., et al. Anion gap as a predictor of trauma outcomes in the older trauma population: correlations with injury severity and mortality. The American Surgeon. 2013;79(11):1203–1206. [PubMed] [Google Scholar]
- 33.Zheng C.-M., Liu W.-C., Zheng J.-Q., et al. Metabolic acidosis and strong ion gap in critically ill patients with acute kidney injury. BioMed Research International. 2014;2014:8. doi: 10.1155/2014/819528.819528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee S. H., Park S., Lee J. W., et al. The anion gap is a predictive clinical marker for death in patients with acute pesticide intoxication. Journal of Korean Medical Science. 2016;31(7):1150–1159. doi: 10.3346/jkms.2016.31.7.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lipnick M. S., Braun A. B., Cheung J. T.-W., Gibbons F. K., Christopher K. B. The difference between critical care initiation anion gap and prehospital admission anion gap is predictive of mortality in critical illness. Critical Care Medicine. 2013;41(1):49–59. doi: 10.1097/CCM.0b013e31826764cd. [DOI] [PubMed] [Google Scholar]
- 36.Mansur A., Klee Y., Popov A. F., et al. Primary bacteraemia is associated with a higher mortality risk compared with pulmonary and intra-abdominal infections in patients with sepsis: a prospective observational cohort study. BMJ Open. 2015;5(1) doi: 10.1136/bmjopen-2014-006616.e006616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spencer S., Calverley P. M. A., Burge P. S., Jones P. W. Impact of preventing exacerbations on deterioration of health status in COPD. The European Respiratory Journal. 2004;23(5):698–702. doi: 10.1183/09031936.04.00121404. [DOI] [PubMed] [Google Scholar]
- 38.Chin Y. R., Lee I. S., Lee H. Y. Effects of hypertension, diabetes, and/or cardiovascular disease on health-related quality of life in elderly Korean individuals: a population-based cross-sectional survey. Asian Nursing Research. 2014;8(4):267–273. doi: 10.1016/j.anr.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Persson J., Holmegaard L., Karlberg I., et al. Spouses of stroke survivors report reduced health-related quality of life even in long-term follow-up: results from Sahlgrenska Academy study on ischemic stroke. Stroke. 2015;46(9):2584–2590. doi: 10.1161/strokeaha.115.009791. [DOI] [PubMed] [Google Scholar]
- 40.Sundh J., Johansson G., Larsson K., et al. Comorbidity and health-related quality of life in patients with severe chronic obstructive pulmonary disease attending Swedish secondary care units. International Journal of Chronic Obstructive Pulmonary Disease. 2015;10:173–183. doi: 10.2147/copd.s74645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee S. J., Son H., Shin S. K. Influence of frailty on health-related quality of life in pre-dialysis patients with chronic kidney disease in Korea: a cross-sectional study. Health and Quality of Life Outcomes. 2015;13, article 70 doi: 10.1186/s12955-015-0270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cuthbertson B. H., Elders A., Hall S., et al. Mortality and quality of life in the five years after severe sepsis. Critical Care. 2013;17(2, article R70) doi: 10.1186/cc12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Of the age-matched sepsis cohort, there was no difference in age, Charlson Comorbidity Index and SOFA score. However, pulmonary sepsis had apparently higher APACHE II(22.1 vs 18.6), prolonged mechanical ventilation days, longer ICU and hospital days. Abdominal-sepsis patients were more prone to develop septic shock.