Abstract
The cardiac ryanodine receptor (RyR2) governs the release of Ca2+ from the sarcoplasmic reticulum, which initiates muscle contraction. Mutations in RyR2 have been linked to ventricular tachycardia (VT) and sudden death, but the precise molecular mechanism is unclear. It is known that when the sarcoplasmic reticulum store Ca2+ content reaches a critical level, spontaneous Ca2+ release occurs, a process we refer to as store-overload-induced Ca2+ release (SOICR). In view of the well documented arrhythmogenic nature of SOICR, we characterized the effects of disease-causing RyR2 mutations on SOICR in human embryonic kidney (HEK)293 cells and found that, at elevated extracellular Ca2+ levels, HEK293 cells expressing RyR2 displayed SOICR in a manner virtually identical to that observed in cardiac cells. Using this cell model, we demonstrated that the RyR2 mutations linked to VT and sudden death, N4104K, R4496C, and N4895D, markedly increased the occurrence of SOICR. At the molecular level, we showed that these RyR2 mutations increased the sensitivity of single RyR2 channels to activation by luminal Ca2+ and enhanced the basal level of [3H]ryanodine binding. We conclude that disease-causing RyR2 mutations, by enhancing RyR2 luminal Ca2+ activation, reduce the threshold for SOICR, which in turn increases the propensity for triggered arrhythmia. Abnormal RyR2 luminal Ca2+ activation likely contributes to the enhanced SOICR commonly observed in various cardiac conditions, including heart failure, and may represent a unifying mechanism for Ca2+ overload-associated VT.
Ventricular tachycardia (VT) is the leading cause of sudden death in patients with heart failure (HF). Delayed afterdepolarizations (DAD) frequently occur in failing hearts and are a major cause of VT, but the reason for the increased incidence of DAD-associated VT in patients with HF is not completely clear. Abnormal Ca2+ handling is believed to be involved in the pathogenesis of VT (1-4). In keeping with this view, mutations in Ca2+ handling proteins, including the cardiac ryanodine receptor (RyR2) and calsequestrin (CASQ2), have been linked to catecholaminergic polymorphic VT (CPVT), which is also thought to be DAD-based (5-10). These similarities suggest that CPVT and DAD-associated VT in patients with HF may share a common arrhythmogenic mechanism. Thus, knowledge gained from investigation of inherited CPVT should lead to a better understanding of the molecular basis of the more commonly occurring VT in patients with HF and other cardiac diseases.
RyR2 is an intracellular Ca2+ release channel located in the sarcoplasmic reticulum (SR) (11). It is a key component of excitation contraction (EC) coupling in cardiac muscle, which is believed to take place via a mechanism known as Ca2+-induced Ca2+ release (CICR) (12, 13). In this process, a small Ca2+ influx through the L-type Ca2+ channel upon membrane depolarization activates the RyR2 channel, resulting in a large Ca2+ release from the SR and subsequent muscle contraction. In addition to this depolarization-stimulated Ca2+ release during normal EC coupling, it is known that when SR Ca2+ content reaches a critical level, spontaneous SR Ca2+ release in the form of Ca2+ waves or Ca2+ oscillations occurs in cardiac cells in the absence of membrane depolarization (14-18). Considering its dependence on the SR Ca2+ store, we refer to this depolarization-independent Ca2+ overload-induced SR Ca2+ release as store-overload-induced Ca2+ release (SOICR).
A number of conditions, such as physical and emotional stresses, digitalis toxicity, elevated extracellular Ca2+, ischemia/reperfusion, etc., can lead to SR Ca2+ overload and subsequent SOICR in cardiac cells (19), and SOICR can activate inward currents. These Ca2+-activated inward currents can alter the surface membrane potential and generate DAD, which in turn can lead to triggered arrhythmia (20). It is important to note that CPVT, an autosomal dominant genetic arrhythmogenic disorder associated with syncope and sudden death, is triggered by emotional and physical stresses, conditions known to induce Ca2+ overload (21). In view of its association with Ca2+ overload and DAD, it is likely that CPVT results from defective SOICR. Consistent with this view, altered SOICR has been implicated in a number of cardiac conditions (19).
The precise mechanisms of how CPVT mutations affect RyR2 channel function and SOICR are largely undefined. Given the prominent role of SOICR in the generation of DAD, it is sensible to propose that CPVT mutations in RyR2 may reduce the threshold for SOICR and thus increase the susceptibility for triggered arrhythmia. Ideally, this hypothesis would be directly tested using cardiac myocytes isolated from patients with CPVT or from animal models of CPVT. However, neither of these approaches is readily available at present. As well, the extremely large size of the RyR2 cDNA (≈15 kb) also excludes the use of adenovirus-mediated gene transfer techniques for introducing the RyR2 mutations into adult cardiac myocytes.
To circumvent these problems, we have developed an alternative cell system for assessing the effect of disease-causing RyR2 mutations on SOICR. We generated stable inducible human embryonic kidney (HEK)293 cell lines expressing the WT RyR2 [RyR2(wt)] and the CPVT mutants. We found that HEK293 cells expressing RyR2(wt) or mutant RyR2 displayed SOICR at elevated extracellular Ca2+ levels in a manner indistinguishable from that observed in cardiac myocytes. Importantly, we demonstrated that CPVT mutations enhanced the propensity for SOICR. We further demonstrated that CPVT mutations increased the RyR2 sensitivity to luminal Ca2+ activation and the basal level of [3H]ryanodine binding. Our studies demonstrate the link between defective luminal Ca2+ activation of RyR2 and CPVT and sudden cardiac death. Enhanced SOICR as a result of augmented RyR2 luminal Ca2+ activation may contribute to Ca2+ overload-associated VT and contractile dysfunction in various cardiac conditions.
Materials and Methods
Site-Directed Mutagenesis and DNA Transfection. The point mutations N4104K, R4496C, and N4895D in the mouse RyR2 were made by the overlap extension method and used to transfect HEK293 cells grown on 100-mm tissue culture dishes using Ca2+ phosphate precipitation as described (22).
Generation of Stable Inducible HEK293 Cell Lines. Flp-In T-REx-293 cells (Invitrogen) were cotransfected with the inducible expression vector pcDNA5/FRT/TO containing either the RyR2(wt) or mutant cDNA and the pOG44 vector encoding the Flp recombinase in 1:5 ratios. Transfected cells were washed 1 day after transfection and allowed to grow in fresh medium for another day. The cells then were washed and replated onto new dishes. After the cells had attached, the growth medium was replaced with a selective medium containing 200 μg/ml hygromycin. The selective medium was changed every 3-4 days until the desired amount of cells was grown. The hygromycin-resistant cells were pooled and stored at -80°C.
Single-Cell Ca2+ Imaging. Intracellular Ca2+ transients in HEK293 cells expressing RyR2(wt) or mutant channels were measured by using single-cell Ca2+ imaging as described (23). Cells were loaded with 5μM Fura-2-acetoxymethyl ester in Krebs-Ringer-Hepes (KRH) buffer plus 0.02% pluronic F-127 (Molecular Probes) and 0.1 mg/ml BSA for 20 min at room temperature. Cells were perfused continuously with KRH buffer containing various concentrations of CaCl2 (0-1 mM) at room temperature. Fura-2 fluorescence was captured every 4 seconds through a Fluar ×20 objective and a Chroma filter set by using the ImageMaster System and the DeltaRAM rapid wavelength-switching illuminator (Photon Technology International, Lawrenceville, NJ).
Preparation of Cell Lysate and [3H]ryanodine Binding. Preparation of cell lysate and equilibrium [3H]ryanodine binding were carried out as described (22).
Single-Channel Recordings. Single-channel analyses were carried out as described (23). Heart phosphatidylethanolamine and brain phosphatidylserine (Avanti Polar Lipids) were combined in a 1:1 ratio (wt/wt), dried under nitrogen gas, and suspended in 30 μl of n-decane at a concentration of 12 mg of lipid per ml. The trans chamber (800 μl) was connected to the head stage input of an Axopatch 200A amplifier (Axon Instruments, Foster City, CA). The cis chamber (1.2 ml) was held at virtual ground. A symmetrical solution containing 250 mM KCl and 25 mM Hepes (pH 7.4) was used for all recordings. Recordings were filtered at 2,500 Hz. Data analyses were carried out using pclamp 8.1 software.
Supporting Information. A detailed Materials and Methods can be found in Supporting Text, which is published as supporting information on the PNAS web site.
Results
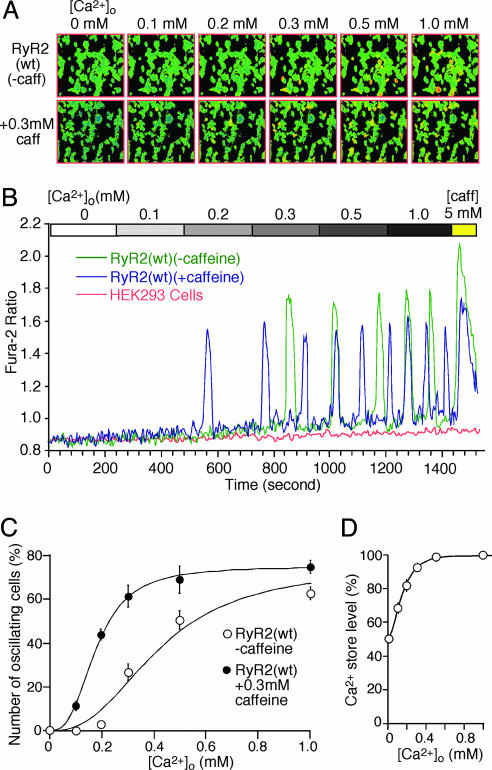
HEK293 Cells Expressing RyR2 Reproduce Cardiac SOICR. To create a cell model for assessing the effect of disease-causing RyR2 mutations on SOICR, we generated a stable tetracyclineinducible HEK293 cell line expressing RyR2(wt). To induce store Ca2+ overload, RyR2(wt) cells were perfused with increasing extracellular Ca2+ concentration ([Ca2+]o) (0-1.0 mM). Ca2+ transients were monitored by using a fluorescence Ca2+ indicator, fura 2 acetoxymethyl ester, and single-cell Ca2+ imaging. As shown in Fig. 1, Ca2+ oscillations appeared in RyR2(wt) cells at ≈0.3 mM [Ca2+]o (Fig. 1 A and B), and the number of Ca2+ oscillating cells increased with increasing [Ca2+]o (Fig. 1C). At [Ca2+]o ≤0.2 mM, few or no Ca2+ oscillating cells were detected. Elevating [Ca2+]o in RyR2(wt) cells slightly increased the resting intracellular Ca2+ concentration and the frequency of Ca2+ oscillations, but had little effect on the amplitude of the oscillations (Fig. 1B). The level of store Ca2+ at each [Ca2+]o was estimated by determining the magnitude of caffeine-(5 mM) induced Ca2+ release. Fig. 1D shows that the level of store Ca2+ increased over the range of [Ca2+]o in which there were few or no Ca2+ oscillations and remained constant over the range of [Ca2+]o in which Ca2+ oscillations occurred. These effects of elevated [Ca2+]o on the store Ca2+ content, frequency, and amplitude of Ca2+ oscillations and the resting intracellular Ca2+ level in RyR2(wt) cells are virtually identical to those observed in cardiac myocytes (24). It should be noted that the occurrence of Ca2+ oscillations in RyR2(wt) cells depended completely on the expression of RyR2. HEK293 cells not expressing RyR2 showed no Ca2+ oscillations under the same conditions (Fig. 1B), indicating that RyR2 is an essential determinant of SOICR.
Fig. 1.
SOICR occurs in HEK293 cells expressing RyR2(wt) at elevated [Ca2+]o. Stable inducible HEK293 RyR2(wt) cells were induced with tetracycline and loaded with 5 μM fura-2 acetoxymethyl ester in Krebs-Ringer-Hepes (KRH) buffer for 20 min at room temperature. Cells were perfused continuously with KRH buffer without (0 mM) or with 0.1, 0.2, 0.3, 0.5, or 1.0 mM CaCl2 or 1.0 mM CaCl2 plus 5 mM caffeine. (A) Single-cell fluorescent Ca2+ images in the presence (Upper) or absence (Lower) of 0.3 mM caffeine at various [Ca2+]o (0-1.0 mM). (B) Fura-2 ratios of representative RyR2(wt) cells in the absence (green trace) and presence (blue trace) of 0.3 mM caffeine and a HEK293 parental cell expressing no RyR2 (pink trace). (C) The fraction (%, mean ± SEM) of cells that display Ca2+ oscillations in the presence (filled circle) and absence (open circle) of 0.3 mM caffeine. The total numbers of cells analyzed for Ca2+ oscillations were 563 (without 0.3 mM caffeine) and 237 (with 0.3 mM caffeine) from three to nine separate experiments. (D) Store Ca2+ content at various [Ca2+]o, which was estimated by measuring the amplitude of caffeine-(5 mM) induced Ca2+ release from oscillating cells and normalized to the maximum level obtained at 1.0 mM [Ca2+]o. Data shown are mean ± SEM from three to seven separate experiments.
SOICR in cardiac myocytes can be modulated by caffeine (25, 26). To determine whether SOICR in RyR2(wt) cells is also sensitive to caffeine modulation, we examined Ca2+ oscillations in these cells while perfusing them with increasing [Ca2+]o and low concentrations of caffeine. As seen in Fig. 1, in the presence of 0.3 mM caffeine, Ca2+ oscillations were detected in ≈10% and ≈40% of the cells perfused with 0.1 and 0.2 mM [Ca2+]o, respectively, although few or no Ca2+ oscillations were observed in the absence of caffeine at these [Ca2+]o (Fig. 1 A-C). These data indicate that caffeine increases the occurrence of SOICR, suggesting that caffeine reduces the threshold for SOICR. In addition, caffeine increases the frequency and reduces the amplitude of Ca2+ oscillations in RyR2(wt) cells (Fig. 1B). These effects of caffeine on the propensity for SOICR and the frequency and amplitude of Ca2+ oscillations in RyR2(wt) cells are nearly identical to those seen in cardiac myocytes (26). Taken together, our results demonstrate that HEK293 cells expressing RyR2 can reproduce cardiac SOICR and thus offer a readily manageable cell model for investigating the impact of disease-causing RyR2 mutations on SOICR.
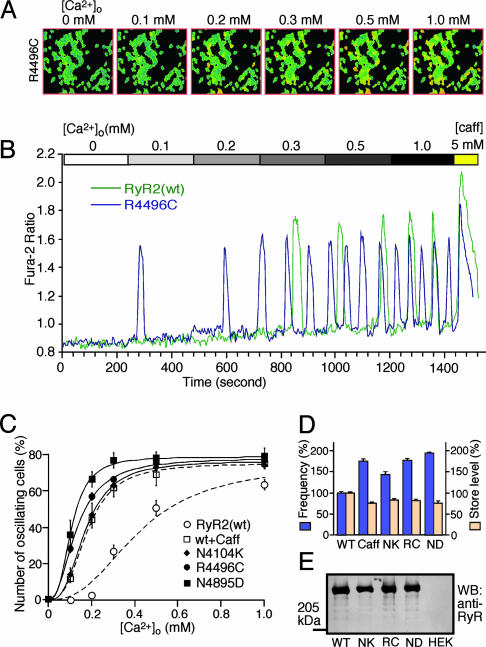
RyR2 Mutations Associated with CPVT Enhance the Propensity for SOICR. To determine whether CPVT mutations alter SOICR, we generated stable inducible HEK293 cell lines expressing the CPVT RyR2 mutants, N4104K, R4496C, and N4895D, and assessed the properties of Ca2+ oscillations induced by elevated [Ca2+]o in each of these cell lines. As shown in Fig. 2, Ca2+ oscillations in R4496C cells appeared at lower [Ca2+]o compared with those in RyR2(wt) cells. In the range of 0.1-0.2 mM [Ca2+]o, in whichfew or no Ca2+ oscillations were observed in RyR2(wt) cells (Fig. 1), Ca2+ oscillations were detected in ≈30-60% of R4496C cells (Fig. 2 A-C). These observations demonstrate that R4496C cells exhibit an enhanced propensity for SOICR. To see whether this is a common feature of the CPVT RyR2 mutants, we performed the same study with N4104K and N4895D cells. Both N4104K and N4895D cells displayed an increased propensity for SOICR (Fig. 2C). Moreover, HEK293 cells expressing CPVT RyR2 mutants showed an increased frequency of Ca2+ oscillations and decreased store Ca2+ content as compared to WT cells (Fig. 2D) (P < 0.002). These effects of the CPVT RyR2 mutations on Ca2+ oscillations and store Ca2+ content are very similar to those of low concentrations of caffeine in WT cells (Fig. 2D) and in cardiac myocytes (26). The expression levels of WT and the CPVT mutants were comparable, as revealed by Western blotting analysis of GST-FKBP12.6 pull-down (Fig. 2E). These observations are consistent with the view that CPVT RyR2 mutations reduce the threshold for SOICR.
Fig. 2.
Effects of CPVT RyR2 mutations on SOICR. Stable inducible HEK293 cells expressing RyR2 mutants were loaded with fura-2-acetoxymethyl ester and perfused continuously with various [Ca2+]o, as described in the legend to Fig. 1. (A) Single-cell fluorescent Ca2+ images of R4496C cells at various [Ca2+]o (0-1.0 mM). (B) Fura-2 ratios of a representative R4496C cell (blue trace) and RyR2(wt) cell (green trace) at various [Ca2+]o.(C) Fraction (%, mean ± SEM) of N4104K (filled diamond), R4496C (filled circle), or N4895D (filled square) cells that display Ca2+ oscillations. The total numbers of cells analyzed for Ca2+ oscillations were 563 for RyR2(wt), 327 for N4104K, 378 for R4496C, and 191 for N4895D from three to nine separate experiments. (D) Frequency of Ca2+ oscillations and store Ca2+ level in HEK293 cells expressing RyR2(wt) in the absence (WT) and presence (Caff) of 0.3 mM caffeine and in HEK293 cells expressing the RyR2 mutants N4104K (NK), R4496C (RC), and N4895D (ND). Both the store Ca2+ content and the frequency of Ca2+ oscillations were determined at 1.0 mM [Ca2+]o. Values were normalized to the WT level (100%). Data shown are mean ± SEM from three to seven separate experiments. RyR2(wt) and the mutant proteins were pulled down by GST-FKBP12.6 from the same amount of cell lysate and Western blotted with an anti-RyR antibody (E).
CPVT RyR2 Mutations Increase the Sensitivity of Single RyR2 Channels to Activation by Luminal Ca2+. Because SOICR is triggered by elevated SR luminal Ca2+, activation of RyR2 by luminal Ca2+ is likely to be involved in SOICR. To explore the molecular basis of the enhanced SOICR observed in CPVT RyR2 mutants, we incorporated single WT and CPVT mutant channels into planar lipid bilayers and examined their response to increasing concentrations of luminal Ca2+. As shown in Fig. 3, a single WT channel exhibited little activity at low cytoplasmic (45 nM) and luminal (45 nM) Ca2+ concentrations (Fig. 3Aa). Raising the luminal Ca2+ concentration to 300 μM increased the open probability (Po) of the WT channel (Figs. 3Ab). The average Po of single WT channels at 300 μM luminal Ca2+ was 0.023 ± 0.007 (mean ± SEM, n = 21) (Fig. 3E). Similar to the WT channels, single CPVT mutant channels displayed little activity at 45 nM cytoplasmic and 45 nM luminal Ca2+ (Figs. 3 Ba, Ca, and Da), but increasing the luminal Ca2+ concentration enhanced the Po of single CPVT mutant channels far more markedly than the WT (Figs. 3 Bb, Cb, and Db). At 300 μM luminal Ca2+, the average Po values of single N4104K, R4496C, and N4895D mutant channels were 0.275 ± 0.051 (n = 7), 0.084 ± 0.017 (n = 14), and 0.308 ± 0.048 (n = 10), respectively, significantly greater than that of single WT channels (Fig. 3E) (P < 0.001). These observations directly demonstrate that CPVT mutations increase the channel sensitivity to activation by luminal Ca2+.
Fig. 3.
CPVT RyR2 mutations increase the sensitivity of single RyR2 channels to luminal Ca2+ activation. Single-channel activities of RyR2(wt) (A), N4104K (B), R4496C (C), and N4895D (D) were recorded in a symmetrical recording solution containing 250 mM KCl and 25 mM Hepes (pH 7.4). EGTA was added to either the cis or trans chamber to determine the orientation of the incorporated channel. The side of the channel to which an addition of EGTA inhibited the activity of the incorporated channel presumably corresponds to the cytoplasmic face. The Ca2+ concentration on both the cytoplasmic and the luminal face of the channel was adjusted to ≈45 nM. The luminal Ca2+ concentration was then increased to various levels by the addition of aliquots of CaCl2 solution. The control current traces for RyR2(wt) or mutants are shown in a, whereas single-channel current traces at 300 μM luminal Ca2+ are depicted in b. The holding potential was -20 mV. Openings are downward. Po, arithmetic mean open time (To), and arithmetic mean closed time (Tc) are indicated on top. A short line to the right of each current trace indicates the baseline. (E) The relationships between Po and luminal Ca2+ concentrations of single RyR2(wt) (open triangle), N4104K (filled diamond), R4496C (filled circle), and N4895D (filled square) are shown. Data points shown are mean ± SEM from 21 RyR2(wt), 7 N4104K, 14 R4496C, and 10 N4895D single channels.
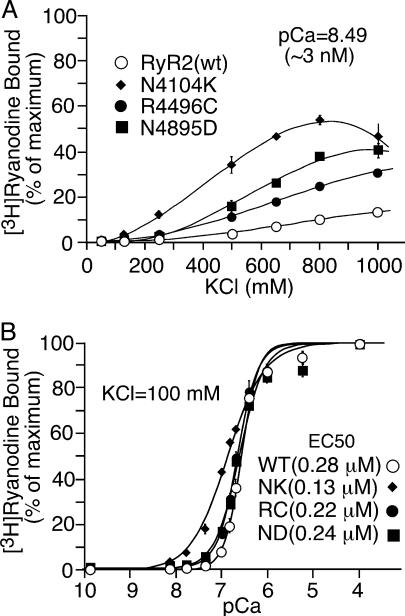
CPVT RyR2 Mutations Enhance the Basal Level of [3H]ryanodine Binding to RyR2. We have previously demonstrated that a CPVT mutation, R4496C, enhances the basal activity of RyR2 (23). To see whether this is a common characteristic of CPVT mutations, we carried out [3H]ryanodine binding to WT and the CPVT mutants in the presence of very low concentrations of Ca2+ (≈3 nM, pCa = 8.49; pCa = -log10[Ca2+]) and increasing concentrations of KCl. As shown in Fig. 4A, the basal level of [3H]ryanodine binding to WT and CPVT mutants increased to different extents with increasing KCl concentrations. Elevating the concentration of KCl slightly increased the basal level of [3H]ryanodine binding to WT but substantially augmented the basal levels of [3H]ryanodine binding to the CPVT mutants. For instance, in the presence of 800 mM KCl, the WT showed 10 ± 0.8% (mean ± SEM, n = 7) of maximum [3H]ryanodine binding, although the CPVT mutants, N4104K, R4496C, and N4895D, exhibited 54 ± 2.2% (n = 3), 25 ± 1.0% (n = 6), and 37 ± 0.7% (n = 3) of maximum [3H]ryanodine binding, respectively, significantly greater than that of the WT (P < 0.0001).
Fig. 4.
Effects of CPVT RyR2 mutations on [3H]ryanodine binding. [3H]ryanodine binding to cell lysate prepared from RyR2(wt) (open circle), N4104K (filled diamond), R4496C (filled circle), and N4895D (filled square) cells was carried out at ≈3nMCa2+, various concentrations of KCl (50-1,000 mM), and 5nM[3H]ryanodine (A), and at various concentrations of Ca2+ (≈0.2-0.1 mM), 100 mM KCl, and 5 nM [3H]ryanodine (B). [3H]Ryanodine binding shown in A was normalized to the binding measured in the presence of 800 mM KCl and 100 μM Ca2+, whereas [3H]ryanodine binding shown in B was normalized to the binding obtained at 100 mM KCl and 100 μMCa2+. Data points shown are mean ± SEM from three to seven experiments.
We have also previously shown that the overall Ca2+ response of the R4496C mutant was comparable to that of the WT (23). Similarly, we found that CPVT mutations, N4104K and N4895D, did not markedly alter the overall Ca2+ response of [3H]ryanodine binding (Fig. 4B). Analysis of the Ca2+ dependence of [3H]ryanodine binding by the Hill equation yielded EC50 values of 0.13 ± 0.009 μM (mean ± SEM, n = 4) for N4104K, 0.22 ± 0.016 μM (n = 4) for R4496C, and 0.24 ± 0.016 μM (n = 6) for N4895D, similar to that for WT (0.28 ± 0.017 μM, n = 15). Differences in [3H]ryanodine binding between WT and the CPVT mutants were mostly observed at low Ca2+ concentrations between ≈20 and ≈200 nM. These observations are consistent with the view that the CPVT mutations result in an increased basal activity of RyR2.
Discussion
The present study has revealed that SOICR, commonly observed in cardiac myocytes, can be reproduced in HEK293 cells expressing RyR2. Using this cell model, we have demonstrated that the RyR2 mutations associated with CPVT, N4104K, R4496C, and N4895D markedly increase the occurrence of SOICR at elevated [Ca2+]o. We have further shown, at the molecular level, that these RyR2 mutations enhance the sensitivity of single RyR2 channels to activation by luminal Ca2+ and augment the basal level of [3H]ryanodine binding. These data indicate that an enhanced luminal Ca2+ activation and basal level of [3H]ryanodine binding are common defects of CPVT RyR2 mutations. Considering the arrhythmogenic characteristics of SOICR, we propose that CPVT RyR2 mutations, by reducing the threshold for SOICR as a result of enhancing luminal Ca2+ activation, increase the susceptibility to VT and sudden death under conditions of Ca2+ overload. Our findings link defective luminal Ca2+ activation of RyR2 to VT and sudden death and suggest RyR2 luminal Ca2+ activation as an alternative target for antiarrhythmic treatment.
Recapitulation of Cardiac SOICR in HEK293 Cells. Investigation of the causative mechanisms of CPVT has been hampered by the lack of animal models for CPVT and methods for introducing large DNA such as the RyR2 cDNA (≈15 kb long) into cardiac myocytes. The present study demonstrates that HEK293 cells expressing RyR2 could be used as an alternative means to investigate the behavior of RyR2 under conditions of Ca2+ overload and the molecular basis of CPVT RyR2 mutations. We have shown that, despite the lack of a number of cardiac-specific Ca2+ handling proteins, HEK293 cells expressing RyR2 produce SOICR at elevated [Ca2+]o in a manner virtually identical to that observed in cardiac cells (Fig. 1). We have also shown that HEK293 cells not expressing RyR2 do not display SOICR at elevated [Ca2+]o (Fig. 1). These observations indicate that RyR2 is essential for SOICR, and that SOICR is not unique to cardiac cells; rather, it reflects the intrinsic properties of RyR2. Hence, HEK293 cells expressing RyR2 provide a readily accessible cell model for molecular analysis of the impact of various CPVT RyR2 mutations on SOICR.
RyR2, a Critical Determinant of the SOICR Threshold. It is well documented that elevated [Ca2+]o produces SR Ca2+ overload, and that once a threshold level of SR Ca2+ content is reached, SOICR occurs. Interestingly, further Ca2+ loading has no effect on SR Ca2+ content. SR Ca2+ content increases only when there is no SOICR (24). Essentially the same relationship between store Ca2+ content and SOICR was observed in HEK293 cells expressing RyR2 (Fig. 1). It is also known that the activity of RyR2 influences the threshold for SOICR in cardiac myocytes. For instance, modest activation of RyR2 by caffeine reduced the threshold for SOICR and SR Ca2+ content, although moderate inhibition of RyR2 by tetracaine increased both the threshold for SOICR and SR Ca2+ content (26, 27). Consistent with this view, we have shown that mutations in RyR2, N4104K, R4496C, and N4895D increased the occurrence of SOICR and the frequency of Ca2+ oscillations and reduced the store Ca2+ content (Fig. 2). Taken together, these observations suggest that RyR2 is a critical determinant of the threshold for SOICR and consequently for the SR Ca2+ content.
Molecular Mechanisms Underlying the Action of CPVT RyR2 Mutations. How do CPVT RyR2 mutations alter the threshold for SOICR? Given that SOICR is triggered by SR Ca2+ overload, and that elevated SR luminal Ca2+ activates RyR2 (28, 29), it is likely that CPVT RyR2 mutations alter the channel sensitivity to activation by luminal Ca2+. In support of this view, we obtained direct evidence that the RyR2 mutations N4104K, R4496C, and N4895D substantially increased the channel sensitivity to activation by luminal Ca2+ (Fig. 3). However, it remains to be determined how these CPVT mutations exert their effects on RyR2 luminal Ca2+ activation. One possibility is that CPVT RyR2 mutations affect domain-domain interactions in RyR2 that mediate luminal Ca2+ activation. CPVT mutations are largely clustered in three domains of RyR2 (21). It has been proposed that interactions among these domains are involved in conformational changes associated with channel gating (30). It is possible that mutations in these domains may weaken the interactions among them and destabilize the closed state of the channel, rendering the channel more sensitive to activation by stimuli. Consistent with this, we found that CPVT RyR2 mutations enhanced basal channel activity (Fig. 4). Another possibility is that CPVT RyR2 mutations affect protein-protein interactions among RyR2 and its associated proteins such as FKBP12.6, CASQ2, triadin, and junctin, which may regulate luminal Ca2+ activation. Wehrens et al. (31) have shown that CPVT RyR2 mutations reduced the affinity of FKBP12.6 binding. On the contrary, however, George et al. (32) have recently demonstrated that CPVT RyR2 mutations augmented SR Ca2+ release in a manner independent of FKBP12.6. Thus, whether CPVT RyR2 mutations alter FKBP12.6 binding requires additional investigations. Regardless of what the exact molecular mechanisms might be, abnormal RyR2 luminal Ca2+ activation may underlie the ultimate outcome of CPVT RyR2 mutations. A detailed understanding of the molecular basis and regulatory mechanism of RyR2 luminal Ca2+ activation will be necessary to appreciate the precise impact of CPVT RyR2 mutations.
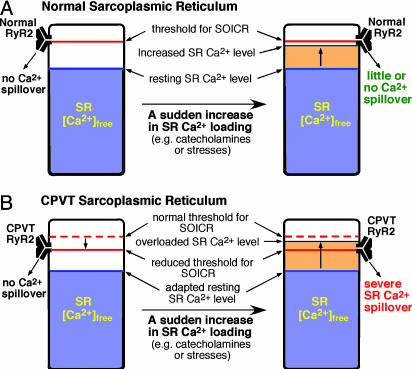
A Proposed Model of CPVT. Based on the results of the present study and those of previous investigations, we propose a simple model to account for CPVT (Fig. 5). In this model, we hypothesize that the threshold for SOICR is primarily determined by RyR2. In normal SR, the threshold for SOICR is higher than the SR free Ca2+ level under both resting and stimulated conditions (catecholamines or stresses). Therefore, there is little or no Ca2+ spillover from the normal SR in either the resting or stimulated states (Fig. 5A). On the other hand, in the CPVT SR, the threshold for SOICR is reduced by mutations in the RyR2 channel. Under resting conditions, the reduced threshold for SOICR is still higher than the resting SR free Ca2+ level, so that there is little or no Ca2+ spillover. However, under stimulated conditions, the CPVT SR is abruptly overloaded with Ca2+. Because of the reduced threshold, SOICR will be more likely to occur during SR Ca2+ loading (Fig. 5B). The resulting large SR Ca2+ spillover can lead to DAD and triggered arrhythmia. It is important to note that, due to SR autoregulation, the resting level of SR free Ca2+ in the CPVT SR might have adapted to a reduced level (33), and that a modest reduction in the threshold for SOICR should not produce a sustained effect on excitation contraction coupling (34). This may explain the absence of functional and structural heart abnormalities in patients with CPVT under resting conditions.
Fig. 5.
A proposed mechanism for CPVT associated with RyR2 mutations. The relationship between the threshold for SOICR and the SR-free Ca2+ level in normal (A) and CPVT SR (B) in the resting and stimulated states is schematically shown. The threshold for SOICR, which is primarily determined by RyR2, is depicted by a red bar. Note that the threshold for SOICR is reduced in the CPVT SR as a consequence of the RyR2 mutations. The SR free Ca2+ level, which is predominantly determined by CASQ2, is represented by the blue area. Note that the resting level of SR-free Ca2+ in the CPVT SR might have adapted to a reduced level due to the existence of SR autoregulation (33). An abrupt increase of SR-free Ca2+ as a result of stimulations by catecholamines or stresses is depicted by the yellow area. When the SR-free Ca2+ level reaches the SOICR threshold, SOICR occurs, leading to a large SR Ca2+ spillover, which in turn can generate DAD and triggered arrhythmia.
SOICR and SR Ca2+ Buffering. It is clear from the proposed model that, besides the threshold for SOICR, another major determinant of the propensity for SOICR is the SR-free Ca2+, which depends largely on SR Ca2+ buffering. Strong SR Ca2+ buffering would slow down the rate of increase of SR free Ca2+ and delay the occurrence of SOICR. On the other hand, in the presence of weak SR Ca2+ buffering, SR-free Ca2+ would be expected to increase rapidly to the threshold level and trigger SOICR readily. Indeed, mutations in CASQ2, a low-affinity high-capacity SR Ca2+-binding protein essential for SR Ca2+ buffering, have also been linked to CPVT (9, 10). Moreover, cardiac myocytes expressing a CPVT CASQ2 mutant that is believed to have no Ca2+ buffering capability exhibited arrhythmic Ca2+ transients and DAD when stimulated with isoproterenol, which increases the loading of SR Ca2+. Intriguingly, the abnormal Ca2+ transient in cardiac myocytes expressing this CASQ2 mutant was restored to normal by loading the cell with citrate, a low-affinity Ca2+ buffer (35, 36), indicating that simply increasing the capacity of SR Ca2+ buffering is sufficient to suppress the Ca2+ overload-induced arrhythmic Ca2+ transients. These observations, together with our data, demonstrate that reducing either the SOICR threshold or the capacity of SR Ca2+ buffering can increase the propensity for SOICR and thus triggered arrhythmias. Hence, our model offers a unifying hypothesis for both RyR2- and CASQ2-linked CPVT.
Implications for VT in Various Cardiac Diseases. VT frequently occurs in a variety of cardiac conditions, such as HF, hypertrophy, and ischemic heart diseases (1-4). Cardiac myocytes and muscles isolated from failing hearts displayed a high incidence of Ca2+ aftertransients, DADs, and triggered activities under conditions that increase SR Ca2+ content (37, 38). Consistent with this, it has also been shown that diastolic SR Ca2+ leak in HF depends steeply on SR Ca2+ load and is greater than that in normal hearts for any given SR Ca2+ load (39). These observations imply that the threshold for SOICR in failing hearts was reduced. A reduced threshold for SOICR has also been observed in hypertensive, hypertrophied, and ischemic/reperfused hearts (19). Thus, a reduced threshold for SOICR is common to many cardiac settings and may contribute to the enhanced propensity for DAD-based VT in these settings. Given the central role of RyR2 in SOICR, altered RyR2 function is likely a major cause of the reduced threshold for SOICR and hence increased susceptibility for VT.
Conclusion
The present study reveals mechanistic insight into the molecular basis of CPVT and proposes a unifying hypothesis for Ca2+ overload-associated VT. We demonstrate that CPVT RyR2 mutations enhance SOICR by increasing the channel sensitivity to activation by luminal Ca2+. Alterations in RyR2 function are likely to contribute to the reduced threshold for SOICR, the high incidence of DAD-associated VT, and the decreased SR Ca2+ content commonly observed in a number of cardiac settings. Therefore, normalizing the threshold for SOICR by controlling the activity of RyR2 offers a promising therapeutic strategy for the treatment not only of CPVT but also of VT in various cardiac conditions.
Supplementary Material
Acknowledgments
We thank Drs. Jonathan Lytton, Andrew Braun, and Henk ter Keurs for helpful discussions; Dr. J. Lytton for the use of the single-cell Ca2+ imaging facility; Jeff Bolstad for critical reading of the manuscript; and Cindy Brown for excellent technical assistance. D.J. and B.X. are recipients of the Alberta Heritage Foundation for Medical Research (AHFMR) Studentship Award, and S.R.W.C. is a Senior Scholar of the AHFMR. This work was supported by research grants from the Canadian Institutes of Health Research and the Heart and Stroke Foundation of Alberta, Northwest Territories, and Nunavut (to S.R.W.C.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: SOICR, store-overload-induced Ca2+ release; VT, ventricular tachycardia; HF, heart failure; DAD, delayed afterdepolarizations; RyR2, cardiac ryanodine receptor; RyR2(wt), WT RyR2; CASQ2, calsequestrin; CPVT, catecholaminergic polymorphic VT; SR, sarcoplasmic reticulum; CICR, Ca2+-induced Ca2+ release; Po, open probability; HEK, human embryonic kidney; [Ca2+]o, extracellular Ca2+ concentration.
References
- 1.Nuss, H. B., Kaab, S., Kass, D. A., Tomaselli, G. F. & Marban, E. (1999) Am. J. Physiol. 277, H80-H91. [DOI] [PubMed] [Google Scholar]
- 2.Zaugg, C. & Buser, P. (2001) Croat. Med. J. 42, 24-32. [PubMed] [Google Scholar]
- 3.Janse, M. (2004) Cardiovasc. Res. 61, 208-217. [DOI] [PubMed] [Google Scholar]
- 4.Pogwizd, S. M. & Bers, D. M. (2004) Trends Cardiovasc. Med. 14, 61-66. [DOI] [PubMed] [Google Scholar]
- 5.Leenhardt, A., Lucet, V., Denjoy, I., Grau, F., Ngoc, D. D. & Coumel, P. (1995) Circulation 91, 1512-1519. [DOI] [PubMed] [Google Scholar]
- 6.Priori, S. G., Napolitano, C., Memmi, M., Colombi, B., Drago, F., Gasparini, M., DeSimone, L., Coltorti, F., Bloise, R., Keegan, R., et al. (2002) Circulation 106, 69-74. [DOI] [PubMed] [Google Scholar]
- 7.Laitinen, P. J., Brown, K. M., Piippo, K., Swan, H., Devaney, J. M., Brahmbhatt, B., Donarum, E. A., Marino, M., Tiso, N., Viitasalo, M., et al. (2001) Circulation 103, 485-490. [DOI] [PubMed] [Google Scholar]
- 8.Tiso, N., Stephan, D. A., Nava, A., Bagattin, A., Devaney, J. M., Stanchi, F., Larderet, G., Brahmbhatt, B., Brown, K., Bauce, B., et al. (2001) Hum. Mol. Genet. 10, 189-194. [DOI] [PubMed] [Google Scholar]
- 9.Lahat, H., Pras, E., Olender, T., Avidan, N., Ben-Asher, E., Man, O., Levy-Nissenbaum, E., Khoury, A., Lorber, A., Goldman, B., et al. (2001) Am. J. Hum. Genet. 69, 1378-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Postma, A. V., Denjoy, I., Hoorntje, T. M., Lupoglazoff, J.-M., Da Costa, A., Sebillon, P., Mannens, M. M. A. M., Wilde, A. A. M. & Guicheney, P. (2002) Circ. Res. 91, 21e-26e. [DOI] [PubMed] [Google Scholar]
- 11.Franzini-Armstrong, C. & Protasi, F. (1997) Physiol. Rev. 77, 699-729. [DOI] [PubMed] [Google Scholar]
- 12.Fabiato, A. (1985) J. Gen. Physiol. 85, 247-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bers, D. M. (2001) Excitation-Contraction Coupling and Cardiac Contractile Force (Kluwer, Dordrecht, The Netherlands), 2nd Ed.
- 14.Kass, R. S. & Tsien, R. W. (1982) Biophys. J. 38, 259-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orchard, C., Eisner, D. & Allen, D. (1983) Nature 304, 735-738. [DOI] [PubMed] [Google Scholar]
- 16.Stern, M., Kort, A., Bhatnagar, G. & Lakatta, E. (1983) J. Gen. Physiol. 82, 119-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wier, W., Kort, A., Stern, M., Lakatta, E. & Marban, E. (1983) 80, 7367-7371. [DOI] [PMC free article] [PubMed]
- 18.Marban, E., Robinson, S. W. & Wier, W. G. (1986) J. Clin. Invest. 78, 1185-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lakatta, E. G. (1992) Cardiovasc. Res. 26, 193-214. [DOI] [PubMed] [Google Scholar]
- 20.Bers, D. M. (2002) Circ. Res. 90, 14-17. [PubMed] [Google Scholar]
- 21.Marks, A., Priori, S., Memmi, M., Kontula, K. & Laitinen, P. (2002) J. Cell Physiol. 190, 1-6. [DOI] [PubMed] [Google Scholar]
- 22.Li, P. & Chen, S. R. (2001) J. Gen. Physiol. 118, 33-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang, D., Xiao, B., Zhang, L. & Chen, S. R. (2002) Circ. Res. 91, 218-225. [DOI] [PubMed] [Google Scholar]
- 24.Diaz, M., Trafford, A., O'Neill, S. & Eisner, D. (1997) J. Physiol. 3-16. [DOI] [PMC free article] [PubMed]
- 25.Lakatta, E., Capogrossi, M., Kort, A. & Stern, M. (1985) Fed. Proc. 44, 2977-2983. [PubMed] [Google Scholar]
- 26.Trafford, A. W., Sibbring, G. C., Diaz, M. E. & Eisner, D. A. (2000) Cell Calcium 28, 269-276. [DOI] [PubMed] [Google Scholar]
- 27.Overend, C. L., O'Neill, S. C. & Eisner, D. A. (1998) J. Physiol. 507, 759-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sitsapesan, R. & Williams, A. (1995) J. Membr. Biol. 146, 133-144. [DOI] [PubMed] [Google Scholar]
- 29.Lukyanenko, V., Gyorke, I. & Gyorke, S. (1996) Pflügers Arch. 432, 1047-1054. [DOI] [PubMed] [Google Scholar]
- 30.Ikemoto, N. & Yamamoto, T. (2000) Trends Cardiovasc. Med. 10, 310-316. [DOI] [PubMed] [Google Scholar]
- 31.Wehrens, X., Lehnart, S., Huang, F., Vest, J., Reiken, S., Mohler, P., Sun, J., Guatimosim, S., Song, L., Rosemblit, N., et al.. (2003) Cell 113, 829-840. [DOI] [PubMed] [Google Scholar]
- 32.George, C. H., Higgs, G. V. & Lai, F. A. (2003) Circ. Res. 93, 531-540. [DOI] [PubMed] [Google Scholar]
- 33.Eisner, D. A., Trafford, A. W., Diaz, M. E., Overend, C. L. & O'Neill, S. C. (1998) Cardiovasc. Res. 38, 589-604. [DOI] [PubMed] [Google Scholar]
- 34.Trafford, A. W., Diaz, M. E., Sibbring, G. C. & Eisner, D. A. (2000) J. Physiol. 522 Pt 2, 259-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terentyev, D., Viatchenko-Karpinski, S., Gyorke, I., Volpe, P., Williams, S. C. & Gyorke, S. (2003) Proc. Natl. Acad. Sci. USA 100, 11759-11764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viatchenko-Karpinski, S., Terentyev, D., Gyorke, I., Terentyeva, R., Volpe, P., Priori, S. G., Napolitano, C., Nori, A., Williams, S. C. & Gyorke, S. (2004) Circ. Res. 94, 471-477. [DOI] [PubMed] [Google Scholar]
- 37.Vermeulen, J., McGuire, M., Opthof, T., Coronel, R., de Bakker, J., Klopping, C. & Janse, M. (1994) Cardiovasc. Res. 28, 1547-1554. [DOI] [PubMed] [Google Scholar]
- 38.Baartscheer, A., Schumacher, C., Belterman, C., Coronel, R. & Fiolet, J. (2003) Cardiovasc. Res. 58, 99-108. [DOI] [PubMed] [Google Scholar]
- 39.Shannon, T. R., Pogwizd, S. M. & Bers, D. M. (2003) Circ. Res. 93, 592-594. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.