ABSTRACT
Neuroinvasive herpesviruses have evolved to efficiently infect and establish latency in neurons. The nervous system has limited capability to regenerate, so immune responses therein are carefully regulated to be nondestructive, with dependence on atypical intrinsic and innate defenses. In this article we review studies of some of these noncanonical defense pathways and how herpesvirus gene products counter them, highlighting the contributions that primary neuronal in vitro models have made to our understanding of this field.
KEYWORDS: herpesviruses, innate immunity, intrinsic immunity, neuroimmunology, neurotropic viruses
INTRODUCTION
In the infected host, virus infection results in two general patterns of infection, acute and persistent (1). Usually acute infection is resolved by the immune system, resulting in viral clearance from the host. The failure to clear an acute infection is often detrimental and may result in severe disease or even death of the host. Some viruses, however, establish persistent infections that are benign or may even benefit the host (2). This détente or balance between host and pathogen results from an effective stalemate between host immunity and viral countermeasures. The nervous system is key for the survival and procreation of the host, with limited capacity for repair and regeneration. It is critical, therefore, that nervous system immune responses be carefully regulated, avoiding massive inflammatory or cell-destructive responses. The nervous system is therefore highly dependent on innate responses to defend against virus infection (3). In turn, the herpesviruses are highly evolved in terms of their capacity to subvert host immunity and to establish lifelong quiescent infection (latency) that can be reactivated. Until relatively recently, the nervous system was thought to be an immune-privileged site. A great deal of recent work, however, has demonstrated that while responses may be muted relative to those in other tissues, there are robust responses in neurons that may not follow the canonical rules established in nonneuronal cells. The terminally differentiated nature and specialized anatomy of neurons has made for significant experimental difficulties in the study of their immunobiology. That said, these unique features of neurons render them fascinating and unusual subjects for study of immunity. In this review, we will focus on studies of neurotropic alphaherpesviruses in cultured primary neurons and how this body of work has elucidated the intrinsic and innate responses of neurons (Fig. 1) that lead to a unique lifelong host-pathogen relationship.
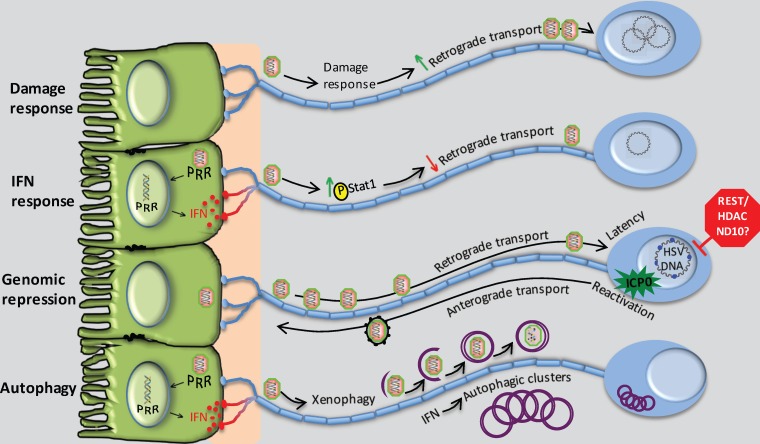
FIG 1.
Intrinsic and innate pathways that modulate alphaherpesvirus infection in peripheral neurons. (From the top) The damage response of neurons may be co-opted by the virus to promote the efficiency of retrograde transport to the cell body. Interferon synthesized by, for example, infected epithelial cells increases levels of phosphorylation of local axonal Stat1, serving to reduce retrograde transport, a response that may be countered by HSV γ34.5. Genomic repression of HSV mediated by intrinsic neuronal factors promotes the establishment and maintenance of latency, which is countered through expression of ICP0. Autophagy induced through IFN-dependent signaling leads to xenophagic clearance of HSV and formation of clusters of autophagosomes that may regulate the latency process.
VIRUS INFECTION OF THE NERVOUS SYSTEM: INTEGRATION OF FORM AND FUNCTION
Viral infections usually begin in peripheral tissues, and some may invade the nervous system, spreading into the peripheral nervous system (PNS) and the central nervous system (CNS). In general, most virus infections do not spread to the CNS because of effective immune responses and multilayer anatomical barriers. Some, however, do enter the nervous system via the bloodstream or by direct infection of nerves that innervate peripheral tissues, resulting in disease (4). Most viruses invading the nervous system are opportunistic or accidental pathogens, but some, most notably the alphaherpesviruses, such as herpes simplex virus 1 (HSV-1), varicella-zoster virus (VZV), and pseudorabies virus (PRV), and other viruses, such as rabies virus (RABV), can invade the nervous system efficiently from peripheral tissues. After entry into PNS axons, virus particles move long distances to the neuronal cell bodies by directed retrograde transport (5, 6). Remarkably, the alphaherpesviruses often establish latent PNS infections in their natural hosts after primary infection of epithelial surfaces (7, 8).
PNS and CNS neurons have specialized signaling and gene expression patterns that maintain the highly polarized morphology for optimal function. The single axons of PNS neurons can be long, containing more than 99% of the neuron's cytoplasm (9). The polarized PNS neurons require long-distance communication between the axon terminals that are in contact with peripheral tissues and the cell bodies in the distant ganglia (9). This axon-to-cell body communication must be finely tuned, so that cell bodies can respond to distant events in a timely fashion. That said, the threshold for lethal consequences such as apoptosis must be high, and the neuronal response to signals from distant axons must be rapid, to avoid loss of irreplaceable neurons.
AXONS SENSE VIRAL INFECTION BY LOCAL AND RAPID PRODUCTION OF NEW AXONAL PROTEINS
A variety of “danger or damage” receptors are well known (10, 11) (12). A subset of mRNAs and the complete protein synthesis machinery are localized to axons in uninfected neurons, far away from the cell body (13). Many of these mRNAs are repressed and can be translated when appropriate local signals are received, allowing for a rapid response (14–20). When axons are damaged, some of the newly made proteins are transported back to the cell bodies, where they stimulate gene expression to repair the damage (19–21). Efficient retrograde herpesvirus capsid transport in axons requires local newly made axonal proteins (20). The newly synthesized proteins in axons after virion entry were identified using bioorthogonal noncanonical amino acid tagging (BONCAT) or click chemistry followed by high-resolution tandem mass spectrometry. These nascent proteins include participants in cytoskeleton remodeling, intracellular trafficking, signaling, and energy metabolism pathways. It was found that axonal injury, when induced prior to viral infection, retards virus transport in these axons by competing for the fast axonal transport complexes (20). This led to the hypothesis that entering viral capsids induce the axonal damage response and quickly repurpose it for more efficient capsid transport to the nucleus.
AXONS HAVE A NONCANONICAL INTERFERON RESPONSE
Unlike epithelial cells, PNS neurons produce little type I interferon (IFN) after HSV-1 infection (22, 23). Nevertheless, prior to, or concurrent with, invasion by herpes virus virions, nerve terminals are bathed in a variety of inflammatory and antiviral cytokines, including type I IFN (IFN-α and IFN-β) and type III IFN (IFN-λ), produced by other surrounding infected cells (8). Previous work on human dorsal root ganglion (DRG) neurons in a chambered culture system showed that IFN-α and IFN-γ, added to the epidermal cell compartment after axonal transmission of HSV-1 infection, inhibited infection and spread (24), although the response of axons to IFN was not addressed. In contrast, other groups have found no antiviral effect against HSV-1 infection when neurites of dorsal root ganglion neurons were pretreated with IFN-α and IFN-β (25).
Recently, our labs simultaneously discovered that type I IFN (IFN-β) acts locally in axons to produce an antiviral effect (26) (27). Preexposure of axons to both type I and type II IFN significantly diminished the number of PRV particles actively moving in the retrograde direction and also reduced the number of cell bodies that were infected (26). In HSV it was shown that the γ34.5 gene likely plays a role in countering this IFN-driven restriction of retrograde trafficking (27). However, PRV does not encode a γ34.5 homolog. Antibodies blocking the type I IFN receptor restored PRV particle axonal transport in IFN-β-treated samples, indicating that IFN-β signaling is responsible for the axonal transport defect. Importantly, exposure of axons to IFN-β induced rapid and local phosphorylation of signal transducer and activator of transcription 1 (STAT1) in axons only. In contrast, axons treated with IFN-γ did not produce locally phosphorylated STAT1. Rather, axonal IFN-γ treatment appeared to transmit a retrograde signal to trigger phosphorylation and translocation of STAT1 (pSTAT1) to the nucleus. Importantly, blocking transcription in cell bodies had no effect on the reduction of viral transport by IFN-β treatment or on the appearance of pSTAT1 in axons. In contrast, inhibiting transcription in the cell bodies reversed the IFN-γ-induced reduction of transport. The activity of IFN-β to reduce particle axonal transport differs therefore from the canonical IFN response because de novo transcription is not required.
VIRAL OFFENSE AND AXON DEFENSE: AN APPARENT PARADOX RESOLVED
A hallmark of alphaherpesvirus infection of their natural hosts is the establishment of a quiescent yet reactivatable infection in PNS neurons. How is this metastable state established? We have found that the outcome after axonal infection could be biased toward a quiescent infection if the number of particles infecting axons was low (28). The idea is that when small numbers of viral genomes are delivered to the cell body nucleus, they tend to be repressed. Since the local intrinsic and innate responses in peripheral tissues function to reduce the number of virus particles available for axonal infection, these processes increase the probability of quiescent infection in PNS neurons (26).
Other intrinsic and longer-established defense mechanisms in neurons play a role in the repression and silencing of alphaherpesvirus gene expression (29). These mechanisms largely center upon a series of repressor functions and complexes that collectively impose silencing of the HSV genome in neurons and other cells. During the establishment of latency, viral DNA is packaged into chromatin and specific histone acetylation patterns which control viral gene expression are formed (30). These are critical for control of HSV latency and reactivation both in vivo and in cultured neuron systems (31). Interestingly, the structure of latent viral chromatin and viral gene expression are modified through expression of infected-cell protein 0 (ICP0) during latency, implicating that ICP0 can function during both lytic and latent infection to overcome intrinsic host defenses (32). Congruent with this idea, neuron-specific microRNA miR-138 can repress ICP0 expression and neuronal survival, suggesting that intrinsic defenses are finely tuned to facilitate the maintenance of latency (33). Several chromatin repressor complexes and epigenetic modification mechanisms have been described, mostly for HSV, but some have been studied in more detail and have attracted significant attention. In particular, the REST/CoREST/LSD1/HDAC complex is believed to be a critical component in regulating latency and reactivation (34), and its repressive activity is efficiently disabled by ICP0 (35). Another intrinsic antiviral defense targeted by ICP0 is mediated by the IFN-inducible ND10 nuclear bodies (36). The E3 ubiquitin ligase activity of ICP0 mediates the degradation of ND10 components, leading to the dispersal of ND10 bodies (37, 38). In the absence of this activity, viral replication is greatly diminished. Importantly, in neurons, the latent HSV genome has been shown to be tightly associated with ND10 bodies (39), representing another key interaction in the repression and derepression of the HSV life cycle.
DEFENSE MEDIATED BY AUTOPHAGY AND OTHER CELL-INTRINSIC RESPONSES
Autophagy and related processes have become a major focus for many investigators in recent years. Autophagy is a highly evolutionarily conserved pathway that, among other functions, serves to eliminate potentially harmful protein aggregates and damaged organelles (40, 41). An emerging theme, however, is that neurons are atypical in their autophagy responses and exhibit unexpected phenotypes when autophagy is modulated (42). The postmitotic nature of neurons renders them highly dependent on autophagy to maintain their integrity. Indeed, ablation of autophagy in neurons results in their death, and defects in the autophagy pathway are implicated in a variety of neurodegenerative disorders (43). Moreover, neuronal autophagy is highly compartmentalized and is insensitive to modulation by standard methods (e.g., starvation and rapamycin), and the autophagosomes form unusual clusters under conditions of virus infection and IFN treatment (42, 44).
In a variety of cells, autophagy and xenophagy (in which the cell engulfs and degrades viable virions to prevent replication) exert both antiviral and proviral effects, depending on the virus (45–48). In addition, autophagy functions broadly in antiviral immunity (49). Also, given the postmitotic status of neurons, xenophagy would be an effective way to clear intracellular pathogens without the collateral damage of an inflammatory or cytotoxic immune response (50). Despite the appeal and much testing of this hypothesis, the role of autophagy in the regulation of neurotropic virus infections is still emerging and controversial. HSV mutants incapable of antagonizing autophagy show attenuation in the nervous system (51). HSV grows to higher titers and is more virulent in mice lacking autophagy machinery in their nervous systems (52). These findings are consistent with the idea that autophagy protects neurons from virus infection. While the replication of these HSV mutants was reduced relative to that of wild-type virus, altered virus replication alone seems insufficient to explain the profound attenuation. This idea is supported by the observation that a virus lacking the γ34.5 gene, which counteracts both IFN responses and autophagy, replicates normally in cultured sensory neurons (53). Consistent with this idea, a knockdown or ablation of autophagy did not affect Sindbis virus replication, but caused increased accumulation of viral proteins, neuronal death, and neurovirulence (54). It is unlikely that protection by the autophagy pathway is solely through direct xenophagy. Rather, autophagy may also be protective through promotion of innate and adaptive immune responses in the host or prolongation of the survival of infected neurons through prevention of accumulation of toxic viral proteins. It is unclear if autophagy and/or xenophagy is directly involved in the establishment, maintenance, and reactivation of herpesvirus latency.
SUMMARY
Neuroinvasive herpesviruses have evolved in the face of atypical intrinsic and innate defenses of neurons, particularly PNS neurons, to promote the reversible silencing of their potent gene expression programs. While these viruses have a remarkable armamentarium of countermeasures against these repressive cellular pathways, they use them judiciously in neurons, perhaps facilitating the establishment, maintenance, and reactivation of latency. For therapeutic purposes, the herpesvirus field has focused largely on development of vaccines that stimulate adaptive immunity and on chemotherapeutic inhibition of virus replication. Given the large burden of latency worldwide that represents a major reservoir of morbidity and mortality, it should be fruitful to focus on the development of specific and potent modulators of these intrinsic and innate defenses to help improve treatment options for herpetic diseases.
ACKNOWLEDGMENTS
We acknowledge Audra Charron for assistance with Fig. 1 and members of our labs for comments on the manuscript.
David A. Leib is supported by grants AI098681 and EY09083 from the NIH. Lynn W. Enquist is supported by grants NS33506 and NS060699 from the NIH.
REFERENCES
- 1.Bloom DC. 2016. Alphaherpesvirus latency: a dynamic state of transcription and reactivation. Adv Virus Res 94:53–80. doi: 10.1016/bs.aivir.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Barton ES, White DW, Cathelyn JS, Brett-McClellan KA, Engle M, Diamond MS, Miller VL, Virgin HW IV. 2007. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature 447:326–329. doi: 10.1038/nature05762. [DOI] [PubMed] [Google Scholar]
- 3.Nair S, Diamond MS. 2015. Innate immune interactions within the central nervous system modulate pathogenesis of viral infections. Curr Opin Immunol 36:47–53. doi: 10.1016/j.coi.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koyuncu OO, Hogue IB, Enquist LW. 2013. Virus infections in the nervous system. Cell Host Microbe 13:379–393. doi: 10.1016/j.chom.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith G. 2012. Herpesvirus transport to the nervous system and back again. Annu Rev Microbiol 66:153–176. doi: 10.1146/annurev-micro-092611-150051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ugolini G. 2011. Rabies virus as a transneuronal tracer of neuronal connections. Adv Virus Res 79:165–202. doi: 10.1016/B978-0-12-387040-7.00010-X. [DOI] [PubMed] [Google Scholar]
- 7.Wilson AC, Mohr I. 2012. A cultured affair: HSV latency and reactivation in neurons. Trends Microbiol 20:604–611. doi: 10.1016/j.tim.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosato P, Leib D. 2015. Neurons versus herpes simplex virus: the innate immune interactions that contribute to a host-pathogen standoff. Future Virol 10:699–714. doi: 10.2217/fvl.15.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein AY, Wang X, Schwarz TL. 2008. Axonal transport and the delivery of pre-synaptic components. Curr Opin Neurobiol 18:495–503. doi: 10.1016/j.conb.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venkatachalam K, Montell C. 2007. TRP channels. Annu Rev Biochem 76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiu I, von Hehn C, Woolf C. 2012. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci 15:1063–1067. doi: 10.1038/nn.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ying Z, Misra V, Verge V. 2014. Sensing nerve injury at the axonal ER: activated Luman/CREB3 serves as a novel axonally synthesized retrograde regeneration signal. Proc Natl Acad Sci U S A 111:16142–16147. doi: 10.1073/pnas.1407462111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vuppalanchi D, Willis DE, Twiss JL. 2009. Regulation of mRNA transport and translation in axons. Results Probl Cell Differ 48:193–224. doi: 10.1007/400_2009_16. [DOI] [PubMed] [Google Scholar]
- 14.Jung H, Yoon BC, Holt CE. 2012. Axonal mRNA localization and local protein synthesis in nervous system assembly, maintenance and repair. Nat Rev Neurosci 13:308–324. doi: 10.1038/nrn3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ben-Yaakov K, Dagan SY, Segal-Ruder Y, Shalem O, Vuppalanchi D, Willis DE, Yudin D, Rishal I, Rother F, Bader M, Blesch A, Pilpel Y, Twiss JL, Fainzilber M. 2012. Axonal transcription factors signal retrogradely in lesioned peripheral nerve. EMBO J 31:1350–1363. doi: 10.1038/emboj.2011.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox LJ, Hengst U, Gurskaya NG, Lukyanov KA, Jaffrey SR. 2008. Intra-axonal translation and retrograde trafficking of CREB promotes neuronal survival. Nat Cell Biol 10:149–159. doi: 10.1038/ncb1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanz S, Perlson E, Willis D, Zheng JQ, Massarwa R, Huerta JJ, Koltzenburg M, Kohler M, van-Minnen J, Twiss JL, Fainzilber M. 2003. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron 40:1095–1104. doi: 10.1016/S0896-6273(03)00770-0. [DOI] [PubMed] [Google Scholar]
- 18.Lin AC, Holt CE. 2008. Function and regulation of local axonal translation. Curr Opin Neurobiol 18:60–68. doi: 10.1016/j.conb.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holt C, Schuman E. 2013. The central dogma decentralized: new perspectives on RNA function and local translation in neurons. Neuron 80:648–657. doi: 10.1016/j.neuron.2013.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koyuncu O, Perlman D, Enquist L. 2013. Efficient retrograde transport of pseudorabies virus within neurons requires local protein synthesis in axons. Cell Host Microbe 13:54–66. doi: 10.1016/j.chom.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lundborg G. 2003. Nerve injury and repair: a challenge to the plastic brain. J Peripher Nerv Syst 8:209–226. doi: 10.1111/j.1085-9489.2003.03027.x. [DOI] [PubMed] [Google Scholar]
- 22.Leib DA, Harrison TE, Laslo KM, Machalek MA, Moorman NJ, Virgin HW. 1999. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J Exp Med 189:663–672. doi: 10.1084/jem.189.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szpara ML, Kobiler O, Enquist LW. 2010. A common neuronal response to alphaherpesvirus infection. J Neuroimmune Pharmacol 5:418–427. doi: 10.1007/s11481-010-9212-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mikloska Z, Cunningham AL. 2001. Alpha and gamma interferons inhibit herpes simplex virus type 1 infection and spread in epidermal cells after axonal transmission. J Virol 75:11821–11826. doi: 10.1128/JVI.75.23.11821-11826.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Svennerholm B, Ziegler R, Lycke E. 1989. Herpes simplex virus infection of the rat sensory neuron. Effects of interferon on cultured cells. Arch Virol 104:153–156. [DOI] [PubMed] [Google Scholar]
- 26.Song R, Koyuncu O, Greco T, Diner B, Cristea I, Enquist L. 2016. Two modes of the axonal interferon response limit alphaherpesvirus neuroinvasion. mBio 7:e02145–15. doi: 10.1128/mBio.02145-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosato PC, Leib DA. 2015. Neuronal interferon signaling is required for protection against herpes simplex virus replication and pathogenesis. PLoS Pathog 11:e1005028. doi: 10.1371/journal.ppat.1005028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koyuncu O, Song R, Greco T, Cristea I, Enquist L. 2015. The number of alphaherpesvirus particles infecting axons and the axonal protein repertoire determines the outcome of neuronal infection. mBio 6:e00276–15. doi: 10.1128/mBio.00276-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knipe DM, Cliffe A. 2008. Chromatin control of herpes simplex virus lytic and latent infection. Nat Rev Microbiol 6:211–221. doi: 10.1038/nrmicro1794. [DOI] [PubMed] [Google Scholar]
- 30.Neumann DM, Bhattacharjee PS, Giordani NV, Bloom DC, Hill JM. 2007. In vivo changes in the patterns of chromatin structure associated with the latent herpes simplex virus type 1 genome in mouse trigeminal ganglia can be detected at early times after butyrate treatment. J Virol 81:13248–13253. doi: 10.1128/JVI.01569-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Messer HG, Jacobs D, Dhummakupt A, Bloom DC. 2015. Inhibition of H3K27me3-specific histone demethylases JMJD3 and UTX blocks reactivation of herpes simplex virus 1 in trigeminal ganglion neurons. J Virol 89:3417–3420. doi: 10.1128/JVI.03052-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raja P, Lee JS, Pan D, Pesola JM, Coen DM, Knipe DM. 2016. A herpesviral lytic protein regulates the structure of latent viral chromatin. mBio 7:e00633-16. doi: 10.1128/mBio.00633-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan D, Flores O, Umbach JL, Pesola JM, Bentley P, Rosato PC, Leib DA, Cullen BR, Coen DM. 2014. A neuron-specific host microRNA targets herpes simplex virus-1 ICP0 expression and promotes latency. Cell Host Microbe 15:446–456. doi: 10.1016/j.chom.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou G, Du T, Roizman B. 2013. HSV carrying WT REST establishes latency but reactivates only if the synthesis of REST is suppressed. Proc Natl Acad Sci U S A 110:E498-506. doi: 10.1073/pnas.1222497110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gu H, Roizman B. 2009. The two functions of herpes simplex virus 1 ICP0, inhibition of silencing by the CoREST/REST/HDAC complex and degradation of PML, are executed in tandem. J Virol 83:181–187. doi: 10.1128/JVI.01940-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maul GG, Guldner HH, Spivack JG. 1993. Modification of discrete nuclear domains induced by herpes simplex virus type 1 immediate early gene 1 product (ICP0). J Gen Virol 74:2679–2690. doi: 10.1099/0022-1317-74-12-2679. [DOI] [PubMed] [Google Scholar]
- 37.Everett RD, Earnshaw WC, Findlay J, Lomonte P. 1999. Specific destruction of kinetochore protein CENP-C and disruption of cell division by herpes simplex virus immediate-early protein Vmw110. EMBO J 18:1526–1538. doi: 10.1093/emboj/18.6.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Sant C, Hagglund R, Lopez P, Roizman B. 2001. The infected cell protein 0 of herpes simplex virus 1 dynamically interacts with proteasomes, binds and activates the cdc34 E2 ubiquitin-conjugating enzyme, and possesses in vitro E3 ubiquitin ligase activity. Proc Natl Acad Sci U S A 98:8815–8820. doi: 10.1073/pnas.161283098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Catez F, Picard C, Held K, Gross S, Rousseau A, Theil D, Sawtell N, Labetoulle M, Lomonte P. 2012. HSV-1 genome subnuclear positioning and associations with host-cell PML-NBs and centromeres regulate LAT locus transcription during latency in neurons. PLoS Pathog 8:e1002852. doi: 10.1371/journal.ppat.1002852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Green DR, Levine B. 2014. To be or not to be? How selective autophagy and cell death govern cell fate. Cell 157:65–75. doi: 10.1016/j.cell.2014.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, Mizushima N, Iwata J, Ezaki J, Murata S, Hamazaki J, Nishito Y, Iemura S, Natsume T, Yanagawa T, Uwayama J, Warabi E, Yoshida H, Ishii T, Kobayashi A, Yamamoto M, Yue Z, Uchiyama Y, Kominami E, Tanaka K. 2007. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 131:1149–1163. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 42.Maday S, Holzbaur EL. 2016. Compartment-specific regulation of autophagy in primary neurons. J Neurosci 36:5933–5945. doi: 10.1523/JNEUROSCI.4401-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. 2006. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 44.Katzenell S, Leib DA. 2016. Herpes simplex virus and interferon signaling induce novel autophagic clusters in sensory neurons. J Virol 90:4706–4719. doi: 10.1128/JVI.02908-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dreux M, Gastaminza P, Wieland SF, Chisari FV. 2009. The autophagy machinery is required to initiate hepatitis C virus replication. Proc Natl Acad Sci U S A 106:14046–14051. doi: 10.1073/pnas.0907344106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Metz P, Chiramel A, Chatel-Chaix L, Alvisi G, Bankhead P, Mora-Rodriguez R, Long G, Hamacher-Brady A, Brady NR, Bartenschlager R. 2015. Dengue virus inhibition of autophagic flux and dependency of viral replication on proteasomal degradation of the autophagy receptor p62. J Virol 89:8026–8041. doi: 10.1128/JVI.00787-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buckingham EM, Carpenter JE, Jackson W, Grose C. 2014. Autophagy and the effects of its inhibition on varicella-zoster virus glycoprotein biosynthesis and infectivity. J Virol 88:890–902. doi: 10.1128/JVI.02646-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alexander DE, Ward SL, Mizushima N, Levine B, Leib DA. 2007. Analysis of the role of autophagy in replication of herpes simplex virus in cell culture. J Virol 81:12128–12134. doi: 10.1128/JVI.01356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paul P, Munz C. 2016. Autophagy and mammalian viruses: roles in immune response, viral replication, and beyond. Adv Virus Res 95:149–195. doi: 10.1016/bs.aivir.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Alexander DE, Leib DA. 2008. Xenophagy in herpes simplex virus replication and pathogenesis. Autophagy 4:101–103. doi: 10.4161/auto.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Orvedahl A, Alexander D, Talloczy Z, Sun Q, Wei Y, Zhang W, Burns D, Leib DA, Levine B. 2007. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe 1:23–35. doi: 10.1016/j.chom.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 52.Yordy B, Iijima N, Huttner A, Leib D, Iwasaki A. 2012. A neuron-specific role for autophagy in antiviral defense against herpes simplex virus. Cell Host Microbe 12:334–345. doi: 10.1016/j.chom.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosato PC, Leib DA. 2014. Intrinsic innate immunity fails to control herpes simplex virus and vesicular stomatitis virus replication in sensory neurons and fibroblasts. J Virol 88:9991–10001. doi: 10.1128/JVI.01462-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Orvedahl A, MacPherson S, Sumpter R Jr, Talloczy Z, Zou Z, Levine B. 2010. Autophagy protects against Sindbis virus infection of the central nervous system. Cell Host Microbe 7:115–127. doi: 10.1016/j.chom.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]