ABSTRACT
Porcine circovirus-associated disease (PCVAD) is clinically manifested by postweaning multisystemic wasting syndrome (PMWS), respiratory and enteric disease, reproductive failure, and porcine dermatitis and nephropathy syndrome (PDNS). Porcine circovirus 2 (PCV2) is an essential component of PCVAD, although an etiologic role in PDNS is not well established. Here, a novel circovirus, designated porcine circovirus 3 (PCV3), was identified in sows that died acutely with PDNS-like clinical signs. The capsid and replicase proteins of PCV3 are only 37% and 55% identical to PCV2 and bat circoviruses, respectively. Aborted fetuses from sows with PDNS contained high levels of PCV3 (7.57 × 107 genome copies/ml), and no other viruses were detected by PCR and metagenomic sequencing. Immunohistochemistry (IHC) analysis of sow tissue samples identified PCV3 antigen in skin, kidney, lung, and lymph node samples localized in typical PDNS lesions, including necrotizing vasculitis, glomerulonephritis, granulomatous lymphadenitis, and bronchointerstitial pneumonia. Further study of archived PDNS tissue samples that were negative for PCV2 by IHC analysis identified 45 of 48 that were PCV3 positive by quantitative PCR (qPCR), with 60% of a subset also testing positive for PCV3 by IHC analysis. Analysis by qPCR of 271 porcine respiratory disease diagnostic submission samples identified 34 PCV3-positive cases (12.5%), and enzyme-linked immunosorbent assay detection of anti-PCV3 capsid antibodies in serum samples found that 46 (55%) of 83 samples tested were positive. These results suggest that PCV3 commonly circulates within U.S. swine and may play an etiologic role in reproductive failure and PDNS. Because of the high economic impact of PCV2, this novel circovirus warrants further studies to elucidate its significance and role in PCVAD.
IMPORTANCE While porcine circovirus 2 (PCV2) was first identified in sporadic cases of postweaning multisystemic wasting syndrome in Canada in the early 1990s, an epidemic of severe systemic disease due to PCV2 spread worldwide in the ensuing decade. Despite being effectively controlled by commercial vaccines, PCV2 remains one of the most economically significant viruses of swine. Here, a novel porcine circovirus (PCV3) that is distantly related to known circoviruses was identified in sows with porcine dermatitis and nephropathy syndrome (PDNS) and reproductive failure. PCV2, which has previously been associated with these clinical presentations, was not identified. High levels of PCV3 nucleic acid were observed in aborted fetuses by quantitative PCR, and PCV3 antigen was localized in histologic lesions typical of PDNS in sows by immunohistochemistry (IHC) analysis. PCV3 was also identified in archival PDNS diagnostic samples that previously tested negative for PCV2 by IHC analysis. The emergence of PCV3 warrants further investigation.
KEYWORDS: porcine circovirus, abortion, porcine dermatitis and nephropathy syndrome
INTRODUCTION
Circoviruses (family Circoviridae, genus Circovirus) are circular, single-stranded DNA viruses that are the smallest known autonomously replicating viruses (1). Circoviruses have an ambisense genome organization containing two major open reading frames (ORFs), rep and cap, on opposite strands of the double-stranded DNA replicative intermediate. While the initial descriptions of circoviruses were mainly from avian species, numerous members of the family Circoviridae have been characterized in fish, insects, and mammals (2–4). Two species of circovirus are known to infect pigs. Porcine circovirus 1 (PCV1) was first identified as a cell culture contaminate and has not been associated with clinical disease (5). In contrast, PCV2 is a ubiquitous, economically significant pathogen that has been associated with a diverse range of clinical diseases (6, 7). Despite the marked difference in pathogenicity between these two porcine circoviruses, the nucleotide sequence identity between the PCV1 and PCV2 rep and cap genes is ∼83% and 67%, respectively (8).
PCV2 was first sporadically identified in pigs with postweaning multisystemic wasting syndrome (PMWS) in Canada in the mid-1990s (7). Epidemics of severe systemic disease, clinically presenting as wasting and failure to thrive, were subsequently observed in Europe and Asia, followed by North America (7, 9–15). Histologic lesions typified by lymphoid depletion and lymphohistiocytic or granulomatous inflammation of multiple organs were described. PMWS diagnosis requires PCV2 antigen detection by immunohistochemistry (IHC) analysis or in situ hybridization (16). PCV2 infection was subsequently associated with other clinical presentations, together termed porcine circovirus-associated disease (PCVAD), which includes PMWS, pneumonia, porcine dermatitis and nephropathy syndrome (PDNS), and reproductive failure (17–21).
The wide range of clinical diseases under the PCVAD umbrella is in part due to coinfecting pathogens. By itself, PCV2 infection is typically subclinical (22). Reproduction of PMWS under controlled conditions has included coinfection with pathogens such as porcine parvovirus (PPV), porcine reproductive and respiratory syndrome virus (PRRSV), and Mycoplasma hyopneumoniae (23, 24). While PDNS is considered a PCVAD because of the association between clinical disease and PCV2 detection, the etiologic role of PCV2 has yet to be established. Clinically, PDNS is distinctive, with apparent well demarcated red-to-dark macules and papules. The kidneys are often enlarged and pale and present cortical petechiae. Histologically, PDNS is characterized by systemic necrotizing vasculitis along with glomerulitis and interstitial nephritis (25–27). The pathogenesis of this systemic vasculitis, as well as the fibrinous glomerulonephritis, is thought to be immune complex mediated. Importantly, PDNS has been reproduced experimentally without PCV2 by using PRRSV and a tissue homogenate containing torque teno virus (TTV) (28).
In 2015, on a farm in North Carolina, sows with chronic reproductive problems, including an above-average sow mortality rate and below-average conception rates, experienced an increased sow mortality rate with clinical signs consistent with PDNS. Concurrently, aborted mummified fetuses collected from sows on the same farm had similar clinical signs. While histological lesions in the sows were consistent with PDNS, the tissues all tested negative for PCV2 by IHC analysis and quantitative PCR (qPCR). Metagenomic sequencing revealed the presence of a novel, genetically divergent circovirus designated PCV3. In addition to this outbreak study, we performed a retrospective study of cases with lesions consistent with PDNS but negative for PCV2 by IHC analysis and evaluated the prevalence of PCV3 nucleic acids in swine diagnostic submission samples and anti-PCV3 antibodies in swine serum samples. The results obtained in these studies show that PCV3 is widely distributed in the U.S. swine population.
RESULTS
Clinical and histological findings of an outbreak of PDNS-like disease on a commercial sow farm.
During June 2015, a commercial swine operation in North Carolina experienced a 10.2% increase in the sow mortality rate and a 0.6% decrease in the conception rate compared to historical farm averages because of an outbreak of PDNS. Clinically, affected sows were anorexic and presented multifocal papules, macules, and superficial dermatitis (Fig. 1a). Tissue samples were submitted to the Iowa State University Veterinary Diagnostic Laboratory (ISUVDL) for diagnostic testing. Histologically, skin lesions were characterized by acute necrotic dermatitis and epidermitis associated with lymphoplasmacytic perivascular cuffs. The kidneys displayed dilated cortical tubules and attenuation and regeneration of the tubular lining epithelium, and large clusters of lymphocytes and macrophages diffusely infiltrated the cortical interstitium and glomeruli. The farm experienced an increase of 1.19 aborted mummified fetuses per litter above the historical average abortion rate. The aborted litters contained mummified fetuses of various gestational ages, consistent with those previously described in PCV2-associated abortion (Fig. 1b) (29). While the gross and histological lesions observed in sows, as well as the presence of abortions, were consistent with PCVAD, all sow tissue samples, including kidney, lymph node, lung, and skin samples, tested negative by IHC analysis and qPCR for PCV2, PRRSV, and influenza A virus (IAV). In addition, fetal tissues were negative for PCV2, PRRSV, and PPV by qPCR.
FIG 1.
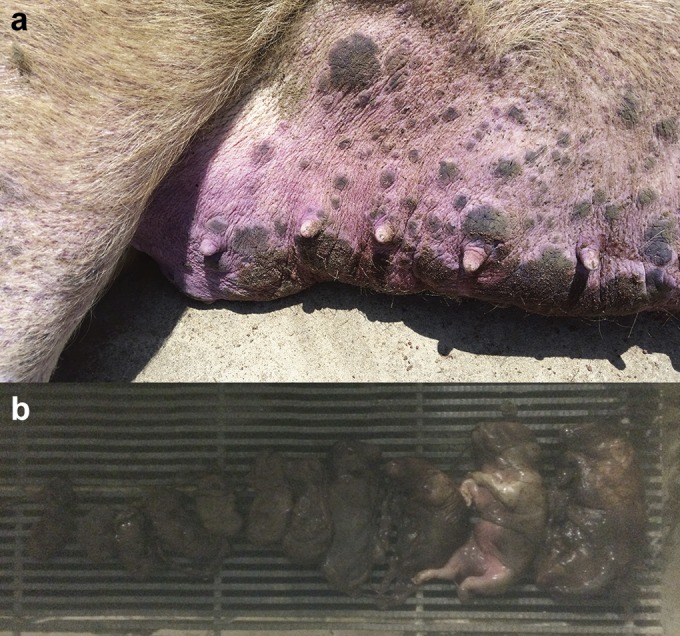
Clinical signs of disease in sows infected with PCV3 at a North Carolina farm. Shown are purple papules and macules (a) and mummified fetuses of various crown-to-rump lengths aborted from sows with lesions similar to those seen in sows with porcine dermatitis and nephropathy syndrome (b).
Metagenomic sequencing.
A tissue homogenate pool prepared from the three fetuses was analyzed by viral metagenomic sequencing. The MiSeq run generated a total of 989,478 reads, with 926,380 mapping to the host (Sus scrofa) genome. The remaining reads were assembled de novo, resulting in 27 contigs. Approximately 54% of the reads mapped to a 1,246-bp contig that was 98% similar to a partial circovirus genome identified in commercial ground pork, PorkNW2/USA/2009 (accession no. HQ738638), when analyzed by BLASTN. The remaining reads showed no similarity to any known eukaryotic virus.
Metagenomic sequencing of a pooled tissue homogenate from the sows with PDNS-like lesions was also performed. De novo assembly of sequences not mapping to the S. scrofa reference (approximately 2.5 million sequences) yielded 735 contigs that were analyzed by BLASTN. Two contigs were ∼97% identical to TTV-1. Assembly with a PCV3 reference (accession no. KT869077) identified four reads mapping to the genome. The remaining reads showed no homology to known eukaryotic viruses.
Genetic analysis.
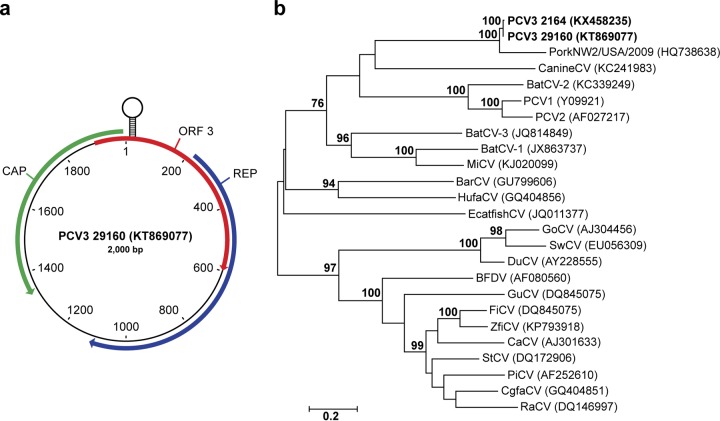
Rolling-circle amplification, followed by PCR and Sanger sequencing of the resulting amplicons, allowed the assembly of a 2,000-nucleotide (nt) circular genome from the fetal tissue homogenate. Open reading frame (ORF) analysis identified three ORFs encoding proteins of >200 amino acids (aa), with two ORFs showing homology to the circovirus rep and cap proteins by BLASTP and oriented in opposite directions (Fig. 2a). Within the 235-nt 5′ intergenic region between the rep and cap ORFs on the rep gene strand was a predicted stem-loop structure with a 9-nt stem-and-loop nonamer identical to PCV1 (TAGTATTAC) (3). Following convention, the A residue at position 8 in the loop nonamer was defined as position 1 in the genome (30).
FIG 2.
The genomic organization and phylogeny of PCV3. (a) The 2,000-nt ambisense genome contains three ORFs encoding >200-aa capsid (CAP) and replicase (REP) proteins and a protein of unknown function (ORF3). (b) Phylogenetic analysis of predicted nucleotide sequences of full-length circovirus genomes. The phylogenetic tree was constructed by maximum-likelihood analysis by using the general time-reversible model with gamma distribution and invariant sites with tree topology evaluated with 1,000 bootstrap replicates. GenBank accession numbers are in parentheses. Previously published sequences used for phylogenetic analysis included barbel circovirus (BarCV), GU799606; bat circovirus-1 (BtCV-1), JX863737; BtCV-2, KC339249; BtCV-3, JQ814849; BFDV, AF080560; canary circovirus, AJ301633; CanineCV, KC241983; chimpanzee feces-associated circovirus, GQ404851; DuCV, AY228555; European catfish circovirus, JQ011377; finch circovirus, DQ845075; GoCV, AJ304456; gull circovirus, DQ845074; HufaCV, GQ404856; mink circovirus, KJ020099; PiCV, AF252610; PCV1, Y09921; PCV2, AF027217; PorkNW2/USA/2009, HQ738638; raven circovirus, DQ146997; starling circovirus, DQ172906; swan circovirus, EU056309; and zebra finch circovirus, KP793918.
The largest ORF encoded a predicted 297-aa protein that by BLASTP was 69.4% identical to a partial replicase protein of Circoviridae PorkNW2/USA/2009 (accession no. ADU77001, 221 aa) and 54% identical to a bat circovirus from China (accession no. AIF76248, 293 aa). The PorkNW2/USA/2009 genome was obtained from commercial pork meat products and includes a complete replicase and partial capsid gene most similar in organization and sequence to that of circoviruses (31). Conserved circovirus replicase and helicase domains were identified by BLASTP from aa 9 to 93 and 162 to 251, respectively, in the rep ORF. Further examination of the rep ORF protein sequence revealed conserved rolling-circle replication (RCR) motifs and a P-loop motif similar to those of goose circovirus (GoCV) and pigeon circovirus (PiCV) (30, 32). Of the three RCR motifs conserved among circoviruses, in PCV3, the FTLNN motif contained a single mutation, present as FTINN. This mutation is seen in other circoviruses such as GoCV (30). The other two RCR motifs, HLQG and YCKK, are present in PCV3 as well. Moreover, three motifs conserved among circovirus replicase proteins but having unknown functions were identified in PCV3, including WWDGY (aa 196 to 200), DDFYGWVP (aa 209 to 216), and DRYP (aa 225 to 228). Interestingly, a canonical start codon was not identified. A GTC codon (encoding valine) is present at 5′ end of the ORF, with the closest in-frame ATG present approximately 400 bp downstream. This alternative start codon was also seen in PorkNW2/USA/2009. Alternative initiation codons have been proposed for a number of avian circoviruses, including GoCV, PiCV, and beak and feather disease virus (BFDV) (33–35).
The putative cap ORF, in the opposite orientation from rep, encodes a 214-aa protein 87% identical to the partial capsid sequence (110 aa) of PorkNW2/USA/2009 and 36 to 37% identical to PCV2 and duck circovirus (DuCV) (233 and 257 aa, respectively) by BLASTP (Fig. 2a). Similar to other circovirus capsid proteins, the N terminus contained numerous arginine residues and was highly basic. A conserved circovirus capsid domain was identified by BLASTP at aa 26 to 173. Additionally, the PCV3 cap protein had no predicted N-linked glycosylation sites but had two predicted O-linked glycosylation sites at aa 146 and 150 (S and T, respectively). This contrasts to PCV2, which has two experimentally verified N-linked glycosylation sites (36).
The third ORF, oriented on the same strand as the predicted rep ORF, encodes a 231-aa protein that is 94% identical to an ORF identified in PorkNW2/USA/2009 and 39% identical to murid herpesvirus M169, a protein of unknown function (37). Similar to that of rep, the initiation codon for ORF3 is unclear. The codon at the 5′ end is TCG (encoding serine). A methionine at ORF3 aa 55 is an alternate possible initiation site, which would yield a 177-aa protein.
Owing to the genetic and structural similarity to the genus Circovirus and the <70% capsid amino acid sequence identity with other species, we propose the novel species as PCV3 (1).
Phylogenetic analysis.
To investigate the evolutionary relationship of PCV3 to other members of the family Circoviridae, genome sequences from 23 members of the family and two PCV3 genomes were analyzed. Analysis of the full circovirus genomes grouped both PCV3 sequences in a clade with PorkNW2/USA/2009 separate from all other members of the genus (Fig. 2b). The phylogeny indicated that PCV3 was most closely related to canine circovirus (CanineCV, accession no. KC241983); however, this relationship lacked strong bootstrap support. The phylogeny also suggests that PCV3 and CanineCV have an ancestor in common with a clade containing PCV1, PCV2, and BatCV-2 (KC339249). With the exception of the human feces-associated circovirus (HufaCV, accession no. GQ404856), mammalian and avian circoviruses belonged to separate, well-supported clades.
Detection of PCV3 by PCR.
To confirm the presence of PCV3 in porcine samples, a 5′ nuclease assay was designed to determine the presence of the PCV3 cap gene. The fetal tissue homogenate samples from the outbreak in North Carolina were strongly positive for PCV3, with cycle threshold (CT) values between 16.7 and 21.3, corresponding to high PCV3 levels of approximately 1.88 × 108 and 7.55 × 106 genome copies (gc)/ml. Tissues from three of the sows with PDNS-like lesions were positive for PCV3, having between 2.13 × 104 and 8.62 × 104 gc/ml. In addition, 30 serum samples submitted to the ISUVDL were analyzed for PCV3 by qPCR. The serum samples were positive for PCV3, with 5.63 × 102 to 2.28 × 104 gc/ml. The serum sample with the highest PCV3 titer was amplified by PCR to generate overlapping amplicons to obtain a second complete PCV3 genome that was 99.0% identical to that of the original North Carolina PCV3 isolate.
Additionally, to investigate the prevalence of PCV3, 271 samples submitted to the ISUVDL for respiratory disease diagnostic testing were analyzed by qPCR. Thirty-four (12.5%) of the samples were positive, with titers of 3.00 × 102 to 1.52 × 107 gc/ml.
Characterization of PCV3-cap MAb 14.
HEK293 cells transfected with pSF-CMV-cap were incubated separately with four different monoclonal antibody (MAb) clonal cell supernatants and screened by immunofluorescence assay (IFA). Fluorescence localized to the nucleus (Fig. 3a), as expected owing to the predicted highly basic nuclear localization signal (38), was observed for clone 14 (MAb 14). No fluorescence was observed in cells transfected with pSF-CMV-Amp (Fig. 3b). In addition, swine testicle (ST) cells infected with PCV2 had no detectable fluorescence.
FIG 3.
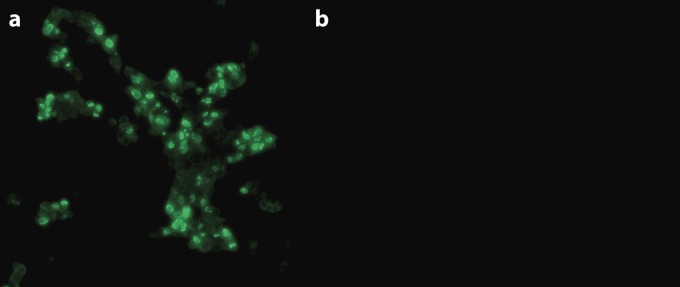
MAbs against PCV3 were generated by immunization of mice with purified N-terminally truncated capsid protein (aa 35 to 214). MAbs were screened by IFA with HEK293 cells transfected with plasmid pSF-CMV-cap bearing a native PCV3 capsid expression cassette (a) or empty plasmid pSF-CMV-AMP (b).
Virus isolation.
Virus isolation was attempted on ST and porcine kidney (PK-15) cells. Cells were inoculated with filtered fetal tissue homogenates and passaged three times. No cytopathic effects were evident, and CT values increased with each successive passage. No fluorescence was evident by IFA with MAb 14.
Histological lesions associated with the presence of PCV3 antigen in PDNS cases.
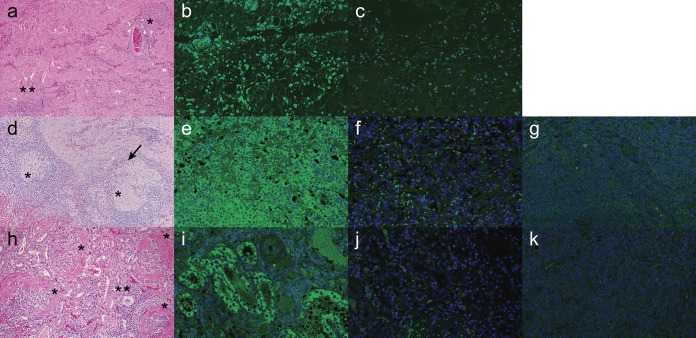
Tissue samples from North Carolina sows with PDNS-like lesions and archived PDNS cases were examined by hematoxylin-and-eosin (H&E) staining and IHC analysis with PCV3 MAb 14. Lungs showed variable degrees of bronchointerstitial pneumonia occasionally complicated by secondary suppurative bronchopneumonia. The small and medium-size airways and small blood vessels were cuffed with peribronchiolar and perivascular aggregates of lymphocytes and plasma cells. Adjacent alveolar septa were infiltrated by lymphocytes and plasma cells. Within alveolar lumina, there was abundant intraluminal edema intermixed with moderate numbers of foamy macrophages, rare multinucleated giant cells, and small clusters of neutrophils. Occasional lymphocytes and scattered macrophages showed moderate intracytoplasmic immunostaining against PCV3. In a section of skin, the dermis and subcutis presented necrotizing vasculitis with fibrinoid change and transmural neutrophilic infiltration, hemorrhage, and fibrin exudation (Fig. 4a). The inflammatory infiltrate often extended into the surrounding dermis and subcutis and often cuffed around vessels and dermal adnexa. Occasionally, the epidermis was hyperplastic with mild orthokeratotic hyperkeratosis. The dermal lymphocytic infiltration showed marked intracytoplasmic immunostaining against PCV3 (Fig. 4b). Minimal background staining was evident when PCV3 MAb 14 was replaced with phosphate-buffered saline (PBS) (Fig. 4c).
FIG 4.
Paraffin-embedded sections of skin (a to c), lymph node (d to g), and kidney (h to k) tissues stained with H&E (a, d, h) or PCV3 MAb 14 (b, e, i) from porcine dermatitis and nephropathy cases. (a) Sections of skin are characterized by necrotizing vasculitis with fibrinoid changes (*) and scattered lymphoplasmacytic dermatitis (**). (b) The dermal lymphocytic infiltration showed marked intracytoplasmic immunostaining against PCV3. (d) There is a multifocal granulomatous lymphadenitis (*) with the presence of multinucleated giant cells (arrow). (e) The follicular and perifollicular lymphocytic population showed diffuse intracytoplasmic staining against PCV3. (h) Kidneys are characterized by the presence of diffuse membranoproliferative glomerulonephritis (*) with minimal interstitial lymphoplasmacytic infiltration (**). (i) The tubular epithelium showed random areas of positive staining against PCV3. Negative staining of each tissue and background controls were performed by elimination of primary PCV3 MAb 14 and replacing it with PBS, followed by a goat anti-mouse secondary antibody (c, f, j). Lymph node and kidney tissues obtained from animals PCV3 negative by qPCR were concurrently stained with PCV3 MAb 14 and the goat anti-mouse secondary antibody to control for nonspecific antibody binding (g, k).
The lymph nodes showed diffuse granulomatous lymphadenitis, characterized by moderate cortical lymphoid depletion, occasionally replaced by histiocytes and numerous multinucleated giant cells (Fig. 4d). The follicular and perifollicular lymphocytic population showed diffuse, intense intracytoplasmic staining against PCV3 (Fig. 4e) compared to background staining, where PCV3 MAb 14 was replaced with PBS (Fig. 4f) or lymph node tissue from a pig PCV3 negative by qPCR (Fig. 4g).
Kidneys presented diffuse membrane proliferative glomerulonephritis (Fig. 4h) characterized by glomerulosclerosis and thickening of the Bowman's capsules, attenuation of the cortical tubules, and variable interstitial fibrosis. The tubules were occasionally ectatic and lined by attenuated epithelium and occasionally presented marked proteinosis. Scattered throughout the sections were small-to-medium-size clusters of lymphocytes and plasma cells in the interstitium. The tubular epithelium showed random areas of positive staining against PCV3 (Fig. 4i). Minimal background fluorescence was observed in slides mock stained with PBS and fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (Fig. 4j) or kidney tissue from a pig PCV3 negative by qPCR (Fig. 4k).
Detection of PCV3 nucleic acid in tissues with PDNS lesions.
To further investigate the etiologic role of PCV3 in PDNS, 48 cases with histological lesions consistent with PDNS that previously tested negative for PCV2 by IHC analysis were evaluated. Tissue scrolls from paraffin-embedded tissue blocks were assayed for PCV3 by qPCR. Forty-five (93.8%) of the cases were positive for PCV3, with viral titers of 1.60 × 104 to 3.47 × 104 gc/ml. To confirm these results, five of the samples with the highest viral titers were analyzed with a PCR assay targeting a 330-bp fragment of the cap gene. The target amplicon was amplified in all of the samples evaluated, and amplicon product sequences showed 100% identity with the PCV3 genome sequence. Tissues from five PCV3 PCR-positive cases were tested by PCV3 IHC analysis. Three of the five were positive.
PCV3 seroprevalence.
The prevalence of anti-PCV3 cap antibodies in swine serum samples were examined by enzyme-linked immunosorbent assay (ELISA) with rPCV3-cap antigen. Eighteen serum samples from 3-week-old pigs obtained from a specific-pathogen-free herd that tested PCV3 negative by qPCR were used as negative controls and had an average absorbance of 0.49. The cutoff value differentiating positive and negative serum samples was determined as 3 standard deviations above the mean of the negative controls (0.87). Serum samples collected from 10 sows 3 months after the PDNS outbreak on the farm in North Carolina were all positive, with an average absorbance of 1.27. Additionally, 27 serum samples from gilts from a farm that supplies replacement animals to the sow farm were also tested, with 17 animals (63%) having absorbances of 0.88 to 1.37. Anti-PCV3 cap antibodies were detected in 47 (56.6%) of 83 samples submitted for unrelated diagnostic testing from multiple states. Of the positive samples, 13 originated from Iowa, 1 was from Indiana, 5 were from Mexico, 4 were from North Carolina, 5 were from Nebraska, 1 was from Oklahoma, and 18 were of unknown origin.
DISCUSSION
First described in 1993 in Europe, PDNS has been reported in numerous countries worldwide (25, 39, 40). Although the prevalence of disease within a herd is typically low (<1%), the mortality rate of afflicted pigs can be high. The incidence of PDNS may exceed that of PMWS in Europe and the United Kingdom (41). Although the etiology of PDNS is unknown, PCV2 nucleic acid is commonly detected in affected pigs by qPCR, while PCV2 antigen is inconsistently detected (26, 42). This has led to speculation about the role of PCV2 in PDNS (43, 44). Here, we identified a highly divergent new species of porcine circovirus, designated PCV3, from mummified fetuses aborted from sows with PDNS-like lesions and from sows that died acutely with clinical signs consistent with PDNS. PCV3 was the only virus identified by metagenomic sequencing of pooled fetal tissue that was confirmed by qPCR. CT values of 16.7 to 21.3 in fetal tissue pools indicate high viral titers of 7.55 × 106 to 1.88 × 108 gc/ml of sample. There is a correlation between the PCV2 titer in the fetuses and reproductive disease, with PCV2 levels of 107 or more PCV2 DNA copies/500 ng of fetal tissue associated with PCV2-associated reproductive failure, including mummification (22, 45–47). On the basis of the amount of PCV3 nucleic acids and tissue distribution detected during this outbreak, we hypothesize a similar correlation between PCV3 and reproductive failure.
PCV3 was also detected by PCR and IHC analysis in the skin, lungs, kidneys, and lymph nodes of sows with PDNS-like lesions. The small amount of PCV3 in the sow serum samples (CT = 27.7 to 29.7) is likely responsible for the low number of PCV3 sequences identified by metagenomic sequencing. PCR, IHC analysis, and metagenomic sequencing all failed to identify PCV2 in the samples collected in North Carolina. These results suggest that PCV3 infection might contribute to the PDNS-like lesions and the presence of abortion and PCV3 in fetuses is the result of vertical transmission. In support of this potential etiologic role for PCV3 in PDNS lesions, screening of archived PCV2 IHC analysis-negative PDNS cases found that PCV3 nucleic acid was highly prevalent (93.8%) and three out of the five cases examined for PCV3 by IHC analysis were positive. We speculate that the relatively low titers of PCV3 in the tissues may limit consistent detection.
Attempts to reproduce PDNS experimentally with PCV2 have been unsuccessful; however, PDNS has been reproduced experimentally in the absence of PCV2 with PRRSV and a tissue homogenate containing TTV (28). Relatively little is known about the clinical significance of TTV infection. TTV is ubiquitous in pigs worldwide (48–50). While TTV is commonly detected in healthy pigs, several studies have suggested that TTV infection moderates disease severity during coinfections. For example, inoculation of pigs with a tissue homogenate containing TTV, followed by PCV2, resulted in PMWS, while monoinfections did not (28). The North Carolina sows with PDNS were infected with both PCV3 and TTV1. The impact of TTV1 coinfection is unknown. It is unclear whether coinfections with genetically diverse small circular DNA viruses such as TTV1 (Anelloviridae), PCV2, and PCV3 influence the development of PDNS. The additive effects on disease severity of coinfections with PCV2 have been demonstrated for PMWS (23, 51–53). A coinfection of PPV and PCV2 has been show to exacerbate disease (23). PCV2 and PRRSV coinfections are a significant component of the porcine respiratory disease complex (52). Infection of a herd with these agents causes severe respiratory disease and economically devastating sow abortions and mortality rates.
The pathogenesis of PDNS, which includes characteristic necrotizing vasculitis, is thought to be a manifestation of an immune complex-mediated disorder involving PCV2 (54, 55). In a case-control study, all pigs with clinical signs of PDNS were PCV2 PCR positive and had PCV2 antibody titers significantly higher than those of clinically normal pigs (53). Examination of the kidneys of PDNS-positive pigs found more fibrinoid deposits in the glomeruli consisting of accumulated IgG1, IgG2, IgM, and complement factors C1q and C3 than in clinically normal pigs. Although PCV2 antigen was identified in the lung tissue of these PDNS pigs by IHC analysis, PCV2 antigen was not identified in the immune complexes. This result is similar to the inconsistent detection of PCV2 in renal tissues of PDNS pigs reported by others (27). Viral infections are known to contribute to immunological disorders, of which Aleutian mink disease (AMD), caused by AMD virus (AMDV), has a pathogenesis similar to that of PDNS (55, 56). The pathogenesis of AMD has been associated with the overproduction of AMDV-specific IgG antibodies (56). A role for PCV3 in a possible immune complex-mediated disorder resulting in the pathogenesis of PDNS needs further study.
PCV2 is one of the most economically significant swine viral pathogens worldwide (57). The most common genotypes associated with PCVAD are PCV2a and PCV2b (58–60). Before 2003, PCV2a was the principal genotype identified in the United States and Canada, although both PCV2a and PCV2b were found internationally (61–63). Around 2003, a drastic shift occurred in the frequency of PCV2 genotypes globally from PCV2a to PCV2b coincident with severe systemic disease associated with PCV2b (61, 64). The worldwide epidemic was successfully controlled by the development of commercial vaccines that contain PCV2a antigen and have been shown to be cross protective (65, 66). More recently, the novel genotype PCV2d, first detected in Switzerland in 1999, spread to China, followed by the United States (67–69). Epidemiological studies suggest that a genotype shift similar to that seen with PCV2b is in progress, with resulting reports of PCV2 vaccine failure and increased clinical disease (70, 71). PCV2d has been implicated in more severe clinical signs and lesions (72).
Circoviruses are genetically diverse and infect a broad range of hosts, with documented cross-species transmission (3). Phylogenetic analysis suggests a closest evolutionary relationship between PCV3 and CanineCV. Interestingly, CanineCV was identified in the liver of a dog displaying necrotizing vasculitis and granulomatous lymphadenitis, both of which were observed in PCV3-infected sows, as well as reported in PCV2 infections (22, 73, 74). Similar to CanineCV, attempts to propagate PCV3 in vitro were unsuccessful. It is unclear whether PCV3 has been evolving undetected in pigs for some time or whether it originated via cross-species transmission or has arisen via recombination between unidentified parental circoviruses. PCV2 is capable of crossing species barriers, causing fatal disease in species other than swine; a recent report identified PCV2 in six mink that died of diarrhea in China (82).
The discovery of a novel porcine circovirus with a likely etiologic role in PDNS and reproductive failure is disconcerting. Retrospective studies suggest that PCV2 caused systemic disease sporadically as earlier as 1985 before becoming an epidemic in the late 1990s. The possibility that PCV3 is on a similar trajectory deserves further research. Importantly, given the approximately 30% identity between the PCV2 and PCV3 capsid proteins, cross protection seems unlikely.
MATERIALS AND METHODS
Ethics statement.
Porcine tissue and serum samples were collected as part of a routine diagnostic investigation by licensed veterinarians and submitted to the Kansas State Veterinary Diagnostic Laboratory or the ISUVDL. Mouse immunizations were performed by following a protocol approved by the Kansas State University Institutional Animal Care and Use Committee.
Clinical outbreak of PDNS.
Metagenomic sequencing was performed with a tissue homogenate consisting of three mummified fetuses originating from a commercial swine operation in North Carolina. The mummified fetuses were aborted from sows with skin lesions consistent with PDNS and were sent to the ISUVDL, where they were negative for PCV2, PRRSV, and PPV by qPCR. Tissue samples were also obtained from sows at the same farm that died with clinical signs of PDNS and were negative for PCV2, PRRSV, and IAV by qPCR. The sows at the farm received multiple doses of a commercial PCV2 vaccine prior to first breeding but were not routinely vaccinated for PCV2 prefarrowing.
PDNS retrospective study.
A retrospective study was conducted with 74 paraffin-embedded fixed tissue samples from 48 cases with histological lesions consistent with PDNS submitted to the ISUVDL between 2010 and 2016. All samples selected for the retrospective study were negative for PCV2 by IHC analysis.
PCV3 seroprevalence and nucleic acid detection in cases not associated with PDNS.
A retrospective serological study was performed to establish the seroprevalence and geographical distribution of PCV3. A total of 150 serum samples, representing at least eight states and Mexico (Iowa [n = 19], Illinois [n = 3], Indiana [n = 1], Minnesota [n = 1], Mexico [n = 5], North Carolina [n = 31], Nebraska [n = 17], Oklahoma [n = 31], and unknown origin [n = 42]) were evaluated by a recombinant PCV3 ELISA. Serum samples (n = 18) from 3-week-old pigs from a specific-pathogen-free research herd that tested negative for PCV3 by qPCR were used as negative controls. The prevalence of PCV3 in U.S. swine was investigated by using 271 lung homogenate, oral fluid, or nasal swab samples submitted to the ISUVDL for respiratory disease diagnostic testing.
Metagenomic sequencing.
Metagenomic sequencing was performed as previously described (75, 76). First-strand cDNA synthesis of viral DNA was performed with the Superscript III first-strand synthesis kit in accordance with the manufacturer's instructions with previously published primers (77). Amplification of the double-stranded cDNA was performed with primers identical to those used for first-strand synthesis but lacking the random hexamer and TaKaRa DNA polymerase.
Genetic analysis.
Raw sequence reads were separated on the basis of barcodes added during library preparation and analyzed in CLC Genomics Workbench (version 7.0). Reads mapping to the host genome (S. scrofa) were removed, and unmapped reads were assembled de novo into contigs. Contigs were evaluated by BLASTN. Sequences were aligned with ClustalW, and phylogeny was inferred by a maximum-likelihood method using the best-fit general time-reversible model with gamma distribution and invariant sites verified by 1,000 bootstrap replicates.
Development of a qPCR assay for specific detection of the PCV3 capsid gene.
Viral DNA was isolated from clinical specimens with the MagMax-96 total nucleic acid isolation kit in accordance with the manufacturer's protocol. Nucleic acid was extracted from formalin-fixed, paraffin-embedded tissues with the QIAamp DNA FFPE Tissue kit as instructed by the manufacturer (Qiagen, Valencia, CA). A 5′ nuclease assay was designed to target a 112-bp region of the PCV3 cap gene nucleic acid in samples with the probe 5′–6-carboxyfluorescein–ACC CCA TGG–Zen–CTC AAC ACA TAT GAC C–Iowa Black–3′, forward primer 5′-AGT GCT CCC CAT TGA ACG-3′, and reverse primer 5′-ACA CAG CCG TTA CTT CAC-3′. The qPCR assay was performed with the Qiagen Quantitect PCR kit under the following conditions: 95°C for 15 min and 45 cycles of 94°C for 15 s and 60°C for 60 s. The sensitivity and specificity of the assay were determined with a dilution series of a plasmid (pSF-CMV-cap) containing the entire PCV3 cap gene cloned into pSF-CMV-AMP (Oxford Genetics, United Kingdom) and PCV2 nucleic acid extracted from a cell culture. The CT values determined from the plasmid dilution series were used to create a standard curve for the calculations to determine the number of genome copies per milliliter.
The complete genome of PCV3 was determined from a fetal tissue homogenate pool from the North Carolina PDNS outbreak and from a sample in the prevalence study by Sanger sequencing of four overlapping amplicons generated with the primers shown in Table 1. The PCR was performed with TaKaRa Taq as follows: 94°C for 4 min, followed by 40 cycles of 94°C for 20 s, 50°C for 30 s, 72°C for 1 min, and 72°C for 5 min. Sequencing to confirm selected PCV3-positive samples was performed with 330-bp internal cap gene primers 5′-CCA CAG AAG GCG CTA TGT C-3′ and 5′-CCG CAT AAG GGT CGT CTT G-3′. The cap gene PCRs were performed with TaKaRa Taq as outlined above. PCR products were Sanger sequenced for verification.
TABLE 1.
Primers used to obtain PCV3 genome sequences
| Primer set | Start position | End position | Forward primer (5′ → 3′) | Tm (°C) | Reverse primer (5′ → 3′) | Tm (°C) |
|---|---|---|---|---|---|---|
| 1 | 74 | 927 | CAC CGT GTG AGT GGA TAT AC | 52.5 | CAA ACC CAC CCT TAA CAG | 50.6 |
| 2 | 1609 | 433 | GTC GTC TTG GAG CCA AGT G | 56.0 | CGA CCA AAT CCG GGT AAG C | 56.1 |
| 3 | 965 | 8 | TGT TGT ACC GGA GGA GTG | 53.9 | TGC CGG GTA ATA CTA GCC | 53.9 |
| 4 | 594 | 1619 | GAA GTT GCG GAG AAG ATG | 50.7 | TCC AAG ACG ACC CTT ATG | 51.1 |
Virus isolation.
Virus isolation was attempted with ST and PK-15 cells maintained in minimal essential medium (MEM) supplemented with l-glutamine and 5% fetal bovine serum (FBS). Cells were seeded onto six-well plates (60 to 80% confluent), and 100 μl of sample was inoculated into 1 ml of viral replacement medium, which consisted of MEM and a penicillin-streptomycin solution. Cells were observed daily for cytopathic effects, and PCV3 growth was monitored by qPCR and IFA.
Cloning, expression, and purification of PCV3 capsid protein.
To create the recombinant PCV3 cap construct, the following primers were designed to amplify a portion of the PCV3 gene encoding aa 35 to 214: F, 5′-AAA AAA GCT AGC GCT GGA ACA TAC TAC ACA-3′; R, 5′-AAA AAA GAA TTC TTA GAG AAC GGA CTTGTA ACG-3′. The 5′ ends of the forward and reverse primers contained NheI and EcoRI restriction sites (underlined), respectively. PCR products were cloned into the pET28a vector (Novagen, Madison, WI) to enable the expression of N-terminally truncated PCV3 cap as an N-terminal six-histidine (His) fusion protein in Escherichia coli. The pET28a-cap plasmid was transformed into E. coli BL21(DE3) cells and expressed as previously described (78). After expression, bacteria were harvested by centrifugation and lysed with the B-PER reagent (Pierce, Rockford, IL) and then Ni-nitrilotriacetic acid agarose (Qiagen, Valencia, CA) purification was completed as specified by the manufacturer. The purity and identity of the recombinant protein were assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis under denaturing conditions and Western blotting detecting the six-His tag at the N-terminal of the recombinant protein.
Production and in vitro characterization of an anti-PCV3 capsid MAb.
BALB/c mice were immunized with purified, truncated capsid protein (35 to 214 aa). The mice were inoculated with 50 μg of antigen mixed with Freund's incomplete adjuvant biweekly for a total of 8 weeks. Subsequently, mouse splenocyte cells were fused with NS-1 myeloma cells. MAbs specific to PCV3 were identified by IFA with HEK293 cells expressing native PCV3 cap. HEK293 cells on a six-well plate maintained in MEM with 5% FBS and antibiotics (ciprofloxacin, penicillin, streptomycin, and gentamicin) were transfected with pSF-CMV-AMP or a plasmid derived from pSF-CMV-AMP that contained the complete PCV3 cap gene cloned into the XhoI restriction site (pSF-CMV-cap). On a six-well plate, 106 cells were transfected with Lipofectamine 2000 (Invitrogen), in accordance with the manufacturer's instructions, and 10 μg of DNA. After 48 h, the plates were fixed with 80% acetone for 10 min at room temperature (RT) and allowed to dry. Transfected cells were incubated with undiluted anti-PCV3-cap MAbs at 37°C for 1 h, followed by three washes with PBS, and incubated with FITC-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) diluted 1:100 in PBS at 37°C for 1 h. Following a final wash, cells were visualized with an Eclipse TE2000-U inverted fluorescence microscope (Nikon). ST cells infected with PCV2 were used to evaluate the specificity of the PCV3 MAbs.
PCV3 detection and IHC analysis in PDNS cases.
Tissues were fixed in 10% neutral buffered formalin at RT, and tissue sections were embedded in paraffin until sectioning. IHC analysis was performed via the standard protocol, and slides were visualized with an LSM 700 confocal scanning microscope (Zeiss).
Development of a recombinant PCV3 capsid ELISA.
The ELISA was performed similar to assays previously described, by using 2 μg/ml purified recombinant PCV3 capsid protein to coat the wells of Corning EIA/RIA high-binding plates (79–81).
Accession number(s).
The full PCV3 genome sequences used in the phylogenetic analyses have been submitted to GenBank under accession numbers KT869077 (strain 29160) and KX458235 (strain 2164).
ACKNOWLEDGMENTS
This research was in part supported by the State of Kansas National Bio and Agro-Defense Facility fund and funds obtained through the USDA Animal Health and Disease Research Program under the provisions of section 1433 of subtitle E, title XIV, of public law 95-113 and the Kansas State Veterinary Diagnostic Laboratory.
Footnotes
Present address: Ben M. Hause, Cambridge Technologies, Worthington, Minnesota, USA.
REFERENCES
- 1.Biagini P, Bendinelli M, Hino S, Kakkola L, Mankertz A, Niel C, Okamoto H, Raidal S, Teo CG, Todd D. 2012. Circoviridae, p 343–349. In King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ (ed), Virus taxonomy: classification and nomenclature of viruses. Ninth report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, New York, NY. [Google Scholar]
- 2.Lőrincz M, Dán Á Láng M, Csaba G, Tóth ÁG Székely C, Cságola A, Tuboly T. 2012. Novel circovirus in European catfish (Silurus glanis). Arch Virol 157:1173–1176. doi: 10.1007/s00705-012-1291-1. [DOI] [PubMed] [Google Scholar]
- 3.Li L, Kapoor A, Slikas B, Bamidele OS, Wang C, Shaukat S, Masroor MA, Wilson ML, Ndjango J-BN, Peeters M, Gross-Camp ND, Muller MN, Hahn BH, Wolfe ND, Triki H, Bartkus J, Zaidi SZ, Delwart E. 2010. Multiple diverse circoviruses infect farm animals and are commonly found in human and chimpanzee feces. J Virol 84:1674–1682. doi: 10.1128/JVI.02109-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garigliany M-M, Börstler J, Jöst H, Badusche M, Desmecht D, Schmidt-Chanasit J, Cadar D. 2015. Characterization of a novel circo-like virus in Aedes vexans mosquitoes from Germany: evidence for a new genus within the family Circoviridae. J Gen Virol 96:915–920. doi: 10.1099/vir.0.000036. [DOI] [PubMed] [Google Scholar]
- 5.Cheung AK. 2003. Comparative analysis of the transcriptional patterns of pathogenic and nonpathogenic porcine circoviruses. Virology 310:41–49. doi: 10.1016/S0042-6822(03)00096-5. [DOI] [PubMed] [Google Scholar]
- 6.Clark EG. 1996. Pathology of postweaning multisystemic wasting syndrome of pigs. Proc West Can Assoc Swine Prod October 1996:22–25. [Google Scholar]
- 7.Harding JCS, Clark EG, Strokappe JH, Willson PI, Ellis JA. 1998. Postweaning multisystemic wasting syndrome: epidemiology and clinical presentation. Swine Health Prod 6:249–254. [Google Scholar]
- 8.Cheung AK. 2003. Transcriptional analysis of porcine circovirus type 2. Virology 305:168–180. doi: 10.1006/viro.2002.1733. [DOI] [PubMed] [Google Scholar]
- 9.Allan GM, McNeilly F, Kennedy S, Daft B, Ellis JA, Haines DM, Meehan BM, Adair BM. 1998. Isolation of porcine circovirus-like viruses from pigs with a wasting disease in the USA and Europe. J Vet Diagn Invest 10:3–10. doi: 10.1177/104063879801000102. [DOI] [PubMed] [Google Scholar]
- 10.Allan G, Meehan B, Todd D, Kennedy S, McNeilly F, Ellis J, Clark EG, Harding J, Espuna E, Botner A, Charreyre C. 1998. Novel porcine circoviruses from pigs with wasting disease syndromes. Vet Rec 142:467–468. [PubMed] [Google Scholar]
- 11.Sato K, Shibahara T, Ishikawa Y, Kondo H, Kubo M, Kadota K. 2000. Evidence of porcine circovirus infection in pigs with wasting disease syndrome from 1985 to 1999 in Hokkaido, Japan. J Vet Med Sci 62:627–633. doi: 10.1292/jvms.62.627. [DOI] [PubMed] [Google Scholar]
- 12.Ellis J, Hassard L, Clark E, Harding J, Allan G, Willson P, Strokappe J, Martin K, McNeilly F, Meehan B, Todd D, Haines D. 1998. Isolation of circovirus from lesions of pigs with postweaning multisystemic wasting syndrome. Can Vet J 39:44–51. [PMC free article] [PubMed] [Google Scholar]
- 13.Segalés J, Sitjar M, Domingo M, Dee S, Del Pozo M, Noval R, Sacristan C, De las Heras A, Ferro A, Latimer KS. 1997. First report of post-weaning multisystemic wasting syndrome in pigs in Spain. Vet Rec 141:600–601. [PubMed] [Google Scholar]
- 14.Kiupel M, Stevenson GW, Mittal SK, Clark EG, Haines DM. 1998. Circovirus-like viral associated disease in weaned pigs in Indiana. Vet Pathol 35:303–307. [DOI] [PubMed] [Google Scholar]
- 15.Choi C, Chae C, Clark EG. 2000. Porcine postweaning multisystemic wasting syndrome in Korean pig: detection of porcine circovirus 2 infection by immunohistochemistry and polymerase chain reaction. J Vet Diagn Invest 12:151–153. doi: 10.1177/104063870001200209. [DOI] [PubMed] [Google Scholar]
- 16.Sorden S. 2000. Update on porcine circovirus and postweaning multisystemic wasting syndrome (PMWS). J Swine Health Prod 8:133–136. [Google Scholar]
- 17.Harding JCS. 2007. Status of porcine circovirus diseases in western Canada. Can Vet J 48:267–268. [PMC free article] [PubMed] [Google Scholar]
- 18.Harms PA, Halbur PG, Sorden SD. 2002. Three cases of porcine respiratory disease complex associated with porcine circovirus type 2 infection. J Swine Health Prod 10:27–30. https://www.aasv.org/shap/issues/v10n1/v10n1p27.pdf. [Google Scholar]
- 19.Kim J, Chung H-K, Chae C. 2003. Association of porcine circovirus 2 with porcine respiratory disease complex. Vet J 166:251–256. doi: 10.1016/S1090-0233(02)00257-5. [DOI] [PubMed] [Google Scholar]
- 20.Rosell C, Segalés J, Plana-Durán J, Balasch M, Rodríguez-Arrioja GM, Kennedy S, Allan GM, McNeilly F, Latimer KS, Domingo M. 1999. Pathological, immunohistochemical, and in-situ hybridization studies of natural cases of postweaning multisystemic wasting syndrome (PMWS) in pigs. J Comp Pathol 120:59–78. [DOI] [PubMed] [Google Scholar]
- 21.Madson DM, Opriessnig T. 2011. Effect of porcine circovirus type 2 (PCV2) infection on reproduction: disease, vertical transmission, diagnostics and vaccination. Anim Health Res Rev 12:47–65. doi: 10.1017/S1466252311000053. [DOI] [PubMed] [Google Scholar]
- 22.Segalés J. 2012. Porcine circovirus type 2 (PCV2) infections: clinical signs, pathology and laboratory diagnosis. Virus Res 164:10–19. doi: 10.1016/j.virusres.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Allan GM, Kennedy S, McNeilly F, Foster JC, Ellis JA, Krakowka SJ, Meehan BM, Adair BM. 1999. Experimental reproduction of severe wasting disease by co-infection of pigs with porcine circovirus and porcine parvovirus. J Comp Pathol 121:1–11. [DOI] [PubMed] [Google Scholar]
- 24.Segalés J, Rosell C, Domingo M. 2004. Pathological findings associated with naturally acquired porcine circovirus type 2 associated disease. Vet Microbiol 98:137–149. doi: 10.1016/j.vetmic.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Smith WJ, Thomson JR, Done S. 1993. Dermatitis/nephropathy syndrome of pigs. Vet Rec 132:47. [DOI] [PubMed] [Google Scholar]
- 26.Thibault S, Drolet R, Germain MC, D'Allaire S, Larochelle R, Magar R. 1998. Cutaneous and systemic necrotizing vasculitis in swine. Vet Pathol 35:108–116. [DOI] [PubMed] [Google Scholar]
- 27.Drolet R, Thibault S, D'Allaire S, Thomson J, Done S. 1999. Porcine dermatitis and nephropathy syndrome (PDNS): an overview of the disease. Swine Health Prod 7:283–285. https://www.aasv.org/shap/issues/v7n6/v7n6p283.pdf. [Google Scholar]
- 28.Krakowka S, Hartunian C, Hamberg A, Shoup D, Rings M, Zhang Y, Allan G, Ellis JA. 2008. Evaluation of induction of porcine dermatitis and nephropathy syndrome in gnotobiotic pigs with negative results for porcine circovirus type 2. Am J Vet Res 69:1615–1622. doi: 10.2460/ajvr.69.12.1615. [DOI] [PubMed] [Google Scholar]
- 29.Pensaert MB, Sanchez RE Jr, Ladekjær-Mikkelsen A-S, Allan GM, Nauwynck HJ. 2004. Viremia and effect of fetal infection with porcine viruses with special reference to porcine circovirus 2 infection. Vet Microbiol 98:175–183. doi: 10.1016/j.vetmic.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Todd D, Weston JH, Soike D, Smyth JA. 2001. Genome sequence determinations and analyses of novel circoviruses from goose and pigeon. Virology 286:354–362. doi: 10.1006/viro.2001.0985. [DOI] [PubMed] [Google Scholar]
- 31.Li L, Shan T, Soji OB, Alam MM, Kunz TH, Zaidi SZ, Delwart E. 2011. Possible cross-species transmission of circoviruses and cycloviruses among farm animals. J Gen Virol 92:768–772. doi: 10.1099/vir.0.028704-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ilyina TV, Koonin EV. 1992. Conserved sequence motifs in the initiator proteins for rolling circle DNA replication encoded by diverse replicons from eubacteria, eucaryotes and archaebacteria. Nucleic Acids Res 20:3279–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niagro FD, Forsthoefel AN, Lawther RP, Kamalanathan L, Ritchie BW, Latimer KS, Lukert PD. 1998. Beak and feather disease virus and porcine circovirus genomes: intermediates between the geminiviruses and plant circoviruses. Arch Virol 143:1723–1744. [DOI] [PubMed] [Google Scholar]
- 34.Phenix KV, Weston JH, Ypelaar I, Lavazza A, Smyth JA, Todd D, Wilcox GE, Raidal SR. 2001. Nucleotide sequence analysis of a novel circovirus of canaries and its relationship to other members of the genus circovirus of the family Circoviridae. J Gen Virol 82:2805–2809. doi: 10.1099/0022-1317-82-11-2805. [DOI] [PubMed] [Google Scholar]
- 35.Bassami MR, Ypelaar I, Berryman D, Wilcox GE, Raidal SR. 2001. Genetic diversity of beak and feather disease virus detected in psittacine species in Australia. Virology 279:392–400. doi: 10.1006/viro.2000.0847. [DOI] [PubMed] [Google Scholar]
- 36.Lv Q, Guo K, Zhang Y. 2014. Current understanding of genomic DNA of porcine circovirus type 2. Virus Genes 49:1–10. doi: 10.1007/s11262-014-1099-z. [DOI] [PubMed] [Google Scholar]
- 37.Zhang W, Li L, Deng X, Kapusinszky B, Delwart E. 2014. What's for dinner?: viral metagenomics of US store bought beef, pork, and chicken. Virology 468-470:303–310. doi: 10.1016/j.virol.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Q, Tikoo S, Babiuk L. 2001. Nuclear localization of the ORF2 protein encoded by porcine circovirus type 2. Virology 285:91–99. doi: 10.1006/viro.2001.0922. [DOI] [PubMed] [Google Scholar]
- 39.White M, Higgins RJ. 1993. Dermatitis nephropathy syndrome of pigs. Vet Rec 132:199. [DOI] [PubMed] [Google Scholar]
- 40.Meehan BM, McNeilly F, McNair I, Walker I, Ellis JA, Krakowka S, Allan GM. 2001. Isolation and characterization of porcine circovirus 2 from cases of sow abortion and porcine dermatitis and nephropathy syndrome. Arch Virol 146:835–842. doi: 10.1007/s007050170152. [DOI] [PubMed] [Google Scholar]
- 41.Gresham A, Giles N, Weaver J. 2000. PMWS and porcine dermatitis nephropathy syndrome in Great Britain. Vet Rec 147:115. [PubMed] [Google Scholar]
- 42.Segalés J, Piella J, Marco E, Mateu-de-Antonio EM, Espuña E, Domingo M. 1998. Porcine dermatitis and nephropathy syndrome in Spain. Vet Rec 142:483–486. [DOI] [PubMed] [Google Scholar]
- 43.Segalés J, Domingo M. 2002. Postweaning multisystemic wasting syndrome (PMWS) in pigs. A review. Vet Q 24:109–124. doi: 10.1080/01652176.2002.9695132. [DOI] [PubMed] [Google Scholar]
- 44.Thomson JR, MacIntyre N, Henderson LE, Meikle CS. 2001. Detection of Pasteurella multocida in pigs with porcine dermatitis and nephropathy syndrome. Vet Rec 149:412–417. doi: 10.1136/vr.149.14.412. [DOI] [PubMed] [Google Scholar]
- 45.Brunborg IM, Jonassen CM, Moldal T, Bratberg B, Lium B, Koenen F, Schönheit J. 2007. Association of myocarditis with high viral load of porcine circovirus type 2 in several tissues in cases of fetal death and high mortality in piglets. a case study. J Vet Diagn Invest 19:368–375. doi: 10.1177/104063870701900405. [DOI] [PubMed] [Google Scholar]
- 46.Hansen MS, Hjulsager CK, Bille-Hansen V, Haugegaard S, Dupont K, Høgedal P, Kunstmann L, Larsen LE. 2010. Selection of method is crucial for the diagnosis of porcine circovirus type 2 associated reproductive failures. Vet Microbiol 144:203–209. doi: 10.1016/j.vetmic.2009.12.038. [DOI] [PubMed] [Google Scholar]
- 47.Madson DM, Patterson AR, Ramamoorthy S, Pal N, Meng XJ, Opriessnig T. 2009. Reproductive failure experimentally induced in sows via artificial insemination with semen spiked with porcine circovirus type 2. Vet Pathol 46:707–716. doi: 10.1354/vp.08-VP-0234-O-FL. [DOI] [PubMed] [Google Scholar]
- 48.McKeown NE, Fenaux M, Halbur PG, Meng XJ. 2004. Molecular characterization of porcine TT virus, an orphan virus, in pigs from six different countries. Vet Microbiol 104:113–117. doi: 10.1016/j.vetmic.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 49.Bigarré L, Beven V, de Boisséson C, Grasland B, Rose N, Biagini P, Jestin A. 2005. Pig anelloviruses are highly prevalent in swine herds in France. J Gen Virol 86:631–635. doi: 10.1099/vir.0.80573-0. [DOI] [PubMed] [Google Scholar]
- 50.Taira O, Ogawa H, Nagao A, Tuchiya K, Nunoya T, Ueda S. 2009. Prevalence of swine torque teno virus genogroups 1 and 2 in Japanese swine with suspected post-weaning multisystemic wasting syndrome and porcine respiratory disease complex. Vet Microbiol 139:347–350. doi: 10.1016/j.vetmic.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 51.Kennedy S, Moffett D, McNeilly F, Meehan B, Ellis J, Krakowka S, Allan G. 2000. Reproduction of lesions of postweaning multisystemic wasting syndrome by infection of conventional pigs with porcine circovirus type 2 alone or in combination with porcine parvovirus. J Comp Pathol 122:9–24. doi: 10.1053/jcpa.1999.0337. [DOI] [PubMed] [Google Scholar]
- 52.Allan GM, McNeilly F, Ellis J, Krakowka S, Meehan B, McNair I, Walker I, Kennedy S. 2000. Experimental infection of colostrum deprived piglets with porcine circovirus 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) potentiates PCV2 replication. Arch Virol 145:2421–2429. doi: 10.1007/s007050070031. [DOI] [PubMed] [Google Scholar]
- 53.Pogranichniy RM, Yoon K-J, Harms PA, Sorden SD, Daniels M. 2002. Case-control study on the association of porcine circovirus type 2 and other swine viral pathogens with postweaning multisystemic wasting syndrome. J Vet Diagn Invest 14:449–456. doi: 10.1177/104063870201400601. [DOI] [PubMed] [Google Scholar]
- 54.Lee Y, Lin C-M, Jeng C-R, Chang H-W, Chang C-C, Pang VF. 2015. The pathogenic role of torque teno sus virus 1 and 2 and their correlations with various viral pathogens and host immunocytes in wasting pigs. Vet Microbiol 180:186–195. doi: 10.1016/j.vetmic.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 55.Wellenberg GJ, Stockhofe-Zurwieden N, de Jong MF, Boersma WJA, Elbers ARW. 2004. Excessive porcine circovirus type 2 antibody titres may trigger the development of porcine dermatitis and nephropathy syndrome: a case-control study. Vet Microbiol 99:203–214. doi: 10.1016/j.vetmic.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 56.Aasted B. 1985. Aleutian disease of mink. Virology and immunology. Acta Pathol Microbiol Immunol Scand Suppl 287:1–47. [PubMed] [Google Scholar]
- 57.Gillespie J, Opriessnig T, Meng X j., Pelzer K, Buechner-Maxwell V. 2009. Porcine circovirus type 2 and porcine circovirus-associated disease. J Vet Intern Med 23:1151–1163. doi: 10.1111/j.1939-1676.2009.0389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Boisséson C, Béven V, Bigarré L, Thiéry R, Rose N, Eveno E, Madec F, Jestin A. 2004. Molecular characterization of porcine circovirus type 2 isolates from post-weaning multisystemic wasting syndrome-affected and non-affected pigs. J Gen Virol 85:293–304. doi: 10.1099/vir.0.19536-0. [DOI] [PubMed] [Google Scholar]
- 59.Gagnon CA, Tremblay D, Tijssen P, Venne M-H, Houde A, Elahi SM. 2007. The emergence of porcine circovirus 2b genotype (PCV-2b) in swine in Canada. Can Vet J 48:811–819. [PMC free article] [PubMed] [Google Scholar]
- 60.Olvera A, Cortey M, Segalés J. 2007. Molecular evolution of porcine circovirus type 2 genomes: phylogeny and clonality. Virology 357:175–185. doi: 10.1016/j.virol.2006.07.047. [DOI] [PubMed] [Google Scholar]
- 61.Gauger PC, Lager KM, Vincent AL, Opriessnig T, Kehrli ME Jr, Cheung AK. 2011. Postweaning multisystemic wasting syndrome produced in gnotobiotic pigs following exposure to various amounts of porcine circovirus type 2a or type 2b. Vet Microbiol 153:229–239. doi: 10.1016/j.vetmic.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 62.Beach NM, Meng X-J. 2012. Efficacy and future prospects of commercially available and experimental vaccines against porcine circovirus type 2 (PCV2). Virus Res 164:33–42. doi: 10.1016/j.virusres.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 63.Patterson AR, Opriessnig T. 2010. Epidemiology and horizontal transmission of porcine circovirus type 2 (PCV2). Anim Health Res Rev 11:217–234. doi: 10.1017/S1466252310000162. [DOI] [PubMed] [Google Scholar]
- 64.Cheung AK, Lager KM, Kohutyuk OI, Vincent AL, Henry SC, Baker RB, Rowland RR, Dunham AG. 2007. Detection of two porcine circovirus type 2 genotypic groups in United States swine herds. Arch Virol 152:1035–1044. doi: 10.1007/s00705-006-0909-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fort M, Sibila M, Allepuz A, Mateu E, Roerink F, Segalés J. 2008. Porcine circovirus type 2 (PCV2) vaccination of conventional pigs prevents viremia against PCV2 isolates of different genotypes and geographic origins. Vaccine 26:1063–1071. doi: 10.1016/j.vaccine.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 66.Opriessnig T, Patterson AR, Madson DM, Pal N, Halbur PG. 2009. Comparison of efficacy of commercial one dose and two dose PCV2 vaccines using a mixed PRRSV-PCV2-SIV clinical infection model 2-3-months post vaccination. Vaccine 27:1002–1007. doi: 10.1016/j.vaccine.2008.11.105. [DOI] [PubMed] [Google Scholar]
- 67.Grierson SS, King DP, Wellenberg GJ, Banks M. 2004. Genome sequence analysis of 10 Dutch porcine circovirus type 2 (PCV-2) isolates from a PMWS case-control study. Res Vet Sci 77:265–268. doi: 10.1016/j.rvsc.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 68.Wang F, Guo X, Ge X, Wang Z, Chen Y, Cha Z, Yang H. 2009. Genetic variation analysis of Chinese strains of porcine circovirus type 2. Virus Res 145:151–156. doi: 10.1016/j.virusres.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 69.Xiao C-T, Halbur PG, Opriessnig T. 2012. Complete genome sequence of a novel porcine circovirus type 2b variant present in cases of vaccine failures in the United States. J Virol 86:12469. doi: 10.1128/JVI.02345-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xiao C-T, Halbur PG, Opriessnig T. 2015. Global molecular genetic analysis of porcine circovirus type 2 (PCV2) sequences confirms the presence of four main PCV2 genotypes and reveals a rapid increase of PCV2d. J Gen Virol 96:1830–1841. doi: 10.1099/vir.0.000100. [DOI] [PubMed] [Google Scholar]
- 71.Grau-Roma L, Stockmarr A, Kristensen CS, Enøe C, López-Soria S, Nofrarías M, Bille-Hansen V, Hjulsager CK, Sibila M, Jorsal SE, Fraile L, Baekbo P, Vigre H, Segalés J, Larsen LE. 2012. Infectious risk factors for individual postweaning multisystemic wasting syndrome (PMWS) development in pigs from affected farms in Spain and Denmark. Res Vet Sci 93:1231–1240. doi: 10.1016/j.rvsc.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 72.Guo L, Fu Y, Wang Y, Lu Y, Wei Y, Tang Q, Fan P, Liu J, Zhang L, Zhang F, Huang L, Liu D, Li S, Wu H, Liu C. 2012. A porcine circovirus type 2 (PCV2) mutant with 234 amino acids in capsid protein showed more virulence in vivo, compared with classical PCV2a/b strain. PLoS One 7:e41463. doi: 10.1371/journal.pone.0041463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li L, McGraw S, Zhu K, Leutenegger CM, Marks SL, Kubiski S, Gaffney P, Dela Cruz FN Jr, Wang C, Delwart E, Pesavento PA. 2013. Circovirus in tissues of dogs with vasculitis and hemorrhage. Emerg Infect Dis 19:534–541. doi: 10.3201/eid1904.121390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Decaro N, Martella V, Desario C, Lanave G, Circella E, Cavalli A, Elia G, Camero M, Buonavoglia C. 2014. Genomic characterization of a circovirus associated with fatal hemorrhagic enteritis in dog, Italy. PLoS One 9:e105909. doi: 10.1371/journal.pone.0105909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hause BM, Collin EA, Anderson J, Hesse RA, Anderson G. 2015. Bovine rhinitis viruses are common in U.S. cattle with bovine respiratory disease. PLoS One 10:e0121998. doi: 10.1371/journal.pone.0121998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Neill JD, Bayles DO, Ridpath JF. 2014. Simultaneous rapid sequencing of multiple RNA virus genomes. J Virol Methods 201:68–72. doi: 10.1016/j.jviromet.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung-Lindell A, Andersson B. 2005. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci U S A 102:12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu P-C, Chen T-Y, Chi J-N, Chien M-S, Huang C. 2016. Efficient expression and purification of porcine circovirus type 2 virus-like particles in Escherichia coli. J Biotechnol 220:78–85. doi: 10.1016/j.jbiotec.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 79.Lin M, Trottier E, Pasick J. 2005. Antibody responses of pigs to defined Erns fragments after infection with classical swine fever virus. Clin Diagn Lab Immunol 12:180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hause B, Collin EA, Peddireddi L, Yuan F, Chen Z, Hesse RA, Gauger PC, Clement T, Fang Y, Anderson G. 2015. Discovery of a novel putative atypical porcine pestivirus in pigs in the United States. J Gen Virol 96:2994–2998. doi: 10.1099/jgv.0.000251. [DOI] [PubMed] [Google Scholar]
- 81.Palinski RM, Chen Z, Henningson JN, Lang Y, Rowland RRR, Fang Y, Prickett J, Gauger PC, Hause BM. 2016. Widespread detection and characterization of porcine parainfluenza virus 1 in pigs in the United States. J Gen Virol 97:281–286. doi: 10.1099/jgv.0.000343. [DOI] [PubMed] [Google Scholar]
- 82.Wang G, Sun N, Tian F, Wen Y-J, Xu C, Li J, Chen Q, Wang J. 2016. Genetic analysis of porcine circovirus 2 from dead minks. J Gen Virol 97:2316–2322. doi: 10.1099/jgv.0.000529. [DOI] [PubMed] [Google Scholar]