Abstract
In a three-generation screen of chemically mutagenized zebrafish, we identified a group of mutations that affect the development and function of hair cells, the mechanically sensitive cells of the inner ear and lateral-line organ. One mutant line, ru920, was discovered in a behavioral screen for defects in the acoustically evoked escape response. Despite apparently normal numbers of hair cells, mutants lack an inner-ear microphonic potential and exhibit reduced labeling of hair cells by a fluorophore that traverses transduction channels. This hair-cell-specific phenotype suggested a defect in the mechanoelectrical transduction apparatus. Positional cloning revealed that the recessive mutation introduces a premature stop codon in the ORF of myosin6b (myo6b), one of the two zebrafish orthologs of the human gene myosin VI. The ru920 line therefore provides an animal model with which to study the role of class VI myosin proteins in mechanotransduction.
Mechanoelectrical transduction in the inner ear is mediated by the hair cell, whose characteristic feature is an apically located hair bundle responsive to mechanical stimuli (1). Because an ear's small complement of hair cells provides little starting material, biochemical and molecular-biological investigations of transduction by hair cells are difficult. Genetic techniques, on the other hand, allow analysis of a system without the constraints imposed by a lack of biological material and have proven useful in identifying proteins essential to hair cells (2). Although many genes required for mechanosensitivity have been identified in Drosophila melanogaster and Caenorhabditis elegans, a vertebrate model is required for mutagenic screens directed specifically at the development and function of hair cells.
The zebrafish, Danio rerio, has emerged as the premier vertebrate system for large-scale mutagenic screens as well as an attractive model for the study of human genetic disorders (3). The inner ear forms rapidly in the externally developing zebrafish embryo and resembles that of other vertebrates (4). Moreover, zebrafish possess a second sensory system that aids in the genetic dissection of hair-cell development and function: the lateral-line organ, an array of hair cells distributed in a stereotypic pattern over the body surface, contributes to schooling, rheotaxis, and the detection of predators and prey (5). The functional unit of the lateral-line organ is the neuromast, an individually innervated rosette of hair cells surrounded by supporting cells in an arrangement similar to that of the sensory epithelia in the inner ear. The characteristic patterning of the lateral-line organ and the external location of its hair cells make this a valuable system for genetic studies and experimental manipulation.
Two extensive genetic screens in the zebrafish characterized several mutants that display abnormal phenotypes during the development of the inner ear and lateral-line organ (6, 7). Analysis of motility mutants revealed additional complementation groups with balance deficiencies indicative of vestibular defects (8). The number of complementation groups identified, the average allele frequency, and the variation rates for different loci suggest that the screens identified only half of the genes mutable to an embryonic or early larval phenotype (9, 10). Because a smaller, focused screen was effective in revealing additional loci essential for visual development (11), we chose to use several assays to evaluate hair-cell development and operation. Sixteen mutant lines were identified and were subjected to additional phenotypic analysis. In this report we detail the phenotype of one line, ru920, and the subsequent cloning of the affected gene.
Materials and Methods
Zebrafish Husbandry and Screening Protocol. Zebrafish were bred and maintained at 28.5°C with standard protocols (12). The ru920 allele was created in the TL strain with the chemical mutagen ethylnitrosourea (13). Offspring of heterozygous ru920 carriers were screened for abnormal startle responses at 5 days postfertilization (dpf) by tapping with a glass probe on the side of a Petri dish containing the larvae. Control responses were elicited with a tactile stimulus provided by a hair. Clutches were also screened for defects in the lateral-line organ after labeling neuromasts at 7 dpf by immersing the larvae in facility water containing 200 μM 4-[4-(diethylamino)styryl]-N-methylpyridinium iodide (4-Di-2-ASP) (Molecular Probes) and 0.1 mg/ml BSA. Larvae were visualized under epifluorescence with a modified fluorescein filter block.
Electrical Recording and Iontophoretic Ejection. Microphonic potentials were measured from the otic vesicles of 7-dpf zebrafish larvae (14). For the iontophoretic ejection of positively charged 4-Di-2-ASP into the otic vesicle, glass microelectrodes were filled with 200 μM fluorophore in standard saline solution and were inserted into the otic vesicles of 5-dpf larvae. We then reversed the -5-nA holding current to +5 nA for up to 20 min, thus iontophoretically expelling 4-Di-2-ASP into the endolymph. Images were acquired through a 40× water-immersion objective lens on a laser-scanning confocal microscope (LSM 510, Zeiss).
Whole-Mount Immunohistochemistry and Phalloidin Labeling. Larvae were fixed, permeabilized, and labeled in blocking solution (protocol 6.12 in ref. 15) with primary antibodies at the following dilutions: 1:5,000 for rabbit anti-parvalbumin 3 serum, 1:50 for mouse 3A10 monoclonal antibodies (Developmental Studies Hybridoma Bank, Iowa City, IA), and 1:200 for anti-myosin VI serum (KA-15; Sigma-Aldrich). Secondary antisera (Alexa Fluor 488 anti-mouse, Alexa Fluor 488 anti-rabbit, or Alexa Fluor 568 anti-rabbit; Molecular Probes) were used at 3 μg/ml. Before observation, 250 μM TO-PRO-3 iodide or 3 units/ml AlexaFluor 568 phalloidin (Molecular Probes) was added for a 30-min incubation period followed by four additional washes. Imaging was performed with a 40× water-immersion objective as described above.
Positional Cloning. Crossing ru920 carriers of the TL strain to WIK fish created a mapping generation. DNA isolation and PCR analysis were conducted (16) with primers (Research Genetics, Huntsville, AL) flanking each of 215 simple sequence-length polymorphisms recommended for bulked-segregant analysis (17). After initial linkage was determined, additional markers were located by searching a simple sequence-length polymorphism database (http://zebrafish.mgh.harvard.edu). PCR analysis was performed by using template DNA isolated from individual mutant and WT larvae and primers (Research Genetics) flanking the simple sequence-length polymorphisms.
Single-nucleotide polymorphisms (SNPs) between the TL and WIK strains were identified in genes and ESTs mapped to the region on the LN54 and T51 radiation hybrid panels (www.zfin.org). Genomic DNA isolated from individual mapping larvae was amplified under the following PCR conditions: 20 mM Tris·HCl (pH 8.8), 2 mM MgSO4, 10 mM KCl, 10 mM (NH4)2SO4, 0.1% (wt/vol) Triton X-100, 100 μg/ml BSA, 200 μM dNTP, 0.6 μM forward primer, 0.6 μM reverse primer, and 1.25 units of PfuTurbo polymerase (BioCrest, Cedar Creek, TX). The PCR was performed for 120 s at 95°C, followed by 35 cycles of 30 s at 95°C, 30 s at 51°C, and 60 s at 72°C, and finishing with 10 min at 72°C. Products were purified by using the QIAquick PCR purification system (Qiagen, Valencia, CA) and were sequenced directly.
To construct a contig of bacterial artificial chromosomes (BACs), we hybridized radioactive probes to filters representing the CHORI-211 genomic library (Children's Hospital Oakland Research Institute, Oakland, CA). Positives were picked from glycerol stocks, and DNA was purified by using Midiprep columns (Qiagen).
Genomic positions for which no recombination events were observed were used to search the first-pass assembly from the zebrafish genome-sequencing project (Assembly06; www.sanger.ac.uk/Projects/D_rerio). The contigs identified by this means were in turn compared to the National Center for Biotechnology Information nonredundant (nr) database (www.ncbi.nih.gov/BLAST) with the blastx algorithm. Identified myo6b exons were amplified from genomic DNA isolated from the mutant and WT progeny of a TL outcross family, created by breeding a TL/WIK ru920 carrier with a TL fish, and were scanned for the causative mutation.
Sequence Analysis and Injections. Cytoplasmic RNA was isolated from 5-dpf WT larvae (18). Gene-specific primers were designed from the available genomic sequence for myo6b and myosin6a (myo6a) found in Assembly06. Sequences of the 5′ and 3′ ends were obtained by using the respective RACE systems (Invitrogen). The remaining coding sequences of myo6a and myo6b were determined by amplifying and directly sequencing 400- to 1,000-bp regions of cDNA representing their respective transcripts. First-strand cDNA synthesis was performed with Super-Script II (Invitrogen). The predicted amino acid sequences of the two zebrafish myosin VI isoforms were compared to those of class VI myosins from the human (GenBank accession no. Q9UM54) and striped bass (GenBank accession nos. AAD52005 and AAD52006) with the clustalx algorithm in megalign (DNASTAR, Madison, WI).
The coding region of myo6b was amplified from cDNA under the reaction conditions described above but with an extension time of 4 min. PCR products were ligated into the CS2++ vector (A. H. Brivanlou, The Rockefeller University, New York), and a mMESSAGE mMACHINE kit (Ambion, Austin, TX) was used to synthesize capped RNA. The extent of rescue was assessed at 3 dpf by comparing the offspring of ru920 carriers injected with ≈200 pg of purified RNA to uninjected WT and mutant larvae by using the 4-Di-2-ASP labeling protocol described above. Genomic DNA was isolated subsequently from the injected larvae for genotyping.
Morpholinos (GeneTools, Philomath, OR) were injected into WT blastulae in amounts of 5-50 ng. One morpholino (myo6b-UTR) was designed to bind in the 5′ UTR of the myo6b transcript (5′-ACCCACAATTACTCCACAGCTATT). A negative control morpholino (myo6b-con) spanned the identical region but contained five mismatched bases (5′-ACgCCACtATTAgTCCtCAGgTATT, with mismatches indicated by lowercase characters). A third morpholino (myo6b-ATG) was designed to bind around the initiation codon of myo6b mRNA (5′-CTTCCCAATCATCCATTGCACCCCAC). The final morpholino (myo6a-ATG) was synthesized to bind the corresponding region in the myo6a transcript (5′-CTTTCCAATCGTCCATTTCACCGCTC, with differences indicated by italic characters).
In Situ Hybridization. Whole-mount in situ hybridization was performed (12) on larvae obtained by breeding homozygous albino zebrafish (University of Oregon, Eugene). Digoxigenin-labeled RNA probes were synthesized according to the manufacturer's instructions (Roche Diagnostics). The myo6b-specific probe included 158 bp of 5′ UTR and the first 232 bp of coding sequence. Hybridizations and washes were performed at 65°C.
Results
Primary Phenotypic Analysis. In a mutagenic screen of zebrafish for animals with defects of the acousticolateralis system, we identified ru920 mutants by their inability to initiate the characteristic C-start avoidance response to vibrational stimuli (19). The only obvious morphological abnormality of most mutant animals was the absence of an inflated swim bladder (Fig. 1A). Although this structure is important for the transmission of acoustic signals to the inner ears of adult zebrafish (20), the lack of an inflated swim bladder did not cause the observed behavioral phenotype in the ru920 line. During screening of F3 clutches from other lines, larvae without inflated swim bladders were regularly observed to respond normally to vibratory stimuli. Moreover, ≈5% of ru920 mutants developed an inflated swim bladder yet remained unable to execute an acoustically induced escape response.
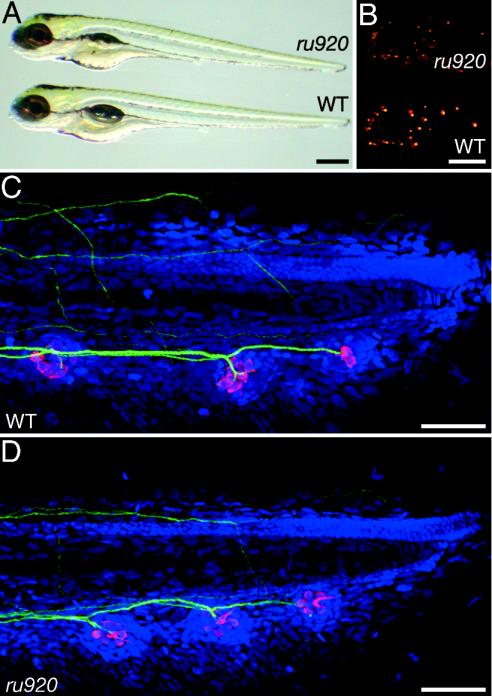
Fig. 1.
Phenotypic characteristics of the ru920 mutation. (A) An ru920 mutant and a WT larva at 7 dpf differ overtly only in the mutant's lack of an inflated swim bladder. (B) Under fluorescent illumination, neuromasts labeled with 4-Di-2-ASP form a stereotypic pattern on the surface of the larvae. The number and patterning of neuromasts in the ru920 mutant are indistinguishable from those of its WT sibling, but the fluorescence intensity is reduced. (C) In a flattened stack of confocal-microscopic sections from a WT larva at 5 dpf, anti-parvalbumin 3 (red) labels the hair cells of the three terminal neuromasts in the posterior lateral-line organ. The 3A10 monoclonal antibody directed against neurofilaments (green) delineates the innervation of each neuromast. TO-PRO-3 (blue) labels cellular nuclei. (D) A similarly prepared mutant larva shows no reproducible differences with respect to the WT larva in neuromast position, afferent innervation, or hair-cell number within each neuromast. (Scale bars: A and B, 500 μm; C and D, 100 μm.)
Because touching a mutant larva with a hair usually elicited an escape response, the defect lay antecedent to the reticulospinal neurons in the reflex arc (21). Unlike their WT siblings, mutants often did not move away from the source of the touch stimulus but instead circled back and swam upside-down. This behavior, which suggests the presence of a vestibular defect, may account for the inviability of mutant fish: most larvae die at 12-15 dpf, when they are required to capture food.
Although mutants appeared to have a full complement of hair cells throughout larval development, their lateral-line hair cells displayed diminished labeling with 4-Di-2-ASP, a cationic fluorophore that enters normal hair cells through mechanoelectrical-transduction channels (22) (Fig. 1 B-D). Similar results were observed when 4-Di-2-ASP was iontophoresed into the inner ears of WT and mutant larvae (Fig. 2).
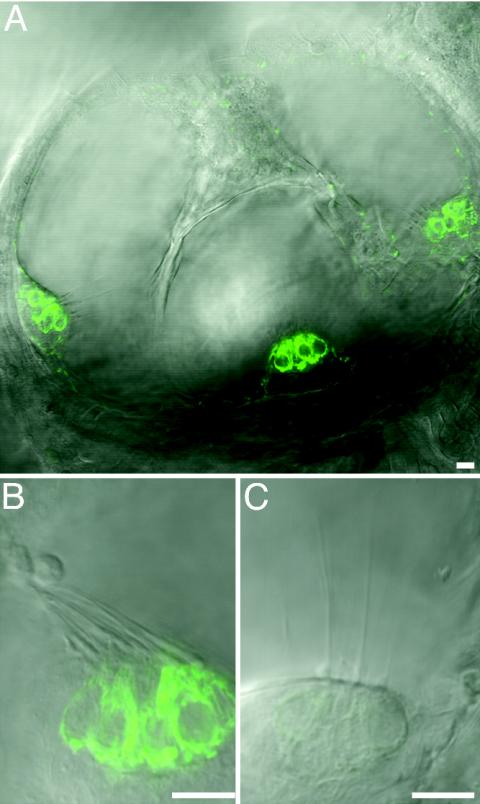
Fig. 2.
Fluorescence and differential-interference-contrast images of inner ears from 5-dpf larvae labeled with 4-Di-2-ASP. (A) In this confocal-microscopic image of a WT fish, 4-Di-2-ASP fluorescence (green) occurs in hair cells of the three semicircular-canal cristae. In higher-magnification views of cristae from posterior semicircular canals of a WT larva (B) and an ru920 mutant (C), the intensity of fluorescence is diminished in the mutant. The confocal settings, including the pinhole size and gain, were matched between B and C. Similar results were obtained for the other sensory epithelia of the inner ear. (Scale bars = 10 μm.)
Microphonic-Potential Recordings. We sought extracellular electrical responses to vibratory stimuli in the otic vesicles of ru920 mutant larvae and WT siblings (Fig. 3). WT ears consistently yielded microphonic responses with peak-to-peak amplitudes of 150-200 μV. These signals were eliminated by the extracellular application of gentamicin, a blocker of the mechanically activated current in hair cells (23), thereby validating mechanotransduction as the source of the electrical response (data not shown). By contrast, we were unable to measure microphonic responses from 10 inner ears of ru920 mutant larvae. In conjunction with the foregoing data, this result implies a deficit in mechanoelectrical transduction throughout the acousticolateralis system.
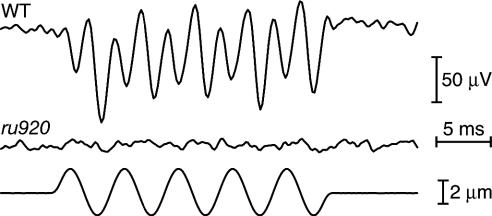
Fig. 3.
Extracellular microphonic responses from the inner ears of larvae at 7 dpf. A ±2-μm vibratory stimulus (Bottom) was delivered by a glass probe applied to the skin overlying the posterior wall of the otic vesicle. A typical extracellular response from a WT larva (Top) has twice the frequency of the mechanical stimulus, owing to the two oppositely oriented populations of hair cells in the zebrafish's maculae. The corresponding signal from an ru920 mutant (Middle) shows no electrical response to mechanical stimulation.
Genetic Mapping and Mutation Detection. The complete penetrance and consistent expressivity of the recessive ru920 mutation facilitated a positional-cloning approach to the identification of its cause. The mutation was initially linked to the simple sequence-length polymorphism z21703 in linkage group 17 (Fig. 4A). Eighty-eight mutants genotyped at other polymorphisms in linkage group 17 revealed that z6561 and z7170 had the smallest mutually exclusive subsets of mutant larvae with recombination events between each marker and the mutation. Genotyping 1,936 mutants identified 37 animals with recombination events between the mutation and z6561 and 20 with crossovers between z7170 and the mutation. This genetic interval was narrowed to 1.26 centimorgans by examining the recombinant larvae at SNPs identified in genes and ESTs previously mapped to the same region of linkage group 17 (Fig. 4B).
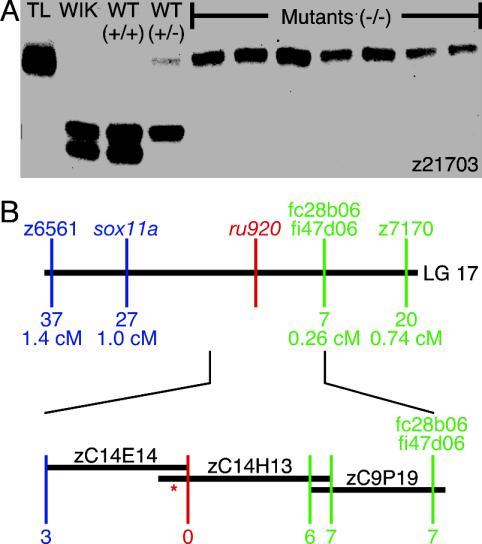
Fig. 4.
Mapping of the ru920 mutation. (A) The original carrier (TL), the WIK fish used to create the mapping family (WIK), and nine mapping offspring were genotyped by amplifying simple sequence-length polymorphism z21703 with PCR and separating the products on an agarose gel. Because only one z21703 allele derived from the TL grandparent is observed in the seven mutant larvae, this allele is linked to the mutation. WT progeny, on the other hand, always carry at least one of the two alleles from the WIK founder. (B) Genotyping mutant larvae at flanking simple sequence-length polymorphisms and newly discovered SNPs localized the mutation within a 1.26-centimorgan segment of linkage group 17 (Upper). The number of recombination events observed between each locus and the mutation and the corresponding genetic distance are indicated. In a contig of BAC inserts defining the critical interval (Lower), the position at which no recombination events were observed is depicted in red. *, Site of the mutation.
Because genes mapped to the region provided no obvious candidates for the affected gene, we undertook a chromosomal walk to create a BAC contig spanning the mutation. DNA probes specific for fc28b06 and fi47d06 were used to screen a library of BAC clones. Polymorphic loci located by using the sequences from each end of the identified BACs were used to orient the genomic inserts with respect to the mutation. The library was screened again with probes directed against the ends of the inserts deemed to be closer to the mutation. This process was repeated until a SNP was identified at which a subset of the z6561 recombinant larvae was observed to have recombination events between the SNP and the mutation, thereby closing the BAC contig around the mutation (Fig. 4B).
All of the recombinant larvae carried two TL alleles at a SNP identified in the sequence corresponding to one end of the zC14E14 BAC insert. We identified one contig from Assembly06, z06s000091, containing this locus. Nineteen distinct regions of the contig encoded segments of a protein with ≈95% identity to the two class VI myosin isoforms from the striped bass (24). We denoted the zebrafish gene associated with ru920 as myo6b because the nucleotide sequence and predicted protein sequence more closely resembled those of the bass gene FMVIB. We designated a related zebrafish gene from Assembly06 as myo6a in view of its greater similarity to the bass gene FMVIA.
Sequence analysis of each ORF indicated that myo6a and myo6b encode 86% identical proteins, each of which is 82% identical to human myosin VI. Both zebrafish proteins have the characteristic features of class VI myosin proteins, including a class-specific 53-aa linker between the head and the neck domains.
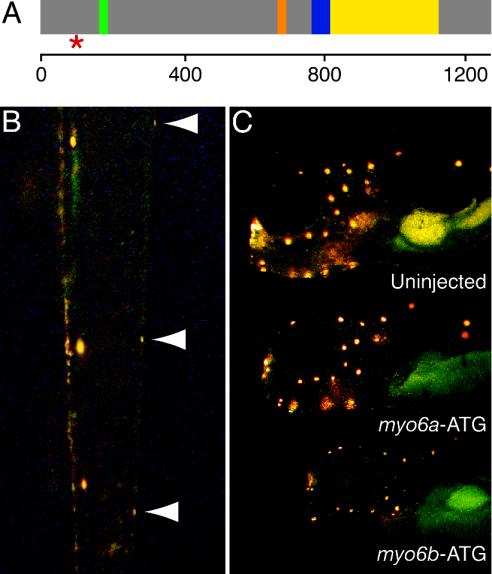
Mutation Confirmation and Further Phenotypic Analysis. Examination of the coding sequence of myo6b from mutant and WT siblings of a TL outcross revealed a single-base change of a thymine to an adenosine in the fifth exon of myo6b. This alteration, which converts a tyrosine codon (TAT) to a stop codon (TAA) 99 aa after the translation start site (Fig. 5A), was not detected in any WT zebrafish strain examined.
Fig. 5.
Confirmation of the role of Myo6b in the ru920 mutation. (A) A diagram of the predicted structure of the Myo6b protein indicates the position of the introduced stop codon (red *) only 99 amino acid residues after the initiation methionine. The ru920 mutation occurs before any of the conserved class VI myosin motifs, including the ATP-binding site (green), the actin-binding site (orange), the 53-aa linker and single IQ motif that interacts with a calmodulin light chain (blue), and the coiled-coil dimerization domain (yellow). The scale at the bottom indicates amino acid position. (B) Injection of in vitro-transcribed mRNA that encodes WT Myo6b rescued the ru920 phenotype. A dorsal view of the tail of a 3-dpf mutant larvae reveals that the mutant has been partially rescued by the exogenous transcript. Neuromasts on the left side of the animal display robust labeling with 4-Di-2-ASP, whereas the three neuromasts on the right side (arrowheads) exhibit the weak labeling characteristic of ru920 mutant hair cells. (C) Inhibiting the translation of Myo6b in WT embryos by injection of a morpholino directed against sequence around the ATG initiation codon phenocopied the ru920 mutation (Bottom). Injection of a morpholino directed against the corresponding region of myo6a (Middle) produced no significant reduction in 4-Di-2-ASP labeling of lateral-line hair cells in comparison with a control animal (Top).
To confirm that the identified mutation causes the observed phenotype, we rescued mutant embryos by injecting in vitro-transcribed RNA that encodes WT zebrafish myosin VIb (Myo6b) into the offspring of heterozygous ru920 carriers. Of 49 injected larvae genotyped as mutants, ≈10% displayed WT levels of fluorescence in all of the neuromasts labeled at 3 dpf with 4-Di-2-ASP. We often observed mutant larvae with normal fluorescent labeling in a fraction of their neuromasts (Fig. 5B), presumably because the exogenous transcripts were restricted to a subset of blastomeres in the injected embryos.
In the inverse experiment, we injected modified antisense oligonucleotides directed against myo6b to recreate the ru920 phenotype in WT larvae. Reduction of Myo6b protein levels by injection of the myo6b-UTR morpholino efficiently reproduced the ru920 phenotype, demonstrated by decreased 4-Di-2-ASP fluorescence in the neuromasts of ≈80% of 269 injected WT larvae. Injection of a control morpholino with five mismatched bases, myo6b-con, yielded no noticeable phenotype, even at 10 times the concentration used for myo6b-UTR. Introduction of a second myo6b-specific morpholino, myo6b-ATG, gave results similar to those obtained with myo6b-UTR (Fig. 5C). Embryos injected with myo6a-ATG, a morpholino directed against the corresponding region of the paralogous gene myo6a, displayed no obvious phenotype. This result provides preliminary evidence that myosin VIa is not required for mechanoelectrical transduction in zebrafish.
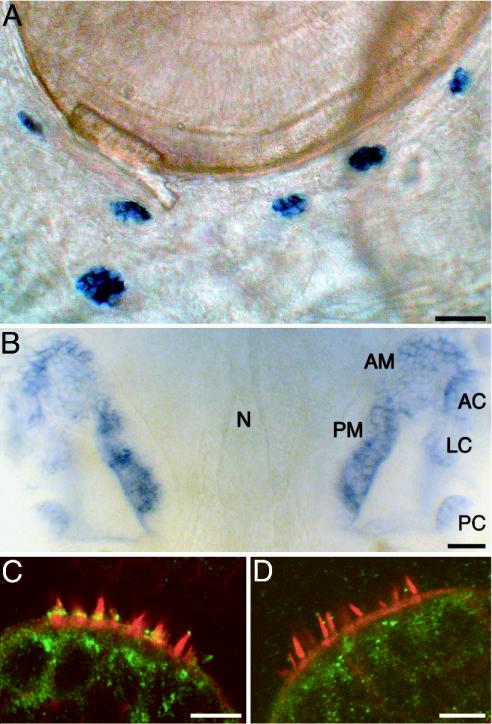
We next analyzed the expression of myo6a and myo6b in zebrafish by whole-mount in situ hybridization and immunohistochemistry. myo6a was broadly expressed, with high levels of mRNA detected in the brain and developing pharyngeal arches of 5-dpf larvae (data not shown). In contrast, the expression of myo6b mRNA was restricted to hair cells of the inner ear and lateral-line organ (Fig. 6 A and B). No labeling was observed when sense probes were used as controls.
Fig. 6.
Localization of myo6b mRNA and Myo6b protein. (A) A 3-dpf larva subjected to whole-mount in situ hybridization illustrates the expression of myo6b mRNA in hair cells of the lateral-line organ. The six neuromasts pictured are members of the infraorbital, opercular, and mandibular lateral lines. (B) In the developing ears of a WT larva at 5 dpf, labeling occurs in the anterior macula (AM) and posterior macula (PM), as well as in the anterior crista (AC), lateral crista (LC), and posterior crista (PC) of the semicircular canals. The notochord (N) delineates the midline in this dorsal view. (C) In a confocal-microscopic section of the medial crista of an 8-dpf WT larva, antibodies against myosin VI (green) intensely label hair cells at the bases of their hair bundles. (D) In an ru920 mutant of the same age, this labeling is absent. The hair bundles were visualized by labeling actin filaments with fluorescent phalloidin (red). (Scale bars: A and B, 50 μm; C and D, 10 μm.)
Polyclonal antibodies raised against a peptide from human myosin VI were used to localize Myo6b in the zebrafish. A band of intense immunolabeling was observed at the base of each apically located hair bundle in the lateral-line organ and inner ear of WT larvae (Fig. 6C). Only one reproducible difference was associated with the mutation: labeling was not observed at the corresponding position in ru920 mutants (Fig. 6D).
Discussion
We have characterized a recessive mutation that disrupts mechanoelectrical transduction in the ear of the zebrafish. ru920 mutant larvae exhibit behavioral phenotypes characteristic of loss of function by aural hair cells: absence of an acoustic startle response and an inability to orient with respect to gravity. Moreover, despite an apparently normal complement of hair cells, mechanical stimuli fail to elicit electrical responses in mutant inner ears. Mutant hair cells throughout the acousticolateralis system are additionally deficient in the internalization of 4-Di-2-ASP, a marker of mechanoelectrical transduction. The defect stems from a stop codon introduced after 99 aa in the normally 1,255-aa protein Myo6b, a zebrafish ortholog of the murine and human myosins VI previously associated with heritable deafness (25, 26). ru920 is almost certainly a null mutation, for the truncated protein lacks every important myosin motif. The rescue of mutant embryos with RNA coding for WT Myo6b and the phenocopying of the mutation with myo6b-specific morpholinos confirm the causative nature of the identified mutation.
Although myosin molecules generally progress toward the barbed ends of actin filaments, myosin VI instead moves toward the pointed ends (27). In view of this unconventional motility, myosin VI has been proposed to serve as a molecular motor during endocytosis and the subsequent transport of organelles away from the plasma membrane (28). A myosin VI splice variant containing a large insert in its tail domain localizes with clathrin-coated vesicles, whereas myosin VI lacking the insert is essential for the ensuing transport of uncoated vesicles to early endosomes (29, 30). As has been proposed for murine myosin VI (31), the lack of Myo6b might disrupt the removal of membrane from the apical surface by clathrin-mediated endocytosis and thereby cause stereociliary fusion. Because Myo6b in the inner ear apparently lacks the large insert, however, it presumably does not associate with clathrin-coated vesicles in this organ. If this is so, the ru920 mutation would not be expected to disrupt an early stage of endocytosis, which accordingly is unlikely to constitute the critical role of myosin VI in hair cells.
An alternative possibility (31) is that Myo6b has an endocytosis-independent role of securing the hair cell's apical plasma membrane to the actin-rich cuticular plate. In the absence of the isozyme, membrane fusion might commence at the bases of adjacent stereocilia and propagate to their tips. Although myosin VI acts as a transporter under low-load conditions, it stalls under high loads (32). Myo6b accordingly might progress down the actin cytoskeletons of stereocilia, pulling the attached apical membrane taut until the increasing load arrests the motor, thereby anchoring the membrane between stereocilia.
Given that only a single myosin VI isoform has been found in mammalian species, myo6a and myo6b probably arose during the genomewide duplication that occurred at the base of the teleostean radiation. The duplication-degeneration-complementation model argues that mutations tend to preserve paralogs by dividing either the sites of action or the functions of an ancestral gene between the paralogs (33). In accordance with this model, myo6a and myo6b appear to have split the expression domain of the ancestral gene, with the latter occurring dominantly in hair cells. The paralogs may also have divided the functions of the ancestral gene, with myosin VIa becoming the transporter for endocytosis and Myo6b assuming the role of membrane anchor. The introduction of exogenous transcripts encoding myosin VIa into the hair cells of ru920 mutants would address whether this is the case.
If Myo6b serves as a membrane anchor in hair cells, what additional proteins might act in concert to connect the apical plasmalemma to actin filaments? Of the known binding partners of myosin VI, only synapse-associated protein 97 (34) has been localized to the ear, where it occurs in the subnuclear and perinuclear regions of inner hair cells in the mammalian cochlea (35). This expression profile argues against an interaction between Myo6b and synapse-associated protein 97 at the stereociliary bases. Disabled 2 (36), a protein that binds the clathrin adapter protein AP-2, likely links longer forms of myosin VI to clathrin-coated vesicles during the early stages of endocytosis. Finally, Gα-interacting protein-interacting-protein-C terminus (30) immunoprecipitates with myosin VI, associates with uncoated vesicles, and interacts with the carboxyl termini of several transmembrane proteins, making it the best candidate for a molecular bridge between myosin VI and the membrane.
This study confirms that myosin VI plays a critical role in mechanotransduction by vertebrate hair cells (25, 26) and provides evidence that compromise of the endocytotic role of myosin VI is not the reason for deficient transduction in mutant animals. The advantages of the zebrafish system, including the ease of introduction of recombinant DNA and in vitro-transcribed RNA and the accessibility of the developing inner ear and lateral-line organ to experimental manipulation, should prove useful in future studies of the specialized role of myosin VI in hair cells.
Note. While this paper was being prepared for publication, another report of the zebrafish myosin VI isoforms appeared (37), with results largely consistent with ours.
Acknowledgments
We thank A. H. Brivanlou for providing the CS2++ vector; J. Fak and S. Nakamura for help in the production and identification of the ru920 mutant line; A. Afolalu and P. Espitia for fish husbandry; and Y. Asai, A. Holtzinger, H. López-Schier, B. M. McDermott, Jr., F. Pataky, and S. Jani for assistance with the maintenance and analysis of mutant lines. J. A. Spudich, T. Noguchi, and members of our research group provided valuable comments on the manuscript. This work was supported by National Institutes of Health Grant DC00241. A.J.H. is an Investigator of the Howard Hughes Medical Institute.
Abbreviations: BAC, bacterial artificial chromosome; 4-Di-2-ASP, 4-(4-(diethylamino)styryl)-N-methylpyridinium iodide; dpf, days postfertilization; SNP, single-nucleotide polymorphism.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database [accession nos. AY691329 (myo6a) and AY691328 (myo6b)].
References
- 1.Hudspeth, A. J. (1989) Nature 341, 397-404. [DOI] [PubMed] [Google Scholar]
- 2.Petit, C., Levilliers, J. & Hardelin, J. P. (2001) Annu. Rev. Genet. 35, 589-646. [DOI] [PubMed] [Google Scholar]
- 3.Dooley, K. & Zon, L. I. (2000) Curr. Opin. Genet. Dev. 10, 252-256. [DOI] [PubMed] [Google Scholar]
- 4.Haddon, C. & Lewis, J. (1996) J. Comp. Neurol. 365, 113-128. [DOI] [PubMed] [Google Scholar]
- 5.Blaxter, J. H. S. & Fuiman, L. A. (1989) in The Mechanosensory Lateral Line: Neurobiology and Evolution, eds. Coombs, S., Görner, P. & Münz, H. (Springer, New York), pp. 481-499.
- 6.Malicki, J., Schier, A. F., Solnica-Krezel, L., Stemple, D. L., Neuhauss, S. C., Stainier, D. Y., Abdelilah, S., Rangini, Z., Zwartkruis, F. & Driever, W. (1996) Development (Cambridge, U.K.) 123, 275-283. [DOI] [PubMed] [Google Scholar]
- 7.Whitfield, T. T., Granato, M., van Eeden, F. J., Schach, U., Brand, M., Furutani-Seiki, M., Haffter, P., Hammerschmidt, M., Heisenberg, C. P., Jiang, Y. J., et al. (1996) Development (Cambridge, U.K.) 123, 241-254. [DOI] [PubMed] [Google Scholar]
- 8.Nicolson, T., Rüsch, A., Friedrich, R. W., Granato, M., Ruppersberg, J. P. & Nüsslein-Volhard, C. (1998) Neuron 20, 271-283. [DOI] [PubMed] [Google Scholar]
- 9.Haffter, P., Granato, M., Brand, M., Mullins, M. C., Hammerschmidt, M., Kane, D. A., Odenthal, J., van Eeden, F. J., Jiang, Y. J., Heisenberg, C. P., et al. (1996) Development (Cambridge, U.K.) 123, 1-36. [DOI] [PubMed] [Google Scholar]
- 10.Driever, W., Solnica-Krezel, L., Schier, A. F., Neuhauss, S. C., Malicki, J., Stemple, D. L., Stainier, D. Y., Zwartkruis, F., Abdelilah, S., Rangini, Z., et al. (1996) Development (Cambridge, U.K.) 123, 37-46. [DOI] [PubMed] [Google Scholar]
- 11.Fadool, J. M., Brockerhoff, S. E., Hyatt, G. A. & Dowling, J. E. (1997) Dev. Genet. 20, 288-295. [DOI] [PubMed] [Google Scholar]
- 12.Westerfield, M., ed. (1995) The Zebrafish Book (Univ. of Oregon Press, Eugene).
- 13.Mullins, M. C., Hammerschmidt, M., Haffter, P. & Nüsslein-Volhard, C. (1994) Curr. Biol. 4, 189-202. [DOI] [PubMed] [Google Scholar]
- 14.Starr, C. J., Kappler, J. A., Chan, D. K., Kollmar, R. & Hudspeth, A. J. (2004) Proc. Natl. Acad. Sci. USA 101, 2572-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jowett, T. (1999) Methods Cell Biol. 59, 63-85. [DOI] [PubMed] [Google Scholar]
- 16.Zhang, J., Talbot, W. S. & Schier, A. F. (1998) Cell 92, 241-251. [DOI] [PubMed] [Google Scholar]
- 17.Shimoda, N., Knapik, E. W., Ziniti, J., Sim, C., Yamada, E., Kaplan, S., Jackson, D., de Sauvage, F., Jacob, H. & Fishman, M. C. (1999) Genomics 58, 219-232. [DOI] [PubMed] [Google Scholar]
- 18.Carninci, P., Nakamura, M., Sato, K., Hayashizaki, Y. & Brownstein, M. J. (2002) BioTechniques 33, 306-309. [DOI] [PubMed] [Google Scholar]
- 19.Kimmel, C. B., Patterson, J. & Kimmel, R. O. (1974) Dev. Psychobiol. 7, 47-60. [DOI] [PubMed] [Google Scholar]
- 20.Bang, P. I., Yelick, P. C., Malicki, J. J. & Sewell, W. F. (2002) J. Neurosci. Methods 118, 177-187. [DOI] [PubMed] [Google Scholar]
- 21.Eaton, R. C., Bombardieri, R. A. & Meyer, D. L. (1977) J. Exp. Biol. 66, 65-81. [DOI] [PubMed] [Google Scholar]
- 22.Meyers, J. R., MacDonald, R. B., Duggan, A., Lenzi, D., Standaert, D. G., Corwin, J. T. & Corey, D. P. (2003) J. Neurosci. 23, 4054-4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kroese, A. B., Das, A. & Hudspeth, A. J. (1989) Hear. Res. 37, 203-217. [DOI] [PubMed] [Google Scholar]
- 24.Breckler, J., Au, K., Cheng, J., Hasson, T. & Burnside, B. (2000) Exp. Eye Res. 70, 121-134. [DOI] [PubMed] [Google Scholar]
- 25.Avraham, K. B., Hasson, T., Steel, K. P., Kingsley, D. M., Russell, L. B., Mooseker, M. S., Copeland, N. G. & Jenkins, N. A. (1995) Nat. Genet. 11, 369-375. [DOI] [PubMed] [Google Scholar]
- 26.Melchionda, S., Ahituv, N., Bisceglia, L., Sobe, T., Glaser, F., Rabionet, R., Arbones, M. L., Notarangelo, A., Di Iorio, E., Carella, M., et al. (2001) Am. J. Hum. Genet. 69, 635-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wells, A. L., Lin, A. W., Chen, L. Q., Safer, D., Cain, S. M., Hasson, T., Carragher, B. O., Milligan, R. A. & Sweeney, H. L. (1999) Nature 401, 505-508. [DOI] [PubMed] [Google Scholar]
- 28.Rock, R. S., Rice, S. E., Wells, A. L., Purcell, T. J., Spudich, J. A. & Sweeney, H. L. (2001) Proc. Natl. Acad. Sci. USA 98, 13655-13659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buss, F., Arden, S. D., Lindsay, M., Luzio, J. P. & Kendrick-Jones, J. (2001) EMBO J. 20, 3676-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aschenbrenner, L., Lee, T. & Hasson, T. (2003) Mol. Biol. Cell 14, 2728-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Self, T., Sobe, T., Copeland, N. G., Jenkins, N. A., Avraham, K. B. & Steel, K. P. (1999) Dev. Biol. 214, 331-341. [DOI] [PubMed] [Google Scholar]
- 32.Altman, D., Sweeney, H. L. & Spudich, J. A. (2004) Cell 116, 737-749. [DOI] [PubMed] [Google Scholar]
- 33.Force, A., Lynch, M., Pickett, F. B., Amores, A., Yan, Y. L. & Postlethwait, J. (1999) Genetics 151, 1531-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu, H., Nash, J. E., Zamorano, P. & Garner, C. C. (2002) J. Biol. Chem. 277, 30928-30934. [DOI] [PubMed] [Google Scholar]
- 35.Davies, C., Tingley, D., Kachar, B., Wenthold, R. J. & Petralia, R. S. (2001) Synapse 40, 258-268. [DOI] [PubMed] [Google Scholar]
- 36.Morris, S. M., Arden, S. D., Roberts, R. C., Kendrick-Jones, J., Cooper, J. A., Luzio, J. P. & Buss, F. (2002) Traffic 3, 331-341. [DOI] [PubMed] [Google Scholar]
- 37.Seiler, C., Ben-David, O., Sidi, S., Hendrich, O., Rüsch, A., Burnside, B., Avraham, K. B. & Nicolson, T. (2004) Dev. Biol. 272, 328-338. [DOI] [PubMed] [Google Scholar]