Summary
It is widely assumed that the complexity of neural circuits enables them to implement diverse learning tasks using just a few, generic forms of synaptic plasticity. In contrast, we report that synaptic plasticity can itself be precisely tuned to the requirements of a learning task. We found that the rules for induction of long-term and single-trial plasticity at parallel fiber-to-Purkinje cell synapses vary across cerebellar regions. In the flocculus, associative plasticity in vitro and in vivo is narrowly tuned for an interval of ~120 ms, which compensates for the specific processing delay for error signals to reach the flocculus during oculomotor learning. In the vermis, which supports a range of behavioral functions, plasticity is induced by a range of intervals, with individual cells tuned for different intervals. Thus, plasticity at a single, anatomically-defined type of synapse can have properties that vary in a way that is precisely matched to function.
Introduction
The algorithm used by a neural circuit to learn is defined by both the circuit-level properties and the rules governing the induction of synaptic plasticity. It has been widely assumed that a few basic synaptic rules, when embedded in the appropriate circuit architecture, can support the full repertoire of learning, in all of its complexity. In contrast, we provide evidence that the synaptic plasticity rules themselves can be highly specialized to match the functional requirements of a learning task.
The fundamental requirement of associative learning is to store information about the correlations between events. Throughout the nervous system, synaptic plasticity mechanisms have been described that can capture the correlations between coincident or nearly coincident events (Feldman, 2012). However, behavioral observations indicate that the brain is also able to associate events that are separated in time, with exquisite temporal precision. For example, during feedback-based learning, a delayed error signal must selectively modify synapses active at the specific, earlier time when the neural command leading to an error was generated, a challenge known as the temporal credit assignment problem (Sutton and Barto, 1981). In throwing a ball, for example, there is a delay of a few hundred milliseconds for the ball to reach the target and visual feedback about the accuracy of the throw to reach the nervous system. Nevertheless, the timing of the finger movements controlling the release of the ball can be learned with remarkable precision - a difference of just 10 ms can cause the throw to be too high or too low (Hore et al., 1996).
Previously described mechanisms of synaptic plasticity do not seem to have the properties to support such temporally precise learning across delays, without additional, circuit-level timing mechanisms. Known mechanisms of associative synaptic plasticity that have precise timing requirements, such as those underlying spike timing dependent plasticity (STDP), are all tuned for neural events occurring within a few tens of milliseconds of each other (Feldman, 2012). Other forms of synaptic plasticity have been described that can associate neural events across longer intervals, but with much less temporal precision (Chen and Thompson, 1995; Debanne et al., 1998; Safo and Regehr, 2008). Therefore, circuit-level mechanisms have been hypothesized to enable known, coincidence-based plasticity mechanisms to support temporally precise learning about events that are separated in time (e.g. Mehta et al., 2002). Here, we show that synaptic plasticity in the cerebellum is itself tuned to meet the dual demands of temporally precise learning in the face of delayed feedback.
During cerebellum-dependent learning, delayed feedback about performance errors is conveyed to the cerebellum by its climbing fiber input. Each spike in a climbing fiber produces a “complex spike” and concomitant calcium influx in its Purkinje cell targets. Repeated pairings of climbing fiber (CF) activation with the activation of parallel fiber synapses onto the Purkinje cells results in depression of the parallel fiber-to-Purkinje cell (PF-to-PC) synapses (Ito, 2001; Linden, 1994). Thus, error signals carried by the climbing fibers are thought to sculpt away, through associative synaptic depression, PF-to-PC synapses that were active around the time that an error was generated.
The induction of plasticity at PF-to-PC synapses has been characterized as having broad timing requirements, with associative synaptic depression induced when climbing fiber and parallel fiber activation are coincident, and also when the climbing fiber activation precedes parallel fiber activation or is delayed by as much as two hundred milliseconds (e.g. Chen and Thompson, 1995; Safo and Regehr, 2008). These studies were conducted almost exclusively in the cerebellar vermis, where the climbing fibers encode error signals of multiple modalities and hence a broad range of feedback delays. This heterogeneity makes it difficult to determine the functionally relevant feedback delay for a given climbing fiber in vitro, and hence to assess the match between the feedback delay and the temporal requirements for the induction of synaptic plasticity. To overcome this challenge, we characterized plasticity at PF-to-PC synapses in a more functionally homogenous region of the cerebellum, the flocculus. We discovered that the rules governing associative plasticity at PF-to-PC synapses in the flocculus are different than in the vermis, and precisely compensate for delays in the error signals in the intact circuit during learning.
Results
Heterogeneity in the rules for long-term plasticity at cerebellar parallel fiber-to-Purkinje cell synapses
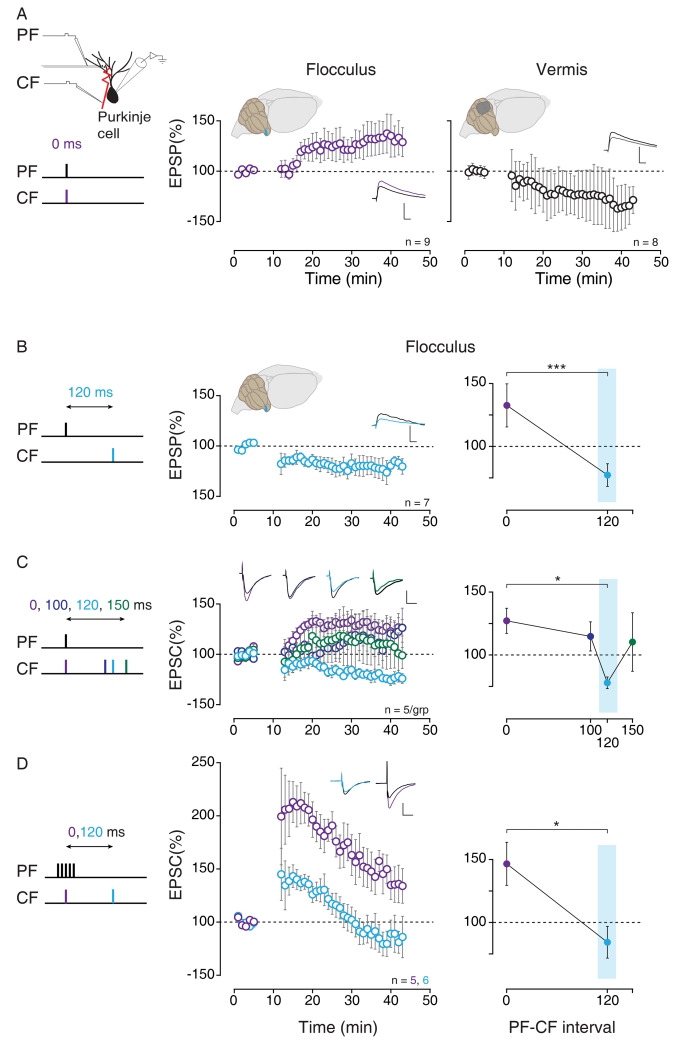
We compared long-term plasticity at PF-to-PC synapses in the cerebellar flocculus with plasticity at PF-to-PC synapses in the cerebellar vermis, in acute slices from adult mice (Fig. 1A-D). We monitored the change in the amplitude of the parallel fiber-elicited EPSP in individual Purkinje cells induced by repeated, coincident pairings of a single stimulus to the parallel fibers and to the climbing fibers (300 times at 1 Hz, parallel fiber - climbing fiber (PF-CF) interval of 0 ms; Fig. 1A, left). Consistent with previous reports (e.g., Hansel et al., 2006; Ito, 2001), coincident stimulation induced robust Long Term Depression (LTD) in slices of the cerebellar vermis (Fig. 1A, right). In the cerebellar flocculus, however, the results were strikingly different. Instead of inducing LTD, coincident activation of parallel fibers and climbing fibers induced Long Term Potentiation (LTP) (Fig. 1A, center). LTP is the non-associative form of plasticity at the PF-PC synapses, which is induced by parallel fiber activation alone in the vermis (Lev-Ram et al., 2002) and the flocculus (Fig S1A). Hence, the rules governing the induction of associative, climbing fiber-driven plasticity at PF-to-PC synapses are different in the cerebellar flocculus and the cerebellar vermis.
Fig. 1. Induction rules for cerebellar LTD are different in the flocculus than the vermis and are tuned to climbing fiber delay.
Long-term plasticity at parallel fiber-to-Purkinje cell (PF-to-PC) synapses was induced with repeated pairings of parallel fiber (PF) and climbing fiber (CF) activation (300 times at 1Hz). The PF-CF pairing interval was varied, as indicated by schematics on left of each panel, and corresponding colors. A) Coincident PF and CF stimulation (0 ms PF-CF interval, purple), induced long-term potentiation (LTP) of PF-to-PC synapses in the cerebellar flocculus (center), but long-term depression (LTD) of PF-to-PC synapses in lobules V/VI of the cerebellar vermis (right). B) Left, center: In the flocculus, LTD was induced when CF stimulation was delayed by 120 ms relative to PF stimulation (light blue). Right: Average EPSP amplitude in the last ten min of current clamp recordings in the flocculus when the PF-CF pairing interval was 0 ms vs. 120 ms. ***p < 0.001, Mann-Whitney. C) Long-term plasticity at PF-to-PC synapses in the flocculus, measured in voltage clamp, for different PF-CF pairing intervals: 0 ms (purple) and 100 ms (dark blue) induced LTP, 120 ms (light blue) induced LTD, and 150 ms (green) induced no significant plasticity. * p < 0.05, Kruskal-Wallis analysis of variance on ranks followed by pairwise Dunn’s test. D) Long-term plasticity at PF-to-PC synapses in the flocculus, induced by pairing trains of PF stimulation (5 at 100 Hz) with CF stimulation (300 times at 1Hz). A PF-CF interval of 0 ms (purple) induced LTP, whereas 120 ms (light blue) induced LTD. * p < 0.05, Mann-Whitney test. n = number of cells. Insets show EPSPs or EPSCs from an example cell just prior to plasticity induction (black), and 30 min later (color); scale bars: 5 mV or 200 pA, 20 ms. All panels are Mean ± S.E.M. (See also Fig. S1A-F))
Long-term associative plasticity in the flocculus in vitro: Temporal requirements matched to behavioral function
Our finding that coincident parallel fiber and climbing fiber stimulation failed to induce LTD in the flocculus was surprising, given the multiple lines of evidence for a role of climbing fiber-triggered LTD in flocculus-dependent learning (e.g., Boyden et al., 2006; Hansel et al., 2006; Ito, 2001; Kimpo et al., 2014; Medina and Lisberger, 2008). However, in vivo, the activation of climbing fibers by performance errors would be delayed relative to the parallel fiber activity that caused the error, rather than coincident. Therefore, we tested whether LTD could be induced in the flocculus when climbing fiber activation was delayed, as would occur in vivo during learning. The function of the flocculus is to regulate the smooth eye movements that stabilize images on the retina (Ito, 2001; du Lac et al., 1995), therefore retinal image motion represents a performance error. Visual feedback about such errors is encoded by climbing fibers in the flocculus at a delay of about 120 ms (Fig. S1B; Maekawa and Simpson, 1973; Raymond and Lisberger, 1998; Stone and Lisberger, 1986). In vivo experiments have suggested that there must be some form of compensation for this delay in order to support the adaptive recalibration of eye movements observed across a range of visual and vestibular stimulus frequencies (Ramachandran and Lisberger, 2005; Raymond and Lisberger, 1998, 2000). More specifically, it can be estimated that it would be optimal for climbing fiber activity to induce plasticity at synapses active ~120 ms earlier (Fig. S1B).
Consistent with this circuit-level constraint on feedback-based learning, robust LTD was induced in floccular slices when parallel fibers were activated 120 ms before the climbing fibers (Fig. 1B). Thus, plasticity at PF-to-PC synapses in the flocculus not only differs from plasticity at PF-to-PC synapses in the vermis, but also compensates for the relevant error signal delay.
These results, from experiments in which PF-to-PC synaptic strength was measured in current clamp (Fig. 1A,B, S1Ci,D), were replicated in additional experiments that measured synaptic strength in voltage clamp (Fig. 1C, S1Cii,D). In addition, the tuning of LTD in the flocculus to the PF-CF interval was tested by comparing four different intervals (Fig. 1C): 0 ms (coincident, purple), 100 ms (dark blue), 120 ms (light blue) and 150 ms (green). Although a climbing fiber delay of 120 ms relative to the parallel fibers induced robust LTD, neither a climbing fiber delay of 100 ms, nor a delay of 150 ms was effective at inducing LTD. Thus, the tuning of LTD for the PF-CF interval was remarkably selective, with a narrow window for associative plasticity of at most a few tens of milliseconds.
To test the generality of this timing requirement, we compared the effectiveness of coincident versus delayed climbing fiber activation when it was paired with a brief, high-frequency train of parallel fiber stimulation (5 PF stimuli at 100 Hz, paired with the CF, 300 times at 1 Hz; Fig 1D). When PF trains were paired with coincident climbing fiber stimulation (PF-CF interval 0 ms), PF-to-PC synapses in the flocculus underwent a robust LTP (Fig. 1D, purple) rather than the LTD that was previously reported in the vermis for similar pairing protocols (Safo and Regehr, 2008; Wang et al., 2000). However, if parallel fiber trains were instead paired with climbing fiber stimulation delayed by 120 ms, there was a transient potentiation of PF-to-PC synapses in the flocculus, followed by LTD (Fig. 1D, light blue). Notably, the synapses that underwent pairing at a PF-CF interval of 120 ms were less potentiated/more depressed than those that underwent coincident PF-CF pairing throughout the entire post-pairing period (Fig. 1D, S1Ciii). LTD was pathway specific (Fig. S1E), as previously described for LTD in the vermis (Ekerot and Kano, 1985; see also Linden, 1994). Moreover, the LTD induced in the flocculus using both single parallel fiber pulses and trains appeared to be expressed post-synaptically, as previously described for cerebellar LTD (e.g., Finch and Augustine, 1998), since there was no change in the paired-pulse ratio (Fig S1D i-iii).
Single-trial, short-term associative plasticity in the flocculus in vitro: Temporal requirements matched to behavioral function
It was recently reported that a single spike in a climbing fiber in vivo can induce plasticity (Kimpo et al., 2014; Medina and Lisberger, 2008; Yang and Lisberger, 2010). We tested whether there was short-term plasticity at the PF-to-PC synapses in response to a single pairing of parallel fiber and climbing fiber activation (see also Brenowitz and Regehr, 2005), which could support the single-trial plasticity observed in vivo during motor learning.
In slices from the mouse cerebellar flocculus, the change in the strength of the PF-to-PC EPSP was measured from test stimuli delivered 1 s before and 1 s after a single pairing of parallel fiber and climbing fiber activation (Fig. 2A, S2A). These single trials were delivered at an inter-trial-interval of 7 s (see Supplemental Experimental Procedures), and the change in the PF-to-PC EPSP measured on single trials was averaged over ~25 trials for each pairing condition. As a control, the PF-CF pairing was replaced by a PF stimulus alone (Fig 2B, S2A-C, no CF), or a climbing fiber stimulus alone (Fig. S2D, no PF), in which case there was no change, on average, in the PF-to-PC EPSP. However, a single pairing of climbing fiber activation 120 ms after parallel fiber activation induced a significant depression of the PF-to-PC EPSP (Fig. 2B, Fig. S2Aii, S2Bii-iv, S2D light blue). This single-trial synaptic plasticity was not only rapidly induced, but also recovered rapidly, returning to baseline prior to the next trial (Fig. S3B). The rapid recovery allowed multiple PF-CF intervals to be tested on the same cell. On average, PF-CF intervals of 0, 100 and 150 ms induced significantly less depression than an interval of 120 (Fig. 2B, see Fig. S2E,F for additional controls). Hence, the temporal specificity for the PF-CF interval in the flocculus was similar for the induction of LTD by multiple PF-CF pairings and for the induction of short-term depression by a single PF-CF pairing.
Figure 2. Similar temporal tuning of single-trial plasticity in the flocculus in vitro and in vivo.
A) The synaptic response in the Purkinje cell to parallel fiber (PF) stimulation was compared before (EPSP1) and after (EPSP3) a single pairing of PF and climbing fiber (CF) stimulation. Stimulus artifact and spike elicited by CF truncated, scale bar is 10 mV. B) Change in the amplitude of EPSP3 relative to EPSP1 (ΔEPSP, mean ± S.E.M.) for different PF-CF pairing intervals and for controls with no CF stimulation (no CF). Single-trial depression of parallel fiber-to-Purkinje cell synapses in the flocculus was selectively induced by a 120-ms PF-CF pairing interval. Number of cells is indicated in each bar. * p < 0.05 repeated measures ANOVA on ranks, followed by pairwise post-hoc Student-Neuman-Keuls comparison. C, D) Trial-to-trial changes in Purkinje cell firing (C) and eye movement responses (D) in vivo during oculomotor learning were precisely timed to compensate for delays in the error signals carried by climbing fibers. Pairs of consecutive trials were analyzed by subtracting the Purkinje cell’s simple spike firing rate or the eye movement response during the second trial of the pair from that on the first trial, averaged across trials of a given type, and then across cells. If there was a spike in the climbing fiber on the first trial of the pair (CF, blue traces), there was a decrease in Purkinje cell firing and a change in the eye movement response on the next trial, at a time corresponding to ~120 ms before the time of the CF spike on the first trial. If there was no spike in the climbing fiber (no CF, black traces), there was no trial-to-trial change. Dotted lines indicate 95% confidence intervals determined by bootstrap. All panels are Mean ± S.E.M. See also Supplemental Experimental Procedures, Fig. S2A-G.
During associative learning in vivo, plasticity of floccular Purkinje cells exhibits precise compensation for feedback delays
The single-trial depression observed at PF-to-PC synapses in response to a single PF-CF pairing in vitro provides a candidate mechanism for trial-to-trial changes in Purkinje cell firing observed in vivo. During oculomotor learning, a spike in a climbing fiber on one trial is associated with a suppression of firing in its Purkinje cell target on the next trial (Kimpo et al., 2014; Medina and Lisberger, 2008; Yang and Lisberger, 2010, 2014). The temporal precision of this single-trial plasticity reported in vivo had appeared to be significantly less than what we found at the PF-to-PC synapses in vitro (hundreds of milliseconds vs. tens of milliseconds). However, previous studies of trial-to-trial changes in vivo had aligned on the trial start time, whereas the in vitro results (Fig. 1, Fig. 2B) suggested that plasticity might occur at synapses active in a specific time window preceding the climbing fiber spike. Therefore, we aligned changes in Purkinje cell firing during VOR learning in rhesus monkeys (Kimpo et al., 2014) to the time of the climbing fiber spike in the preceding trial (Fig. 2C, Fig. S2G, Supplemental Experimental Procedures). If there was no spike in a given climbing fiber on one trial, then there was no detectable change in the firing rate of its Purkinje cell target on the next trial (Fig. 2C, no CF, black trace). However, if there was a spike in the climbing fiber on one trial, then there was a reduction in the Purkinje cell firing rate on the next trial (Fig. 2C, CF, blue trace). Remarkably, the reduction in firing occurred at a time within the trial corresponding to ~120 ms prior to the time of the climbing fiber spike on the previous trial, and then rapidly returned to baseline before the time of the climbing fiber spike. This suggests that in vivo, a climbing fiber spike triggers plasticity at synapses active ~120 ms earlier, and not at synapses active coincidently. Accompanying the changes in Purkinje cell firing were adaptive changes in eye velocity that were also precisely timed (Fig. 2D), and slightly delayed relative to the altered Purkinje cell responses, suggesting a causal relationship.
Thus, in vivo, single-trial, climbing fiber-triggered plasticity in the flocculus of rhesus monkeys has temporal properties similar to the single-trial depression of the PF-to-PC synapses in floccular slices from mice. Both have the temporal precision to precisely compensate, to within a few tens of milliseconds, for the ~120 ms delay in the error signals carried by climbing fibers during eye movements in both species (Fig. S1B). In addition, it suggests that there is sufficient temporal precision in the patterns of activation of the parallel fibers to provide a substrate for the temporally precise plasticity (Fujita, 1982; Medina and Mauk, 2000; Yamazaki and Tanaka, 2009; but see Johansson et al., 2014).
Heterogeneity in the rules for single-trial, short-term plasticity at cerebellar parallel fiber-to-Purkinje cell synapses
As observed for LTD, we found that the induction of single-trial depression at the PF-to-PC synapses is governed by different rules in the cerebellar vermis than in the flocculus. However, by leveraging the ability to test an individual cell with multiple PF-CF intervals, we uncovered temporal precision of the single-trial plasticity in the vermis comparable to that in the flocculus.
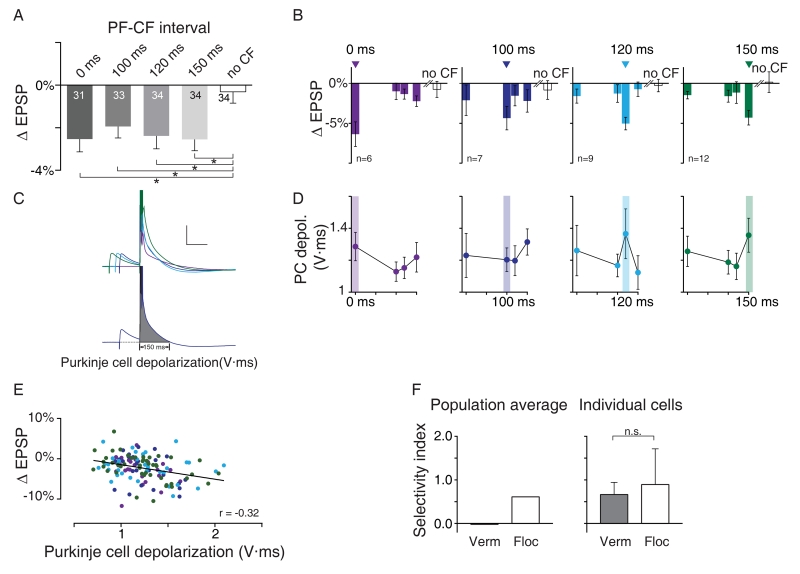
In contrast to the flocculus, a broad range of PF-CF intervals was effective at inducing single-trial synaptic depression at PF-to-PC synapses in the vermis. A single, coincident PF-CF pairing (Fig. 3A, 0 ms), as well as climbing fiber stimulation delayed by intervals as long as 150 ms relative to parallel fiber stimulation, induced significant depression at PF-to-PC synapses, as measured in the population average (Fig. 3A; Fig. S3A). This broad temporal window for the induction of single-trial depression at PF-to-PC synapses in the vermis is similar to what has been described previously for long-term depression (LTD) at the same synapses (e.g., Safo and Regehr, 2008). However, broad tuning at the population level masked precise temporal requirements for the single-trial plasticity in individual cells.
Figure 3. In the vermis, single-trial depression at the parallel fiber-to-Purkinje cell synapses is induced by a range of parallel fiber-climbing fiber pairing intervals.
A) Single-trial synaptic plasticity in the vermis, measured as in Fig 2A,B. * p < 0.05, repeated measures ANOVA, followed by pairwise post-hoc Student-Neuman-Keuls comparison. B) Cells in A were sorted according to the PF-CF interval that induced the maximum depression, the “preferred interval” (arrowheads). There was no significant depression at any PF-CF interval except the preferred interval, for any subgroup of cells (repeated measures ANOVA), n = number of cells. C) Top: traces from a single cell, showing different amount of Purkinje cell depolarization for different PF-CF pairing intervals (indicated by color). This cell showed maximal depression for a PF-CF interval of 150 ms. Spike truncated. Bottom: grey shading indicates the area measured to quantify the Purkinje cell depolarization. D) Purkinje cell depolarization for each PF-CF interval in each sub-group of cells; cells sorted as in B according to the interval inducing the greatest depression (highlighted). E) Synaptic depression (Δ EPSP) in each cell for each PF-CF interval was correlated with the amount of Purkinje cell depolarization during PF-CF pairing. See also Fig. S3D. F) A selectivity index (SI), quantifying the tuning of single-trial depression for the PF-CF pairing interval was calculated for the population averages shown in Fig 2B, 3A, and for the individual cells contributing to those averages, SI= (DepressionPreferred − Avg. DepressionNon–Preferred)/DepressionPreferred), (see Supplemental Experimental Procedures). The population average (left) reveals no selectivity in the vermis (selectivity index very close to zero), in contrast to the flocculus; however, the selectivity index for individual cells in the vermis was not significantly different from that in the flocculus (right, Mann-Whitney test). All panels are Mean ± S.E.M. See also Fig. S3A-D.
The cells recorded in the vermis (Fig. 3A) were sorted according to their preferred PF-CF interval, i.e. the one that produced the biggest single-trial depression (Fig. 3B). Notably, cells that preferred a given interval underwent no significant depression at any of the other PF-CF intervals (Fig. 3B, repeated measures ANOVA).
The tuning of plasticity to the PF-CF interval was quantified with a selectivity index, SI (Fig. 3F):
where DPref = depression at the preferred interval, and DNonPref = average depression at non-preferred intervals.
At the population level, the selectivity index was close to zero in the vermis, indicating almost no selectivity, whereas the selectivity index was 0.6 for the flocculus, indicating considerable selectivity (Fig. 3F, left). However, when the selectivity index was calculated for individual cells, the mean was greater than 0.6 in both regions, and not significantly different between the two regions (Fig. 3F, right). Thus, individual cells in both regions were tuned for the PF-CF interval. The key difference between regions is that in the flocculus, the majority of cells prefer the same, behaviorally relevant climbing fiber delay of 120 ms, whereas in lobules V/VI of the vermis, different cells prefer different intervals.
We leveraged this variability across cells to look for electrophysiological correlates of the preferred interval for inducing synaptic depression. Cells that preferred different PF-CF intervals had no detectable difference in the baseline EPSP amplitude or spatial localization within lobules V and VI (Fig. S3Ci,ii). However, one electrophysiological signature was identified that predicted the amount of depression. In individual cells, the extent to which the Purkinje cell depolarized during the complex spike elicited by CF stimulation varied with the PF-CF interval (Fig. 3D), and the PF-CF interval that induced the greatest depolarization of the Purkinje cell tended to be the one that induced the greatest single-trial depression (Fig. 3D,E, S3D). Previously, the size of the complex spike has been correlated with the amplitude of the calcium transient and the induction of plasticity (e.g., Kitamura and Häusser, 2011; Mathy et al., 2009; Wang et al., 2000; Yang and Lisberger, 2014). Our results extend these previous observations by showing that the size of the complex spike, as measured by the amount of depolarization, varies with the PF-CF interval.
Discussion
Our findings prompt a rethinking of the common assumption that the properties of synaptic plasticity are uniform across an anatomically-defined type of synapse. Given the “crystalline” cytoarchitecture of the cerebellum, it has been widely assumed that the properties of synaptic plasticity that have been described in the vermis apply throughout the cerebellum. However, there are known differences in gene expression, Purkinje cell firing rates, and behavioral functions of different cerebellar regions (Cerminara et al., 2015), and a report that some regions exhibit less PF-to-PC LTD than others (Wadiche and Jahr, 2005). Our results suggest that all regions of the cerebellum may undergo PF-to-PC LTD, but the rules for the induction of this plasticity may be different. We found different rules for depression of the PF-to-PC synapses in different functional regions of the cerebellum (flocculus vs. vermis; Fig 1A, 2B, 3A), and even different cells within a small region (lobules V/VI of vermis; Fig 3B).
In the vermis, associative synaptic depression can be induced by a wide range of delays between parallel fiber and climbing fiber activation. This wide range of effective delays may reflect the wide range of delays in the error signals carried by the different climbing fiber inputs to the cerebellar vermis, which supports behavioral functions ranging from postural to cognitive (Menghini et al., 2013; Stoodley et al., 2012). For example, climbing fiber inputs to lobules V/VI of the vermis, where the in vitro studies of single-trial synaptic plasticity shown in Fig. 3A-F were conducted, carry signals ranging from spinal afferent signals with latencies as short as 10-30 ms (Berthoz and Llinas, 1974), to cognitive signals with presumably much longer latencies (Menghini et al., 2013).
In the flocculus, tuning to the PF-CF interval was much more uniform, so that it was evident in the population average as well as in individual cells. Depression was reliably induced by a climbing fiber delay of 120 ms, and not by coincident climbing fiber activation or by climbing fiber delays just a few tens of milliseconds greater or less than 120 ms. Tuning for the same, 120 ms delay was observed in the flocculus over a wide range of conditions: using trains of parallel fiber stimuli as well as single parallel fiber stimuli during LTD induction, in separate experiments measuring LTD in current clamp and voltage clamp, for short-term as well as long-term plasticity, and in vivo as well as in vitro. This tuning for the PF-CF interval provides a synaptic mechanism for the circuit to solve a major component of the temporal credit assignment problem, by precisely compensating, to within a few tens of milliseconds, for delays in the feedback about errors carried by climbing fibers in vivo (Fig. S4A, B).
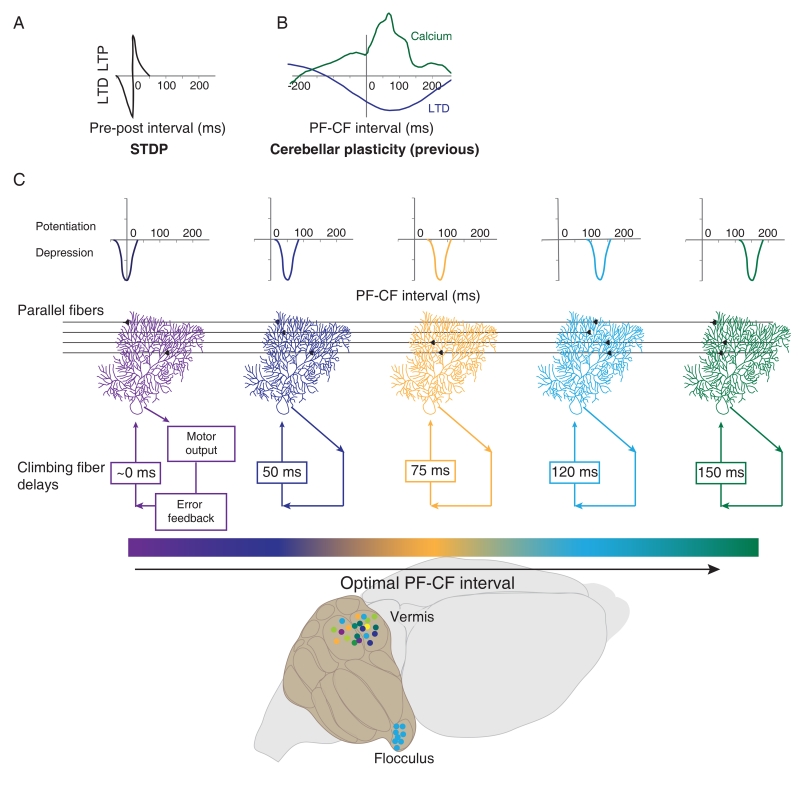
The synaptic plasticity we describe expands the known repertoire of cellular mechanisms available to neural circuits for making associations between non-coincident events over a wide range of time intervals (Fig. 4A, B). Other, known forms of plasticity with precise timing requirements for induction, such as STDP, are tuned for inter-event intervals close to coincident (Fig. 4A). Such forms of plasticity provide a mechanism for distinguishing the order in which neurons fire, and hence causality. In contrast, the narrow tuning of plasticity at the PF-to-PC synapses to much longer intervals provides a mechanism, not just for distinguishing the order, but also for encoding the precise timing between neural events.
Figure 4. Synaptic mechanisms for learning a range of behaviorally relevant temporal correlations.
A) Schematic based on previous findings of spike timing-dependent plasticity (STDP), showing precise induction of plasticity for short inter-event intervals, centered around coincident pre- and post-synaptic inputs (Bi and Poo, 1998; Markram et al., 1997). B) Schematic based on previous findings in the cerebellum, illustrating calcium increases (green; Wang et al., 2000), and synaptic plasticity (LTD, blue; Safo and Regehr, 2008) induced by a broad range of PF-CF intervals, extending out to hundreds of milliseconds. C) Top: Schematic of plasticity in the cerebellum, based on current data, showing each cell narrowly tuned to a single PF-CF interval, and different cells tuned for different intervals, together spanning a range of >100 ms. Schematics in A-C are on the same time scale. Bottom: In each region of the cerebellum, the population of cells (colored circles) may tile the space of functionally relevant intervals for the behaviors supported by that region. In regions like the flocculus, with a uniform error-signal delay, cells (light blue circles) are more uniformly tuned to a single, behaviorally-relevant interval (See also Fig. S4A, B).
The discovery of precise tuning of synaptic plasticity to a behaviorally-relevant inter-event interval was facilitated by the uniform and well-characterized function of the cerebellar flocculus. However, each region of the cerebellum may contain an array of synaptic detectors that are tuned, either through evolution or through metaplasticity, so that the population tiles the range of parallel fiber-climbing fiber intervals relevant for the behaviors supported by that region (Fig 4C). It is intriguing to speculate that such tuning may be a more widespread property of synapses throughout the brain. In the particular case of Purkinje cells, all synapses onto a given cell may be tuned to the same interval, determined by the characteristic delay of the error signals carried by the single climbing fiber input to that cell. However for other types of neurons, even different synapses onto the same cell might obey different plasticity rules, if the synapses are part of different circuits. Thus, a diversity of synaptic learning rules may work in conjunction with circuit-level mechanisms to learn the temporal correlations between events in the natural world.
Experimental Procedures
Supplementary Material
Highlights.
Synaptic plasticity rules are not uniform, but tuned to specific circuit function.
Different rules at different cerebellar parallel fiber-to-Purkinje cell synapses.
Synaptic plasticity can precisely compensate for circuit delays of >100ms.
Provides a mechanism for solving temporal credit assignment problem.
Acknowledgements
We thank J. Burnett for his support through the BioX NeuroVentures Fund; W. Newsome, S. Lisberger, L. Giocomo, L. Frank, B. Nguyen-Vu, T. Manninen, A. Shakhawat, J. Bant, and A. Hayer for helpful input; M. Fu for technical help; and E. Knudsen and L. Giocomo for use of equipment. This work was supported by NIH RO1 NS072406 and RO1 DC004154. A.S. was supported by a Katherine McCormick postdoctoral fellowship. H.L.P. was supported by NSF graduate research fellowship DGE-114747 and NSF IGERT traineeship 0801700.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions. AS and JR planned the experiments, AS performed and analyzed the in vitro experiments, HP analyzed the in vivo data, AS and JR wrote the paper.
References
- Berthoz A, Llinas I. Exp. Brain Res. 1974;20:385–401. doi: 10.1007/BF00237383. [DOI] [PubMed] [Google Scholar]
- Bi GQ, Poo MM. J. Neurosci. 1998;18:10464–10472. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Katoh A, Pyle JL, Chatila TA, Tsien RW, Raymond JL. Selective engagement of plasticity mechanisms for motor memory storage. Neuron. 2006;51:823–834. doi: 10.1016/j.neuron.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Brenowitz SD, Regehr WG. Associative short-term synaptic plasticity mediated by endocannabinoids. Neuron. 2005;45:419–431. doi: 10.1016/j.neuron.2004.12.045. [DOI] [PubMed] [Google Scholar]
- Cerminara NL, Lang EJ, Sillitoe RV, Apps R. Redefining the cerebellar cortex as an assembly of non-uniform Purkinje cell microcircuits. Nat. Rev. Neurosci. 2015;16:79–93. doi: 10.1038/nrn3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Thompson RF. Temporal specificity of long-term depression in parallel fiber-Purkinje synapses in rat cerebellar slice. Learn. Mem. 1995;2:185–198. doi: 10.1101/lm.2.3-4.185. [DOI] [PubMed] [Google Scholar]
- Debanne D, Gahwiler BH, Thompson SM. Long-term synaptic plasticity between pairs of individual CA3 pyramidal cells in rat hippocampal slice cultures. J. Physiol. 1998;507:237–247. doi: 10.1111/j.1469-7793.1998.237bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekerot CF, Kano M. Long-term depression of parallel fibre synapses following stimulation of climbing fibres. Brain Res. 1985;342:357–360. doi: 10.1016/0006-8993(85)91136-9. [DOI] [PubMed] [Google Scholar]
- Feldman DE. The spike-timing dependence of plasticity. Neuron. 2012;75:556–571. doi: 10.1016/j.neuron.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch EA, Augustine GJ. Local calcium signalling by inositol-1,4,5-trisphosphate in Purkinje cell dendrites. Nature. 1998;396:753–756. doi: 10.1038/25541. [DOI] [PubMed] [Google Scholar]
- Fujita M. Adaptive filter model of the cerebellum. Biol. Cybern. 1982;45:195–206. doi: 10.1007/BF00336192. [DOI] [PubMed] [Google Scholar]
- Hansel C, de Jeu M, Belmeguenai A, Houtman SH, Buitendijk GHS, Andreev D, De Zeeuw CI, Elgersma Y. alphaCaMKII Is essential for cerebellar LTD and motor learning. Neuron. 2006;51:835–843. doi: 10.1016/j.neuron.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Hore J, Watts S, Tweed D. Errors in the control of joint rotations associated with inaccuracies in overarm throws. J. Neurophysiol. 1996;75:1013–1025. doi: 10.1152/jn.1996.75.3.1013. [DOI] [PubMed] [Google Scholar]
- Ito M. Cerebellar Long-Term Depression : Characterization, signal transduction, and functional roles. Physiol Rev. 2001;81:1143–1195. doi: 10.1152/physrev.2001.81.3.1143. [DOI] [PubMed] [Google Scholar]
- Johansson F, Jirenhed D, Rasmussen A, Zucca R, Hesslow G. Memory trace and timing mechanism localized to cerebellar Purkinje cells. Proc Natl Acad Sci U S A. 2014;111:14930–14934. doi: 10.1073/pnas.1415371111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimpo RR, Rinaldi JM, Kim CK, Payne HL, Raymond JL. Gating of neural error signals during motor learning. Elife. 2014;3:1–23. doi: 10.7554/eLife.02076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K, Häusser M. Dendritic calcium signaling triggered by spontaneous and sensory-evoked climbing fiber input to cerebellar Purkinje cells in vivo. J. Neurosci. 2011;31:10847–10858. doi: 10.1523/JNEUROSCI.2525-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Lac S, Raymond JL, Sejnowski TJ. Learning and memory in the vestibulo-ocular reflex. Annu. Rev. Neurosci. 1995;409:409–441. doi: 10.1146/annurev.ne.18.030195.002205. [DOI] [PubMed] [Google Scholar]
- Lev-Ram V, Wong ST, Storm DR, Tsien RY. A new form of cerebellar long-term potentiation is postsynaptic and depends on nitric oxide but not cAMP. Proc. Natl. Acad. Sci. U. S. A. 2002;99:8389–8393. doi: 10.1073/pnas.122206399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DJ. Long-term synaptic depression in the mammalian brain. Neuron. 1994;12:457–472. doi: 10.1016/0896-6273(94)90205-4. [DOI] [PubMed] [Google Scholar]
- Maekawa K, Simpson JL. Climbing fiber responses evoked in vestibulocerebellum of rabbit from visual system. J. Neurophysiol. 1973;36:649–666. doi: 10.1152/jn.1973.36.4.649. [DOI] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M, Sakmann B. Science. 1997;275:213–215. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- Mathy A, Ho SSN, Davie JT, Duguid IC, Clark BA, Häusser M. Encoding of Oscillations by Axonal Bursts in Inferior Olive Neurons. Neuron. 2009;62:388–399. doi: 10.1016/j.neuron.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Lisberger SG. Links from complex spikes to local plasticity and motor learning in the cerebellum of awake-behaving monkeys. Nat. Neurosci. 2008;11:1185–1192. doi: 10.1038/nn.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Mauk MD. Computer simulation of cerebellar information processing. Nat. Neurosci. 2000;3:1205–1211. doi: 10.1038/81486. [DOI] [PubMed] [Google Scholar]
- Mehta MR, Lee AK, Wilson MA. Role of experience and oscillations in transforming a rate code into a temporal code. Nature. 2002;417:741–746. doi: 10.1038/nature00807. [DOI] [PubMed] [Google Scholar]
- Menghini D, Di Paola M, Murri R, Costanzo F, Caltagirone C, Vicari S, Petrosini L. Cerebellar vermis abnormalities and cognitive functions in individuals with Williams syndrome. Res. Dev. Disabil. 2013;34:2118–2126. doi: 10.1016/j.ridd.2013.03.026. [DOI] [PubMed] [Google Scholar]
- Ramachandran R, Lisberger SG. Normal performance and expression of learning in the vestibulo-ocular reflex (VOR) at high frequencies. J. Neurophysiol. 2005;93:2028–2038. doi: 10.1152/jn.00832.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond JL, Lisberger SG. Neural learning rules for the vestibulo-ocular reflex. J. Neurosci. 1998;18:9112–9129. doi: 10.1523/JNEUROSCI.18-21-09112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond JL, Lisberger SG. Hypotheses about the neural trigger for plasticity in the circuit for the vestibulo-ocular reflex. Prog. Brain Res. 2000;124:235–246. doi: 10.1016/S0079-6123(00)24020-X. [DOI] [PubMed] [Google Scholar]
- Safo P, Regehr WG. Timing dependence of the induction of cerebellar LTD. Neuropharmacology. 2008;54:213–218. doi: 10.1016/j.neuropharm.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone LS, Lisberger SG. Detection of tracking errors by visual climbing fiber inputs to monkey cerebellar flocculus during pursuit eye movements. Neurosci. Lett. 1986;72:163–168. doi: 10.1016/0304-3940(86)90073-x. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: An fMRI study. Neuroimage. 2012;59:1560–1570. doi: 10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RS, Barto AG. Toward a modern theory of adaptive networks: expectation and prediction. Psychol. Rev. 1981;88:135–170. [PubMed] [Google Scholar]
- Wadiche JI, Jahr CE. Patterned expression of Purkinje cell glutamate transporters controls synaptic plasticity. Nat. Neurosci. 2005;8:1329–1334. doi: 10.1038/nn1539. [DOI] [PubMed] [Google Scholar]
- Wang SS, Denk W, Häusser M. Coincidence detection in single dendritic spines mediated by calcium release. Nat. Neurosci. 2000;3:1266–1273. doi: 10.1038/81792. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Tanaka S. Computational models of timing mechanisms in the cerebellar granular layer. Cerebellum. 2009;8:423–432. doi: 10.1007/s12311-009-0115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Lisberger SG. Learning on multiple timescales in smooth pursuit eye movements. J. Neurophysiol. 2010;104:2850–2862. doi: 10.1152/jn.00761.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Lisberger SG. Purkinje-cell plasticity and cerebellar motor learning are graded by complex-spike duration. Nature. 2014;510:529–532. doi: 10.1038/nature13282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.