Abstract
Previous studies demonstrated the presence of oxytocin (OT) and oxytocin receptors (OTRs) in the heart. The present work provides results supporting a potential role of OT in cardiomyogenesis. Here, we show a maximal OT and OTR protein level in the developing rat heart at day 21 of gestation and postnatal days 1-4, when cardiac myocytes are at a stage of intense hyperplasia. Between postnatal days 1 and 66, OT decreased linearly in all heart chambers (4.1- to 6.6-fold). Correspondingly, immunocytochemistry demonstrated that OTRs, which were eminent in postnatal cardiomyocytes, declined with age to low levels in adults. Interestingly, in coronary vasculature, OTRs developed in endothelial cells at postnatal days 12 and 22 and achieved a plateau in adult rats. These findings suggest that OT can be involved in developmental formation of the coronary vessels. In vivo, the OT/OTR system in the fetal heart was sensitive to the actions of retinoic acid (RA), recognized as a major cardiac morphogen. RA treatment produced a significant increase (2- to 3-fold) both in the OT concentration and in the OT mRNA levels. Ex vivo, an OT antagonist inhibited RA-mediated cardiomyocyte differentiation of P19 embryonic stem cells. The decline of cardiac OT expression from infancy to adulthood of the rat and changes in cell types expressing OTR indicate a dynamic regulation of the OT system in the heart rather than constitutive expression. The results support the hypothesis that RA induces cardiomyogenesis by activation of the cardiac OT system.
Keywords: heart development, oxytocin receptors, retinoic acid, oxytocin antagonist, cardiomyocyte differentiation
Oxytocin (OT), recognized traditionally as a reproductive hormone with a major role in childbirth and lactation, is produced in high concentrations in the hypothalamic supraoptic nucleus and paraventricular nucleus, then transported from these source nuclei to the posterior pituitary by neurosecretion (1). Longitudinal studies of neural and hormonal circuits activated by experimental volume expansion have identified OT as a major regulator of cardiovascular functions (reviewed in refs. 2 and 3). OT, injected peripherally, causes a decrease of arterial pressure (4) while reducing both heart rate and the force of contractions in isolated atria (5). OT acts via neuroendocrine/endocrine/paracrine pathways to release atrial natriuretic peptide (ANP) from the heart (6, 7). ANP is a potent diuretic, natriuretic, and vasorelaxant hormone that is also involved in cell growth regulation. In addition, we have demonstrated that in isolated, perfused hearts, an OT antagonist (OTA) blocks basal ANP release (7), suggesting the presence of local OT in the heart. Further study revealed cardiac OT synthesis (8), with OT being detected in the medium of cultured neonatal rat cardiomyocytes (9).
Recently, we showed that P19 embryonic carcinoma cells, a model of mouse embryonic stem cells, express OT receptors (OTRs), and OT stimulates the differentiation of these cells into beating cell colonies expressing ANP mRNA and cardiomyocyte-specific markers (10). Other substances reported to induce cardiomyogenesis in the P19 cell model are DMSO, retinoic acid (RA), 3,3′,5 triiodo-L-thyronine, and dynorphin (reviewed in ref. 11). The importance of OT was demonstrated when OTA completely inhibited the formation of beating cells, not only in OT- but also in DMSO-induced culture (10). Another unique feature of OT is that, in addition to differentiation of embryonic P19 cells, this peptide can induce the cardiomyogenic differentiation of adult stem/progenitor cells (12). Recently, it was reported that Sca-1-positive cells (stem cell antigen) isolated from the adult mouse heart, when treated with OT, express genes of cardiac transcription factors and contractile proteins and display sarcomeric structure and spontaneous beating (12). Ultimately, though, it has to be established whether in vivo OT regulates cardiomyocyte differentiation of embryonal or somatic stem cells. However, the data are sparse on any role for OT during development, and in this respect, nothing is known about the OT system in the heart and vasculature. For these reasons, elucidation of the role of the OT/OTR system and its upstream and downstream signaling pathways needs to be pursued. The OT gene contains response elements for RA (13). These elements are functional because the OT uterine gene responds to RA treatments in vivo and ex vivo in cultured cells (14). OT expression, therefore, might be downstream of RA-induced P19 cardiac differentiation as well. Consequently, in our present studies, we investigated whether the OT system is altered in the developing heart, similarly to other genes involved in cardiomyocyte differentiation (reviewed in ref. 15), such as transforming growth factor β1 (16), insulin-like growth factor 1 (17), and erythropoietin (18). Furthermore, we examined the hypothesis that the OT system acts in concert with RA during heart development in vivo, and ex vivo in the P19 cardiomyocyte differentiation model.
Materials and Methods
Animals. Experiments were performed in accordance with guidelines of the Canadian Council on Animal Care with the approval of the Ethics Committee of the Centre Hospitalier de l'Université de Montréal. Pregnant and nonpregnant female and male Sprague-Dawley rats were obtained from Charles River Breeding Laboratories along with male and nonpregnant female rats at postnatal day (PD) 66. After delivery, 1-, 4-, and 22-day-old pups were killed by decapitation, and their hearts were rapidly excised. To examine the response of the fetal cardiac OT system to RA, pregnant rats at day 21 of gestation (E21, embryonic day 21) were injected (i.p.) with 5 mg of RA and killed 7 h later to isolate the fetal heart.
RIA. OT was extracted from heart tissue with heat-activated Vycor glass beads (no. 7930 mesh 140, Corning). The RIA was developed in our laboratory by using specific antibodies generated against OT nonapeptide (8). Synthetic OT (Peninsula Laboratories) was labeled with Na125I by the lactoperoxidase method, and 200 μl of sample or standards (0-200 pg) was incubated with 100 μl of antibody (1:80,000) for 24 h at 4°C. Then, 100 μl of 125I-OT (3,000 cpm) was added to each tube and incubated for 48 h at 4°C. Antibody-bound radioactivity was separated from free radioactivity by adding 1 ml of dextran-coated charcoal and centrifuged in 4°C at 1,000 × g for 45 min. The radioactivity in the supernatant was then measured in a γ counter. Standard curves generated with 125I-OT demonstrated a sensitivity of 0.1 pg, linearity in the range of 0.25-200 pg/ml, and inter- and intraassay coefficients of variation of 17.7% and 6.8%, respectively. The cross-reactivity of the antibody was <1% with arginine-vasopressin and vasotocin.
Immunocytochemistry (ICC). The tissues of rats at PDs 6, 12, 22, and 66 were fixed by heart perfusion with 4% formaldehyde and 0.1% picric acid in 0.1 M phosphate buffer, pH 7.4 (19), embedded in wax, and cut into 5-μm sections. For comparison, embryonic, postnatal, and adult rat tissues were fixed by immersion in the same fixative solution and treated in the same way. Before ICC, OTR antigenic sites were retrieved by immersing the sections in 0.1 M citrate buffer, pH 6.0, heated to 90°C for 20 min, and allowed to cool slowly to room temperature. Goat anti-OTR (no. Sc-8102, Santa Cruz Biotechnology) or rabbit anti-ANP antibodies diluted 1/300 in blocking solution (no. 00-8020, Zymed) were revealed by either the biotin-streptavidin method (no. 95-999, Histostain-DS, Zymed) or with rabbit anti-goat IgG-horseradish peroxidase (no. A 5420, Sigma-Aldrich) as reported in ref. 19. Controls obtained by the omission of primary antibodies were negative, emphasizing the specificity of ICC.
Autoradiography. Iodinated [d(CH2)5,1Tyr(Me)2,Thr4,Orn8,Tyr-NH2]-vasotocin (Peninsula Laboratories), a highly specific OTA, served as a ligand in binding experiments (7). The binding densities were measured by PhosphorImager software in four sections for total binding and four sections in the presence of 10-6 to 10-12 M OTA or OT, respectively. The binding values in the presence of each unlabeled peptide were averaged and subtracted from total binding for specific binding values.
Culture, Differentiation, and Staining of P19 Cells. P19 cells were propagated and differentiated according to the procedures of Rudnicki and McBurney (20), with minor modifications (10). Briefly, 0.25 × 106 cells were allowed to aggregate for 4 days in nonadhesive, bacteriological grade Petri dishes (6-cm diameter) containing 5 ml of complete medium supplemented with 10-7 M OT or RA (10-7 M and 10-8 M) in the presence or absence of 10-7 M OTA. On day 4, the aggregates were transferred to tissue culture grade vessels (10-cm-diameter dishes or 24-well plates) and cultured in complete medium. The cell populations were analyzed on days 10-14 of the differentiation protocol for beating cell colonies with a Zeiss inverted microscope (Zeiss IM) equipped with phase-contrast objectives and an MC 100 camera. For staining with rhodamine123 (Sigma), day-4 aggregates were distributed in 24-well culture plates and grown until day 8. Then, dye was added to the culture medium at a final concentration of 1 μg/ml for 45 min, after which the cells were washed extensively with PBS and cultured for 48 h without the dye. The dye retained by cells in each well was measured with a fluorescence micro-plate reader (SPECTRA Max Gemini, Molecular Devices) at 505 nm for excitation and 534 nm for emission.
RT-PCR and Northern Blotting. Total cellular RNA was extracted with TRIzol Reagent (Invitrogen). First-strand cDNA was synthesized in a final volume of 40 μl containing first-strand buffer, 3 μg of cellular RNA, 4 μl of hexanucleotide primers (Amersham Pharmacia), and avian myeloblastosis virus reverse transcriptase (12 units/μg of RNA, Invitrogen). The first-strand cDNA (5 μl) was then used for PCR amplification with OT, OTR, or GAPDH exon-specific oligonucleotide primers in a Robocycler Gradient 40 thermocycler (Stratagene). Conditions for RT-PCR analysis of GAPDH, rat OT, and OTR transcripts (7, 8) and mouse OTR (10) have already been described. OTR expression also was investigated in the human heart by using cDNA of Human Cardiovascular System MTC Panel (no. 636749, Clontech). OTR forward primer 5′-1470 (bp 1470-1489) and reverse primer 3′-1860 (bp 1860-1841) were designed from the sequence of human OTR, GenBank accession no. NM-00096. For all PCR studies, the number of cycles used was within the linear range of amplification.
The retinal dehydrogenase 1 gene expression was analyzed by Northern blotting in total RNA from the rat fetal heart and kidneys (E21) as described in ref. 21.
Western Blot Analysis. Aliquots (20 μg of protein) were subjected to SDS/PAGE under reducing conditions, followed by electro-transfer onto pure nitrocellulose membrane (Hybond-C, Amersham Pharmacia). Molecular size calibration was achieved with Broad Standard Solution (Bio-Rad). The nitrocellulose blots were blocked overnight with 5% nonfat milk in TBS (20 mM Tris·Cl, pH 8.0/140 mM NaCl/1% BSA/0.1% Tween 20), then probed with goat anti-OTR antibody (1:1,000) for 2 h at room temperature. Antibody incubations and washes were performed in TBS throughout. Detection was realized by enhanced chemiluminescence with an Amersham-Pharmacia ECL kit and an appropriate peroxidase-conjugated secondary antibody (10, 19).
Statistics. The data are expressed as means ± SEM of a minimum of five different subjects. Statistical comparisons were made by using prism 3 (GraphPad Software, San Diego). Data from the RIA studies were analyzed by one-way ANOVA. Differences were assessed by the Newman-Keuls multiple comparison test and examined with the linear trend formula. Comparisons between two groups were done by unpaired Student's t test.
Results
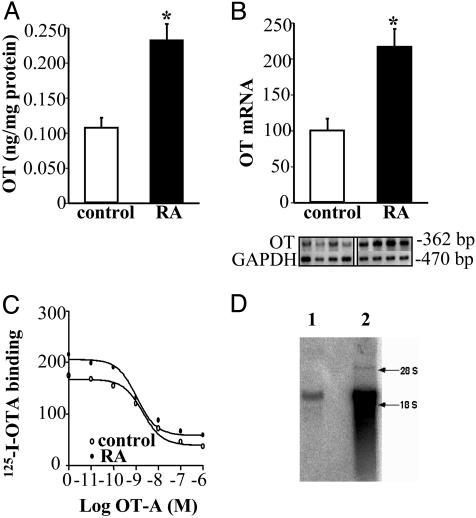
Identification and Regulation of the OT System in the Fetal Rat Heart. Using a specific antibody that selectively recognizes the amidated form of OT, RIA revealed that the heart produces biologically active OT at least at the end of fetal life (preterm, E21). At this time, total cardiac OT content (105.0 ± 4.9 pg) was 1.6 times lower than that in the whole hypothalamus (168.5 ± 0.5 pg, n = 8; P < 0.01). Seven hours after injection with RA to pregnant rats (5 mg s.c.), the cardiac OT concentration increased in the fetus (E21, 0.11 ± 0.02 vs. 0.23 ± 0.03 ng/mg of protein, P < 0.01, Fig. 1A) of pregnant rats, indicating dynamic OT regulation in the fetal heart. The detection of OT transcript by RT-PCR analysis pointed to the fetal heart as the site of OT synthesis. This synthesis was regulated in the fetal heart at the transcriptional level because OT mRNA expression levels increased 2.6-fold (P < 0.05, Fig. 1B) after RA treatment. RA-stimulated cardiac OT can induce autocrine/paracrine effects because of OT-binding sites present in fetal cardiac sections. This finding is illustrated by quantitative autoradiography with 125I-OTA bound to fetal heart sections and dose-dependently inhibited by unlabeled OTA (Fig. 1C). Analysis of the competition binding curves revealed a single class of high-affinity binding sites in the fetal rat heart (Kd = 1.05 nM), but the difference of 125I-OTA binding in fetal heart sections from RA-treated and control rats was not significant. Northern blot analysis of total RNA from the fetal heart showed the presence of retinal dehydrogenase type 1 transcripts (Fig. 1D), indicating that it has enzymatic machinery to synthesize RA and suggesting that endogenous RA can regulate heart morphogenesis by changes of OT gene expression during development.
Fig. 1.
RA activates OT systems in the fetal heart (E21). OT concentration was measured by RIA (A, n = 11; *, P < 0.05) and OT mRNA by semiquantitative RT-PCR (B, n = 8; *, P < 0.05). (C) 125I-OTA binding to the fetal sections demonstrated by autoradiography. The representative competition curve of 125I-OTA binding to the fetal heart sections by unlabeled OTA is shown. (D) Retinal dehydrogenase type 1 mRNA detected by Northern blotting in the fetal heart (lane 1) and kidney (lane 2).
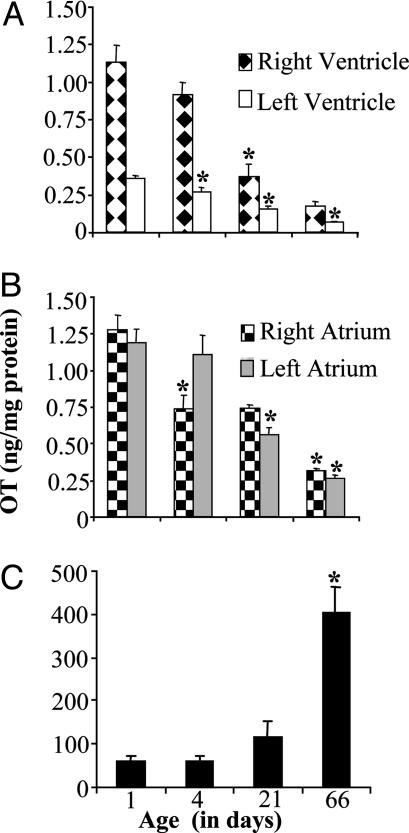
Dynamic Regulation of the OT System in Rats During Postnatal Development. OT concentration in the rat heart was studied from PD 1 to PD 66, when the rats were able to reproduce. The results of OT RIA in dissected heart chambers are shown in Fig. 2 A and B. In rats at PD 1, OT concentration was not different in the right ventricle (1.12 ± 0.12 ng/mg of protein), right atrium (1.28 ± 0.10 ng/mg of protein), and left atrium (1.12 ± 0.10 ng/mg of protein), but was significantly higher than in the left ventricle (0.35 ± 0.02 ng/mg of protein, P < 0.001). Data processing by trend analysis indicated that from PD 1 to PD 66, OT decreased linearly in the right ventricle (6.6-fold; R = 0.63; P < 0.0001), right atrium (4.1-fold; R = 0.76; P < 0.0001), left atrium (4.6-fold; R = 0.68; P < 0.0001), and left ventricle (5.4-fold; R = 0.038; P = 0.017). In effect, rats at PD 66 had similar OT concentrations in the right atrium (0.31 ± 0.02 ng/mg of protein) and left atrium (0.26 ± 0.02 ng/mg of protein), lower in the right ventricle (0.18 ± 0.03 ng/mg of protein, P < 0.01 and P < 0.05 vs. right atrium and left atrium, respectively), and lowest in the left ventricle (0.07 ± 0.01 ng/mg of protein; P < 0.001 vs. all other chambers). As shown in Fig. 1C, OT in the hypothalamus was regulated in the opposite direction from the heart and increased linearly from PD 1 to PD 66 (6.7-fold; R = 0.53; P < 0.0001).
Fig. 2.
Changes in OT concentration during rat postnatal development measured by RIA in the heart chambers (A, atria; B, ventricles; n = 6-12; *, P < 0.05, indicating increase vs. preceding age group) and hypothalamus (C; n = 6-8; *, P < 0.05).
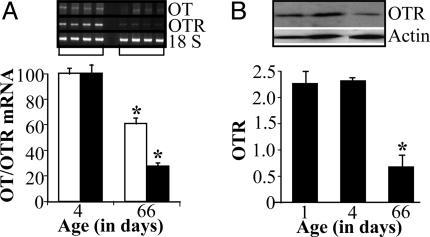
As seen in Fig. 3, OT down-regulation is displayed at the transcript level by a significant reduction of OT mRNA from PD 1 to PD 66 in the left atrium. This OT decrease in the heart was associated with OTR mRNA down-regulation (Fig. 3). A similar regulation was observed in the right atrium and ventricles (data not shown). OTR mRNA down-regulation was effectively transmitted to the protein level, as demonstrated by specific antibody binding to OTR by Western blot analysis (Fig. 3B).
Fig. 3.
Atrial OT and OTR during rat maturation. (A) RT-PCR for OT and OTR mRNA in the left atria of newborn (PD 4) and adult (PD 66) rats, n = 6; *, P < 0.05. (B) Corresponding Western blot analysis of OTR expression in the left atrium, n = 4; *, P < 0.05.
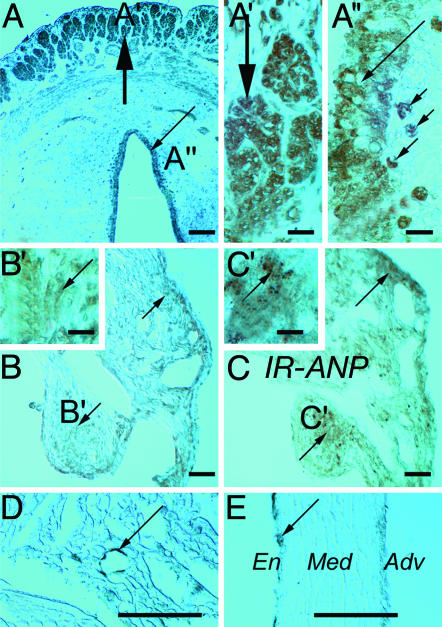
OTR Localization in the Maturing Rat Heart. As a positive standard, OTR immunoreactivity was first detected in the uterus and fallopian tube (Fig. 4A), where smooth muscle cells (Fig. 4A′), epithelial cells, and stroma macrophages were positively stained (Fig. 4A″). A stronger diffused reaction was noted at PD 6, especially in the atrial (Fig. 4 B and B′), but less in ventricular cardiomyocytes. OTR density was similar to ANP regional distribution in the heart, with the highest values in the atria (Fig. 4 C and C′) and much lower values in the ventricles. In adult atria, a relatively weak OTR immunoreaction was found in cardiomyocytes (Fig. 4D) compared with intensive OTR labeling of the coronary vasculature (long arrow in Fig. 4D). Consistent with previous observations in the rat aorta (19), OTR immunostaining was detected in the endothelium of coronary arteries (Fig. 4E). Interestingly, at PD 6, newborn rat endothelial cells appeared unstained, but 30-50% of them were OTR-positive on PD 12 and PD 22.
Fig. 4.
ICC staining. (A-A″) OTR ICC staining (arrows) in adult rat fallopian tubes at low (A) and higher (A′ and A″) magnifications. Heavy arrows, smooth muscles; long thin arrows, epithelial cells; small arrows, stroma macrophages. OTR ICC sites (arrows) in atrial cardiomyocytes at low (B) and high (B′) magnification of a 6-day-old rat heart. For comparison, ANP immunoreactivity (IR-ANP) is shown at low (C) and higher (C′) magnification of adjacent section. Adult rat atrium OTR ICC sites (arrows) are shown in D, and coronary artery is in E. Long arrows, endothelial cells; Adv, tunica adventicia; En, endothelial cells; Med, tunica media. (×40 in A-C; ×405 in A′, A″, B′, and C′; ×160 in D and E. Bar = 100 μm in A-E; bar = 10 μm in A′, A″, B′, and C′.)
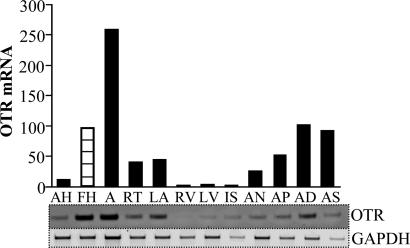
OTR Expression in the Human Heart. We investigated whether human hearts express OTR mRNA and whether the changes of the OT system in cardiac development are limited to rodents. The RT-PCR analysis of cDNA from the human heart clearly demonstrated elevated OTR transcripts in the fetal heart compared with corresponding samples of adult heart (Fig. 5). In the adult human heart, highest OTR expression was in the right auricle and left auricle, sites rich in ANP, supporting our hypothesis (based on morphological findings) that the OT system works in the heart by stimulation of ANP release (7).
Fig. 5.
OTR mRNA expression in human adult heart (AH) and fetal heart (FH) detected by RT-PCR. The studies were performed in cDNA samples from human hearts (Human Cardiovascular System MTC Panel no. 636749, Clontech). (Lower) Representative PhoshorImager-scanned bands of PCR products amplified with specific primers for OTR and GAPDH used as internal standard. (Upper) The mean OTR value from three independent amplifications after adjusting the GAPDH value. A, aorta; RT, right atrium; LA, left atrium; RV, right ventricle; LV, left ventricle; IS, interventricular septum; AN, atrioventricular node; AP, apex of the heart; AD, right auricle; AS, left auricle.
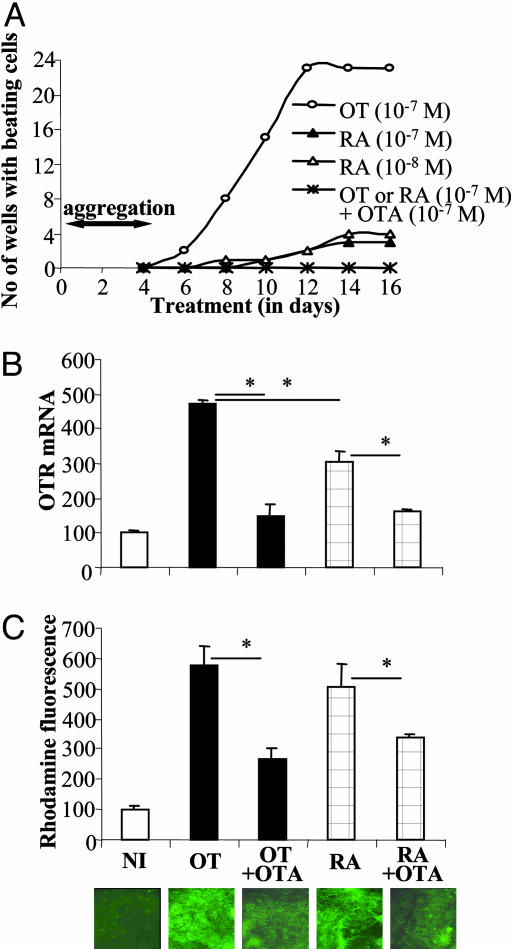
OT Influence on RA-Mediated Cardiomyocyte Differentiation of P19 Cells. To identify the functionality of RA-induced effects on the OT system in the developing heart, we performed experiments of cardiomyocyte differentiation. As presented in Fig. 6A, RA at 10-8 and 10-7 M concentrations induced cardiomyogenesis of P19 cells. RA, however, had relatively lower potency (4 times lower) compared with 10-7 M OT. RA-mediated cardiomyocyte differentiation of P19 cells was inhibited by the presence of 10-7 M OTA. OT treatment of P19 cells resulted in significantly higher OTR expression compared with RA treatment (Fig. 6B). Because both OT- and RA-induced increases in OTR mRNA expression were neutralized in the presence of OTA, these observations suggest that the level of OTR activation is a critical factor in the strength of cardiomyogenesis in P19 cells. As illustrated in Fig. 6A, treatments with both RA and OT similarly induced rhodamine123 retention in differentiated P19 cells, indicating increased mitochondrial activities. This effect was abolished when these inducers were combined with OTA.
Fig. 6.
Cardiomyogenic effect of OT and RA in P19 cells is suppressed by OTA. (A) Time course of appearance of beating cell colonies upon treatment with OT and RA in presence and absence of OTA. The number of wells containing beating cell colonies was calculated in a 24-well tissue culture plate at 2-day intervals. The results are means of three independent differentiation experiments. (B) RT-PCR analysis of OTR gene transcript in undifferentiated and induced cultures. Cell aggregates were exposed to OT (10-7 M) or RA (10-7 M) in the absence or presence of OTA (10-7 M) from day 0 to day 4, and RNA was extracted at day 14 of the differentiation protocol. OTR transcript also was evaluated in undifferentiated cells grown in monolayers (NI). Levels of OTR mRNA were adjusted by dividing by corresponding GAPDH mRNA and then expressed as the percentage of the NI value. Results are the means ± SEM of five independent studies (*, P < 0.05). (C) Monitoring of P19 cell differentiation into cardiomyocytes by using selective vital staining with rhodamine123 in noninduced and OT- and RA-induced cultures. On day 8, the cells were stained for 45 min with rhodamine123 (1 μg/ml), washed, and again cultured without dye for 48 h. Then, the retained dye was fluorimetrically quantified for each well, and the results are reported as the means ± SEM of 24 determinations (*, P < 0.05).
Discussion
In support of the action of OT in cardiomyogenesis, we found elevated OT and OTR protein levels in the fetal heart at E21 and PDs 1-4, when cardiac myocytes are at a stage of intense hyperplasia. ICC revealed that in the rat heart at PD 6, OTRs were localized predominantly in atrial cardiomyocytes, but in the adult rat, intense OTR staining was observed in the endothelium of coronary arteries. OT transcripts in the fetal heart are sensitive to the stimulatory actions of RA, recognized as a major cardiac morphogen. Because OTA inhibited RA-mediated cardiomyocyte differentiation of P19 embryonic stem cells, the results support the hypothesis that RA induces cardiomyogenesis by activation of the cardiac OT system.
The importance of the OT system during rat development is validated by the observation that OT and OTR are expressed in the fetal rat heart and decreased to relatively low levels in adulthood. In rats, starting from E18, in situ hybridization studies localized OT neurons in the dorsal portion of the supraoptic nucleus of the hypothalamus, whereas its ventricular portion is occupied by arginine vasopressin neurons. Fewer OT-labeled cells were found in the developing paraventricular nucleus (22). OT-specific binding sites were detected first in the brain at E14 and before OT expression (23). Even after OT is expressed in the fetal rat brain, OT peptide precursor is not completely processed to OT nanopeptide until after birth (24). Our data suggest that cardiac OT is functional in fetal life, which contrasts with low OT synthesis (25), and the low concentration of biologically active OT in the hypothalamus, as we are reporting here. It might be speculated that OT production in the heart during fetal life and early maturation can supplement low OT production in hypothalamic nuclei. This hypothesis is indicated by high OT plasma level in newborn rats exceeding those in adult rats (26).
Identification of OTR in the cardiomyocytes of newborn rats supports the hypothesis that OT plays a role in the development of these cells. However, under our experimental conditions, we failed to demonstrate immunoreactive OTR in the fetal heart, despite evidence of OT binding sites in the fetal cardiac sections. It should be noted that OTR immunoreaction in cardiomyocytes is fixation-sensitive, and our attempts to detect immunoreactive OTR in the embryonic and early postnatal stages in immersion-fixed hearts were unsuccessful. We cannot provide results from perfused fetal cardiac tissue, but we can state with certainty that OTR immunoreaction, which was eminent in postnatal heart cardiomyocytes, declines with age to low levels in adults. Interestingly, in coronary vasculature, OTRs develop in endothelial cells postnatally, stained evidently at PDs 12 and 22, achieving a plateau in adult rats. These findings suggest that OT can be involved in developmental formation of the coronary vessels. Supporting this hypothesis, Thibonnier et al. (27) have reported that OT stimulates proliferation of cultured endothelial cells.
OT differentiation capacity indicates its influence on cellular growth. Indeed, the antiproliferative effect of OT, mediated by OTR, has been documented in breast cancer cells (28) and other tumors (29, 30). In contrast to its action on tumoral cells, a mitogenic action of OT by phosphorylation and activation of mitogen-activated protein kinase has been described in myometrium (31). OT stimulates the proliferation of thymocytes (32, 33), the prostate epithelium (34), and trophoblasts (35). OT has also been reported to enhance myoepithelial cell differentiation and proliferation in the mouse mammary gland (36). OT increases the rate of myoblast fusion and myotubule formation (37). Interestingly, most transcription factors identified so far in the heart are also present in other muscle cells, and myoblast transplantation to the injured heart improves regional systolic heart function (38). Assuming similarities in the differentiation mechanisms of skeletal and cardiac muscles and the expression (15) of both OT and OTR in these cells (8, 37), we can speculate that OT plays a role in cardiac muscle regeneration.
The P19 mouse embryonal carcinoma cell line gives rise to the formation of cell derivatives of all three germ layers and seems to differentiate by the same mechanisms as normal embryonic stem cells (11). The cardiomyogenic capacity of RA suggests that transcription factors of the steroid-thyroid-RA superfamily could be involved in the cardiac differentiation of P19 cells (39). RA, the active form of vitamin A (retinol), regulates vertebrate growth and development. RA receptors mediate RA-induced expression of genes with roles in cell differentiation. Likewise, cardiac malformations are observed in the developing heart of vitamin A-deficient rats, and these defects can be prevented by vitamin A administration to pregnant rats at specific times during gestation (40). Excess RA also can cause heart defects, and malformations of cardiac structures are often formed in infants whose mothers took the vitamin-A analogue 13-cis-RA during the first week of pregnancy (41). Experiments have shown also the ability of 3,3′,5-triiodo-L-thyronine (T3) to induce cardiomyogenesis (42) by specific thyroid receptor, influencing gene expression of P19 cells (43). We have evidence that this reaction is inhibited by OTA. However, the mechanisms responsible for triggering cardiomyogenic genes by RA and T3 in the embryonal cells are still unknown, as is the mode of OT action with respect to the cardiomyogenic program in P19 cells.
Evidence that OT plays a physiological role in fetal life and the early postnatal period is sparse and controversial. Exogenously induced OT elevation has been reported to impair the development of rat offspring at birth and during postnatal stages (44). In this context, OT has been shown to influence the developing heart; OT administered in excess to the fetus may inhibit cardiac growth in humans and rats (45, 46). Conversely, OTR suppression by OTA in the early stage of chicken egg development leads to cardiac malformation in the embryos, which suggests the importance of OT in the cardiac development of birds.¶ More recent findings indicate that early postnatal OT administration in the rat has lifelong effects, resulting in increased body weight, body fatness in adulthood, and, interestingly, a lifelong decline of arterial blood pressure (47).
In summary, the present study demonstrates that the OT system in the heart is up-regulated in the fetus at term and in the early postnatal period and is sensitive to RA actions during fetal life. Correspondingly, the RA effect leading to differentiation of P19 cells into a cardiomyocyte phenotype is mediated by the OT system. Assuming that the OT system is functional in somatic stem cell precursors capable of differentiating into cardiomyocytes, we suggest that OT has potential in cardiomorphogenesis or cardioregenerative activities.
Acknowledgments
We acknowledge the editorial work of Mr. Ovid Da Silva and the secretarial assistance of Mrs. Antoinette Paolitto. This work was supported by Canadian Institutes of Health Research and Canadian Heart and Stroke Foundation Grants MOP-53217 (to J.G. and M.J.), NET SRD-63193 (to J.G., M.J., and J.P.), and MOP 62926 (to P.B.) and National Institutes of Health Grant MH-51853 (to S.M.M.).
Abbreviations: OT, oxytocin; OTR, OT receptor; RA, retinoic acid; ANP, atrial natriuretic peptide; OTA, OT antagonist; En, embryonic day n; ICC, immunocytochemistry; PD n, postnatal day n.
Footnotes
Widmer, H., Durroux, T., Kempf, H., Mouillac, B., Gasc, J. M. & Barberis, C., Abstracts of 1999 World Congress on Neurohypophysial Hormones, Aug. 28-Sept. 6, 1999, Edinburgh, p. 94 (abstr.).
References
- 1.Gimpl, G. & Fahrenholz, F. (2001) Physiol. Rev. 81, 629-683. [DOI] [PubMed] [Google Scholar]
- 2.McCann, S. M., Antunes-Rodrigues, J., Jankowski, M. & Gutkowska, J. (2002) Prog. Brain Res. 130, 309-328. [DOI] [PubMed] [Google Scholar]
- 3.Antunes-Rodrigues, J., de Castro., M, Elias, L. L., Valenca, M. M. & McCann, S. M. (2004) Physiol. Rev. 8, 169-208. [DOI] [PubMed] [Google Scholar]
- 4.Petersson, M., Alster P., Lundberg, T. & Uvnas-Moberg, K. (1996) Physiol. Behav. 60, 1311-1315. [DOI] [PubMed] [Google Scholar]
- 5.Favaretto, A. L., Ballejo, G. O., Albuquerque-Araujo, W. I., Gutkowska, J., Antunes-Rodrigues, J. & McCann, S. M. (1997) Peptides 18, 1377-1381. [DOI] [PubMed] [Google Scholar]
- 6.Haanwinckel, M. A., Elias, L. K., Favaretto, A. L., Gutkowska, J., McCann, S. M. & Antunes-Rodrigues, J. (1995) Proc. Natl. Acad. Sci. USA 92, 7902-7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutkowska, J., Jankowski, M., Lambert, C., Mukaddam-Daher, S., Zingg, H. H. & McCann, S. M. (1997) Proc. Natl. Acad. Sci. USA 94, 11704-11709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jankowski, M., Hajjar, F., Al Kawas, S., Mukaddam-Daher, S., Hoffman, G., McCann, S. M. & Gutkowska, J. (1998) Proc. Natl. Acad. Sci. USA 95, 14558-14563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gutkowska, J., Jankowski, M., Mukaddam-Daher, S. & McCann, S. M. (2000) Braz. J. Med. Biol. Res. 33, 625-633. [DOI] [PubMed] [Google Scholar]
- 10.Paquin, J., Danalache, B. A., Jankowski, M., McCann, S. M. & Gutkowska, J. (2002) Proc. Natl. Acad. Sci. USA 99, 9550-9555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Heyden, M. A. G. & Defize, L. H. K. (2003) Cardiovasc. Res. 58, 292-302. [DOI] [PubMed] [Google Scholar]
- 12.Matsuura, K., Nagai, T., Nishigaki, N., Oyama, T., Nishi, J., Wada, H., Sano, M., Toko, H., Akazawa, H., Sato, T., et al. (2004) J. Biol. Chem. 279, 11384-11391. [DOI] [PubMed] [Google Scholar]
- 13.Richard, S. & Zingg, H. H. (1991) J. Biol. Chem. 266, 21428-21433. [PubMed] [Google Scholar]
- 14.Larcher, A., Neculcea, J., Chu, K. & Zingg, H. H. (1995) Mol. Cell. Endocrinol. 114, 69-76. [DOI] [PubMed] [Google Scholar]
- 15.Heng, B. C., Haider, H. K., Sim, E. K-W., Cao, T. & Ng, S. C. (2004) Cardiovasc. Res. 62, 34-42. [DOI] [PubMed] [Google Scholar]
- 16.Engelmann, G. L., Boehm, K. D., Birchenall-Roberts, M. C. & Rusetti, F. W. (1992) Mech. Dev. 38, 85-97. [DOI] [PubMed] [Google Scholar]
- 17.Reiss, K., Cheng, W., Ferber, A., Kajstura, J., Li, P., Li. B., Olivetti, G., Homcy, C. J., Baserega, R. & Anversa, P. (1996) Proc. Natl. Acad. Sci. USA 93, 8630-8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu, X., Lin, C.-S., Costantini, F. & Noguchi, T. (2001) Blood 98, 475-477. [DOI] [PubMed] [Google Scholar]
- 19.Wang, D., Marcinkiewicz, M., Rachelska, G., Gutkowska, J. & Jankowski, M. (2003) Cardiovasc. Res. 57, 186-194. [DOI] [PubMed] [Google Scholar]
- 20.Rudnicki, M. A. & McBurney, M. W. (1987) in Teratocarcinomas and Embryonic Stem Cells: A Practical Approach, ed. Robertson, E. J. (IRL, Oxford), pp. 19-49.
- 21.Labrecque, J., Dumas, F., Lacroix, A. & Bhat, P. V. (1995) Biochem. J. 305, 681-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trembleau, A., Ugrumov, M., Roche, D. & Calas, A. (1995) Brain Res. Bull. 37, 437-448. [DOI] [PubMed] [Google Scholar]
- 23.Tribollet, E., Charpak, S., Schmidt, A., Dubois-Dauphin, M. & Dreifuss, J. J. (1989) J. Neurosci. 9, 1764-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alstein, M., Whitnall, M. H., House, S., Key, S. & Gainer, H. (1988) Peptides 9, 87-105. [DOI] [PubMed] [Google Scholar]
- 25.Almazan, G., Lefebvre, D. L. & Zingg, H. H. (1989) Dev. Brain Res. 45, 69-75. [DOI] [PubMed] [Google Scholar]
- 26.Hartman, R. D., Rosella-Dampman, L. M., Emmert, S. E. & Summy-Long, J. Y. (1986) Endocrinology 119, 1-11. [DOI] [PubMed] [Google Scholar]
- 27.Thibonnier, M., Conarty, D. M., Preston, J. A., Plesnicher, C. L., Dweik, R. A. & Erzurum, S. C. (1999) Endocrinology 140, 1301-1309. [DOI] [PubMed] [Google Scholar]
- 28.Cassoni, P., Sapino, A., Fortunati, N., Munaron, L., Chini, B. & Bussolati, G. (1997) Int. J. Cancer 72, 340-344. [DOI] [PubMed] [Google Scholar]
- 29.Cassoni, P., Sapino, A., Stella, A., Fortunati, N. & Bussolati, G. (1998) Int. J. Cancer 77, 695-700. [DOI] [PubMed] [Google Scholar]
- 30.Cassoni, P., Fulcheri, E., Carcangiu, M. L., Stella, A., Deaglio, S. & Bussolati, G. (2000) J. Pathol. 190, 470-477. [DOI] [PubMed] [Google Scholar]
- 31.Ohmichi, M., Koike, K., Nohara, A., Kanda, Y., Sakamoto, Y., Zhang, Z. X., Hirota, K. & Miyake, A. (1995) Endocrinology 136, 2082-2087. [DOI] [PubMed] [Google Scholar]
- 32.Martens, H., Kecha, O., Charlet-Renard, C., Defresne, M. P. & Geenen, V. (1998) Neuroendocrinology 67, 282-289. [DOI] [PubMed] [Google Scholar]
- 33.Geenen, V., Kecha, O., Brilot, F., Charlet-Renard, C. & Martens, H. (1999) Neuroimmunomodulation 6, 115-125. [DOI] [PubMed] [Google Scholar]
- 34.Plecas, B., Popovic, A., Jovovic, D. & Hristic, M. (1992) J. Endocrinol. Invest. 15, 249-253. [DOI] [PubMed] [Google Scholar]
- 35.Cassoni, P., Sapino, A., Munaron, L., Deaglio, S., Chini, B., Graziani, A., Ahmed, A. & Bussolati, G. (2001) Endocrinology 142, 1130-1136. [DOI] [PubMed] [Google Scholar]
- 36.Sapino, A., Macri, L., Tonda, L. & Bussolati, G. (1993) Endocrinology 133, 838-842. [DOI] [PubMed] [Google Scholar]
- 37.Breton, C., Haenggeli, C., Barberis, C., Heitz, F., Bader, C. R., Bernheim, L. & Tribollet, E. (2002) J. Clin. Endocrinol. Metab. 87, 1415-1418. [DOI] [PubMed] [Google Scholar]
- 38.Thompson, R. B., Emani, S. M., Davis, B. H., van den Bos, E. J., Morimoto, Y., Craig, D., Glower, D. & Taylor, D. A. (2003) Circulation 108, Suppl. 1, 264-271. [DOI] [PubMed] [Google Scholar]
- 39.Edwards, M. K. S. & McBurney, M. W. (1983) Dev. Biol. 98, 187-191. [DOI] [PubMed] [Google Scholar]
- 40.Wilson, J. G., Roth, C. B. & Warkanay, J. (1953) Am. J. Anat. 92, 189-217. [DOI] [PubMed] [Google Scholar]
- 41.Lammer, E. J., Chen, D. T., Hoaer, R. M., Agnish, N. D., Benke P. J., Braun, J. T., Curry C. J., Fernhoff, P. M., Grix, A. W., Jr., & Lott, I. T. (1985) N. Engl. J. Med. 313, 837-841. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez, E. R., Tan, C. D., Onwuta, U. S., Yu, Z. X., Ferrans, V. J. & Parrillo, J. E. (1994) Biochem. Biophys. Res. Commun. 205, 652-658. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez, E. R., Tan, C. D., Onwuta, U. S. & Parrillo, J. E. (1994) Biochem. Biophys. Res. Commun. 205, 1899-1906. [DOI] [PubMed] [Google Scholar]
- 44.Boer, G. J. (1993) Neurotoxicol. Teratol. 15, 383-389. [DOI] [PubMed] [Google Scholar]
- 45.Chard, T., Boyd, N. R., Forsling, M. L., McNeilly, A. S. & Landon, J. (1970) J. Endocrinol. 48, 223-234. [DOI] [PubMed] [Google Scholar]
- 46.Schriefer, J. A., Lewis, P. R. & Miller, J. W. (1982) Biol. Reprod. 27, 362-368. [DOI] [PubMed] [Google Scholar]
- 47.Olausson, H., Uvnäs-Moberg, K. & Sohlström, A. (2003) Am. J. Physiol. 284, E475-E480. [DOI] [PubMed] [Google Scholar]