ABSTRACT
CD8+ T cells are crucial components of immunity and play a vital role in recovery from West Nile virus (WNV) infection. Here, we identify a previously unrecognized function of interleukin-17A (IL-17A) in inducing cytotoxic-mediator gene expression and promoting CD8+ T cell cytotoxicity against WNV infection in mice. We find that IL-17A-deficient (Il17a−/−) mice are more susceptible to WNV infection and develop a higher viral burden than wild-type (WT) mice. Interestingly, the CD8+ T cells isolated from Il17a−/− mice are less cytotoxic and express lower levels of cytotoxic-mediator genes, which can be restored by supplying recombinant IL-17A in vitro and in vivo. Importantly, treatment of WNV-infected mice with recombinant IL-17A, as late as day 6 postinfection, significantly reduces the viral burden and increases survival, suggesting a therapeutic potential for IL-17A. In conclusion, we report a novel function of IL-17A in promoting CD8+ T cell cytotoxicity, which may have broad implications in other microbial infections and cancers.
IMPORTANCE Interleukin-17A (IL-17A) and CD8+ T cells regulate diverse immune functions in microbial infections, malignancies, and autoimmune diseases. IL-17A is a proinflammatory cytokine produced by diverse cell types, while CD8+ T cells (known as cytotoxic T cells) are major cells that provide immunity against intracellular pathogens. Previous studies have demonstrated a crucial role of CD8+ T cells in recovery from West Nile virus (WNV) infection. However, the role of IL-17A during WNV infection remains unclear. Here, we demonstrate that IL-17A protects mice from lethal WNV infection by promoting CD8+ T cell-mediated clearance of WNV. In addition, treatment of WNV-infected mice with recombinant IL-17A reduces the viral burden and increases survival of mice, suggesting a potential therapeutic. This novel IL-17A–CD8+ T cell axis may also have broad implications for immunity to other microbial infections and cancers, where CD8+ T cell functions are crucial.
KEYWORDS: CD8 T cell, IL-17A, West Nile virus
INTRODUCTION
West Nile virus (WNV) is a neurotropic flavivirus primarily transmitted to humans by infected mosquitoes, but it can also be acquired through blood transfusion, organ transplantation, and congenital infection (1). After mosquito inoculation, WNV infects keratinocytes and skin-resident dendritic cells (Langerhans cells), and the latter cells can carry virus to draining lymph nodes and cause viremia (2, 3). Subsequently, WNV disseminates to peripheral organs, such as the spleen and liver, and then to the spinal cord and brain. WNV can cause neuronal injury and death, potentially leading to encephalitis, meningitis, and poliomyelitis (1). As of now, no vaccine or specific treatment is available for neurological sequelae of human WNV infection.
Despite intensive investigations over the past 15 years, the immunopathogenesis of WNV infection is still not well understood. In brief, type I interferons (IFNs) (4, 5), the complement system (6), and humoral immunity (7, 8) limit viremia and control WNV dissemination to the brain. Components of cell-mediated immunity, including CD4+ (9) and CD8+ (10) T cells, have been shown to clear WNV from the central nervous system (CNS) and to limit viral persistence. In contrast, the roles of neutrophils, NK cells, and γδ-T cells are still unclear (11–13). Cytokine signaling of interleukin-23 (IL-23) (14), gamma interferon (IFN-γ) (15), and IL-1β (16) has been shown to protect against WNV infection, whereas IL-10 (17) and IL-22 (18) have been shown to favor WNV pathogenicity. The role of tumor necrosis factor alpha (TNF-α) remains elusive (19, 20), and the functions of many other cytokines have not been studied in WNV infection.
IL-17A, a major cytokine of the IL-17 family, was identified in 1993 as cytotoxic T lymphocyte antigen 8 (21). Previous studies have demonstrated that IL-17A signaling regulates diverse immune functions, including the expression of various inflammatory cytokines and chemokines, activation and recruitment of leukocytes, and production of antibodies (22, 23). IL-17A has often been described as a mediator of inflammation (24) with a prominent role in allergic and autoimmune diseases, including multiple sclerosis (25, 26), rheumatoid arthritis (27), psoriasis (28), asthma (29), and Crohn's disease (30). However, the role of IL-17A may be either beneficial or detrimental in the host response to bacterial and fungal infections. For instance, IL-17A may enhance neutrophil recruitment and protect against bacterial and fungal pathogens, such as Klebsiella pneumoniae and Escherichia coli (31–33), Listeria monocytogenes (34), Mycobacterium tuberculosis (24), Francisella tularensis (35), Chlamydia muridarum (36), Candida albicans (37, 38), and Pneumocystis jirovecii (39). Conversely, IL-17A may facilitate toxoplasmosis (40) and certain fungal infections (41). The role of IL-17A in viral infection is also not clear. For example, genetically constructed vaccinia virus (VV) expressing IL-17A (VVIL-17A) caused more severe disease in mice (42), but VVIL-17A was also reported to be less virulent, and IL-17-deficient (Il17a−/−) mice were more susceptible to VV infection (43). In addition, IL-17A was implicated in priming T cell responses during lymphocytic choriomeningitis virus (LCMV) hepatitis (44) and mediating the immunopathogenicity of viral infections, such as influenza virus (45), respiratory syncytial virus (46, 47), murine encephalomyelitis virus (48), and hepatitis B virus (49) infections.
We previously reported that Toll-like receptor 7 (TLR7) mediates IL-23-dependent protective immune responses against WNV infection in mice (14). IL-23 is known as a prime regulator for stabilization and maintenance of CD4+ T helper 17 (Th17) cells, which are the major cell type secreting IL-17A (50–52). Since IL-17A is also described as a mediator of CNS inflammation (25, 26) and a factor contributing to blood-brain barrier permeability (53), we hypothesized that IL-17A may play a crucial role in the host immune response to WNV infection. Indeed, here, we report that IL-17A protects mice from lethal WNV infection and demonstrate a novel function of the cytokine in promoting CD8+ T cell cytotoxicity.
RESULTS
WNV infection induces expression of Il17a and Il17ra in humans and mice.
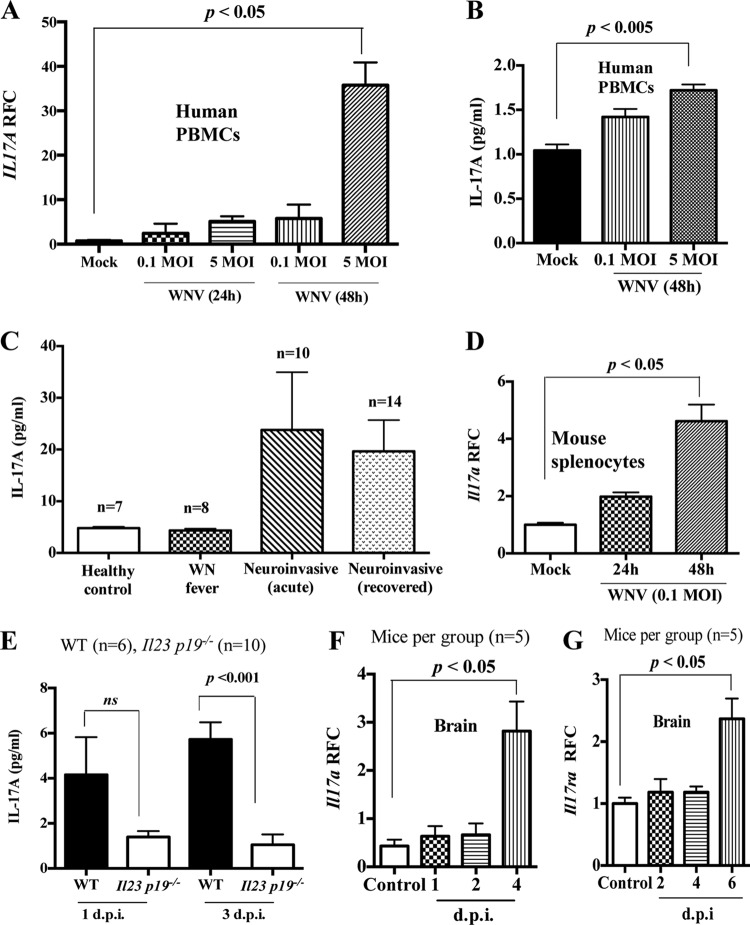
We previously reported that WNV induces IL-23 production in mice in a TLR7-dependent manner (14). Considering the role of IL-23 in Th17 cell stabilization and IL-17A production (52), we hypothesized that IL-17A may play a role in WNV infection. To test this, we measured the expression of Il17a in human cells infected with WNV in vitro. Human peripheral blood mononuclear cells (hPBMCs) isolated from healthy human volunteers without a history of WNV infection were infected with WNV (multiplicity of infection [MOI] = 0.1 and 5) for 24 h and 48 h in vitro. WNV-infected hPBMCs were collected, total RNA was isolated, and cDNA synthesis and quantitative real-time PCR (qPCR) were performed to measure transcripts of Il17a and cellular β-actin as a housekeeping gene. The qPCR results showed that Il17a gene expression was upregulated in WNV-infected hPBMCs (Fig. 1A), which was further confirmed by measuring IL-17A production in hPBMC culture supernatants (Fig. 1B) by an enzyme-linked immunosorbent assay (ELISA). To relate these in vitro results to WNV infection in humans, we used ELISA to measure the production of IL-17A in the sera of human cases with active WNV infection (fever or neuroinvasive disease) or with a history of recovery from neuroinvasive WNV disease and healthy controls who had no history of WNV infection. The cases with active disease and those with a longstanding history of neuroinvasive WNV disease showed a trend of levels of IL-17A in sera higher than those in WNV fever cases and healthy controls (Fig. 1C), with no difference between the last two. These results demonstrate that WNV infection induces the production of IL-17A in humans and suggest that the cytokine may play a role in WNV infection.
FIG 1.
WNV induces expression of Il17a and Il17ra in both humans and mice. (A) Il17a transcripts were measured by qPCR and expressed as RFC after normalization to cellular β-actin in human PBMCs infected with WNV for 24 h or 48 h. (B) IL-17A production in culture supernatant of WNV-infected hPBMCs measured by ELISA. (C) Levels of IL-17A in sera of human WNV patients and healthy controls measured by ELISA. (D) RFC of Il17a transcripts after normalization to cellular β-actin in mouse splenocytes (MOI = 0.1). (E) IL-17A production measured by ELISA in plasma of Il23p19−/− mice and their littermate WT control mice (7 to 9 weeks old) infected with WNV (1,000 PFU i.p.). (F and G) WT (C57BL/6) mice were infected with WNV (1,000 PFU i.p.), and expression of Il17a (F) and Il17ra (G) transcripts was measured in brain tissue by qPCR. Shown are means and standard errors of the mean (SEM). The data represent the results of two independent experiments performed in triplicate and analyzed by one-way ANOVA. (E, F, and G) The data represent the results of two independent experiments (n = 5 mice/group) analyzed by a two-tailed Student t test; ns, no significant difference (P > 0.05).
To expand upon these findings, we used a mouse model of WNV infection because it reflects various aspects of human WNV disease (14, 17, 54). Splenocytes isolated from C57BL/6J mice were infected with WNV (MOI = 0.1) in vitro for 24 h and 48 h, and the expression of the Il17a gene was measured by qPCR. Similar to hPBMCs, Il17a transcript levels were upregulated at both 24 and 48 h postinfection (hpi) in mouse splenocytes infected with WNV in vitro (Fig. 1D). To further measure Il17a expression in mice and to test whether its production was IL-23 dependent, we intraperitoneally (i.p.) infected a group of wild-type (WT) littermates and IL-23-deficient (Il23p19−/−) mice (both were in a mixed C57BL/6 × 129 background) with 1,000 PFU of WNV and measured IL-17A protein in plasma by ELISA. The results showed that WNV infection in mice induced IL-17A production (Fig. 1E), but the level of the cytokine was undetectable in serum samples from mock-infected control mice (data not shown). Moreover, there was approximately 80% reduction in Il17a expression in Il23p19−/− mice at 3 days postinfection (dpi), suggesting that IL-17A production during WNV infection in mice largely depends on IL-23 signaling (Fig. 1E). Since astrocytes, microglia, and brain-infiltrating immune cells express functional interleukin-17 receptor A (IL-17RA) under brain-inflammatory conditions (55), we measured the expression of Il17a and Il17ra genes in brains of WNV-infected mice. For this, we infected a group of WT mice with WNV (1,000 PFU i.p.), sacrificed them at various time points to collect the brains, and measured levels of Il17a and Il17ra transcripts by qPCR. Indeed, there was significantly upregulated expression of both the Il17a (Fig. 1F) and Il17ra (Fig. 1G) genes in brains of WNV-infected mice compared to uninfected controls. Collectively, these results indicate that WNV infection elevates the expression of both Il17a and Il17ra, suggesting a possible role of IL-17A in WNV infection.
IL-17A protects mice from lethal WNV infection.
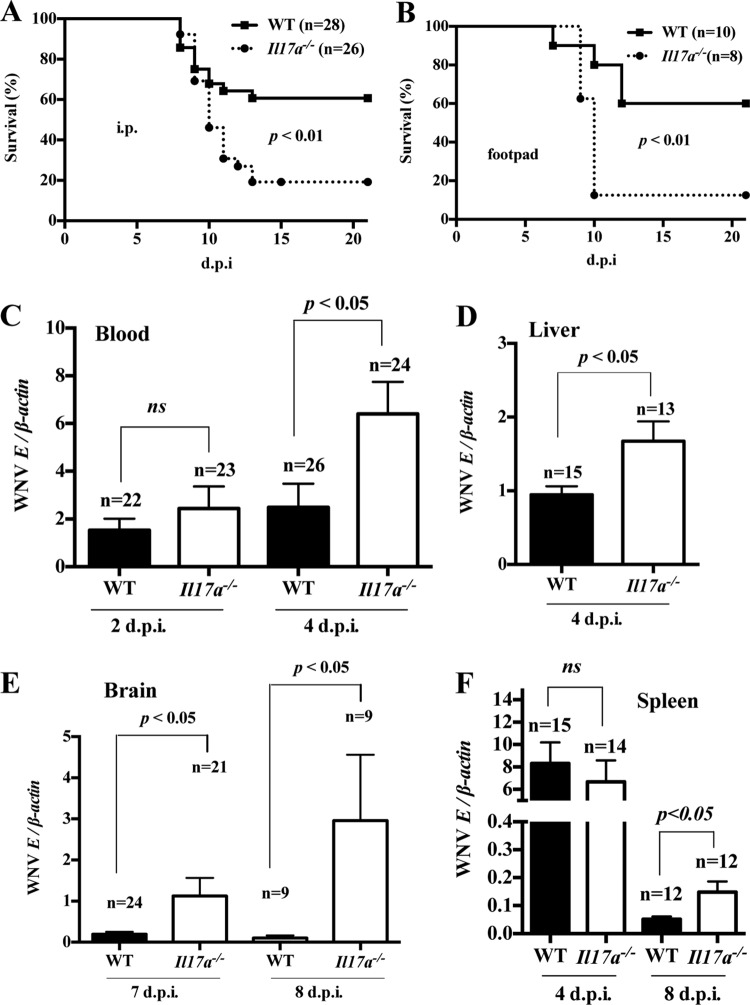
To investigate the role of IL-17A in WNV pathogenesis, we used a mouse model of WNV encephalitis (14). IL-17A-deficient (Il17a−/−) and WT control (7- to 8-week-old, sex-matched, strain C57BL/6J) mice were challenged via i.p. injection with 1,000 PFU of WNV (14), a dose that kills approximately 40 to 50% of WT animals. Morbidity and mortality were monitored twice daily for 21 days. We found strikingly greater susceptibility of Il17a−/− mice (20% survival) than of WT control mice (60% survival) to lethal WNV infection (Fig. 2A). To exclude the possibility of an inoculation-route-specific response, we also challenged Il17a−/− and WT mice with 100 PFU of WNV via the footpad (17) and performed survival analysis. Similar to the i.p. route, footpad inoculation showed that Il17a−/− mice were more susceptible to WNV infection (Fig. 2B). Together, these data suggest that IL-17A protects mice from lethal WNV infection.
FIG 2.
Il17a−/− mice are more susceptible to WNV infection. Seven- to 9-week-old WT (C57BL/6J) and Il17a−/− mice were infected with WNV via the i.p. (1,000 PFU) or footpad (100 PFU) route and monitored for mortality twice daily for 21 days; survival percentages were compared using the Kaplan-Meier survival and log-rank tests. (A and B) Survival curves after i.p. inoculation (A) and footpad inoculation (B). (C to F) In i.p.-inoculated mice, qPCR was performed to measure WNV-E RNA in blood (C), liver (D), brain (E), and spleen (F), with viral burdens expressed as the ratio of WNV-E RNA copies to cellular β-actin transcripts. The ratios of viral loads between WT and Il17a−/− mice (means and SEM) were compared by two-tailed Student t tests; ns, no significant difference (P > 0.05).
To further study the role of IL-17A in controlling WNV infection, we compared the virological profiles of WNV-infected Il17a−/− and WT mice. Measurement of WNV viremia by qPCR revealed no difference at 2 dpi; however, a 3-fold increase in the transcript level of the WNV envelope (WNV-E) gene (WNVE) was observed at 4 dpi in WNV-infected Il17a−/− mice compared to WT controls (Fig. 2C). To assess the viral burden in peripheral organs, we sacrificed WNV-infected mice; collected liver, spleen, and brain samples at selected time points; and performed a qPCR analysis. Compared to WT controls, we found an approximately 2-fold increase in WNVE transcripts in the livers of Il17a−/− mice (Fig. 2D). Consistent with the survival results, there were about 6-fold (at 7 dpi) and 30-fold (at 8 dpi) increases in WNVE transcripts in the brains of WNV-infected Il17a−/− mice compared to WT controls (Fig. 2E). Although there was no difference in the viral burdens in spleens of WT versus Il17a−/− mice at 4 dpi, Il17a−/− mice had significantly (about 3-fold) higher levels of WNVE transcripts at 8 dpi (Fig. 2F). These data demonstrate that mice deficient in IL-17A develop a higher viral burden in blood and liver at 4 dpi and have deficient clearance of WNV from the brain and spleen at 8 dpi, leading to greater WNV susceptibility. Collectively, these results indicate that IL-17A plays a protective role during WNV infection.
WNV infection promotes leukocyte infiltration into brains of Il17a−/− mice.
IL-17A has been shown to recruit leukocytes, including neutrophils, during microbial infections (31, 32) and under inflammatory conditions (56). Since we observed a higher WNV burden in brains of Il17a−/− mice, we asked if this was related to IL-17A-mediated control of leukocyte infiltration into the brain during WNV infection. To investigate this, we performed confocal microscopy to detect WNV-E antigen, CD45 (a panleukocyte marker), and CD11b (a microglial and macrophage marker) in brain sections of WNV-infected Il17a−/− and WT mice sacrificed at 6 dpi. We focused on the olfactory bulb, because we have shown that this brain region is most sensitive to WNV infection (14, 17, 19). Consistent with the qPCR measurement of WNVE RNA in brain tissue (Fig. 2E), the confocal imaging revealed more WNV-E antigens in the brains of WNV-infected Il17a−/− mice than in WT controls (Fig. 3A and B). Similar results were also obtained in other brain regions, including the cerebral cortex, brain stem, cerebellum, and striatum (data not shown). Unexpectedly, confocal imaging results showed more CD45+ (Fig. 3A) and CD11b+ (Fig. 3B) leukocytes in the brains of WNV-infected Il17a−/− mice than in WT controls. To confirm these data and further quantify brain-infiltrating immune cells, we performed flow cytometric analysis of brain leukocytes isolated from WT and Il17a−/− mice infected (i.p.) with 1,000 PFU of WNV for 6 days. We characterized CD45+, CD11b+, CD3+ CD4+, and CD3+ CD8+ cell populations, as previously described (14). Consistent with the confocal imaging results, we observed a trend toward elevation of all the leukocyte populations in the brains of WNV-infected Il17a−/− mice compared to WT controls (Fig. 3C). To test whether the greater leukocyte infiltration into WNV-infected Il17a−/− mouse brains was affected by leukocyte expansion or differentiation in the periphery, we compared leukocyte populations in spleens of Il17a−/− and WT mice infected with WNV. There was no difference in the leukocyte populations in spleens of WNV-infected Il17a−/− and those of WT mice (data not shown), suggesting that more leukocytes in the brain may not be due to the possible effects of IL-17A on leukocyte expansion in the periphery.
FIG 3.
Leukocyte infiltration into the CNS is elevated in WNV-infected Il17a−/− mice. Seven- to 9-week-old WT (C57BL/6J) and Il17a−/− mice were challenged i.p. with WNV (1,000 PFU). (A and B) PBS-perfused brains were isolated at 6 dpi, and WNV antigen (green signal) and CD45 (leukocyte common antigen; red) (A) or CD11b (macrophage and microglial marker; red) (B) was detected with a Nikon A1R confocal microscope (original magnification, ×20). DAPI (blue signal) was used as a nuclear counterstain; representative images are shown. (C) Brain leukocytes isolated at 6 dpi were characterized by flow cytometry after probing with antibodies against WNV-E, CD45, CD4, CD8, and CD11b. The signal colors used in the dot plots and the percentages of positive cells within gated populations are shown on the right. The data represent the results of two independent experiments (n = 5 mice per group for each experiment). Boxes indicate CD45hi cells, upper circles indicate CD3+ T cells, and lower circles indicate CD11b+ cells. (D and E) Expression of Ccl5 (D) and Ccr5 (E) genes at the mRNA level was measured by qPCR in the blood of WNV-infected mice at 2 and 4 dpi. (F to H) mRNA levels of Ccl5 (F), Cxcl10 (G), and Cxcr3 (H) genes were measured by qPCR in brains of WNV-infected mice at 8 dpi. The gene expression data were normalized to cellular β-actin mRNA and compared by two-tailed Student t tests; ns, no significant difference (P > 0.05).
To further dissect the mechanism by which more leukocytes migrate into the brains of WNV-infected Il17a−/− mice, we performed qPCR to measure the expression of selected chemokine genes (Cxcl1, Cxcl10, and Ccl5) known to mediate recruitment of leukocytes. There was significantly elevated expression of Ccl5 (Fig. 3D) and its receptor, Ccr5 (Fig. 3E), in the blood of WNV-infected Il17a−/− mice at 4 dpi but no difference in the expression of other chemokines, such as Cxcl1 and Cxcl10 (data not shown). In addition, there was a significant increase in Ccl5 expression (Fig. 3F) in the brains of WNV-infected Il17a−/− mice at 8 dpi but no difference in the expression of other leukocyte-recruiting chemokines or chemokine receptors, such as Cxcl10 (Fig. 3G) and Cxcr3 (Fig. 3H). These results may imply a link between deficient IL-17A and higher Ccl5 expression that could contribute to more leukocyte homing to the brains of Il17a−/− mice during the course of WNV infection.
IL-17A does not affect innate inflammatory responses or antibody production.
WNV infection induces potent type I IFN responses in mice, which play a critical role in controlling both viremia and encephalitis (4). We tested by qPCR and ELISA whether IL-17A deficiency alters type I IFN expression during WNV infection. The qPCR results showed no difference in Ifn-α expression in the blood of Il17a−/− versus WT control mice at 4 dpi (Fig. 4A). Similarly, no difference in the expression of the Ifn-β gene was observed in blood (Fig. 4B), spleen (Fig. 4C), liver (Fig. 4D), and brain (Fig. 4E) samples from WNV-infected Il17a−/− versus WT control mice measured at various time points. To further confirm these results, we also measured IFN-β protein in the plasma of WNV-infected WT and Il17a−/− mice at 3 dpi by ELISA and found no difference in IFN-β expression (Fig. 4F). These results suggest that the type I IFN response remains unaltered in Il17a−/− mice during WNV infection. We also assessed the possible role of IL-17A in inflammatory responses during WNV infection by measuring inflammatory cytokine expression in plasma by ELISA. Again, there was no difference in levels of IL-1β, IL-6, IL-10, IFN-γ, IL-12 p40, and TNF-α in plasma from WNV-infected Il17a−/− versus WT control mice at both 1 and 3 dpi (Fig. 4G to L). In addition, no significant difference in the expression of these cytokines was detected by qPCR in brains of WNV-infected Il17a−/− versus WT control mice at 8 dpi (data not shown). We next asked if IL-17A has direct antiviral activity against WNV infection, which has been shown for some other cytokines, such as TNF-α (20, 57) and IL-6 (58). However, no effect on replication of WNV was observed in Raw 264.7 cells (mouse macrophages) (Fig. 4M) and Neuro 2a cells (mouse neuroblasts) (Fig. 4N) that were pretreated with mouse recombinant IL-17A (1 to 100 ng/ml), suggesting that IL-17A may not have a direct antiviral effect against WNV replication.
FIG 4.
Antiviral, inflammatory, and antibody responses of Il17a−/− mice during WNV infection. Seven- to 9-week-old WT (C57BL/6J) and Il17a−/− mice were infected (i.p.) with 1,000 PFU of WNV. Blood, plasma, and tissue samples were collected at the indicated time points for cytokine and anti-WNV-E IgM measurement. (A to E) Expression of Ifn-α gene transcripts in blood (A) and expression of Ifn-β gene transcripts in blood (B), spleen (C), liver (D), and brain (E) were measured by qPCR (normalized to cellular β-actin mRNA). (F to L) Protein levels of IFN-β, IL-1β, IL-6, IL-10, IFN-γ, IL-12p40, and TNF-α in plasma were measured by ELISA. (M and N) Replication of WNV was analyzed by qPCR in Raw 264.7 (M) and Neuro 2a (N) cells that were pretreated with mouse recombinant IL-17A (1 to 100 ng/ml) for 6 h, followed by infection with WNV (MOI = 1) for 24 h. (O) Anti-WNV-E IgM in plasma was measured by ELISA. The data (means and SEM) represent the results of at least two independent experiments performed in triplicate and analyzed by one-way ANOVA (M and N) or two-tailed Student t tests (A to L and O); ns, no significant difference (P > 0.05).
Besides type I IFN, the humoral immune response also plays an important role in clearance of WNV from the blood and peripheral organs and limits viral dissemination to the CNS (7). Although the role of IL-17A in humoral immunity is not well understood, it has been shown that B cells express IL-17RA (59), whereas Th17 cells (major IL-17A producers) promote antibody production by B cells (60). To test the possible effect of IL-17A in humoral immune responses during WNV infection, we compared WNV-E-specific IgM antibody production in WNV-infected Il17a−/− and WT mice by ELISA. Il17a−/− and WT mice produced similar levels of anti-WNV-E IgM when measured at 3, 5, and 7 dpi (Fig. 4O). These results collectively demonstrate that the higher viral load in Il17a−/− mice is likely not due to altered type I IFN, inflammatory cytokines, antibody responses, or a direct antiviral effect of IL-17A.
IL-17A facilitates CD8+ T cell cytotoxicity.
Brain-infiltrating leukocytes play a vital role in clearing WNV from the CNS during WNV infection (9, 10, 14). In particular, CD8+ T cells are crucial for clearance of WNV from the CNS and spleen (10, 12, 61, 62). Despite a modest elevation trend of brain-infiltrating CD8+ T cells in Il17a−/− mice, the viral burden in the brains of these mice was higher than in WT controls (Fig. 2E). In addition, Il17a−/− mice were also deficient in clearing WNV from the spleen (Fig. 2F). Therefore, we hypothesized that CD8+ T cells in Il17a−/− mice may be functionally defective in their ability to clear WNV-infected target cells. To test this, we infected WT and Il17a−/− mice i.p. with a sublethal dose of WNV (100 PFU) to prolong the course of WNV infection. This is important, because CD8+ T cells play a major role in clearing WNV-infected cells during the later phase (days 8 to 12) of infection (10, 61), and most Il17a−/− mice infected with a higher dose (e.g., 1,000 PFU or more) develop severe disease and die during this period. At 10 dpi, the mice were sacrificed, and splenic CD8+ T cells were purified using a negative antibody selection method. The purified effector CD8+ T cells were then cocultured with the target cells (MC57GLWNV-E) or control cells (MC57GLvector) for 4 h. The target cells express the ectodomain of WNV-E in a pcDNA3.1 vector, while the control cells express only the parent vector (10). The cytotoxicity of effector CD8+ T cells to WNV-specific target cells was assessed by measuring the quantity of intracellular lactate dehydrogenase released into culture supernatants from the lysed target cells. Strikingly, the cytotoxicity assay showed about 2-fold reduction in cytotoxicity of CD8+ T cells isolated from WNV-infected Il17a−/− mice in comparison to WNV-infected WT mice (Fig. 5A). These results demonstrate that CD8+ T cells from Il17a−/− mice failed to mount an effective target cell-specific cytotoxic response during WNV infection.
FIG 5.
Reduced cytotoxicity of CD8+ T cells from Il17a−/− mice. Seven- to 9-week-old WT (C57BL/6J) and Il17a−/− mice were infected (i.p.) with WNV at 100 PFU for 10 days. (A) Purified splenic CD8+ T cells were cocultured with target (MC57GLWNV-E) or control (MC57GLvector) cells at a 50:1 effector/target ratio for 4 h, and cytotoxicity was assayed by measuring the release of intracellular lactate dehydrogenase in culture supernatants. (B to I) RFC in transcripts of perforin-1 (B and F), granzyme A (C and G), granzyme B (D and H), and FasL (E and I) in blood, spleen, liver, and brain (B to E) and splenic CD8+ T cells or CD8− cells (F to I) from WT or Il17a−/− mice measured by qPCR (normalized to cellular β-actin mRNA). (J and K) RFC in the expression of perforin-1 (J) and granzyme A (K) in the spleens of CHIKV-infected (105 PFU i.p.) WT and Il17a−/− mice was measured at 12 dpi by qPCR. The data (means and SEM) in panels A to I represent the results of three independent experiments (n = 3 mice/group); the data in panels J and K represent the results of two independent experiments (n = 3 mice/group). ns, no significant difference (P > 0.05).
Cytotoxic CD8+ T cells employ granule (e.g., perforin and granzyme) exocytosis and Fas-Fas ligand (FasL)-dependent mechanisms to kill target cells (63). To test whether IL-17A regulates the expression of cytotoxicity mediator genes, we performed qPCR assays in blood, spleens, livers, and brains of WT and Il17a−/− mice that were infected with WNV (100 PFU) for 10 days. Interestingly, the expression levels of perforin-1 (Fig. 5B), granzyme A (Fig. 5C), granzyme B (Fig. 5D), and Fas-ligand (FasL) (Fig. 5E) were significantly lower in most of these tissues collected from Il17a−/− mice than in those from WT controls. Although CD8+ cells are the major cells that express these cytotoxic mediators, other cells, such as NK cells, may also express the genes and contribute to cytotoxic effector function by a mechanism similar to that of CD8+ T cells. To specifically test if the attenuated cytotoxicity of CD8+ T cells in Il17a−/− mice was due to the lower expression of these cytotoxic-mediator genes, we performed qPCR to measure the expression of perforin-1, granzyme A, granzyme B, and FasL in CD8+ T cells purified from spleens of WNV-infected WT and Il17a−/− mice at 10 dpi. Consistent with the cytotoxicity assay results, the qPCR showed that CD8+ T cells isolated from the Il17a−/− mice had significantly reduced expression of perforin-1 (Fig. 5F), granzyme A (Fig. 5G), granzyme B (Fig. 5H), and FasL (Fig. 5I) compared to WT controls. To test if these cytotoxicity mediators were also less expressed in other immune cells in Il17a−/− mice, we performed qPCR assays on CD8-negative (CD8−) splenocytes (mixed immune cells, including NK cells) isolated from WNV-infected WT and Il17a−/− mice at 10 dpi. In contrast to the CD8+ cells, we found no difference in the expression of perforin-1, granzyme A, granzyme B, and FasL genes in CD8− splenocytes from Il17a−/− mice (Fig. 5F to I). Whereas in WNV-infected WT mice the expression of these cytotoxicity marker genes was significantly higher in CD8+ than CD8− cells, no such differences were detected between CD8+ and CD8− cells isolated from Il17a−/− mice (Fig. 5F to I). In addition, there was no difference in the expression of these cytotoxicity marker genes between uninfected Il17a−/− and WT mice (data not shown). To further test whether the IL-17A-mediated cytotoxicity mediator expression is specific to WNV or common across viral infections, we measured the expression of cytotoxicity mediator genes in WT and Il17a−/− mice infected with chikungunya virus (CHIKV), a single-stranded RNA virus belonging to the genus Alphavirus of the family Togaviridae. Similar to WNV, we found significantly reduced expressions of perforin-1 (Fig. 5J) and granzyme A (Fig. 5K) in the splenocytes isolated from CHIKV-infected Il17a−/− mice compared to WT controls. Collectively, these results suggest that IL-17A promotes the expression of the cytotoxicity mediators and facilitates CD8+ T cell cytotoxicity during infections with WNV and other viruses, such as CHIKV.
IL-17A induces cytotoxic-mediator gene expression in a CD4+ T cell-independent manner.
The role of IL-17A in regulating the expression of cytotoxic mediators in CD8+ T cells has not been previously reported. To further investigate and confirm this, we isolated splenocytes from WNV-infected WT mice at 8 dpi; cultured them with recombinant IL-17A (50 ng/ml) ex vivo for 24 h; and measured the expression of perforin-1, granzyme A, granzyme B, and FasL genes by qPCR assay. The treatment with recombinant IL-17A significantly upregulated the expression of perforin-1, granzyme A, and granzyme B, but not FasL (Fig. 6A to D), in the splenocytes isolated from WNV-infected WT mice. Similar results were obtained when splenocytes were isolated from WNV-infected Il17a−/− mice and treated with recombinant IL-17A ex vivo (Fig. 6A to D). To assess the possible role of CD4+ T cells in IL-17A-mediated expression of cytotoxic mediators, we depleted CD4+ T cells from the splenocytes isolated from WNV-infected Il17a−/− and WT mice at 8 dpi and cultured CD4− splenocytes with recombinant IL-17A (50 ng/ml) ex vivo for 24 h. The supply of recombinant IL-17A induced expression of the cytotoxic mediators even in the absence of CD4+ T cells (Fig. 6A to D), suggesting that IL-17A-mediated induction of cytotoxic-mediator expression in CD8+ T cell is independent of CD4+ T cells.
FIG 6.
IL-17A promotes expression of cytotoxic-mediator genes independently of CD4+ T cells. (A to D) Seven- to 9-week-old WT (C57BL/6J) and Il17a−/− mice (n = 6 mice/group) were infected (i.p.) with 100 PFU of WNV and sacrificed at 8 dpi to isolate splenocytes and CD8+ T cells. Splenocytes or CD4+ T cell-depleted splenocytes (CD4− splenocytes) were cultured with or without recombinant mouse IL-17A (rIL17A) (50 ng/ml) for 24 h ex vivo, and RFC of transcripts of perforin-1 (A), granzyme A (B), granzyme B (C), and FasL (D) was measured by qPCR (normalized to cellular β-actin mRNA). (E and F) Splenocytes (E) or purified CD8+ T cells (F) were cultured ex vivo with or without recombinant IL-17A (50 ng/ml) for 24 h, and expression (RFC) of Act-1 was by measured qPCR. (G) RFC of indicated genes in splenocytes cultured ex vivo for 24 h with or without rIL17A (50 ng/ml) in the presence of Bay-11-7082 (4 μM) or dimethyl sulfoxide (DMSO) as a vehicle control (<0.05%). All the data (means and SEM) represent the results of two independent experiments (n = 3 mice/group) compared by two-tailed Student t tests. (A to F) All the data from WT and Il17a−/− mice were normalized to the respective mock-treated controls. *, P < 0.05; **, P < 0.005; ns, not significant (P > 0.05).
IL-17A exerts its function through IL-17A receptor (IL-17R), which is a heterodimeric receptor complex of IL-17RA and IL-17RC. Although the signaling pathway downstream of IL-17R is not completely understood, one common pathway that has been characterized is the activation of the classical NF-κB pathway (23, 64). Upon binding to IL-17A, the SEFIR (similar expression to fibroblast growth factor genes and IL-17 receptor) domain of IL-17R recruits the signaling adaptor ACT-1, which further recruits an essential upstream activator of the classical NF-κB pathway called TRAF6 (64). To test if IL-17A signaling in WNV-infected cells occurs via ACT-1, we measured the expression of Act-1 in splenocytes and CD8+ T cells isolated from WNV-infected mice and treated ex vivo with recombinant IL-17A, as described above. The qPCR results showed that IL-17A treatment upregulated the expression of the Act-1 gene in splenocytes (Fig. 6E) and CD8+ T cells (Fig. 6F) isolated from WNV-infected mice. To further test if IL-17A-mediated expression of cytotoxicity mediators involves NF-κB activation, we treated splenocytes isolated from WNV-infected WT mice with recombinant IL-17A in the presence of Bay-11-7082 (an inhibitor of NF-κB) and measured the expression of the cytotoxicity mediators, as described above. Consistent with previous reports (65, 66), Bay-11-7082 inhibited IL-17A-mediated Cxcl1 expression, which involves the NF-κB pathway. However, inhibition of NF-κB did not inhibit but, interestingly, further upregulated the IL-17A-mediated expression of perforin-1, granzyme A, and granzyme B (Fig. 6G). These data indicate that IL-17A-mediated expression of cytotoxicity mediators may involve ACT-1 but may occur independently of the NF-κB pathway, which requires further investigation.
IL-17A promotes the expression of cytotoxic-mediator genes in CD8+ T cells.
IL-17R is expressed by virtually all cell types and tissues examined (59, 67, 68). CD8+ T cells also express IL-17R (59, 69); however, the functional role of this receptor in CD8+ T cell biology has not yet been recognized. To test if IL-17A may directly promote cytotoxic-mediator expression in CD8+ T cells, we treated CD8+ T cells purified (∼80 to 90% pure) from spleens of WNV-infected WT mice with the recombinant IL-17A (50 ng/ml) ex vivo for 24 h and then measured the expression of cytotoxicity mediators by qPCR. IL-17A treatment significantly induced the expression of perforin-1 (Fig. 7A), granzyme A (Fig. 7B), and granzyme B (Fig. 7C), but not FasL (Fig. 7D), in CD8+ T cells purified from WNV-infected WT mice. Similar results were also obtained in CD8+ T cells purified from WNV-infected Il17a−/− mice (Fig. 7A to D). In a separate experiment, we cultured splenocytes from WNV-infected Il17a−/− and WT mice with recombinant IL-17A (50 ng/ml) ex vivo for 24 h, separated CD8+ T cells from CD8− cells, and then measured the expression of cytotoxic mediators by qPCR. Consistently, IL-17A treatment induced the expression of cytotoxic mediators in CD8+ T cells (data not shown), but not in CD8− cells (Fig. 7A to D). To further confirm the role of IL-17A in promoting the expression of the cytotoxic mediators in brain CD8+ T cells in vivo, we infected Il17a−/− mice with WNV (100 PFU), treated them with recombinant IL-17A (2.5 μg per mouse at 6 dpi), and performed flow cytometric analysis of brain leukocytes. The results showed that treatment of WNV-infected Il17a−/− mice with recombinant IL-17A significantly induced the production of perforin and granzyme A in brain-infiltrating CD8+ T cells (Fig. 7E). Taken together, these results suggest that IL-17A promotes the expression of cytotoxic-mediator genes in CD8+ T cells during WNV infection in mice.
FIG 7.
IL-17A induces cytotoxicity mediator gene expression in CD8+ T cells. (A to D) Seven- to 9-week-old WT (C57BL/6J) and Il17a−/− mice (n = 6) were infected (i.p.) with 100 PFU of WNV and sacrificed at 8 dpi to collect their spleens, followed by magnetic separation of CD8+ T cells. (A to D) RFC of transcripts of perforin-1 (A), granzyme A (B), granzyme B (C), and FasL (D) was measured by qPCR in CD8+ or CD8− T cells cultured ex vivo for 24 h with or without recombinant mouse IL-17A (50 pg/ml). (E) Eight-week-old Il17a−/− mice (n = 4 per group) infected with WNV (100 PFU i.p.) were treated i.p. with recombinant IL-17A (2.5 μg/mouse) or PBS (control) at 6 dpi and sacrificed at 8 dpi to characterize brain leukocytes by flow cytometry. The mean fluorescence intensities of perforin and granzyme A within the gated CD45hi CD8+ cells (green) are shown on the right. All the data (means and SEM) represent the results of two independent experiments compared by two-tailed Student t tests. (A to D) All the data from WT and Il17a−/− mice were normalized to the respective mock-treated controls. ns, no significant difference (P > 0.05).
IL-17A has therapeutic potential against WNV infection.
Since CD8+ T cells are essential to clear WNV from the CNS at a later phase of infection, we hypothesized that recombinant IL-17A treatment in vivo may offer protection from WNV infection by promoting cytotoxicity of CD8+ T cells. To test if IL-17A may serve as a therapeutic reagent to treat WNV infection in mice, we infected WT female mice with WNV (100 PFU) via the i.p. route. At 6 dpi, mice were injected with carrier-free mouse recombinant IL-17A (eBioscience) or phosphate-buffered saline (PBS) as a control via the i.p. route for survival analysis. Mice that received recombinant IL-17A showed a significantly increased survival rate compared to the PBS-treated control mice (Fig. 8A). Consistent with the survival results, mice treated with recombinant IL-17A also showed lower viral burdens in the brain than the PBS-treated control mice at 8 dpi (Fig. 8B). To test if the effect of the recombinant IL-17A treatment involves promotion of cytotoxicity of CD8+ T cells, we also measured the expression of the cytotoxicity mediators in splenic CD8+ and CD8− T cells. Consistent with reduced viral burden and increased survival, administration of recombinant IL-17A in WNV-infected mice induced expression of the cytotoxic mediators in CD8+ T cells, but not in CD8− T cells (Fig. 8C to F), which was in agreement with our in vitro results. In addition, we also detected increased expression of granzyme A, granzyme B, and FasL in brain tissues of WNV-infected mice after IL-17A treatment (Fig. 8G). Collectively, these results suggest a novel and promising therapeutic role of IL-17A in facilitating WNV clearance by promoting CD8+ T cell cytotoxicity.
FIG 8.
Recombinant IL-17A treatment reduces the WNV burden, increases cytotoxicity markers, and increases survival of WNV-infected mice. WT (C57BL/6J) mice (8-week-old females) were infected with 100 PFU of WNV via the i.p. route. At 6 dpi, the mice were injected i.p. (2.5 μg/mouse) with carrier-free mouse rIL17A or PBS. (A) Survival percentages were compared using the Kaplan-Meier survival and log-rank tests. (B to G) The viral burden in the brain (B) and the transcripts of perforin-1, granzyme A, granzyme B, and FasL in splenic CD8+ and CD8− T cells (C to F) and the brain (G) were measured by qPCR and compared by two-tailed Student t tests. ns, no significant difference (P > 0.05).
DISCUSSION
This study reveals a novel role of IL-17A in facilitating WNV clearance by inducing the expression of cytotoxic-mediator genes and promoting CD8+ T cell cytotoxicity. Specifically, we report here that (i) WNV induces IL-17A expression in both mice and humans; (ii) Il17a−/− mice generate a higher viral burden and are more susceptible to WNV infection; (iii) CD8+ T cells purified from Il17a−/− mice are less cytotoxic and express lower levels of cytotoxic mediators, i.e., perforin-1, granzyme A, granzyme B, and FasL; and, most importantly, (iv) in vivo supply of recombinant IL-17A as late as day 6 postinfection significantly reduces the viral burden in the brain and increases the survival rate of WNV-infected mice, suggesting a therapeutic potential of IL-17A.
IL-17A is a pleiotropic cytokine that plays key roles in infection (70), inflammation (71), and autoimmune diseases (72). Further, it regulates the expression of a number of cytokines, including IL-1β, IFN-γ, and TNF-α (22, 73). Considering their critical roles in WNV pathogenesis (15, 16, 19, 20), it is plausible that IL-17A may control the expression of these cytokines during WNV infection. However, we did not detect significant differences in the expression of IL-1β, IFN-γ, and TNF-α in WNV-infected Il17a−/− mice compared to WT controls. These results suggest the likelihood that IL-17A-mediated protective immunity against WNV infection may not be associated with functions controlled by these cytokines. Previous studies have suggested that type I IFNs (antiviral cytokines), which can potently suppress IL-17A expression (74, 75), play a prominent role in controlling WNV infection (4, 5). Again, we did not detect any change in the expression of type I IFNs in Il17a−/− mice versus WT control mice during WNV infection. Moreover, IL-17A has been suggested to regulate the humoral immune response (60, 76, 77); however, we did not see such an effect during WNV infection in mice. Despite unaltered inflammatory response, type I IFN expression, and humoral immune response, Il17a−/− mice generated higher viral burdens, suggesting that IL-17A-mediated protective immunity during WNV infection is independent of these immune responses.
WNV invades the CNS and infects neurons and CNS-resident cells, such as microglia and astrocytes, which leads to production of chemokines and cytokines that recruit peripheral leukocytes into the brain (10, 14, 78, 79). We previously reported that IL-23 has an important role in recruiting CD11b+ monocytes and macrophages into the CNS to control WNV infection (14). Considering the role of IL-23 in producing IL-17A, and the role of both of these cytokines in inducing and sustaining leukocyte recruitment to infected sites (80, 81), we hypothesized that leukocyte migration into the CNS would be reduced in Il17a−/− mice. Surprisingly, we detected a modestly elevated leukocyte influx into WNV-infected Il17a−/− mouse brains by both flow cytometry and confocal microscopy. In dissecting the mechanism, we found that the expression of Ccl5 (whose product is also known as RANTES) was elevated in both the CNS and the peripheral tissues of WNV-infected Il17a−/− mice compared to WT controls. CCL5 plays a protective role in WNV, influenza virus, and parainfluenza virus infections by promoting leukocyte trafficking to infected tissues (78, 82). Also, Ccl5 expression sustains CD8+ T cell responses during influenza virus (82), parainfluenza virus (82), and chronic LCMV (83) infections. The upregulation of Ccl5 in Il17a−/− mice may be due to several reasons. First, the higher viral load in Il17a−/− mice could trigger stronger Ccl5 expression (84, 85). Second, the presence of IL-17A in WT mice may suppress Ccl5 expression, since IL-17A can downregulate Ccl5 expression (86, 87). Although increased Ccl5 expression may account for a modest elevation in brain-infiltrating leukocytes in Il17a−/− mice, it did not result in protection of the mice from WNV infection, implying a possible functional defect in brain-infiltrating effector leukocytes in Il17a−/− mice.
It has been reported that mice treated with anti-IL-17A antibody show an approximately 20% reduced survival rate compared to the control mice after a lethal WNV challenge (88). However, no difference in the WNV burden was observed between anti-IL-17A antibody-treated and control mice (88). In contrast, our results clearly demonstrate that Il17a−/− mice are more susceptible to WNV infection. The discrepancy between these two studies may be due to the transient effects of anti-IL-17A treatment compared to complete genetic deficiency of IL-17A in Il17a−/− mice. In addition, we cannot rule out the possibility that the genetic deficiency of IL-17A may have other, secondary immune system effects that may also contribute to the different outcomes compared to temporarily blocking IL-17A signaling by antibody administration. More to this point, γδ-T cells produce IL-17A in the early phase, whereas CD4+ Th17 cells become the major IL-17A producer during the late phase of infection (34). Thus, the administration of anti-IL-17A antibody in an early phase of WNV infection (0 and 5 dpi) (88) may not have sufficiently blocked IL-17A production and function during the later phase of infection. Indeed, we found that IL-17A was not essential for controlling viremia at 2 dpi but became critical for reducing viremia at 4 dpi and clearing viruses from the spleen and brain at 8 dpi. Thus, it appears that IL-17A-mediated protective immunity against WNV infection predominantly occurs during the late phase of infection, which requires further investigation.
CD8+ T cells play a critical role in clearance of viruses from the CNS (10, 89, 90). CD8+ T cells can control viral infection directly by inducing apoptosis of virus-infected cells via perforin, granzyme, or Fas-FasL interactions (63, 91) or indirectly by immune-mediated noncytolytic clearance of viruses from neurons by producing cytokines, such as TNF-α and IFN-γ (89, 90). Mice deficient in the expression of cytotoxic mediators, such as perforin and FasL, or cytokines, such as TNF-α and IFN-γ, exhibit increased mortality and viral burden in the CNS and peripheral organs following WNV infection (12, 15, 20, 61, 62, 92). However, we did not detect any significant difference in the expression of cytokines, including TNF-α and IFN-γ, between WNV-infected WT and Il17a−/− mice, suggesting that IL-17A-mediated control of WNV by CD8+ T cells may occur independently of the effects of these cytokines. We report here that CD8+ T cells isolated from Il17a−/− mice have significantly reduced cytotoxicity compared to WT controls, which may explain the higher viral burden and lower survival rates of Il17a−/− mice, despite elevated leukocyte infiltration (including CD8+ T cells) into the CNS. While we are not certain whether CD8+ T cells predominantly contribute to WNV clearance by causing cytolysis of WNV-infected neurons due to lower major histocompatibility complex class I (MHC-I) expression in these cells (93, 94), it is likely that CD8+ T cells can cause cytolysis of WNV-infected nonneural cells, such as infiltrating macrophages and neutrophils, and microglia in the CNS, thus facilitating virus clearance (10, 12, 13, 62). In line with reduced cytotoxicity, CD8+ T cells from Il17a−/− mice had significantly reduced expression of perforin-1, granzyme A, granzyme B, and FasL in most of the tissues examined during WNV infection. However, the induction of FasL by recombinant IL-17A was differentially affected in our experiments. For instance, in vitro IL-17A treatment did not induce the expression of FasL in CD8+ T cells, while it was induced after in vivo administration of IL-17A. This discrepancy is likely due to the experimental conditions, or it may reflect different roles of IL-17A in regulating the expression of these cytotoxic mediators, which requires further investigation. Although NK cells (a type of CD8− cells) also express these genes and mediate the apoptosis of target cells, we did not detect a difference in the expression of cytotoxic mediators in CD8− cell populations isolated from WNV-infected WT and Il17a−/− mouse spleens. This indicates that NK cells may not play a prominent role in IL-17A-mediated cytotoxicity during WNV infection. This notion is consistent with the previous reports that NK cells have little or no role in controlling WNV infection (12). Thus, our data suggest that IL-17A signaling protects mice from WNV infection by upregulating the expression of cytotoxic mediators, thereby promoting CD8+ T cell cytotoxicity. It is worthy of note that CD8+ T cells have also been shown to have an immunopathological role when mice were infected intravenously with a high dose (108 PFU) of WNV (strain Sarafend), resulting in 100% mortality with a 6-day mean survival time (95). However, such an immunopathology role of CD8+ T cells was likely not due to their cytotoxic functions, because these cells are usually activated after 1 week postinfection. In support of this, the same study and many other reports also showed a recovery role of CD8+ T cells when mice were infected with low doses (102 to 103 PFU) of WNV, which is similar to the current study (12, 92, 95–98). In addition, Th17 cells and IL-17A have been implicated in inflammation and immunopathology associated with autoimmune diseases (25–27) and some virus-induced chronic CNS diseases (48, 99, 100), suggesting that Th17/IL-17A axis may have different implications under such immunopathological and chronic inflammatory conditions.
A wide variety of immune cells, including CD4+ Th17 cells, γδ-T cells, NK T cells, and CD8+ T cells, can produce IL-17A (23, 88). In addition, the IL-17A receptor (IL-17R) is expressed ubiquitously in virtually all cell types and tissues examined (67, 68, 86). Although CD8+ T cells can produce IL-17A and also express its cognate receptor (59, 69), the link between IL-17A and CD8+ T cell cytotoxic function has not been previously recognized. In theory, it is possible that IL-17A may affect CD8+ T cell development and/or function by acting on these cells either directly or indirectly through other cell types. Since we found similar levels of CD8+ T cells and CD4+/CD8+ ratios in splenocytes isolated from Il17a−/− and WT mice infected with WNV, it appears that CD8+ T cell development remains unaltered in Il17a−/− mice and that IL-17A may largely control CD8+ T cell function during WNV infection. This hypothesis was confirmed by ex vivo treatment of splenocytes with recombinant IL-17A isolated from WNV-infected Il17a−/− and WT mice, which showed upregulation of perforin-1, granzyme A, and granzyme B expression in CD8+ T cells by IL-17A in a CD4+ T cell-independent manner. These results suggest that IL-17A can induce the expression of cytotoxic mediators by acting directly on CD8+ T cells. Importantly, administration of a single dose of recombinant IL-17A to WNV-infected mice even as late as 6 dpi induced the expression of cytotoxic mediators in CD8+ T cells, dramatically reduced the viral burden in the brain, and increased the survival rate, suggesting a promising therapeutic role of IL-17A against WNV infection.
The role of IL-17A in promoting the expression of cytotoxic mediators in CD8+ T cells has not been previously reported, and the signaling mechanism by which IL-17A mediates the expression of cytotoxicity mediators and regulates CD8+ T cell cytotoxicity is not currently understood. Both CD4+ Th17 cells and γδ-T cells, the major cells that produce IL-17A, have been previously shown to promote CD8+ T cell cytotoxicity during infection (101, 102) and autoimmune disease (103) and in cancers (104, 105). Also, NK cells treated with IL-17A upregulate the expression of perforin and granzyme genes and cytotoxic functions (106), which provides additional evidence that IL-17A signaling can induce the expression of cytotoxicity mediators. These reports support our current findings and suggest that IL-17A may facilitate cytotoxicity of CD8+ T cells under diverse disease conditions.
In conclusion, this study uncovered a novel function of IL-17A in promoting CD8+ T cell cytotoxicity during WNV infection in mice. Further studies are warranted to better understand the regulation of IL-17A production during WNV infection and to dissect the specific mechanism by which IL-17A induces the expression of cytotoxicity mediator genes and promotes CD8+ T cell cytotoxicity in the context of WNV infection and other diseases, which may help to exploit novel IL-17A-based therapeutic strategies.
MATERIALS AND METHODS
Ethics statement and biosafety.
Written informed consent was obtained from all human volunteers and human WNV cases prior to inclusion in this study. The protocol for human subjects was reviewed and approved by the University of Southern Mississippi (USM) Institutional Review Board (protocol CH-R11120601). All animal experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee at USM (protocol 12041201). All the in vitro experiments and animal studies involving live WNV were performed by certified personnel in biosafety level 3 (BSL3) laboratories following standard biosafety protocols approved by the USM Institutional Biosafety Committee.
Virus stock and animal studies.
The low-passage WNV isolate CT2741 (107) was provided by John F. Anderson at the Connecticut Agricultural Experiment Station. The WNV stocks used in this study were prepared by propagating the viruses in Vero cells through a single passage and titrated in Vero cells by plaque-forming assay as previously described (54). Vero cells (ATCC CCL-81) were cultured in a 37°C incubator with 5% CO2 in Dulbecco's modified Eagle medium (DMEM) (Life Technologies) supplemented with 10% fetal bovine serum (FBS). Il17a−/− mouse breeding pairs (C57BL/6J background) were provided by Richard A. Flavell at the Yale University School of Medicine, and WT control mice (C57BL/6J) were purchased from the Jackson Laboratory (Bar Harbor, ME). Il23p19−/− breeding pairs on a mixed C57BL/6 × 129 background were obtained from the Mutant Mouse Regional Resource Center (MMRRC). The mice were housed under standard conditions in the animal facility at USM. Gender-matched 7- to 9-week-old Il17a−/− and WT control mice were infected with 1,000 PFU of WNV by i.p. injection in 100 μl of PBS containing 5% gelatin (14). For footpad inoculation, 100 PFU of WNV in 50 μl PBS containing 1% FBS was injected into the mouse footpad after isoflurane anesthesia (17). The infected animals were observed twice daily for up to 21 days for morbidity and mortality.
Cell culture and in vitro infection.
hPBMCs were isolated from the blood of healthy human volunteers using Ficoll-Paque Plus (GE Healthcare). To isolate murine splenocytes, healthy C57BL/6J mice (7 weeks old) were euthanized and spleens were collected to make a single-cell suspension. After red blood cell lysis, both mouse splenocytes and hPBMCs were purified and cultured in DMEM (Life Technologies) supplemented with 10% FBS, 2 mM l-glutamine, and 1% nonessential amino acids. The cells were infected with WNV (MOI = 0.1, 1, or 5) and collected in Trireagent (Molecular Research Center) at 24 h and 48 h for total RNA extraction.
qPCR.
Total RNA was extracted from cells or animal tissues (i.e., blood, spleen, and brain) using a Trireagent or RNeasy kit with on-column DNA digestion (Qiagen). First-strand cDNA was synthesized using an iScript cDNA synthesis kit (Bio-Rad). WNVE RNA copy numbers were quantified using probe-based qPCR and normalized to the cellular β-actin gene, as previously described (54). qPCR assays for cytokine, chemokine, and other immunological marker genes were performed using SYBR green supermix (Bio-Rad), and data are presented either as the relative fold change (RFC) by the ΔΔCT method using β-actin as a housekeeping gene or as a copy number target gene/cellular β-actin ratio. Primer sequences for mouse (54) and human (108) β-actin were previously described. Primer sequences for human Il17a (F, 5′-TGTGATCTGGGAGGCAAAGT-3′; R, 5′-GATCTCTTGCTGGATGGGGA-3′) and mouse Il17a (F, 5′-TCTCCACCGCAATGAAGACC-3′; R, 5′-TTTCCCTCCGCATTGACACA-3′), perforin-1 (F, 5′-TGTTCCTCCTGGGCCTTTTC-3′; R, 5′-CCATACACCTGGCACGAACT-3′), granzyme-A (F, 5′-CACGTGAGGGGGATCTACAAC-3′; R, 5′-TCTCCCCCATCCTGCTACTC-3′), granzyme-B (F, 5′-TGCTACTGCTGACCTTGTCTC-3′; R, 5′-CCATGTAGGGTCGAGAGTGG-3′), fasL (F, 5′-GAACTGGCAGAACTCCGTGA-3′; R, 5′-TGAGTGGGGGTTCCCTGTTA-3′), Ifn-α (F, 5′-TTCCCCTGACCCAGGAAGAT-3′; R, 5′-CTTCTGCTCTGACCACCTCC-3′), Ifn-β (F, 5′-TGTCCTCAACTGCTCTCCAC-3′; R, 5′-ATCTCTGCTCGGACCACCAT-3′), and Act-1 (F, 5′-GAGGACGAGCATGGCTTACA-3′; R, 5′-TGGCATTTGGGAAGAGCACA-3′) were designed using NCBI's primer-designing tool and synthesized by Integrated DNA Technologies.
ELISA.
IL-17A, IFN-β, IL-1β, IL-6, IL-10, IFN-α, IL-12p40, TNF-α, and anti-WNV-E IgM antibody in the plasma of WNV-infected (1,000 PFU i.p.) mice were measured using an ELISA kit (R&D Systems) following the manufacturer's instructions. The levels of IL-17A in culture media and the sera of human WNV cases and healthy controls were measured with an ELISA kit from Enzo Life Sciences.
Confocal microscopy.
Brains were collected from WNV-infected (1,000 PFU i.p.) mice after intracardial PBS perfusion, fixed overnight in 4% paraformaldehyde (PFA) at 4°C, and cryoprotected in sucrose. Paramedian sagittal sections (25 μm) of the brain were preblocked for 30 min at room temperature and then probed overnight at 4°C with a combination of primary antibodies against CD11b, CD45, and WNV antigen (anti-WNV antibody was provided by John F. Anderson; the other antibodies were purchased from BD Biosciences). After a PBS wash, the sections were probed with appropriate fluorescently labeled secondary antibodies for 1 h at room temperature, counterstained with DAPI (4′,6-diamidino-2-phenylindole) (Invitrogen), and mounted in fluorescent mounting medium (ProLong Gold). Images were acquired in independent channels using a Nikon A1R confocal microscope.
Flow cytometry.
Brains were collected from WNV-infected (1,000 PFU i.p.) mice after intracardial PBS perfusion and processed into a single-cell suspension. Brain leukocytes were isolated using a discontinuous Percoll gradient (GE Healthcare) and probed with CD45, CD4, CD8, and CD11b (BD Biosciences or eBioscience). After staining, the cells were washed two times in flow cytometry buffer (FCB) (PBS with 2% FBS) and fixed in 4% PFA for 15 min. For intracellular staining, cells were permeabilized and probed with antibodies against perforin and granzyme A (eBioscience). The cells were then washed and resuspended in FCB. Data were acquired on a flow cytometer (BD LSRFortessa) and analyzed with FlowJo or FACSDiva software (BD Biosciences).
CD8+ T cell isolation and cytotoxicity assay.
Spleens were collected from WNV-infected (100 PFU i.p.) mice at 10 dpi. Splenic CD8+ T cells were isolated by negative antibody selection with magnetic beads using the mouse CD8+ T Lymphocyte Enrichment Set-DM (BD Biosciences). The purity of CD8+ T cells was examined by flow cytometry after staining with fluorescently labeled anti-CD3 and anti-CD8 antibodies (eBioscience). The cytotoxicity of CD8+ T cells was measured as described previously (10) with some modifications. Briefly, purified CD8+ T cells (∼80 to 90% purity) were cocultured for 4 h in 96-well plates with target cells expressing the ectodomain of WNV-E (MC57GLWNV-E) or control cells containing the expression vector (MC57GLvector) (given by Michael S. Diamond) with effector-to-target cell ratios of 50:1. CD8+ T cell cytotoxicity was measured using an LDH Cytotoxicity Detection kit (Thermo Scientific). Transcripts of perforin-1, granzyme A, granzyme B, and FasL genes in purified CD8+ T cells and CD8− cells were measured by qPCR, as described above.
Ex vivo and in vivo IL-17A treatment assay.
For ex vivo studies, splenocytes were isolated from WNV-infected (100 PFU i.p.) mice at 8 dpi, and splenic CD8+ T cells were purified as described above. In some experiments, CD4+ T cells were depleted from splenocytes using CD4 Magnetic Particles-DM (BD Biosciences). The splenocytes, purified CD8+ T cells, or CD4− splenocytes were cultured for 24 h in the presence of mouse recombinant IL-17A (50 ng/ml; eBioscience). In some experiments, splenocytes were cultured for 24 h in the presence of mouse recombinant IL-17A (50 ng/ml) and subjected to CD8+ T cell purification. Expression of perforin-1, granzyme A, granzyme B, and FasL in splenocytes, CD8+ T cells, and splenic CD8− cells was measured by qPCR, as described above.
For in vivo IL-17A treatment and survival studies, WT female mice (8 weeks old) were inoculated with WNV (100 PFU) via the i.p. route. At 6 dpi, the mice were treated (2.5 μg/mouse) i.p. with carrier-free mouse recombinant IL-17A (eBioscience) or PBS and monitored daily for mortality and morbidity for up to 21 days. Randomly selected mice were euthanized at 8 dpi, and the WNV burden in the brain and the expression of the cytotoxic mediators in splenic CD8+ and CD8− T cells were measured by qPCR as described above.
Statistical analyses.
Data were analyzed using a two-tailed Student t test or analysis of variance (ANOVA) in GraphPad Prism (GraphPad Software; version 6), with a P value of <0.05 considered statistically significant.
ACKNOWLEDGMENTS
We thank John F. Anderson (Connecticut Agricultural Experiment Station) for providing WNV (CT2741) and Michael S. Diamond (Washington University in St. Louis School of Medicine) for providing MC57GLWNV-E and MC57GLvector cells. We also thank Sharon Sims (Mississippi State Department of Health) and Parminder Vig (University of Mississippi Medical Center) for providing serum samples from human WNV cases and the Mississippi-IDeA Network of Biomedical Research Excellence for the use of research facilities.
This work was supported in part by restricted funds to the Wilson Research Foundation, Jackson, MS; the University of Southern Mississippi startup fund; and a Lucas Endowment award (F.B.). P.W. was supported by National Institutes of Health grants AI099625 and AI103807.
REFERENCES
- 1.Colpitts TM, Conway MJ, Montgomery RR, Fikrig E. 2012. West Nile Virus: biology, transmission, and human infection. Clin Microbiol Rev 25:635–648. doi: 10.1128/CMR.00045-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim PY, Behr MJ, Chadwick CM, Shi PY, Bernard KA. 2011. Keratinocytes are cell targets of West Nile virus in vivo. J Virol 85:5197–5201. doi: 10.1128/JVI.02692-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ye C, Abraham S, Wu H, Shankar P, Manjunath N. 2011. Silencing early viral replication in macrophages and dendritic cells effectively suppresses flavivirus encephalitis. PLoS One 6:e17889. doi: 10.1371/journal.pone.0017889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samuel MA, Diamond MS. 2005. Alpha/beta interferon protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. J Virol 79:13350–13361. doi: 10.1128/JVI.79.21.13350-13361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lazear HM, Pinto AK, Vogt MR, Gale M Jr, Diamond MS. 2011. Beta interferon controls West Nile virus infection and pathogenesis in mice. J Virol 85:7186–7194. doi: 10.1128/JVI.00396-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehlhop E, Diamond MS. 2006. Protective immune responses against West Nile virus are primed by distinct complement activation pathways. J Exp Med 203:1371–1381. doi: 10.1084/jem.20052388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diamond MS, Shrestha B, Marri A, Mahan D, Engle M. 2003. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J Virol 77:2578–2586. doi: 10.1128/JVI.77.4.2578-2586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diamond MS, Sitati EM, Friend LD, Higgs S, Shrestha B, Engle M. 2003. A critical role for induced IgM in the protection against West Nile virus infection. J Exp Med 198:1853–1862. doi: 10.1084/jem.20031223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sitati EM, Diamond MS. 2006. CD4+ T-cell responses are required for clearance of West Nile virus from the central nervous system. J Virol 80:12060–12069. doi: 10.1128/JVI.01650-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shrestha B, Diamond MS. 2004. Role of CD8+ T cells in control of West Nile virus infection. J Virol 78:8312–8321. doi: 10.1128/JVI.78.15.8312-8321.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welte T, Lamb J, Anderson JF, Born WK, O'Brien RL, Wang T. 2008. Role of two distinct gammadelta T cell subsets during West Nile virus infection. FEMS Immunol Med Microbiol 53:275–283. doi: 10.1111/j.1574-695X.2008.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shrestha B, Samuel MA, Diamond MS. 2006. CD8+ T cells require perforin to clear West Nile virus from infected neurons. J Virol 80:119–129. doi: 10.1128/JVI.80.1.119-129.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bai F, Kong KF, Dai J, Qian F, Zhang L, Brown CR, Fikrig E, Montgomery RR. 2010. A paradoxical role for neutrophils in the pathogenesis of West Nile virus. J Infect Dis 202:1804–1812. doi: 10.1086/657416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Town T, Bai F, Wang T, Kaplan AT, Qian F, Montgomery RR, Anderson JF, Flavell RA, Fikrig E. 2009. Toll-like receptor 7 mitigates lethal West Nile encephalitis via interleukin 23-dependent immune cell infiltration and homing. Immunity 30:242–253. doi: 10.1016/j.immuni.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shrestha B, Wang T, Samuel MA, Whitby K, Craft J, Fikrig E, Diamond MS. 2006. Gamma interferon plays a crucial early antiviral role in protection against West Nile virus infection. J Virol 80:5338–5348. doi: 10.1128/JVI.00274-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramos HJ, Lanteri MC, Blahnik G, Negash A, Suthar MS, Brassil MM, Sodhi K, Treuting PM, Busch MP, Norris PJ, Gale M Jr. 2012. IL-1beta signaling promotes CNS-intrinsic immune control of West Nile virus infection. PLoS Pathog 8:e1003039. doi: 10.1371/journal.ppat.1003039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bai F, Town T, Qian F, Wang P, Kamanaka M, Connolly TM, Gate D, Montgomery RR, Flavell RA, Fikrig E. 2009. IL-10 signaling blockade controls murine West Nile virus infection. PLoS Pathog 5:e1000610. doi: 10.1371/journal.ppat.1000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang P, Bai F, Zenewicz LA, Dai J, Gate D, Cheng G, Yang L, Qian F, Yuan X, Montgomery RR, Flavell RA, Town T, Fikrig E. 2012. IL-22 signaling contributes to West Nile encephalitis pathogenesis. PLoS One 7:e44153. doi: 10.1371/journal.pone.0044153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. 2004. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med 10:1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- 20.Shrestha B, Zhang B, Purtha WE, Klein RS, Diamond MS. 2008. Tumor necrosis factor alpha protects against lethal West Nile virus infection by promoting trafficking of mononuclear leukocytes into the central nervous system. J Virol 82:8956–8964. doi: 10.1128/JVI.01118-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rouvier E, Luciani MF, Mattei MG, Denizot F, Golstein P. 1993. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol 150:5445–5456. [PubMed] [Google Scholar]
- 22.Song X, Qian Y. 2013. The activation and regulation of IL-17 receptor mediated signaling. Cytokine 62:175–182. doi: 10.1016/j.cyto.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Gu C, Wu L, Li X. 2013. IL-17 family: cytokines, receptors and signaling. Cytokine 64:477–485. doi: 10.1016/j.cyto.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witowski J, Ksiazek K, Jorres A. 2004. Interleukin-17: a mediator of inflammatory responses. Cell Mol Life Sci 61:567–579. doi: 10.1007/s00018-003-3228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McFarland HF, Martin R. 2007. Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol 8:913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- 26.Zepp J, Wu L, Li X. 2011. IL-17 receptor signaling and T helper 17-mediated autoimmune demyelinating disease. Trends Immunol 32:232–239. doi: 10.1016/j.it.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Berg WB, Miossec P. 2009. IL-17 as a future therapeutic target for rheumatoid arthritis. Nat Rev Rheumatol 5:549–553. doi: 10.1038/nrrheum.2009.179. [DOI] [PubMed] [Google Scholar]
- 28.Raychaudhuri SP. 2013. Role of IL-17 in psoriasis and psoriatic arthritis. Clin Rev Allergy Immunol 44:183–193. doi: 10.1007/s12016-012-8307-1. [DOI] [PubMed] [Google Scholar]
- 29.Newcomb DC, Peebles RS Jr. 2013. Th17-mediated inflammation in asthma. Curr Opin Immunol 25:755–760. doi: 10.1016/j.coi.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siakavellas SI, Bamias G. 2012. Role of the IL-23/IL-17 axis in Crohn's disease. Discov Med 14:253–262. [PubMed] [Google Scholar]
- 31.Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, Odden AR, Shellito JE, Bagby GJ, Nelson S, Kolls JK. 2005. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med 202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. 2001. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med 194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. 2007. Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J Immunol 178:4466–4472. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- 34.Hamada S, Umemura M, Shiono T, Tanaka K, Yahagi A, Begum MD, Oshiro K, Okamoto Y, Watanabe H, Kawakami K, Roark C, Born WK, O'Brien R, Ikuta K, Ishikawa H, Nakae S, Iwakura Y, Ohta T, Matsuzaki G. 2008. IL-17A produced by gammadelta T cells plays a critical role in innate immunity against Listeria monocytogenes infection in the liver. J Immunol 181:3456–3463. doi: 10.4049/jimmunol.181.5.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin Y, Ritchea S, Logar A, Slight S, Messmer M, Rangel-Moreno J, Guglani L, Alcorn JF, Strawbridge H, Park SM, Onishi R, Nyugen N, Walter MJ, Pociask D, Randall TD, Gaffen SL, Iwakura Y, Kolls JK, Khader SA. 2009. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity 31:799–810. doi: 10.1016/j.immuni.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang X, Gao L, Lei L, Zhong Y, Dube P, Berton MT, Arulanandam B, Zhang J, Zhong G. 2009. A MyD88-dependent early IL-17 production protects mice against airway infection with the obligate intracellular pathogen Chlamydia muridarum. J Immunol 183:1291–1300. doi: 10.4049/jimmunol.0803075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang W, Na L, Fidel PL, Schwarzenberger P. 2004. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis 190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 38.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, Filler SG, Masso-Welch P, Edgerton M, Gaffen SL. 2009. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med 206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rudner XL, Happel KI, Young EA, Shellito JE. 2007. Interleukin-23 (IL-23)-IL-17 cytokine axis in murine Pneumocystis carinii infection. Infect Immun 75:3055–3061. doi: 10.1128/IAI.01329-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guiton R, Vasseur V, Charron S, Arias MT, Van Langendonck N, Buzoni-Gatel D, Ryffel B, Dimier-Poisson I. 2010. Interleukin 17 receptor signaling is deleterious during Toxoplasma gondii infection in susceptible BL6 mice. J Infect Dis 202:427–435. doi: 10.1086/653738. [DOI] [PubMed] [Google Scholar]
- 41.Zelante T, De Luca A, Bonifazi P, Montagnoli C, Bozza S, Moretti S, Belladonna ML, Vacca C, Conte C, Mosci P, Bistoni F, Puccetti P, Kastelein RA, Kopf M, Romani L. 2007. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol 37:2695–2706. doi: 10.1002/eji.200737409. [DOI] [PubMed] [Google Scholar]
- 42.Patera AC, Pesnicak L, Bertin J, Cohen JI. 2002. Interleukin 17 modulates the immune response to vaccinia virus infection. Virology 299:56–63. doi: 10.1006/viro.2002.1400. [DOI] [PubMed] [Google Scholar]
- 43.Kohyama S, Ohno S, Isoda A, Moriya O, Belladonna ML, Hayashi H, Iwakura Y, Yoshimoto T, Akatsuka T, Matsui M. 2007. IL-23 enhances host defense against vaccinia virus infection via a mechanism partly involving IL-17. J Immunol 179:3917–3925. doi: 10.4049/jimmunol.179.6.3917. [DOI] [PubMed] [Google Scholar]
- 44.Jie Z, Liang Y, Hou L, Dong C, Iwakura Y, Soong L, Cong Y, Sun J. 2014. Intrahepatic innate lymphoid cells secrete IL-17A and IL-17F that are crucial for T cell priming in viral infection. J Immunol 192:3289–3300. doi: 10.4049/jimmunol.1303281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crowe CR, Chen K, Pociask DA, Alcorn JF, Krivich C, Enelow RI, Ross TM, Witztum JL, Kolls JK. 2009. Critical role of IL-17RA in immunopathology of influenza infection. J Immunol 183:5301–5310. doi: 10.4049/jimmunol.0900995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mukherjee S, Lindell DM, Berlin AA, Morris SB, Shanley TP, Hershenson MB, Lukacs NW. 2011. IL-17-induced pulmonary pathogenesis during respiratory viral infection and exacerbation of allergic disease. Am J Pathol 179:248–258. doi: 10.1016/j.ajpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Almeida Nagata DE, Demoor T, Ptaschinski C, Ting HA, Jang S, Reed M, Mukherjee S, Lukacs NW. 2014. IL-27R-mediated regulation of IL-17 controls the development of respiratory syncytial virus-associated pathogenesis. Am J Pathol 184:1807–1818. doi: 10.1016/j.ajpath.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hou W, Kang HS, Kim BS. 2009. Th17 cells enhance viral persistence and inhibit T cell cytotoxicity in a model of chronic virus infection. J Exp Med 206:313–328. doi: 10.1084/jem.20082030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Q, Zhou J, Zhang B, Tian Z, Tang J, Zheng Y, Huang Z, Tian Y, Jia Z, Tang Y, van Velkinburgh JC, Mao Q, Bian X, Ping Y, Ni B, Wu Y. 2013. Hepatitis B virus induces IL-23 production in antigen presenting cells and causes liver damage via the IL-23/IL-17 axis. PLoS Pathog 9:e1003410. doi: 10.1371/journal.ppat.1003410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stritesky GL, Yeh N, Kaplan MH. 2008. IL-23 promotes maintenance but not commitment to the Th17 lineage. J Immunol 181:5948–5955. doi: 10.4049/jimmunol.181.9.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O'Shea JJ, Cua DJ. 2009. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol 10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. 2003. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem 278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 53.Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. 2007. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med 13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bai F, Wang T, Pal U, Bao F, Gould LH, Fikrig E. 2005. Use of RNA interference to prevent lethal murine West Nile virus infection. J Infect Dis 191:1148–1154. doi: 10.1086/428507. [DOI] [PubMed] [Google Scholar]
- 55.Das Sarma J, Ciric B, Marek R, Sadhukhan S, Caruso ML, Shafagh J, Fitzgerald DC, Shindler KS, Rostami A. 2009. Functional interleukin-17 receptor A is expressed in central nervous system glia and upregulated in experimental autoimmune encephalomyelitis. J Neuroinflammation 6:14. doi: 10.1186/1742-2094-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Griffin GK, Newton G, Tarrio ML, Bu DX, Maganto-Garcia E, Azcutia V, Alcaide P, Grabie N, Luscinskas FW, Croce KJ, Lichtman AH. 2012. IL-17 and TNF-alpha sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J Immunol 188:6287–6299. doi: 10.4049/jimmunol.1200385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ruggiero V, Antonelli G, Gentile M, Conciatori G, Dianzani F. 1989. Comparative study on the antiviral activity of tumor necrosis factor (TNF)-alpha, lymphotoxin/TNF-beta, and IL-1 in WISH cells. Immunol Lett 21:165–169. doi: 10.1016/0165-2478(89)90054-0. [DOI] [PubMed] [Google Scholar]
- 58.Moore TC, Bush KL, Cody L, Brown DM, Petro TM. 2012. Control of early Theiler's murine encephalomyelitis virus replication in macrophages by interleukin-6 occurs in conjunction with STAT1 activation and nitric oxide production. J Virol 86:10841–10851. doi: 10.1128/JVI.01402-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, Cohen JI, Spriggs MK. 1995. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity 3:811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 60.Yuan J, Cao AL, Yu M, Lin QW, Yu X, Zhang JH, Wang M, Guo HP, Liao YH. 2010. Th17 cells facilitate the humoral immune response in patients with acute viral myocarditis. J Clin Immunol 30:226–234. doi: 10.1007/s10875-009-9355-z. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y, Lobigs M, Lee E, Mullbacher A. 2004. Exocytosis and Fas mediated cytolytic mechanisms exert protection from West Nile virus induced encephalitis in mice. Immunol Cell Biol 82:170–173. doi: 10.1046/j.0818-9641.2004.01227.x. [DOI] [PubMed] [Google Scholar]
- 62.Shrestha B, Diamond MS. 2007. Fas ligand interactions contribute to CD8+ T-cell-mediated control of West Nile virus infection in the central nervous system. J Virol 81:11749–11757. doi: 10.1128/JVI.01136-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shresta S, Pham CT, Thomas DA, Graubert TA, Ley TJ. 1998. How do cytotoxic lymphocytes kill their targets? Curr Opin Immunol 10:581–587. doi: 10.1016/S0952-7915(98)80227-6. [DOI] [PubMed] [Google Scholar]
- 64.Gaffen SL. 2009. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol 9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang F, Kao CY, Wachi S, Thai P, Ryu J, Wu R. 2007. Requirement for both JAK-mediated PI3K signaling and ACT1/TRAF6/TAK1-dependent NF-kappaB activation by IL-17A in enhancing cytokine expression in human airway epithelial cells. J Immunol 179:6504–6513. doi: 10.4049/jimmunol.179.10.6504. [DOI] [PubMed] [Google Scholar]
- 66.Ribaux P, Ehses JA, Lin-Marq N, Carrozzino F, Boni-Schnetzler M, Hammar E, Irminger JC, Donath MY, Halban PA. 2007. Induction of CXCL1 by extracellular matrix and autocrine enhancement by interleukin-1 in rat pancreatic beta-cells. Endocrinology 148:5582–5590. doi: 10.1210/en.2007-0325. [DOI] [PubMed] [Google Scholar]
- 67.Moseley TA, Haudenschild DR, Rose L, Reddi AH. 2003. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev 14:155–174. doi: 10.1016/S1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 68.Yao Z, Spriggs MK, Derry JM, Strockbine L, Park LS, VandenBos T, Zappone JD, Painter SL, Armitage RJ. 1997. Molecular characterization of the human interleukin (IL)-17 receptor. Cytokine 9:794–800. doi: 10.1006/cyto.1997.0240. [DOI] [PubMed] [Google Scholar]
- 69.Lindemann MJ, Hu Z, Benczik M, Liu KD, Gaffen SL. 2008. Differential regulation of the IL-17 receptor by gammac cytokines: inhibitory signaling by the phosphatidylinositol 3-kinase pathway. J Biol Chem 283:14100–14108. doi: 10.1074/jbc.M801357200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Iwakura Y, Nakae S, Saijo S, Ishigame H. 2008. The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol Rev 226:57–79. doi: 10.1111/j.1600-065X.2008.00699.x. [DOI] [PubMed] [Google Scholar]
- 71.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Onishi RM, Gaffen SL. 2010. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology 129:311–321. doi: 10.1111/j.1365-2567.2009.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maione F, Paschalidis N, Mascolo N, Dufton N, Perretti M, D'Acquisto F. 2009. Interleukin 17 sustains rather than induces inflammation. Biochem Pharmacol 77:878–887. doi: 10.1016/j.bcp.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 74.Tilg H, Moschen AR, Kaser A. 2009. Suppression of interleukin-17 by type I interferons: a contributing factor in virus-induced immunosuppression? Eur Cytokine Netw 20:1–6. [DOI] [PubMed] [Google Scholar]
- 75.Curtis MM, Way SS, Wilson CB. 2009. IL-23 promotes the production of IL-17 by antigen-specific CD8 T cells in the absence of IL-12 and type-I interferons. J Immunol 183:381–387. doi: 10.4049/jimmunol.0900939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grund LZ, Komegae EN, Lopes-Ferreira M, Lima C. 2012. IL-5 and IL-17A are critical for the chronic IgE response and differentiation of long-lived antibody-secreting cells in inflamed tissues. Cytokine 59:335–351. doi: 10.1016/j.cyto.2012.04.045. [DOI] [PubMed] [Google Scholar]
- 77.Tarlinton D. 2008. IL-17 drives germinal center B cells? Nat Immunol 9:124–126. doi: 10.1038/ni0208-124. [DOI] [PubMed] [Google Scholar]
- 78.Glass WG, Lim JK, Cholera R, Pletnev AG, Gao JL, Murphy PM. 2005. Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival in West Nile virus infection. J Exp Med 202:1087–1098. doi: 10.1084/jem.20042530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hussmann KL, Fredericksen BL. 2014. Differential induction of CCL5 by pathogenic and non-pathogenic strains of West Nile virus in brain endothelial cells and astrocytes. J Gen Virol 95:862–867. doi: 10.1099/vir.0.060558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shahrara S, Pickens SR, Dorfleutner A, Pope RM. 2009. IL-17 induces monocyte migration in rheumatoid arthritis. J Immunol 182:3884–3891. doi: 10.4049/jimmunol.0802246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roussel L, Houle F, Chan C, Yao Y, Berube J, Olivenstein R, Martin JG, Huot J, Hamid Q, Ferri L, Rousseau S. 2010. IL-17 promotes p38 MAPK-dependent endothelial activation enhancing neutrophil recruitment to sites of inflammation. J Immunol 184:4531–4537. doi: 10.4049/jimmunol.0903162. [DOI] [PubMed] [Google Scholar]
- 82.Kohlmeier JE, Miller SC, Smith J, Lu B, Gerard C, Cookenham T, Roberts AD, Woodland DL. 2008. The chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infections. Immunity 29:101–113. doi: 10.1016/j.immuni.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Crawford A, Angelosanto JM, Nadwodny KL, Blackburn SD, Wherry EJ. 2011. A role for the chemokine RANTES in regulating CD8 T cell responses during chronic viral infection. PLoS Pathog 7:e1002098. doi: 10.1371/journal.ppat.1002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saito T, Deskin RW, Casola A, Haeberle H, Olszewska B, Ernst PB, Alam R, Ogra PL, Garofalo R. 1997. Respiratory syncytial virus induces selective production of the chemokine RANTES by upper airway epithelial cells. J Infect Dis 175:497–504. doi: 10.1093/infdis/175.3.497. [DOI] [PubMed] [Google Scholar]
- 85.Matsukura S, Kokubu F, Kubo H, Tomita T, Tokunaga H, Kadokura M, Yamamoto T, Kuroiwa Y, Ohno T, Suzaki H, Adachi M. 1998. Expression of RANTES by normal airway epithelial cells after influenza virus A infection. Am J Respir Cell Mol Biol 18:255–264. doi: 10.1165/ajrcmb.18.2.2822. [DOI] [PubMed] [Google Scholar]
- 86.Andoh A, Fujino S, Bamba S, Araki Y, Okuno T, Bamba T, Fujiyama Y. 2002. IL-17 selectively down-regulates TNF-alpha-induced RANTES gene expression in human colonic subepithelial myofibroblasts. J Immunol 169:1683–1687. doi: 10.4049/jimmunol.169.4.1683. [DOI] [PubMed] [Google Scholar]
- 87.Shen F, Ruddy MJ, Plamondon P, Gaffen SL. 2005. Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17- and TNF-alpha-induced genes in bone cells. J Leukoc Biol 77:388–399. [DOI] [PubMed] [Google Scholar]
- 88.Welte T, Aronson J, Gong B, Rachamallu A, Mendell N, Tesh R, Paessler S, Born WK, O'Brien RL, Wang T. 2011. Vgamma4+ T cells regulate host immune response to West Nile virus infection. FEMS Immunol Med Microbiol 63:183–192. doi: 10.1111/j.1574-695X.2011.00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Binder GK, Griffin DE. 2001. Interferon-gamma-mediated site-specific clearance of alphavirus from CNS neurons. Science 293:303–306. doi: 10.1126/science.1059742. [DOI] [PubMed] [Google Scholar]
- 90.Griffin DE. 2010. Recovery from viral encephalomyelitis: immune-mediated noncytolytic virus clearance from neurons. Immunol Res 47:123–133. doi: 10.1007/s12026-009-8143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Harty JT, Badovinac VP. 2002. Influence of effector molecules on the CD8(+) T cell response to infection. Curr Opin Immunol 14:360–365. doi: 10.1016/S0952-7915(02)00333-3. [DOI] [PubMed] [Google Scholar]
- 92.Shrestha B, Pinto AK, Green S, Bosch I, Diamond MS. 2012. CD8+ T cells use TRAIL to restrict West Nile virus pathogenesis by controlling infection in neurons. J Virol 86:8937–8948. doi: 10.1128/JVI.00673-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kimura T, Griffin DE. 2000. The role of CD8(+) T cells and major histocompatibility complex class I expression in the central nervous system of mice infected with neurovirulent Sindbis virus. J Virol 74:6117–6125. doi: 10.1128/JVI.74.13.6117-6125.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mucke L, Oldstone MB. 1992. The expression of major histocompatibility complex (MHC) class I antigens in the brain differs markedly in acute and persistent infections with lymphocytic choriomeningitis virus (LCMV). J Neuroimmunol 36:193–198. doi: 10.1016/0165-5728(92)90050-U. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang Y, Lobigs M, Lee E, Mullbacher A. 2003. CD8+ T cells mediate recovery and immunopathology in West Nile virus encephalitis. J Virol 77:13323–13334. doi: 10.1128/JVI.77.24.13323-13334.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brien JD, Uhrlaub JL, Nikolich-Zugich J. 2007. Protective capacity and epitope specificity of CD8(+) T cells responding to lethal West Nile virus infection. Eur J Immunol 37:1855–1863. doi: 10.1002/eji.200737196. [DOI] [PubMed] [Google Scholar]
- 97.Shrestha B, Ng T, Chu HJ, Noll M, Diamond MS. 2008. The relative contribution of antibody and CD8+ T cells to vaccine immunity against West Nile encephalitis virus. Vaccine 26:2020–2033. doi: 10.1016/j.vaccine.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brien JD, Daffis S, Lazear HM, Cho H, Suthar MS, Gale M Jr, Diamond MS. 2011. Interferon regulatory factor-1 (IRF-1) shapes both innate and CD8(+) T cell immune responses against West Nile virus infection. PLoS Pathog 7:e1002230. doi: 10.1371/journal.ppat.1002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hou W, Jin YH, Kang HS, Kim BS. 2014. Interleukin-6 (IL-6) and IL-17 synergistically promote viral persistence by inhibiting cellular apoptosis and cytotoxic T cell function. J Virol 88:8479–8489. doi: 10.1128/JVI.00724-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Savarin C, Stohlman SA, Hinton DR, Ransohoff RM, Cua DJ, Bergmann CC. 2012. IFN-gamma protects from lethal IL-17 mediated viral encephalomyelitis independent of neutrophils. J Neuroinflammation 9:104. doi: 10.1186/1742-2094-9-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xu S, Han Y, Xu X, Bao Y, Zhang M, Cao X. 2010. IL-17A-producing gammadeltaT cells promote CTL responses against Listeria monocytogenes infection by enhancing dendritic cell cross-presentation. J Immunol 185:5879–5887. doi: 10.4049/jimmunol.1001763. [DOI] [PubMed] [Google Scholar]
- 102.Hamada H, Garcia-Hernandez MDL, Reome JB, Misra SK, Strutt TM, McKinstry KK, Cooper AM, Swain SL, Dutton RW. 2009. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J Immunol 182:3469–3481. doi: 10.4049/jimmunol.0801814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ankathatti Munegowda M, Deng Y, Chibbar R, Xu Q, Freywald A, Mulligan SJ, van Drunen Littel-van den Hurk S, Sun D, Xiong S, Xiang J. 2011. A distinct role of CD4+ Th17- and Th17-stimulated CD8+ CTL in the pathogenesis of type 1 diabetes and experimental autoimmune encephalomyelitis. J Clin Immunol 31:811–826. doi: 10.1007/s10875-011-9549-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ankathatti Munegowda M, Deng Y, Mulligan SJ, Xiang J. 2011. Th17 and Th17-stimulated CD8(+) T cells play a distinct role in Th17-induced preventive and therapeutic antitumor immunity. Cancer Immunol Immunother 60:1473–1484. doi: 10.1007/s00262-011-1054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C. 2009. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity 31:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Al Omar S, Flanagan BF, Almehmadi M, Christmas SE. 2013. The effects of IL-17 upon human natural killer cells. Cytokine 62:123–130. doi: 10.1016/j.cyto.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 107.Anderson JF, Andreadis TG, Vossbrinck CR, Tirrell S, Wakem EM, French RA, Garmendia AE, Van Kruiningen HJ. 1999. Isolation of West Nile virus from mosquitoes, crows, and a Cooper's hawk in Connecticut. Science 286:2331–2333. doi: 10.1126/science.286.5448.2331. [DOI] [PubMed] [Google Scholar]
- 108.Kozaci DL, Chernajovsky Y, Chikanza IC. 2007. The differential expression of corticosteroid receptor isoforms in corticosteroid-resistant and -sensitive patients with rheumatoid arthritis. Rheumatology 46:579–585. [DOI] [PubMed] [Google Scholar]