ABSTRACT
A recombinant strain HCV1 (hepatitis C virus [HCV] genotype 1a) gpE1/gpE2 (E1E2) vaccine candidate was previously shown by our group to protect chimpanzees and generate broad cross-neutralizing antibodies in animals and humans. In addition, recent independent studies have highlighted the importance of conserved neutralizing epitopes in HCV vaccine development that map to antigenic clusters in E2 or the E1E2 heterodimer. E1E2 can be purified using Galanthis nivalis lectin agarose (GNA), but this technique is suboptimal for global production. Our goal was to investigate a high-affinity and scalable method for isolating E1E2. We generated an Fc tag-derived (Fc-d) E1E2 that was selectively captured by protein G Sepharose, with the tag being removed subsequently using PreScission protease. Surprisingly, despite the presence of the large Fc tag, Fc-d E1E2 formed heterodimers similar to those formed by GNA-purified wild-type (WT) E1E2 and exhibited nearly identical binding profiles to HCV monoclonal antibodies that target conserved neutralizing epitopes in E2 (HC33.4, HC84.26, and AR3B) and the E1E2 heterodimer (AR4A and AR5A). Antisera from immunized mice showed that Fc-d E1E2 elicited anti-E2 antibody titers and neutralization of HCV pseudotype viruses similar to those with WT E1E2. Competition enzyme-linked immunosorbent assays (ELISAs) showed that antisera from immunized mice inhibited monoclonal antibody binding to neutralizing epitopes. Antisera from Fc-d E1E2-immunized mice exhibited stronger competition for AR3B and AR5A than the WT, whereas the levels of competition for HC84.26 and AR4A were similar. We anticipate that Fc-d E1E2 will provide a scalable purification and manufacturing process using protein A/G-based chromatography.
IMPORTANCE A prophylactic HCV vaccine is still needed to control this global disease despite the availability of direct-acting antivirals. Previously, we demonstrated that a recombinant envelope glycoprotein (E1E2) vaccine (genotype 1a) elicited cross-neutralizing antibodies from human volunteers. A challenge for isolating the E1E2 antigen is the reliance on GNA, which is unsuitable for large scale-up and global vaccine delivery. We have generated a novel Fc domain-tagged E1E2 antigen that forms functional heterodimers similar to those with native E1E2. Affinity purification and removal of the Fc tag from E1E2 resulted in an antigen with a nearly identical profile of cross-neutralizing epitopes. This antigen elicited anti-HCV antibodies that targeted conserved neutralizing epitopes of E1E2. Owing to the high selectivity and cost-effective binding capacity of affinity resins for capture of the Fc-tagged rE1E2, we anticipate that our method will provide a means for large-scale production of this HCV vaccine candidate.
KEYWORDS: envelope glycoproteins, epitopes, hepatitis C virus, neutralizing antibodies, vaccines
INTRODUCTION
Hepatitis C virus (HCV) infection remains a major global health concern, with more than 150 million people infected worldwide (1). The recent approval of direct-acting antivirals has greatly improved patient outcomes, with high cure rates (2). However, the high cost of direct-acting antivirals is likely to limit the number of patients that receive these treatments in developed nations and, particularly, low- to middle-income regions (3). In addition, significant drug treatment challenges include the identification of chronically HCV-infected individuals who are unaware of their status (4), as well as the potential for reinfection after cure for high-risk groups (5). For these reasons, the development of a prophylactic HCV vaccine is critical in an effort toward the elimination of this major global disease.
A major challenge in the development of a prophylactic HCV vaccine is the high diversity of the virus and immune evasion in the infected host (6). Thus, selection of the appropriate immunogen for an HCV vaccine is critical to generate HCV neutralizing antibodies (nAbs) that target conserved epitopes of HCV. nAbs that primarily target the HCV envelope glycoproteins E1 and E2 have been identified during natural HCV infection (7–10). However, more recent studies have highlighted a protective role for HCV nAbs during the acute phase of infection that are associated with recovery (11–13).
Much attention in recent years has been focused on nAbs present in HCV patient sera and cross-neutralizing monoclonal antibodies (MAbs) isolated from patients and immunized animals (10, 14). Both patient serum Ig and MAbs prevent chronic HCV infection in the passively immunized chimeric human liver SCID/uPa mouse model and in chimpanzees (15–18). Cross competition and epitope mapping analyses have defined at least five clusters of overlapping conformational cross-neutralizing epitopes. Three clusters (antigenic domains B, C, and D) of conformational epitopes map to the E2 protein, and two clusters (designated antigenic regions [AR] 4 and 5) map to the E1E2 heterodimer (reviewed in reference 19). Our understanding of these epitopes has been further enhanced by two recent reports of the crystal structure of the core E2 domain (20, 21). Collectively, these studies have provided insight for rational vaccine design aimed at targeting these conserved epitopes (19). However, a significant challenge in HCV vaccine development is the underlying complexity of the folding and interaction of the E1 and E2 glycoproteins. E1 and E2 are generated following cleavage of the HCV polypeptide precursor by a host signal peptidase in the endoplasmic reticulum (ER) membrane. E1 and E2 are highly glycosylated proteins, and their correct folding is interdependent (22–24) and requires ER chaperones (25–27). Studies have also highlighted the importance of key residues within the transmembrane domains of E1 and E2 (28–31), as well as N-linked glycosylation sites (25, 32), for heterodimer formation. Recombinant E1E2 (expressed and isolated from mammalian cell culture) can be isolated as a noncovalently linked heterodimer. This form has been thought to represent the native state, whereas a high-molecular-weight disulfide-linked aggregate form may represent misfolded E1E2 (26, 29, 33). However, disulfide-linked E1E2 complexes have been detected in isolated HCV virions, suggesting that oligomerization of E1E2 may occur during virus assembly and secretion (34).
Previous work from our group has shown that chimpanzees vaccinated with an E1E2 antigen derived from the strain HCV1 (genotype 1a) had significantly reduced rates of chronic infection following challenge with either homologous or heterologous 1a virus. Sterilizing immunity was observed in some of these vaccinated animals (35–37). A phase I dose-ranging clinical trial was conducted on healthy human volunteers and demonstrated the induction of anti-E1E2 antibodies (determined by an enzyme immunoassay) and strong T-helper cell responses (38). Follow-up studies demonstrated that antibodies present in sera from vaccinated individuals could impair infection in vitro by HCV genotype 1a as well as other diverse genotypes (39–41). The neutralization of diverse genotypes from this HCV vaccine candidate was supported by further epitope mapping and competition enzyme-linked immunosorbent assay (ELISA) analyses of sera from vaccinated individuals that demonstrated the targeting of multiple cross-neutralizing epitopes (42).
The aim of the current study was to investigate a new method to purify E1E2 in an effort to improve upon the existing Galanthis nivalis lectin agarose (GNA)-based purification scheme thus facilitating vaccine production and global delivery (35, 37, 43). We were able to express and isolate a novel E1E2 antigen from an Fc-tagged precursor protein from Chinese hamster ovary (CHO) cells. We performed ELISAs with well-described HCV MAbs and compared the results to those obtained with GNA-isolated wild-type (WT) E1E2. Following vaccination of mice with E1E2 antigens, we examined serum samples for HCV nAbs and the recognition of cross-neutralizing epitopes by competition ELISAs with the same panel of HCV MAbs. Our results support the notion that the Fc tag-derived (Fc-d) E1E2 exhibits features nearly identical to those of the WT E1E2 antigen in terms of representation of cross-neutralizing epitopes and immunogenicity. We anticipate that the Fc-d E1E2 antigen will be amenable for large-scale production as a candidate HCV vaccine.
RESULTS
Native folding and purification of E1E2 from an Fc-tagged precursor.
Previously, GNA was used as an enrichment step for the isolation of recombinant E1E2 and E2 proteins (35, 37, 43). This method was also used successfully to obtain high-purity E1E2 for vaccination of human volunteers in a phase I HCV vaccine trial (38). However, isolation of E1E2 using GNA poses a significant challenge due to its lack of specificity (GNA binds to all branched high-mannose glycoproteins) and low target protein affinity (∼3 mg of target glycoprotein/ml of GNA). We sought to improve upon this method by developing a process that would have high specificity and high target protein binding capacity.
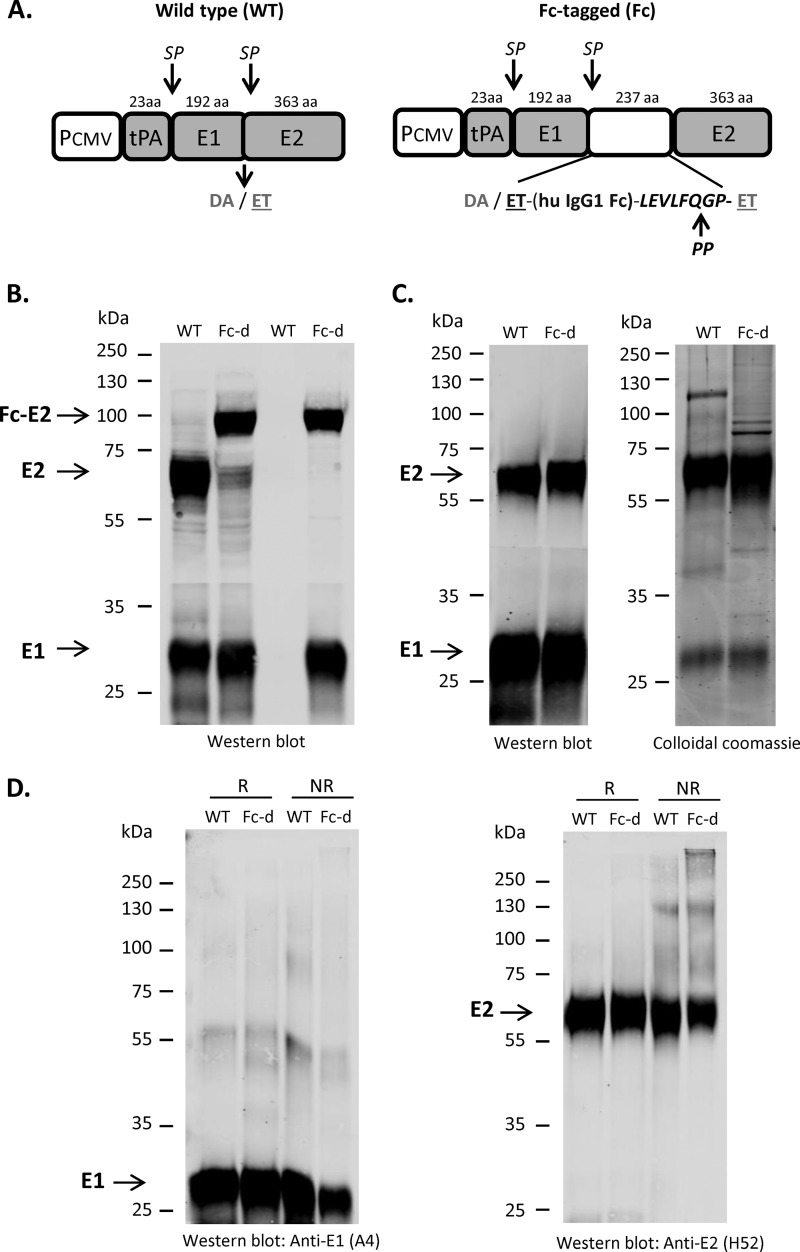
The constructs used for E1E2 expression are shown in Fig. 1A. The full-length E1E2 sequence from H77c (GenBank accession no. AF009606) was inserted downstream of the tissue plasminogen activator (tPA) leader sequence and a cytomegalovirus (CMV) promoter. To generate an affinity-tagged form of E1E2, the Fc domain from human IgG1 was inserted at the junction between E1 and E2. Both WT and Fc-tagged E1E2 could be precipitated by GNA, whereas only the latter was precipitated by protein G Sepharose (Fig. 1B). Importantly, the precipitated Fc-E2 protein was associated with E1, demonstrating that the Fc tag did not interfere with heterodimer formation.
FIG 1.
Purification of E1E2 from an Fc-tagged precursor. (A) Schematic representation of wild-type (WT) and Fc-tagged constructs and polypeptide processing. The E1E2 H77c (GenBank accession no. AF009606) polypeptide was expressed under the control of the CMV promoter (PCMV) and preceded by the signal sequence from tissue plasminogen activator (tPA). For Fc-tagged E1E2, a duplication of the E2 N-terminal amino acids (384 and 385) (ET) was inserted, followed by the human IgG1 Fc tag (hu IgG1 Fc) and a PreScission protease (PP) recognition sequence (LEVLFQGP). Sizes of the polypeptide regions are shown at the top (aa, amino acids) as well as cleavage sites by signal peptidase (SP). (B) Capture of WT or Fc tag-derived (Fc-d) E1E2 from CHO cell extracts was performed with GNA and protein G Sepharose, respectively, and proteins were separated by SDS-PAGE and blotted with anti-E1 (A4) and anti-E2 (H52) monoclonal antibodies (MAbs). (C) Purified E1E2 antigens (with PP-mediated Fc tag removal step for Fc-d) were separated by SDS-PAGE. (Left) Western blot with anti-E1 (A4) and anti-E2 (H52) MAbs (1 μg loaded per lane); (right) Coomassie brilliant blue G250 (2 μg loaded per lane). (D) Wild-type and Fc tag-derived E1E2 antigens (1 μg/lane) were denatured at 95°C for 5 min in Laemmli buffer with (R) or without (NR) 1% β-mercaptoethanol. Samples were separated by SDS-PAGE and blotted with ant-E1 (A4) and anti-E2 (H52) MAbs.
As outlined in Materials and Methods, Fc-d E1E2 (with the Fc tag removed) was purified and compared to WT E1E2 isolated with GNA. Both methods resulted in a highly enriched E1E2 antigen that contained a minor fraction of contaminant proteins (Fig. 1C). The yields between the two methods were similar at the scale of the isolations performed (∼1 mg of E1E2 per 100 g of CHO cells). Consistent with findings from previous studies (26, 29), SDS-PAGE and Western blot analyses performed with reducing versus nonreducing Laemmli buffer supported the idea that the vast majority of the isolated E1E2 antigens were in noncovalently linked complexes (Fig. 1D). Western blots using anti-calnexin, anti-GRP78, and anti-glutathione S-transferase (anti-GST) antibodies confirmed that the final E1E2 antigens did not contain detectable levels of ER chaperones (and thus were likely folded correctly) or residual His6-GST-human rhinovirus protease 3C (HRV3C) protease used in the digestion step to cleave the Fc tag from the E1E2 antigen (data not shown).
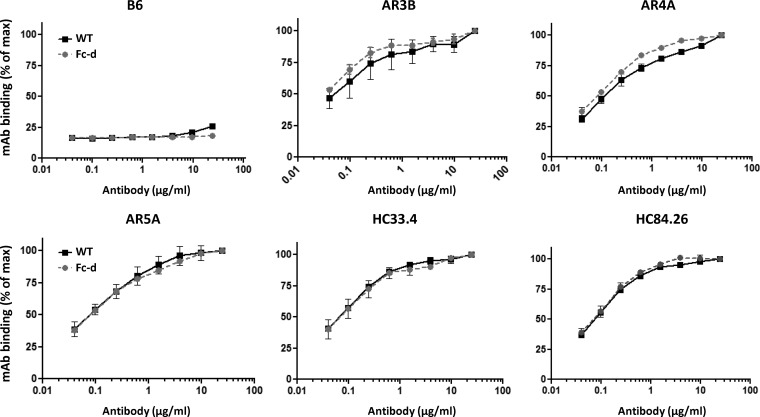
We next addressed whether Fc-d E1E2 was comparable to WT E1E2 in terms of neutralizing epitopes present within E2 and E1E2. Because the Fc domain is a large tag (237 amino acids [aa]), it is possible that expression of the Fc-tagged E2 protein may influence its folding and/or association with E1 in the ER. E1E2 antigens were used to coat ELISA plates and probed using E2-specific (HC33.4, HC84.26, and AR3B) and E1E2-specific (AR4A and AR5A) cross-neutralizing MAbs. HC33.4, HC84.26, and AR3B target the disparate regions of E2 that form the cluster of differentiation 81 (CD81) receptor binding site (CD81bs) and are capable of binding soluble E2. HC33.4 recognizes a linear epitope in E2 (aa 409 to 423) (15, 16, 44, 45). AR3B and HC84.26 are directed to conformation-specific E2 epitopes. However, HC84.26 additionally recognizes an E2 linear synthetic peptide (aa 433 to 447) (44). AR4A and AR5A recognize unique conformational epitopes outside the CD81bs and are capable of binding native E1E2 but not E2 or E1 alone (15). We observed that WT and Fc-d E1E2 antigens bound E2- and E1E2-specific antibodies in nearly identical manners (Fig. 2). These data support the idea that, similar to the WT, Fc-d E1E2 maintains correct folding and presents conformation-specific E2 and E1E2 cross-neutralizing epitopes.
FIG 2.
Binding of HCV cross-neutralizing monoclonal antibodies to purified E1E2 antigens. Wild-type or Fc tag-derived antigens were used to coat ELISA plates in triplicate and probed with increasing concentrations of neutralizing human HCV monoclonal antibodies. Binding of E2 MAbs was detected by anti-human alkaline phosphatase-conjugated secondary antibody and p-nitrophenylphosphate substrate. The optical densities at 405 to 495 nm (normalized to the maximum, 24 μg/ml, of each MAb concentration tested) from two independent experiments are shown plotted versus MAb concentration (log10 micrograms per milliliter). E2-specific antibodies included HC33.4, HC84.26, and AR3B. E1E2-specific antibodies included AR4A and AR5A. The control human anti-HIV IgG1 was B6 (normalized to the maximum dose of AR3B).
The Fc-d E1E2 heterodimer elicits nAbs in mice.
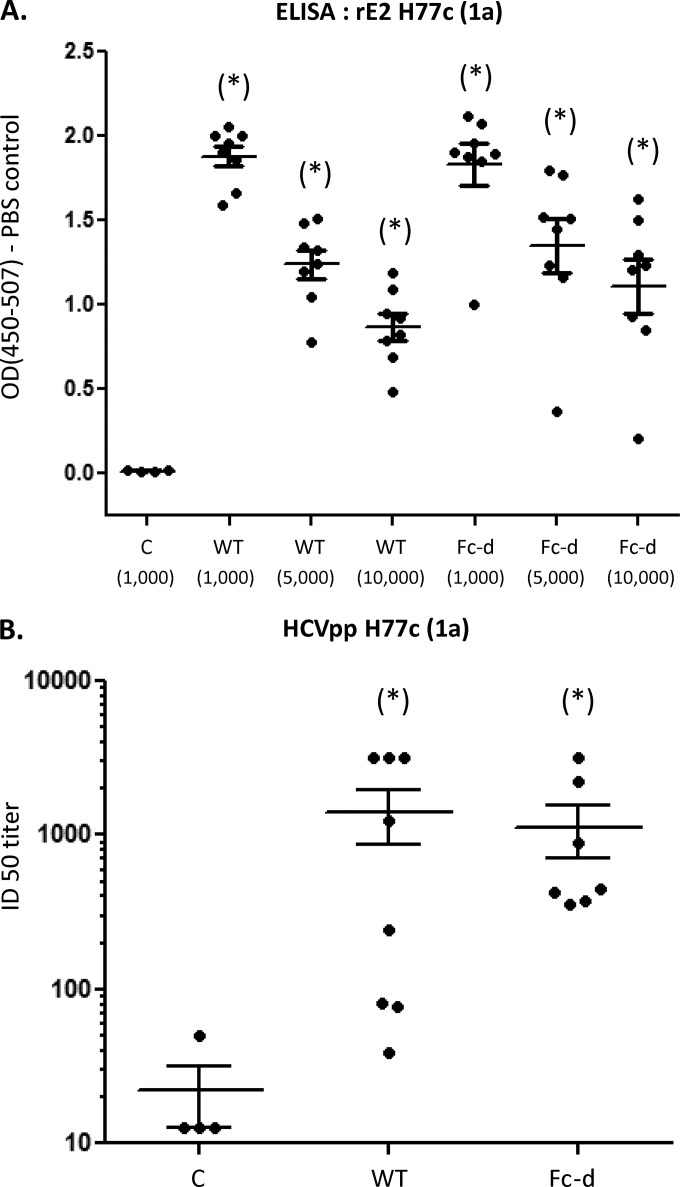
Eight CB6F1 mice were immunized with WT or Fc-d E1E2 (2 μg per injection) in a 1:1 ratio with adjuvant (75 μg of alum and 7.5 μg of monophosphoryl lipid A [MPLA]). Four animals were injected with buffer containing adjuvant as controls. Animals received a total of three injections at 2-week intervals. Sera obtained from terminal bleeds were examined for homologous anti-E2 activity by ELISA plates coated with recombinant E2 HCV1 (1a). Sera from WT and Fc-d E1E2-vaccinated mice exhibited strong anti-E2 titers for E2 HCV1 at all dilutions examined (1,000-, 5,000-, and 10,000-fold) (Fig. 3A). For each dilution examined, sera from mice vaccinated with WT and Fc-d E1E2 were statistically elevated from controls (P < 0.05, one-way analysis of variance [ANOVA] and Tukey's post hoc test) (Fig. 3A). Mean absorbance values between sera from mice vaccinated with WT and Fc-d E1E2 exhibited no statistical difference at each dilution examined.
FIG 3.
Neutralizing antibodies induced by vaccination with wild-type and Fc tag-derived E1E2. (A) Recombinant E2 HCV1 (1a) (amino acids 384 to 661; GenBank accession no. M62321.1) was used to coat ELISA plates in triplicate and probed with postvaccination mouse sera. Binding of E2-specific antibodies in sera from WT and Fc-d E1E2-vaccinated mice (1,000-, 5,000-, and 10,000-fold dilutions) compared to sera from control-vaccinated mice (1,000-fold dilution) were detected by anti-mouse horseradish peroxidase-conjugated secondary antibody and peroxidase substrate. The optical densities at 450 to 507 nm (OD450-507) (means and SEM) from three independent experiments are shown plotted versus serum dilution. (B) Neutralization of HCVpp H77c (1a) entry was performed using 2-fold dilutions (1:25 to 1:1,600) of pre- and postvaccination sera, and the ID50s were determined (shown as the reciprocal value of the dilution to achieve 50% neutralization). The group mean with SEM is shown from a representative of two independent experiments. Vaccinated mouse groups: control (C), buffer plus alum/MPL; WT, GNA agarose-derived E1E2 H77c plus alum/MPL; and Fc-d, Fc tag-derived E1E2 H77c plus alum/MPL. *, P < 0.05 respective to control by one-way ANOVA by Kruskal-Wallis and Dunn's post hoc tests.
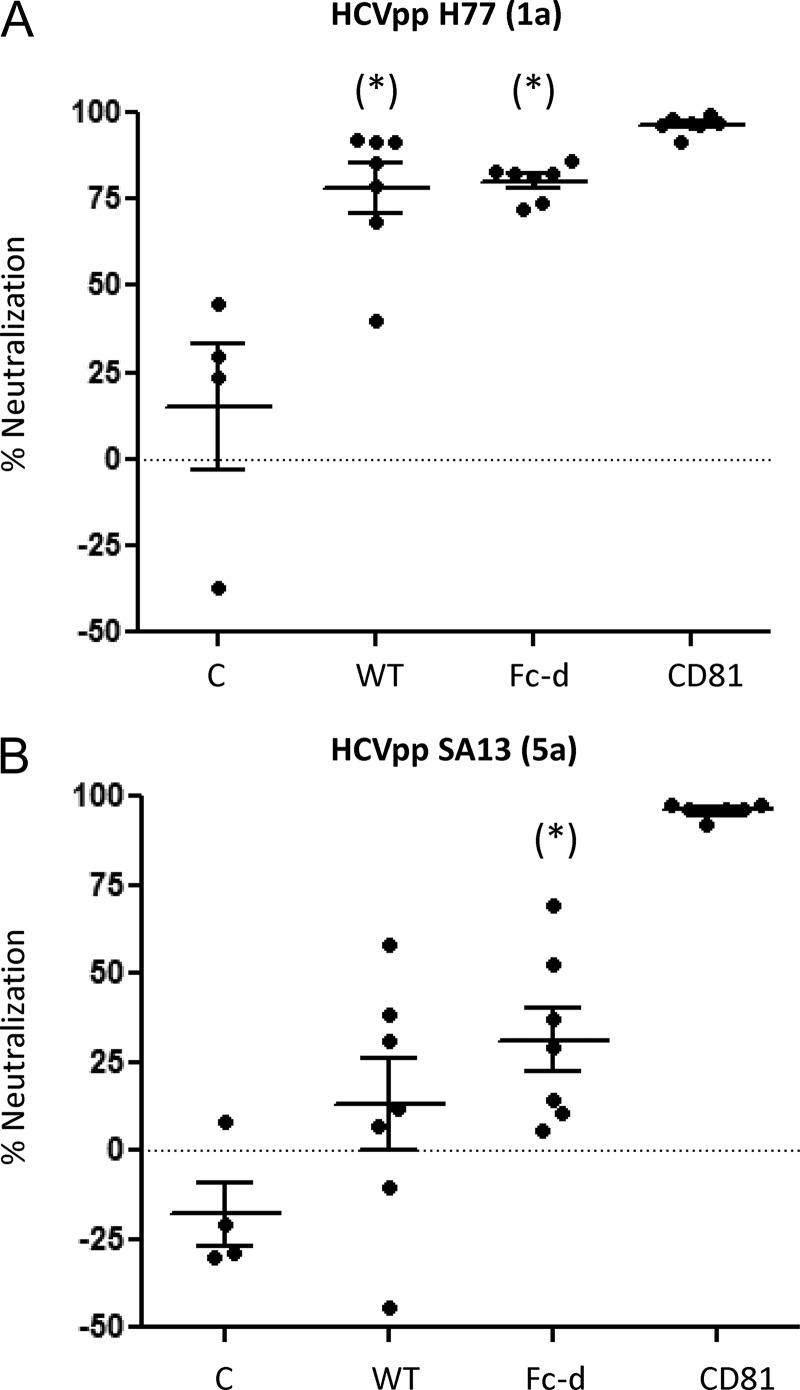
Pre- and postvaccination serum samples were evaluated for the ability to inhibit the infectivity of HCV pseudotype virus (HCVpp) H77 in Huh7.5 cells. Twofold serial dilutions (1:25 to 1:1,600) of sera were examined, and the inhibitory dose to achieve 50% neutralization (ID50) was represented as the reciprocal value of the dilution (Fig. 3B). WT and Fc-D E1E2 exhibited similar group mean ID50s, 1,412 ± 541 and 1,134 ± 428, respectively, that were each significantly different from that of the control group. Fc-d E1E2-vaccinated mice exhibited less variability in ID50s (minimum, 360; maximum, 3,200) than the WT group (minimum, 39; maximum, 3,200). We next compared the neutralization response of homologous genotype 1a (H77) to that of heterologous genotype 5a (SA13) HCVpp. With sera diluted 1:50, WT and Fc-d groups showed very similar mean neutralizations of HCVpp H77, at 78.3% ± 7.2% and 80.2% ± 2.0%, respectively (Fig. 4A). Fc-d E1E2-vaccinated animals exhibited a neutralization of HCVpp SA13 (mean, 31% ± 9%) significantly different from that of controls. However, the WT group did not show neutralization toward SA13 that was significantly different from that of the control group (Fig. 4B). We were unable to detect neutralization activity within the Fc-d E1E2 group using further dilutions of the sera.
FIG 4.
Comparison of neutralizing antibodies toward homologous (1a) and heterologous (5a) HCVpp. Neutralization of homologous HCVpp H77 (1a) and heterologous HCVpp SA13 (5a) was performed using pre- and postvaccination sera (1:50), and the group means with SEM were plotted from representatives of two independent experiments. Positive control, anti-CD81 MAb (1 μg/ml). *, P < 0.05 respective to control by one-way ANOVA and Tukey post hoc test.
Antisera from E1E2-vaccinated mice compete for binding with cross-neutralizing HCV MAbs.
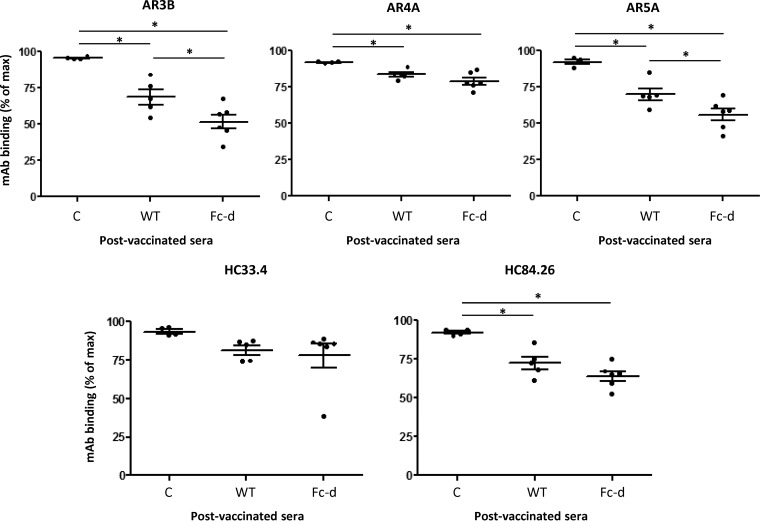
The observed neutralization responses of WT and Fc-d E1E2-vaccinated mice for HCVpp H77 (1a) and HCVpp SA13 (5a) indicated a dominance toward homologous (1a) E1E2 epitopes. To determine if anti-E1E2 antibodies from WT and Fc-d E1E2-vaccinated animals targeted conserved epitopes to well-characterized HCV neutralizing MAbs, we performed competition ELISAs according to a previous study (42). Antisera from control animals exhibited negligible competition with each of the MAbs examined: >90% MAb binding (normalized to maximum binding in the absence of any sera) (Fig. 5). Vaccination with WT and Fc-d E1E2 significantly reduced AR3B, AR4A, AR5A, and HC84.26 binding compared to that with control sera (P < 0.05, one-way ANOVA and Tukey's post hoc test). For HC33.4, neither WT nor Fc-d E1E2 impaired the binding of this MAb significantly compared to controls.
FIG 5.
Mouse antisera compete for the binding of HCV cross-neutralizing MAbs to E1E2. Competition studies with mouse antisera and a panel of cross-neutralizing human HCV antibodies were done. Microtiter wells containing GNA-purified E1E2 H77c were incubated with diluted postvaccination antiserum (1:100) in triplicate for 1 h, followed by incubation with the indicated MAb. Binding of the MAbs was detected with anti-human alkaline phosphatase-conjugated secondary antibodies. The percentages of MAb binding were calculated relative to the amount of MAb bound in the absence of antiserum. Shown are mean values for each group ± the range from two independent experiments. Vaccinated mouse groups: control (C), buffer plus alum/MPL; WT, GNA agarose-derived E1E2 H77c plus alum/MPL; Fc-d, Fc tag-derived E1E2 H77c plus alum/MPL. E2-specific antibodies included HC33.4, HC84.26, and AR3B. E1E2-specific antibodies included AR4A and AR5A. *, P < 0.05 by one-way ANOVA and Tukey's post hoc test. At n = 2, SEM is actually the same as the range.
For both the AR4A and HC84.26 MAbs, binding was reduced to similar extents in animals vaccinated with WT and Fc-derived E1E2 (for AR4A, means of 83.4 and 78.8%, respectively; for HC84.26, means of 72.3 and 63.8%, respectively). For AR3B and AR5A, Fc-d E1E2 vaccination impaired MAb binding to a greater extent than WT vaccination (for AR3B, means of 68.6 and 51.5%, respectively; for AR5A, means of 69.8 and 55.9%, respectively) (P < 0.05, one-way ANOVA and Tukey's post hoc test). These findings suggest that WT and Fc-d E1E2-vaccinated mice did develop anti-HCV antibodies that target conserved neutralizing epitopes. However, it is noted that our competition ELISA likely exhibits high sensitivity since the neutralization of heterologous HCVpp SA13 (5a) was considerably lower than that of HCVpp H77 (1a) from sera of WT and Fc-d E1E2-vaccinated mice (Fig. 4).
DISCUSSION
The expression and purification of E1E2 are very challenging owing to its complexity of folding, glycosylation, and requirement for ER chaperones (22, 26, 32). Furthermore, because E1 and E2 transmembrane domain residues are required for heterodimer formation (28, 30, 31), the highest probability to achieve properly folded E1E2 is expression of the full-length membrane-anchored proteins. Previously, small affinity tags, such as the tandem Strep (33) and FLAG (46) tags, were integrated successfully into HCV genomes without negatively affecting viral replication, assembly, and release. Surprisingly, we encountered difficulties in the use of these smaller tags for E1E2 purification. We found that the polyhistidine (His6) and tandem Strep tag II (IBA Lifesciences) constructs (as similarly designed in Fig. 1A) failed to enrich E1E2 heterodimers with nickel-nitrilotriacetic acid (Ni-NTA) and Streptactin affinity resins, respectively (unpublished data). This did not appear to be the result of a lack of expression of the E1E2 constructs in CHO cells. Rather, we speculate that capture of the E1E2 antigens may have been limited due to the accessibility of these tags under native purification conditions. In contrast to the results obtained with small affinity tags, we observed that the insertion of an Fc domain tag at the E1-E2 junction resulted in the formation of Fc-E2, which, despite its large size, was still able to interact with E1. This permitted the selective capture of Fc-tagged E1E2 heterodimers using protein G Sepharose (Fig. 1B and C). Subsequent removal of the Fc tag by protease cleavage then produced an E1E2 heterodimer indistinguishable from that produced from earlier purification methods using GNA chromatography as determined by ELISA with HCV neutralizing MAbs (Fig. 2). An analysis of escape variant forms of Fc-d E1E2 antigens is currently in progress to confirm critical binding residues of neutralizing epitopes and evaluate the robustness of the purification process.
In this study, we performed small-scale purifications of WT and Fc-d E1E2 antigens, which generated similar yields (∼1 mg of E1E2 per 100 g of CHO cell pellet). However, we anticipate that the purification scheme for Fc-tagged rE1E2 will generate significantly better yields upon scale-up and offer several advantages for manufacturing. WT E1E2 has been isolated previously using the mannose-binding lectin GNA (35, 37, 43). GNA was originally described to have specificity for Manα (1, 3) and Man-containing oligosaccharides (47), and members of this family interact with viral glycoproteins (48). Although GNA enriches E1E2 from cell extracts, this interaction is not specific and additional contaminant proteins bound to GNA must be removed by subsequent purification steps. In addition, GNA-coupled agarose has several disadvantages for large-scale purification of E1E2. First, GNA agarose exhibits a relatively low target protein binding capacity (∼3 mg of target/ml of resin). Therefore, scale-up requires a cost-prohibitive volume of media. Second, GNA agarose does not have an established life span for reuse. Therefore, a limited number of purification cycles are possible before replacement of the medium. Third, because GNA is isolated from snow drop bulbs, a consistent supply of this resin (at large scale) is unreliable.
In contrast to GNA, recombinant protein A- or G-based chromatography media are readily available and widely used for commercial purification of monoclonal antibodies (49, 50). Protein A medium exhibits high selectivity, a high flow rate, and a cost-effective binding capacity for antibodies and Fc-fusion proteins (dynamic capacity ranging from 15 to 50 mg of antibody/ml of resin) (51). In addition, protein A columns can be sanitized with dilute sodium hydroxide (0.05 to 0.2 N) without loss of activity. The usable lifetime of the resins can exceed 100 cycles with minimal loss of binding capacity. Lastly, if more stringent sanitization conditions are required, an alkali-stabilized protein A-derived ligand is available that can withstand harsh sanitization solutions such as 0.1 to 0.5 N sodium hydroxide (MAbSelect Sure; GE Healthcare). For these reasons, we anticipate that our Fc-d E1E2 construct should be amenable to large-scale production utilizing protein A chromatography media.
Many discrete MAbs with broad cross-neutralizing activity have been isolated and characterized (10, 14), including some that recognize overlapping regions of the E2 protein as well as residues in the CD81bs (45, 52). In this study, we used discrete MAbs (HC33.4, HC84.26, and AR3B) that bind to various regions of the CD81bs to block the E2-CD81 interaction (16, 44, 45, 52). In addition, we used monoclonal AR4A and AR5A antibodies that target discrete epitopes outside the CD81bs and that do not interfere with CD81 binding (15). Both of these MAbs bind to the native E1E2 heterodimer but not to denatured E1E2 or to E2 or E1 alone. AR4A and AR5A can bind to E1E2 simultaneously without competition, and each is broadly neutralizing (15). ELISAs showed that that GNA and Fc-d E1E2 antigens exhibited nearly identical binding profiles for all MAbs examined (HC33.4, HC84.26, AR3B, AR4A, and AR5A) (Fig. 2). In addition, sera from Fc-d E1E2-vaccinated mice generated highly comparable anti-E2 antibodies (determined by rE2 ELISA) and neutralizing responses to homologous HCVpp H77 (1a) compared with GNA E1E2 (Fig. 3A and B).
In competition ELISAs, antisera from Fc-d E1E2-vaccinated mice exhibited significantly higher competition for AR3B and AR5A than did those from WT E1E2-vaccinated animals, indicating enhanced immunogenicity for these epitopes in the Fc-d E1E2 antigen (Fig. 5). As noted previously (42), we saw less competition with AR4A relative to the other MAbs, and there was no difference in competition between WT E1E2 and Fc-d E1E2 (Fig. 5). Interestingly, we observed significant competition of vaccinated sera with HC84.26 but not HC33.4, which can recognize linear E2 epitopes within aa 433 to 447 and aa 409 to 423, respectively (44, 45). The lack of significant competition with HC33.4 was somewhat surprising because previously characterized murine anti-HCV MAbs have tended to target linear E2 epitopes (53, 54) and we observed significant binding of HC33.4 to both GNA E1E2 and Fc-d E1E2 heterodimers (Fig. 2). However, it is well known that vaccine antigenicity is not always consistent with vaccine immunoreactivity. Indeed, our competition ELISA indicated that WT and Fc-d E1E2-vaccinated mice exhibited antibodies that target cross-neutralizing epitopes. However, these data did not correlate with robust neutralization of heterologous HCVpp SA13 (5a) (Fig. 4). This finding may relate to differences in immunogenicity of HCV glycoprotein antigens in mice compared to other hosts. As mentioned above, murine MAbs isolated through immunization with E2 proteins have tended to target linear epitopes (53, 54), and reduced efficacy toward heterologous genotypes has been reported (55). Another plausible explanation for the lower neutralization of HCVpp SA13 (5a) from vaccinated mice is the immunization schedule. In this study, we used an accelerated vaccination schedule that was considerably shorter in duration than in previous studies that reported cross-neutralizing antibodies from guinea pigs (56), goats (42), chimpanzees (57), and humans (38, 39) vaccinated with HCV1 E1E2 (1a) antigen. Studies to address the optimal vaccination schedule, adjuvant, and antigen dose of our recombinant E1E2 antigen are in progress.
We anticipate that our novel Fc-d E1E2 antigen is an attractive candidate for an HCV vaccine and will provide an improved scalable method from previous GNA-based methods. In this study, Fc-d E1E2 was shown to display a highly comparable profile of neutralizing epitopes to WT (GNA-extracted) E1E2 by ELISA with cross-neutralizing MAbs (Fig. 2). Furthermore, Fc-d E1E2-vaccinated mice developed neutralization titers to HCVpp H77 (1a) that were similar to those with WT E1E2 (Fig. 3). To date, virtually all successful viral vaccines have been based on the induction of nAbs, usually via the targeting of viral coat proteins (58). For HCV, 1a E1E2 was shown previously to reduce the carrier rate in immunized chimpanzees and generate sterilizing immunity in some individuals in response to homologous and heterologous 1a virus challenges (35, 36, 57). Furthermore, E1E2 is the only candidate vaccine to demonstrate broad nAb responses from vaccinated human volunteers (38, 39, 41, 42). Soluble forms of E2 can be produced from mammalian and insect cells (20, 21, 59, 60), and rational vaccine design has been proposed based on E2 cross-neutralizing epitopes (19). However, it is important to note that cross-neutralizing epitopes that contain E1 and E2 residues, such as those recognized by the broadly neutralizing MAbs AR4A and AR5A (15), are not represented in soluble E2 proteins. Furthermore, recombinant 1b E1, but not recombinant E2, provided protection to immunized chimpanzees from homologous genotype 1b viral challenge (61). For these reasons, we believe that E1E2 remains a prime candidate for HCV vaccine development.
MATERIALS AND METHODS
Cell cultures and antibodies.
CHO cells stably expressing recombinant E1E2 constructs from the genotype 1a H77c strain (GenBank accession no. AF009606) were propagated in Iscove's modified Dulbecco's medium (Thermo Fisher Scientific, Waltham, MA) containing 10% heat-inactivated fetal bovine serum (FBS) (Thermo Fisher Scientific), 0.1 mM/0.016 mM sodium hypoxanthine/thymidine (HT supplement; Thermo Fisher Scientific), 0.002 mM methotrexate, and 100 U/ml of penicillin and 100 μg/ml of streptomycin (PenStrep; Invitrogen, Carlsbad, CA). Huh7.5 cells were propagated in Dulbecco's modified Eagle's medium (Thermo Fisher Scientific) containing 10% heat-inactivated fetal bovine serum (Omega Scientific, Tarzana, CA), 0.1 mM nonessential amino acids (Invitrogen), and PenStrep (Invitrogen). The MAb mouse anti-cluster of differentiation 81 (CD81) clone JS-81 (BD Biosciences, Franklin Lakes, NJ), mouse isotype control IgG1 (R&D Systems, Minneapolis, MN), anti-HCV MAbs (HC33.4, HC84.26, AR3B, AR4A, and AR5A), and human anti-HIV antibody B6 have been described previously (15, 16, 44, 45). A4 and H52 were provided by Jean Dubuisson (62, 63).
Expression and purification of recombinant E1E2 antigens.
The E1E2 glycoprotein coding region from H77c (genotype 1a) (GenBank accession no. AF009606; amino acids 192 to 746), preceded by the signal peptide sequence for tissue plasminogen activator (tPA), was inserted into the SpeI/MluI site of the pTRIP lentiviral vector bearing an internal ribosome entry site (IRES)-AcGFP reporter (64). For the Fc-tagged E1E2 construct, a duplication of amino acids 384 to 385 (ET) was inserted at the N terminus of E2, followed by the human IgG1 Fc tag (227 amino acids) and a PreScission protease/human rhinovirus protease 3C (HRV3C) sequence (LEVLFQGP) (Fig. 1A). Lentiviral particles were generated in HEK-293 T cells according to a previous method (64), and CHO cells were transduced with packaged lentivirus. GFP-positive CHO cells expressing WT or Fc-tagged recombinant E1E2 were sorted by flow cytometry using a BD FACSAria III cell sorter (BD Biosciences) and then suspension adapted in PROCHO4 medium (Lonza, Walkersville, MD) with 6% FBS in 250-ml shaker flasks (Corning, Corning, NY) and expanded in 3-liter spinner flasks (Corning).
Recombinant WT E1E2 was purified from CHO cell extracts using GNA (Vector Laboratories, Burlingame, CA) according to a previous study (37). The GNA eluate fraction was loaded on to a hydroxyapatite (HAP) column (Bio-Rad, Hercules, CA; 158-8000), and the flowthrough was concentrated with a 50,000-molecular-weight cutoff centrifugal filter unit (EMD Millipore, Billerica, MA). For Fc-d E1E2, the CHO cell extract was applied to protein G Sepharose 4 Fast Flow (GE Healthcare, Piscataway, NJ) and washed with 10 mM sodium phosphate–80 mM NaCl–0.1% TX-100 (pH 6.8), and the resin was digested with His6-GST-HRV3C protease (Thermo Fisher Scientific) overnight at 4°C. The digested material was applied to glutathione Sepharose 4B (GE Healthcare) to remove the protease, and the flowthrough was applied to HAP with a final concentration as described for WT E1E2.
Immunization of mice and serum samples.
Female CB6F1 mice (Charles River Laboratories, Montreal, QC, Canada) (5 to 7 weeks old) used for vaccination experiments were cared for in accordance with the Canadian Council on Animal Care guidelines. Experimental methods were reviewed and approved by the University of Alberta Health Sciences Animal Welfare Committee. Recombinant E1E2 H77 antigens (2 μg) were mixed in a 1:1 ratio with 75 μg of alum and 7.5 μg of monophosphoryl lipid A (MPLA Vaccigrade) (Invivogen, San Diego, CA) (30-μl final injection volume). Mice were injected intramuscularly at days 0, 14, and 28. Prevaccination serum was collected at day 0 and postvaccination sera (terminal bleeds) were obtained at day 42. Sera were collected after centrifugation of the samples at 5,000 × g for 15 min. Sera were heat inactivated by incubation at 56°C for 30 min and stored in aliquots at −80°C until use.
ELISA. (i) E1E2 ELISA.
Microtiter plates (Corning) were coated with E1E2 antigens (100 ng/well) in carbonate buffer (15 mM sodium carbonate, 35 mM sodium bicarbonate [pH 9.6]) overnight at 4°C. Plates were washed with phosphate-buffered saline containing 0.2% Tween 20 (PBST) and blocked for 1 h in 4% bovine serum albumin (Sigma-Aldrich, St. Louis, MO) in PBST. E2-specific MAbs (HC33.4, HC84.26, and AR3B) (16, 44, 45), E1E2-specific MAbs (AR4A and AR5A) (15), or control (B6) (16) MAb (50 μl/well) was added for 1 h and detected by an anti-human alkaline phosphatase-conjugated secondary antibody (1:10,000; Jackson Immuno Research, West Grove, PA) and p-nitrophenylphosphate (Millipore Sigma) substrate. Absorbance (405 to 495 nm) was read using an Enspire plate reader (Perkin-Elmer, Waltham, MA).
(ii) E2 ELISA.
Microtiter plates were coated with E2 (amino acids 384 to 661) HCV1 (GenBank accession no. M62321.1) and blocked in 4% bovine serum albumin as described for the E1E2 ELISA. Antisera from vaccinated mice (terminal bleeds) were diluted in PBST and added to the plates for 1 h (50 μl/well). E2-specific antibodies from mouse antisera were detected by a horseradish peroxidase-conjugated goat anti-mouse secondary antibody (1:10,000; Cedarlane Laboratories, Burlington, ON, Canada) and peroxidase substrate (KPL, Gaithersburg, MD). Absorbance was read at 450 to 570 nm. Absorbance values from three independent experiments are expressed as means ± standard errors of the means (SEM). E2 HCV1 was kindly provided by Joseph Marcotrigiano.
(iii) Competition ELISA.
Mouse antisera (terminal bleeds) were assessed for competition with conformation-specific E2 MAbs for E1E2 binding according to a method described previously (42). Briefly, microtiter plates (Corning) were coated with GNA-purified WT E1E2 H77c in carbonate buffer overnight at 4°C and blocked in 1% casein (Sigma-Aldrich) in PBST. Diluted mouse antisera were incubated for 1 h in E1E2-coated wells. HCV-specific (HC33.4, HC84.26, AR3B, AR4A, and AR5A) or control (B6 anti-HIV) MAbs were added at a concentration normally resulting in 70% maximal binding. Binding of HCV-specific MAbs was detected by an anti-human alkaline phosphatase-conjugated secondary antibody (1:10,000; Jackson Immuno Research) and p-nitrophenylphosphate (Millipore Sigma) substrate. Absorbance was read at 405 to 495 nm. Values were calculated as the percentage of MAb binding relative to the MAb bound in the absence of antiserum. Data are plotted as means ± standard error of the means from three independent experiments.
HCVpp production and neutralization assay.
HCV pseudotype virus (HCVpp) expressing a luciferase reporter was generated as described previously (65). For neutralization assays, human hepatoma cells (Huh7.5) were plated on poly-lysine-coated 96-well plates 1 day prior to infection. HCVpp was diluted 1:10 and premixed with heat-inactivated diluted sera for 1 h at 37°C, followed by addition to Huh7.5 cells. Six hours postinfection, the antibody-virus inoculum was replaced with fresh culture medium. Cells were processed 48 h postinfection using the Bright-glo luciferase assay system (Promega, Madison, WI). Luminescence was measured using an Enspire plate reader (PerkinElmer). Neutralization activity was calculated using the following formula: percent neutralization = (pre-post)/pre × 100 where pre-post represents the luciferase activity done after incubating with either the pre- or postvaccination sera. For ID50 titers, 2-fold dilutions of sera from 1:25 to 1:1,600 were examined. Titers were expressed as a reciprocal of the dilution calculated to neutralize 50% of the virus. If 50% neutralization was not achieved at the highest concentration examined (1:25), then the next highest concentration was assigned to the sample (i.e., 1:12.5). Similarly, if the lowest dilution examined (1:1,600) resulted in >50% neutralization, we assigned the next dilution for this sample (i.e., 1:3,200).
ACKNOWLEDGMENTS
We thank Jean Dubuisson for providing A4 and H52, Charles Rice for providing the pTRIP-IRES-AcGFP vector and other HCV-related reagents, and Jens Bukh and Francois-Loic Cosset for providing reagents for HCVpp production.
M.H. is a recipient of a Canada Excellence Research Chair.
REFERENCES
- 1.Hajarizadeh B, Grebely J, Dore GJ. 2013. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol 10:553–562. doi: 10.1038/nrgastro.2013.107. [DOI] [PubMed] [Google Scholar]
- 2.Chung RT, Baumert TF. 2014. Curing chronic hepatitis C—the arc of a medical triumph. N Engl J Med 370:1576–1578. doi: 10.1056/NEJMp1400986. [DOI] [PubMed] [Google Scholar]
- 3.Callaway E. 2014. Hepatitis C drugs not reaching poor. Nature 508:295–296. doi: 10.1038/508295a. [DOI] [PubMed] [Google Scholar]
- 4.Cox AL. 2015. Global control of hepatitis C virus. Science 349:790–791. doi: 10.1126/science.aad1302. [DOI] [PubMed] [Google Scholar]
- 5.Midgard H, Bjoro B, Maeland A, Konopski Z, Kileng H, Damas JK, Paulsen J, Heggelund L, Sandvei PK, Ringstad JO, Karlsen LN, Stene-Johansen K, Pettersson JH, Dorenberg DH, Dalgard O. 2016. Hepatitis C reinfection after sustained virological response. J Hepatol 64:1020–1026. doi: 10.1016/j.jhep.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Rosen HR. 2013. Emerging concepts in immunity to hepatitis C virus infection. J Clin Invest 123:4121–4130. doi: 10.1172/JCI67714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farci P, Shimoda A, Coiana A, Diaz G, Peddis G, Melpolder JC, Strazzera A, Chien DY, Munoz SJ, Balestrieri A, Purcell RH, Alter HJ. 2000. The outcome of acute hepatitis C predicted by the evolution of the viral quasispecies. Science 288:339–344. doi: 10.1126/science.288.5464.339. [DOI] [PubMed] [Google Scholar]
- 8.Logvinoff C, Major ME, Oldach D, Heyward S, Talal A, Balfe P, Feinstone SM, Alter H, Rice CM, McKeating JA. 2004. Neutralizing antibody response during acute and chronic hepatitis C virus infection. Proc Natl Acad Sci U S A 101:10149–10154. doi: 10.1073/pnas.0403519101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Hahn T, Yoon JC, Alter H, Rice CM, Rehermann B, Balfe P, McKeating JA. 2007. Hepatitis C virus continuously escapes from neutralizing antibody and T-cell responses during chronic infection in vivo. Gastroenterology 132:667–678. doi: 10.1053/j.gastro.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Keck ZY, Foung SK. 2011. Neutralizing antibody response to hepatitis C virus. Viruses 3:2127–2145. doi: 10.3390/v3112127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lavillette D, Morice Y, Germanidis G, Donot P, Soulier A, Pagkalos E, Sakellariou G, Intrator L, Bartosch B, Pawlotsky JM, Cosset FL. 2005. Human serum facilitates hepatitis C virus infection, and neutralizing responses inversely correlate with viral replication kinetics at the acute phase of hepatitis C virus infection. J Virol 79:6023–6034. doi: 10.1128/JVI.79.10.6023-6034.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osburn WO, Fisher BE, Dowd KA, Urban G, Liu L, Ray SC, Thomas DL, Cox AL. 2010. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology 138:315–324. doi: 10.1053/j.gastro.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pestka JM, Zeisel MB, Blaser E, Schurmann P, Bartosch B, Cosset FL, Patel AH, Meisel H, Baumert J, Viazov S, Rispeter K, Blum HE, Roggendorf M, Baumert TF. 2007. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C Proc Natl Acad Sci U S A 104:6025–6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sautto G, Tarr AW, Mancini N, Clementi M. 2013. Structural and antigenic definition of hepatitis C virus E2 glycoprotein epitopes targeted by monoclonal antibodies. Clin Dev Immunol 2013:450963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giang E, Dorner M, Prentoe JC, Dreux M, Evans MJ, Bukh J, Rice CM, Ploss A, Burton DR, Law M. 2012. Human broadly neutralizing antibodies to the envelope glycoprotein complex of hepatitis C virus. Proc Natl Acad Sci U S A 109:6205–6210. doi: 10.1073/pnas.1114927109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Law M, Maruyama T, Lewis J, Giang E, Tarr AW, Stamataki Z, Gastaminza P, Chisari FV, Jones IM, Fox RI, Ball JK, McKeating JA, Kneteman NM, Burton DR. 2008. Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nat Med 14:25–27. doi: 10.1038/nm1698. [DOI] [PubMed] [Google Scholar]
- 17.Meuleman P, Bukh J, Verhoye L, Farhoudi A, Vanwolleghem T, Wang RY, Desombere I, Alter H, Purcell RH, Leroux-Roels G. 2011. In vivo evaluation of the cross-genotype neutralizing activity of polyclonal antibodies against hepatitis C virus. Hepatology 53:755–762. doi: 10.1002/hep.24171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morin TJ, Broering TJ, Leav BA, Blair BM, Rowley KJ, Boucher EN, Wang Y, Cheslock PS, Knauber M, Olsen DB, Ludmerer SW, Szabo G, Finberg RW, Purcell RH, Lanford RE, Ambrosino DM, Molrine DC, Babcock GJ. 2012. Human monoclonal antibody HCV1 effectively prevents and treats HCV infection in chimpanzees. PLoS Pathog 8:e1002895. doi: 10.1371/journal.ppat.1002895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong L, Jackson KN, Wilson IA, Law M. 2015. Capitalizing on knowledge of hepatitis C virus neutralizing epitopes for rational vaccine design. Curr Opin Virol 11:148–157. doi: 10.1016/j.coviro.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan AG, Whidby J, Miller MT, Scarborough H, Zatorski AV, Cygan A, Price AA, Yost SA, Bohannon CD, Jacob J, Grakoui A, Marcotrigiano J. 2014. Structure of the core ectodomain of the hepatitis C virus envelope glycoprotein 2. Nature 509:381–384. doi: 10.1038/nature13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong L, Giang E, Nieusma T, Kadam RU, Cogburn KE, Hua Y, Dai X, Stanfield RL, Burton DR, Ward AB, Wilson IA, Law M. 2013. Hepatitis C virus E2 envelope glycoprotein core structure. Science 342:1090–1094. doi: 10.1126/science.1243876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brazzoli M, Helenius A, Foung SK, Houghton M, Abrignani S, Merola M. 2005. Folding and dimerization of hepatitis C virus E1 and E2 glycoproteins in stably transfected CHO cells. Virology 332:438–453. doi: 10.1016/j.virol.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 23.Meunier JC, Fournillier A, Choukhi A, Cahour A, Cocquerel L, Dubuisson J, Wychowski C. 1999. Analysis of the glycosylation sites of hepatitis C virus (HCV) glycoprotein E1 and the influence of E1 glycans on the formation of the HCV glycoprotein complex. J Gen Virol 80(Part 4):887–896. doi: 10.1099/0022-1317-80-4-887. [DOI] [PubMed] [Google Scholar]
- 24.Voisset C, Dubuisson J. 2004. Functional hepatitis C virus envelope glycoproteins. Biol Cell 96:413–420. doi: 10.1016/j.biolcel.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Choukhi A, Ung S, Wychowski C, Dubuisson J. 1998. Involvement of endoplasmic reticulum chaperones in the folding of hepatitis C virus glycoproteins. J Virol 72:3851–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubuisson J, Rice CM. 1996. Hepatitis C virus glycoprotein folding: disulfide bond formation and association with calnexin. J Virol 70:778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liberman E, Fong YL, Selby MJ, Choo QL, Cousens L, Houghton M, Yen TS. 1999. Activation of the grp78 and grp94 promoters by hepatitis C virus E2 envelope protein. J Virol 73:3718–3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cocquerel L, Meunier JC, Op de Beeck A, Bonte D, Wychowski C, Dubuisson J. 2001. Coexpression of hepatitis C virus envelope proteins E1 and E2 in cis improves the stability of membrane insertion of E2. J Gen Virol 82:1629–1635. doi: 10.1099/0022-1317-82-7-1629. [DOI] [PubMed] [Google Scholar]
- 29.Deleersnyder V, Pillez A, Wychowski C, Blight K, Xu J, Hahn YS, Rice CM, Dubuisson J. 1997. Formation of native hepatitis C virus glycoprotein complexes. J Virol 71:697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Falson P, Bartosch B, Alsaleh K, Tews BA, Loquet A, Ciczora Y, Riva L, Montigny C, Montpellier C, Duverlie G, Pecheur EI, le Maire M, Cosset FL, Dubuisson J, Penin F. 2015. Hepatitis C virus envelope glycoprotein E1 forms trimers at the surface of the virion. J Virol 89:10333–10346. doi: 10.1128/JVI.00991-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel J, Patel AH, McLauchlan J. 2001. The transmembrane domain of the hepatitis C virus E2 glycoprotein is required for correct folding of the E1 glycoprotein and native complex formation. Virology 279:58–68. doi: 10.1006/viro.2000.0693. [DOI] [PubMed] [Google Scholar]
- 32.Goffard A, Callens N, Bartosch B, Wychowski C, Cosset FL, Montpellier C, Dubuisson J. 2005. Role of N-linked glycans in the functions of hepatitis C virus envelope glycoproteins. J Virol 79:8400–8409. doi: 10.1128/JVI.79.13.8400-8409.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Catanese MT, Uryu K, Kopp M, Edwards TJ, Andrus L, Rice WJ, Silvestry M, Kuhn RJ, Rice CM. 2013. Ultrastructural analysis of hepatitis C virus particles. Proc Natl Acad Sci U S A 110:9505–9510. doi: 10.1073/pnas.1307527110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vieyres G, Thomas X, Descamps V, Duverlie G, Patel AH, Dubuisson J. 2010. Characterization of the envelope glycoproteins associated with infectious hepatitis C virus. J Virol 84:10159–10168. doi: 10.1128/JVI.01180-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choo QL, Kuo G, Ralston R, Weiner A, Chien D, Van Nest G, Han J, Berger K, Thudium K, Kuo C, Kansopon J, McFarland J, Tabrizi A, Ching K, Moss B, Cummins LB, Houghton M, Muchmore E. 1994. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc Natl Acad Sci U S A 91:1294–1298. doi: 10.1073/pnas.91.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Houghton M. 2011. Prospects for prophylactic and therapeutic vaccines against the hepatitis C viruses. Immunol Rev 239:99–108. doi: 10.1111/j.1600-065X.2010.00977.x. [DOI] [PubMed] [Google Scholar]
- 37.Ralston R, Thudium K, Berger K, Kuo C, Gervase B, Hall J, Selby M, Kuo G, Houghton M, Choo QL. 1993. Characterization of hepatitis C virus envelope glycoprotein complexes expressed by recombinant vaccinia viruses. J Virol 67:6753–6761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frey SE, Houghton M, Coates S, Abrignani S, Chien D, Rosa D, Pileri P, Ray R, Di Bisceglie AM, Rinella P, Hill H, Wolff MC, Schultze V, Han JH, Scharschmidt B, Belshe RB. 2010. Safety and immunogenicity of HCV E1E2 vaccine adjuvanted with MF59 administered to healthy adults. Vaccine 28:6367–6373. doi: 10.1016/j.vaccine.2010.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Law JL, Chen C, Wong J, Hockman D, Santer DM, Frey SE, Belshe RB, Wakita T, Bukh J, Jones CT, Rice CM, Abrignani S, Tyrrell DL, Houghton M. 2013. A hepatitis C virus (HCV) vaccine comprising envelope glycoproteins gpE1/gpE2 derived from a single isolate elicits broad cross-genotype neutralizing antibodies in humans. PLoS One 8:e59776. doi: 10.1371/journal.pone.0059776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ray R, Meyer K, Banerjee A, Basu A, Coates S, Abrignani S, Houghton M, Frey SE, Belshe RB. 2010. Characterization of antibodies induced by vaccination with hepatitis C virus envelope glycoproteins. J Infect Dis 202:862–866. doi: 10.1086/655902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stamataki Z, Coates S, Abrignani S, Houghton M, McKeating JA. 2011. Immunization of human volunteers with hepatitis C virus envelope glycoproteins elicits antibodies that cross-neutralize heterologous virus strains. J Infect Dis 204:811–813. doi: 10.1093/infdis/jir399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong JA, Bhat R, Hockman D, Logan M, Chen C, Levin A, Frey SE, Belshe RB, Tyrrell DL, Law JL, Houghton M. 2014. Recombinant hepatitis C virus envelope glycoprotein vaccine elicits antibodies targeting multiple epitopes on the envelope glycoproteins associated with broad cross-neutralization. J Virol 88:14278–14288. doi: 10.1128/JVI.01911-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heile JM, Fong YL, Rosa D, Berger K, Saletti G, Campagnoli S, Bensi G, Capo S, Coates S, Crawford K, Dong C, Wininger M, Baker G, Cousens L, Chien D, Ng P, Archangel P, Grandi G, Houghton M, Abrignani S. 2000. Evaluation of hepatitis C virus glycoprotein E2 for vaccine design: an endoplasmic reticulum-retained recombinant protein is superior to secreted recombinant protein and DNA-based vaccine candidates. J Virol 74:6885–6892. doi: 10.1128/JVI.74.15.6885-6892.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keck Z, Wang W, Wang Y, Lau P, Carlsen TH, Prentoe J, Xia J, Patel AH, Bukh J, Foung SK. 2013. Cooperativity in virus neutralization by human monoclonal antibodies to two adjacent regions located at the amino terminus of hepatitis C virus E2 glycoprotein. J Virol 87:37–51. doi: 10.1128/JVI.01941-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keck ZY, Xia J, Wang Y, Wang W, Krey T, Prentoe J, Carlsen T, Li AY, Patel AH, Lemon SM, Bukh J, Rey FA, Foung SK. 2012. Human monoclonal antibodies to a novel cluster of conformational epitopes on HCV E2 with resistance to neutralization escape in a genotype 2a isolate. PLoS Pathog 8:e1002653. doi: 10.1371/journal.ppat.1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prentoe J, Bukh J. 2011. Hepatitis C virus expressing flag-tagged envelope protein 2 has unaltered infectivity and density, is specifically neutralized by flag antibodies and can be purified by affinity chromatography. Virology 409:148–155. doi: 10.1016/j.virol.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 47.Shibuya N, Goldstein IJ, Van Damme EJ, Peumans WJ. 1988. Binding properties of a mannose-specific lectin from the snowdrop (Galanthus nivalis) bulb. J Biol Chem 263:728–734. [PubMed] [Google Scholar]
- 48.Balzarini J, Hatse S, Vermeire K, Princen K, Aquaro S, Perno CF, De Clercq E, Egberink H, Vanden Mooter G, Peumans W, Van Damme E, Schols D. 2004. Mannose-specific plant lectins from the Amaryllidaceae family qualify as efficient microbicides for prevention of human immunodeficiency virus infection. Antimicrob Agents Chemother 48:3858–3870. doi: 10.1128/AAC.48.10.3858-3870.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fahrner RL, Knudsen HL, Basey CD, Galan W, Feuerhelm D, Vanderlaan M, Blank GS. 2001. Industrial purification of pharmaceutical antibodies: development, operation, and validation of chromatography processes. Biotechnol Genet Eng Rev 18:301–327. doi: 10.1080/02648725.2001.10648017. [DOI] [PubMed] [Google Scholar]
- 50.Liu HF, Ma J, Winter C, Bayer R. 2010. Recovery and purification process development for monoclonal antibody production. MAbs 2:480–499. doi: 10.4161/mabs.2.5.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghose S, Hubbard B, Cramer SM. 2007. Binding capacity differences for antibodies and Fc-fusion proteins on protein A chromatographic materials. Biotechnol Bioeng 96:768–779. doi: 10.1002/bit.21044. [DOI] [PubMed] [Google Scholar]
- 52.Owsianka AM, Timms JM, Tarr AW, Brown RJ, Hickling TP, Szwejk A, Bienkowska-Szewczyk K, Thomson BJ, Patel AH, Ball JK. 2006. Identification of conserved residues in the E2 envelope glycoprotein of the hepatitis C virus that are critical for CD81 binding. J Virol 80:8695–8704. doi: 10.1128/JVI.00271-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ball JK, Tarr AW, McKeating JA. 2014. The past, present and future of neutralizing antibodies for hepatitis C virus. Antiviral Res 105:100–111. doi: 10.1016/j.antiviral.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mancini N, Diotti RA, Perotti M, Sautto G, Clementi N, Nitti G, Patel AH, Ball JK, Clementi M, Burioni R. 2009. Hepatitis C virus (HCV) infection may elicit neutralizing antibodies targeting epitopes conserved in all viral genotypes. PLoS One 4:e8254. doi: 10.1371/journal.pone.0008254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sabo MC, Luca VC, Prentoe J, Hopcraft SE, Blight KJ, Yi M, Lemon SM, Ball JK, Bukh J, Evans MJ, Fremont DH, Diamond MS. 2011. Neutralizing monoclonal antibodies against hepatitis C virus E2 protein bind discontinuous epitopes and inhibit infection at a postattachment step. J Virol 85:7005–7019. doi: 10.1128/JVI.00586-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stamataki Z, Coates S, Evans MJ, Wininger M, Crawford K, Dong C, Fong YL, Chien D, Abrignani S, Balfe P, Rice CM, McKeating JA, Houghton M. 2007. Hepatitis C virus envelope glycoprotein immunization of rodents elicits cross-reactive neutralizing antibodies. Vaccine 25:7773–7784. doi: 10.1016/j.vaccine.2007.08.053. [DOI] [PubMed] [Google Scholar]
- 57.Meunier JC, Gottwein JM, Houghton M, Russell RS, Emerson SU, Bukh J, Purcell RH. 2011. Vaccine-induced cross-genotype reactive neutralizing antibodies against hepatitis C virus. J Infect Dis 204:1186–1190. doi: 10.1093/infdis/jir511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burton DR. 2002. Antibodies, viruses and vaccines. Nat Rev Immunol 2:706–713. doi: 10.1038/nri891. [DOI] [PubMed] [Google Scholar]
- 59.Krey T, d'Alayer J, Kikuti CM, Saulnier A, Damier-Piolle L, Petitpas I, Johansson DX, Tawar RG, Baron B, Robert B, England P, Persson MA, Martin A, Rey FA. 2010. The disulfide bonds in glycoprotein E2 of hepatitis C virus reveal the tertiary organization of the molecule. PLoS Pathog 6:e1000762. doi: 10.1371/journal.ppat.1000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Whidby J, Mateu G, Scarborough H, Demeler B, Grakoui A, Marcotrigiano J. 2009. Blocking hepatitis C virus infection with recombinant form of envelope protein 2 ectodomain. J Virol 83:11078–11089. doi: 10.1128/JVI.00800-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verstrepen BE, Depla E, Rollier CS, Mares G, Drexhage JA, Priem S, Verschoor EJ, Koopman G, Granier C, Dreux M, Cosset FL, Maertens G, Heeney JL. 2011. Clearance of genotype 1b hepatitis C virus in chimpanzees in the presence of vaccine-induced E1-neutralizing antibodies. J Infect Dis 204:837–844. doi: 10.1093/infdis/jir423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dubuisson J, Hsu HH, Cheung RC, Greenberg HB, Russell DG, Rice CM. 1994. Formation and intracellular localization of hepatitis C virus envelope glycoprotein complexes expressed by recombinant vaccinia and Sindbis viruses. J Virol 68:6147–6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patel AH, Wood J, Penin F, Dubuisson J, McKeating JA. 2000. Construction and characterization of chimeric hepatitis C virus E2 glycoproteins: analysis of regions critical for glycoprotein aggregation and CD81 binding. J Gen Virol 81:2873–2883. doi: 10.1099/0022-1317-81-12-2873. [DOI] [PubMed] [Google Scholar]
- 64.Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, Rice CM. 2011. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 472:481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hsu M, Zhang J, Flint M, Logvinoff C, Cheng-Mayer C, Rice CM, McKeating JA. 2003. Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proc Natl Acad Sci U S A 100:7271–7276. doi: 10.1073/pnas.0832180100. [DOI] [PMC free article] [PubMed] [Google Scholar]