Abstract
AIM
To assess the effect of sofosbuvir (SOF) based regimens on glycemic and lipid control.
METHODS
This is a retrospective analysis of hepatitis C virus (HCV)-infected patients treated and cured with a SOF regimen [SOF/ribavirin/interferon, SOF/simeprevir, or SOF/ledipasvir (LDV) ± ribavirin] from January 2014 to March 2015. Patients with hemoglobin A1C (HbA1C) and lipid panels within six months before and six months after therapy were identified and included in our study. Due to the known hemolytic effect of ribavirin, HbA1C was obtained a minimum of three months post-treatment for the patients treated with a ribavirin regimen. Medical history, demographics, HCV genotype, pre-therapy RNA, and liver biopsies were included in our analysis. The patients who started a new medication or had an adjustment of baseline medical management for hyperlipidemia or diabetes mellitus (DM) were excluded from our analysis.
RESULTS
Two hundred and thirty-four patients were reviewed, of which 60 patients met inclusion criteria. Sixty-three point three percent were male, 26.7% were Caucasian, 41.7% were African American and 91.7% were infected with hepatitis C genotype 1. Mean age was 60.6 ± 6.7 years. Thirty-nine patients had HbA1C checked before and after treatment, of which 22 had the diagnosis of DM type 2. HbA1C significantly decreased with treatment of HCV (pretreatment 6.66% ± 0.95% vs post-treatment 6.14% ± 0.65%, P < 0.005). Those treated with SOF/LDV had a lower HbA1C response than those treated with other regimens (0.26% ± 0.53% vs 0.71% ± 0.83%, P = 0.070). Fifty-two patients had pre- and post-treatment lipid panels; there was a significant increase in low-density lipoprotein (LDL) and total cholesterol (TC) after treatment (LDL: 99.5 ± 28.9 mg/dL vs 128.3 ± 34.9 mg/dL, P < 0.001; TC: 171.6 ± 32.5 mg/dL vs 199.7 ± 40.0 mg/dL, P < 0.001). Pre-treatment body-mass index (BMI) did not differ from post-treatment BMI (P = 0.684).
CONCLUSION
Eradication of HCV with a SOF regimen resulted in a significant drop in HbA1C and an increase in LDL and TC post therapy.
Keywords: Hepatitis C, Sofosbuvir, Hyperlipidemia, Hemoglobin A1c, Low-density lipoprotein
Core tip: In our retrospective study, we evaluated the changes in glucose and lipid metabolism in a group of hepatitis C patients treated and cured with a sofosbuvir-containing regimen. We used hemoglobin A1c (HgA1c) and lipid panels to assess those two parameters. Six months post eradication, we found a statistically significant drop in HgA1c and an increase in low-density lipoprotein and total cholesterol. The use of HgA1c, although not perfect, is easy to understand and is frequently used by primary care doctors as a tool to assess glucose control.
INTRODUCTION
Hepatitis C virus (HCV) is a leading cause of chronic liver disease, with a prevalence of infection in the United States of approximately 1.6%[1]. It remains the leading cause of death from liver disease and is the leading indication for liver transplantation in the United States despite recent medical advances in HCV therapy. HCV can cause major alterations in insulin resistance (IR) and lipid homeostasis[2-4]. HCV infection has been associated with the development of diabetes mellitus type 2[5,6] as well as a 3.5-fold increase in the prevalence of glucose alterations in non-diabetics[7]. The pathogenesis between HCV and IR seems to be multifactorial, with cytokine upregulation and direct interactions between viral particles and insulin signaling pathways[8-12]. In the traditional pegylated interferon (PegIFN) based regimens, IR was associated with decreased sustained virological response (SVR)[13]; multiple studies have shown an association between viral suppression or clearance and improvement of IR[14-17].
Along with IR, steatosis is also very common in patients infected with HCV[18]. The exact mechanism has not been fully elucidated, but host lipid alterations seem to play a major role. The virus utilizes very low-density lipoproteins (LDLs) to infect hepatocytes and several other lipid secretory mechanisms to perpetuate replication[4]. Several proteins, including Seipin and the HCV core protein, have been shown to alter the production of free fatty acids, as well as the proper excretion of lipids, increasing steatosis in the host[19-21]. Hypocholesterolemia is another finding that seems to be closely related to HCV replication mechanisms. After successful treatment with interferon-based therapy, it has been shown that hypocholesterolemia was resolved, with significant increases in LDL, triglycerides, and cholesterol[22,23].
The era of direct-acting antiviral (DAA) agents has increased SVR rates to over 90%, with dramatically improved side effect profiles[24-30]. IR and lipid alterations do not seem to affect treatment outcomes, and there is limited data on the effects of DAA therapy on metabolic and lipid profiles. A recent study evaluating the effects of sofosbuvir (SOF) and ribavirin (RBV) therapy in a mostly non-diabetic population demonstrated fluctuations in LDL levels throughout treatment, with elevations in LDL in patients achieving SVR as well as a small decrease in hemoglobin A1C (HbA1C) levels (5.58% ± 0.08% to 5.45% ± 0.91%; P = 0.0046)[31]. Additional data regarding metabolic alterations after therapy with the new DAA is scarce. In this retrospective study, the effects of HCV eradication on glucose and lipid metabolism in patients treated with SOF-based regimens at WRNMMC from 2014 to 2015 were assessed.
MATERIALS AND METHODS
Eligibility criteria
Patients aged 18 years or older with confirmed infections with HCV (by RNA) treated and cured at our institution with any combination of a NS5B inhibitor (SOF), NS5A inhibitor [ledipasvir (LDV)], protease inhibitor (Simeprevir), RBV and PegIFN from January 2014 to March 2015 were eligible for the study. Electronic records were reviewed to look for patients with a HbA1C and/or lipid panel drawn before and after therapy. A total of 234 patient charts were reviewed.
HbA1C
The HbA1C closest to starting day of HCV therapy (up to six months pre-therapy) and the closest HbA1C post-therapy (up to six months) were included in our analysis. RBV is known to cause hemolysis, with remarkable drops in hemoglobin. Since HbA1C is closely related to red blood cell lifespan and could be altered by RBC destructions and anemia, the HbA1C in this population was selected between three to six months post therapy. Hemoglobin levels were reviewed pre-therapy and post-therapy and added to the analysis.
In patients with the diagnosis of diabetes mellitus type 2, a review of concomitant hypoglycemic medications was performed. All clinic encounters up to a year prior to starting HCV therapy, during therapy, and up to six months post therapy were reviewed, looking for adjustments of medications that could have altered HbA1C values. Those patients who started a medication or had an adjustment of baseline medical management were removed from the analysis. Patients on stable doses of oral hypoglycemic medications or insulin regimens during the study period were included. Attempts to account for diet and exercise regimens were beyond the scope of this analysis.
Lipids
Lipid panels closest to the starting and end dates of therapy (up to six months pre and post therapy) were included. All samples were drawn during the morning. However, due to the retrospective nature of the analysis, fasting could not be confirmed for all patients. Patients on stable doses of lipid-lowering agents were included, while patients started on new medications or with adjustments during the study period were removed from the analysis.
Data collection
Basic demographic and clinical information was collected for all patients, including age, gender, race, body-mass index (BMI) pre-and post-HCV therapy, and specific HCV anti-viral therapy used. Pre-therapy aspertate aminotransferase (AST), alanine transaminase (ALT), alkaline phosphatase, total bilirubin, albumin, total protein, Hepatitis C RNA, Hepatitis C genotype, and liver biopsy staging were also included. Patients with other liver diseases, such as hemochromatosis, Wilson disease, and alcoholic liver disease, were excluded from the analysis. A total of 60 patients were included in the final analysis. The study protocol was approved by the Institutional Review Board of Walter Reed National Military Medical Center.
Statistical analysis
Data were collated and analyzed using statistical software package IBM SPSS Statistics 21.0 (IBM, Armonk, New York). Continuous data was reported as
means ± SDs. Paired t-test was used to compare variables measured before and after HCV treatment. Student’s t test was used to analyze between group comparisons of continuous data including the change in HbA1C, HA1C, LDL, and high density lipoprotein (HDL) over the HCV treatment. A probability value of less than 0.05 was considered statistically significant. The statistical review of the study was performed by a biomedical statistic.
RESULTS
A total of 234 patients were treated for HCV during the study period. Of these, 60 patients met the inclusion criteria. Their average age was 60.6 ± 6.7 years; 26.7% were Caucasian, 41.7% were African American, and 63.3% were male. Clinical history in the cohort was significant for diabetes (38.3%) and hyperlipidemia (HLD) (33.3%). Patients had a mean viral load of 4.7 × 106 ± 7.6 × 106; 50.0% were infected with genotype 1a, and 26.7% were infected with genotype 1b. The mean pre-treatment AST was 61.0 ± 49.3 units/L, ALT 72.1 ± 56.2 units/L, alkaline phosphatase 102.3 ± 71.3 units/L, total bilirubin 0.8 ± 1.5 mg/dL, albumin 4.2 ± 0.4 g/dL, and total protein 7.4 ± 0.7 g/dL.
All patients were treated with a SOF-based regimen. Of the 23 patients with diabetes, 15 were treated with a stable dose of anti-diabetic medications, most commonly metformin. Of the 20 patients with HLD, 12 were taking a statin, and only three had their statins held during therapy (Table 1).
Table 1.
Patient characteristics (n = 60)
| Male (n = 38) | 63.3% |
| Female (n = 22) | 36.7% |
| Race | |
| Caucasian (n = 16) | 26.7% |
| African American (n = 25) | 41.7% |
| Hispanic (n = 3) | 5.0% |
| Asian (n = 2) | 3.3% |
| Not listed (n = 14) | 23.3% |
| Mean age ± SD | 60.6 ± 6.7 |
| Diabetic (n = 23) | 38.3% |
| Hyperlipidemia (n = 20) | 33.3% |
| Hypertension (n = 42) | 70.0% |
| Treatment | |
| Sofosbuvir/ribavarin/interferon (n = 21) | 35.0% |
| Sofosbuvir/simeprevir (n = 11) | 18.3% |
| Sofosbuvir/ledipesvir (n = 23) | 38.3% |
| Sofosbuvir/ribavirin (n = 4) | 8.3% |
| Sofosbuvir (n = 1) | 1.7% |
| Biopsy stage (n = 49) | |
| 1 (n = 8) | 13.3% |
| 2 (n = 21) | 35.0% |
| 3 (n = 5) | 8.3% |
| 4 (n = 15) | 25.0% |
| Statin use (n = 20) | 20.0% |
| Statin held during tx (n = 3) | 5.0% |
| Mean viral load | 4746471 ± 7641768 |
| Mean ALT | 72.1 ± 56.2 |
| Genotype | |
| 1a (n = 30) | 50.0% |
| 1b (n = 16) | 26.7% |
| 1 undistinguished (n = 9) | 15.0% |
| 2 (n = 2) | 3.3% |
| 3 (n = 3) | 5.0% |
ALT: Alanine transaminase.
A total of 39 patients had pre- and post-treatment HbA1C measured. Overall, there was a significant drop in HbA1C during treatment (Figure 1). This was not accompanied by a significant decrease in BMI (pre-treatment 28.86 ± 5.15 kg/m2, post treatment 28.48 ± 4.72 kg/m2, P = 0.683). There was no significant difference in HbA1C effect between males and females (P = 0.793). There was no significant difference in HbA1C drop between genotype 1a and 1b (P = 0.605). Although not statically significant, patients with a history of diabetes tended to have a larger drop in HbA1C than those without diabetes, and Caucasians tended to have a larger drop in HA1C than African Americans. Patients aged 65 and older were less likely to have a drop in their HbA1C with treatment (younger than 65, 0.68% ± 0.75%, 65 and older, -0.01% ± 0.47%, P = 0.0187). Sixteen of these patients were treated in conjunction with ribavirin; this did not have a significant effect on HbA1C change (drop in HbA1C with ribavirin 0.44% ± 0.76%; without ribavirin 0.68% ± 0.74%, P = 0.342). Patients with a high viral load (> 6000000 copies) tended to have a larger drop in HbA1C with treatment (high VL 0.87% ± 0.97%, low VL 0.40% ± 0.62%, P = 0.080).
Figure 1.
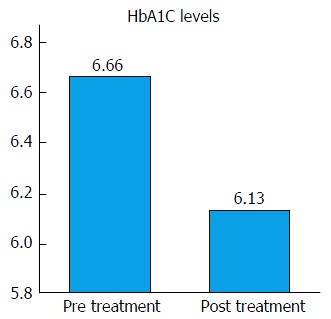
Effects of hepatitis C eradication on hemoglobin A1C. Vertical axis represents HbA1C levels in mg/dL. HbA1C significantly decreased after eradication of hepatitis C virus (pretreatment 6.66 ± 0.95 mg/dL vs post-treatment 6.14 ± 0.65 mg/dL, P < 0.005). HbA1C: Hemoglobin A1C.
Fifty-two patients had pre- and post-treatment lipid panels measured. Overall, there was a significant increase in LDL and total cholesterol (TC) with minimal change in HDL (pre 52.8 ± 18.3 mg/dL, post 51.4 ± 18.5 mg/dL, P = 0.699) and triglycerides (pre 132.1 ± 99.7 mg/dL, post 129.8 ± 80.8 mg/dL, P = 0.853) (Figure 2). Patients with a history of HLD did not have significantly larger increase in LDL than those without a history of hyperlipidemia (HLD 27.8 ± 30.7 mg/dL, non-HLD 30.4 ± 44.6 mg/dL, P = 0.810). Patient’s age 65 or older did not have a significantly larger increase in LDL than younger patients (65 or older 22.4 ± 32.3 mg/dL, younger 30.4 ± 37.1 mg/dL, P = 0.511). Caucasians and African Americans had similar increases in LDL (Caucasians 35.7 ± 36.0 mg/dL, African Americans 33.4 ± 35.5 mg/dL, P = 0.847). Those treated with SOF + ledipisvir tended to have a larger increase in LDL than those treated with other regimens (SOF + LED 36.7 ± 39.3 mg/dL, other therapy 22.1 ± 33.1 mg/dL, P = 0.157). Treatment regimen including interferon did not affect LDL increase (P = 0.755). High VL (> 6000000 copies) prior to treatment did not affect significantly impact the increase in LDL (P = 0.221). Patients with hepatitis C genotype 3 (n = 3) on average had an increase in LDL of 59 mg/dL (pre 87.3 mg/dL, post 146.3 mg/dL) and an increase in TC of 60 mg/dL (pre 148.7 mg/dL, post 208.7 mg/dL).
Figure 2.
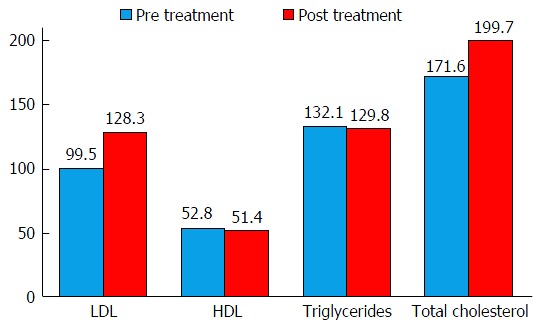
Effects of hepatitis C eradication on lipids. Vertical axis represents lipid levels in mg/dL. LDL and total cholesterol were significantly higher post hepatitis C eradication (LDL: 99.5 ± 28.9 mg/dL vs 128.3 ± 34.9 mg/dL, P < 0.001; total cholesterol 171.6 ± 32.5 mg/dL vs 199.7 ± 40.0 mg/dL, P < 0.001). No significant changes were noted for HDL and triglycerides. LDL: Low-density lipoprotein; HDL: High-density lipoprotein.
DISCUSSION
The main finding of this retrospective study was a significant decrease in HbA1C up to six months post-HCV eradication. The mechanism responsible for this improvement in glycemic control is unknown although likely multifactorial. It is well known that HCV alters glucose metabolism by inducing inflammatory cascades and promoting IR. Defects in pathways important in hepatic gluconeogenesis such as Pi3K and AKT phosphorylation have been reported in patients infected with HCV. Insulin receptor substrates 1 and 2 are closely related to the Pi3K/AKT pathways; these two receptors are key components in the development of IR in patients infected with HCV. The virus can degrade these two receptors, directly affecting the PI3K/AKT pathways[32-34]. Eradication of the virus restores homeostasis of these pathways, leading to an improvement in IR.
In the interferon/RBV era, several studies have demonstrated an improvement of IR with SVR. Early work by Thompson et al[17] demonstrated a 10% decrease in IR in genotype1 patients who achieved SVR, which was supported by the more recent results from Chien et al[35] that showed a significant decrease in HOMA-IR at EOT after eradication of the virus with this combination. Similarly, a study by Meissner et al[31] demonstrated a small but significant decrease in HbA1c in patients treated with SOF/RBV (5.58% ± 0.08% to 5.45% ± 0.91%; P = 0.0046). While the majority of these patients were non-diabetic or pre-diabetics, the patients included in this analysis had a significantly higher rate of diabetes, at 56%. When compared to the non-diabetic patients, the diabetics had a greater improvement in HbA1C. Gender, race, HCV genotype, and HCV RNA did not affect HbA1C drop.
In a subgroup analysis, patients treated with the SOF/LDV had a lower drop in HbA1C when compared to SOF/RBV and SOF/SIM groups. One possible explanation is the relationship between the new DAA and its target. The non-structural proteins of the virus NS5A and NS5B are key components in the activation of inflammatory cascades promoting insulin resistance[34]. It is plausible that the interaction of the medication or the duration of therapy alters the effects of insulin resistance, although further study is required.
Although this study was not designed to identify the long-term implications of hepatitis C eradication in glucose control, it is possible that these changes could have long-term implications regarding medical management. One of 16 patients on medical therapy for diabetes required a decrease in insulin therapy post viral eradication and another was taken off completely of therapy. The savings from a drop of even 0.5% of HbA1c are significant, and many oral hypoglycemics maximum efficacy is only a 1% improvement in Hba1c. Even more important than potential cost savings are the implications of better glucose control in the development of microvascular and macrovascular disease as small drops in HbA1C can alter the course of these complications. Primary care physician should monitor diabetic patients post HCV eradication to assess if changes in medical management are required and to prevent complications such as hypoglycemia.
The implications of insulin resistance, especially in diabetic patients infected with HCV, are well established. Huang et al[36] showed an increased risk of liver disease progression to cirrhosis in HCV-infected patients with diabetes. Hui et al[3] demonstrated that insulin resistance was an independent predictor for the degree of fibrosis and fibrosis progression in HCV-infected patients. Everhart et al[37] showed that not only hepatic steatosis was associated with liver disease progression, but also the degree of insulin resistance. They suggested that addressing these two issues might modify disease progression[37]. Taking into account this information and the results of our study, we should consider adding diabetic HCV-infected patients to the high-risk group that would benefit from priority in treatment.
These results also correlate with previous studies evaluating the effects of HCV eradications and lipids. An increase in TC and LDL post therapy was demonstrated irrespective of anti-viral therapy or genotype. Chronic infection with HCV has been implicated in the development of hypolipidemia[38,39]. A reversal of these findings has been reported in patients treated with INF/RBV regimens, as well as SOF/RBV regimens that have achieved SVR, suggesting this is most likely related to viral clearance rather than a medication effect[22,23,31]. The implications of these alterations in cardiovascular and cerebrovascular disease are beyond the scope of this retrospective study but should be further investigated.
The study does have several limitations including its retrospective nature and the small number of patients. Even though all lipids were drawn during the morning time, fasting was unable to be confirmed. Other parameters that could have altered the results, such as dietary changes and exercise, were not available. Medication reconciliation was not directly obtained, but an evaluation of several encounters from the electronic medical record from different providers was performed, looking for adequate medication reconciliation. The length of analysis was also limited to six months post-HCV therapy, so an analysis of the long-term implications of these results cannot be made.
This analysis did strengthen the knowledge pertaining to the metabolic effects of SOF-based regimens and confirmed that eradication of the virus could have extra-hepatic benefits. Even though HOMA-IR is a more direct measurement of IR, HbA1C is a more practical parameter that can be used to assess glucose control, and this study confirmed an improvement in HgA1c with SVR.
In conclusion, this study showed a significant drop in HbA1C up to six months after the eradication of HCV with SOF-based regimens. Future studies are needed to see if this change is sustainable. The effects of virus eradication on lipid panels were also determined, and they confirmed previous analyses that showed an increase in lipid panels, including LDL and TC, with SVR. This study suggests that physicians treating HCV patients should reassess preventive medicine measures after therapy, as the benefits of eradicating HCV may extend beyond eliminating the effects of chronic liver inflammation.
COMMENTS
Background
The hepatitis C virus (HCV) is a leading cause of chronic liver disease, with a prevalence of infection in the United States of approximately 1.6%. It is the leading cause of death from liver disease and is the leading indication for liver transplantation in the United States. Chronic hepatitis C infection (CHC) is known to induce systemic changes regarding glucose control and lipid metabolism. Glycemic balance can be affected by direct effect over insulin activation cascades and as a systemic response to inflammatory cytokines. Patients with CHC developed diabetes mellitus earlier than non-infected patients. Lipid metabolism is also affected due to known impaired lipid secretions associated with the infectious mechanism of the virus (possible use of lipid receptor to infect hepatocytes). Steatosis is another major finding in patients infected with CHC.
Research frontiers
Hepatitis C therapy has changed drastically in the last four years. The authors are now able to achieve cure rates of over 90%, with minimal side effects. The long-term implications of these new agents are still unclear, and previous studies have shown mixed results regarding alterations in glucose and lipid control after eradication. There is limited data regarding the newer anti-viral agents and their effects on metabolic derangements. The study attempts to assess the metabolic changes associated with these new agents.
Innovations and breakthroughs
Similar studies evaluating metabolic changes associated with hepatitis C eradication have used HOMA-IR as a surrogate of glucose homeostasis. Although this is a very accurate way of assessing glucose changes post hepatitis C eradication, its use on a daily clinic encounter is limited. In the study, the authors used HgA1c as a surrogate for glucose homeostasis. This laboratory test is easy to use and is well known by non-gastroenterology/hepatology providers. This laboratory test is more practical for daily clinic encounters. Previous studies on patients treated and cured with interferon and Ribavirin have shown alterations in lipid homeostasis, similar to the results. As the data on lipid alterations with the new direct antiviral agents is limited, the study adds to the knowledge on non-hepatic effects associated with a sustained virological response.
Applications
As reported in the study, several patients required adjustments in their hyperglycemic regimens, and one patient was completely taken off medication. The study suggests that shortly after completing hepatitis C therapy, primary care doctors should monitor diabetic patients, under medical management, to assess if changes to their medications are needed. Although the study was not meant to assess long-term effects, changes in HbA1c, as seen in the study, can add benefits in cost savings, as well as prevent microvascular and macrovascular disease. The changes seen in lipid homeostasis are worrying and require further investigation. These patients are still at risk of developing other liver diseases, such as non-alcoholic fatty liver disease. Primary care doctors should implement close monitoring of lipids after hepatitis C eradication, and those who meet the criteria for therapy should be treated accordingly.
Terminology
NS5A: Non-structural protein 5A. This protein plays a key role in HCV replication. It is one of the main targets for some of the new direct acting antiviral agents; NS5B: Non-structural protein 5B. Involved in Hepatitis C RNA replication. Main target for some of the new anti-viral agents, such as Sofosbuvir; Sustain virological response: Patients with undetectable hepatitis C viral load 12 wk after completing hepatitis C therapy.
Peer-review
The manuscript is well presented and of interest and the results can contribute to increase the knowledge of this topic.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study protocol was reviewed and approved by the Walter Reed National Military Medical Center Institutional Review Board.
Informed consent statement: Informed consent was not provided by patients. The Walter Reed National Military Medical Center Institutional Review Board provided a waiver of informed consent authorizing the use of de-identified patient data for research purpose.
Conflict-of-interest statement: No potential conflicts of interest to report.
Data sharing statement: Technical appendix, statistical code, and dataset available from the corresponding author at amilcar.l.moralescardona.mil@mail.mil.
Peer-review started: August 23, 2016
First decision: September 6, 2016
Article in press: October 24, 2016
P- Reviewer: Aghakhani A, Blanco JR, Gutierrez JA, Hwang SG, Rezaee-Zavareh MS S- Editor: Ji FF L- Editor: A E- Editor: Li D
References
- 1.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moucari R, Asselah T, Cazals-Hatem D, Voitot H, Boyer N, Ripault MP, Sobesky R, Martinot-Peignoux M, Maylin S, Nicolas-Chanoine MH, et al. Insulin resistance in chronic hepatitis C: association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology. 2008;134:416–423. doi: 10.1053/j.gastro.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Hui JM, Sud A, Farrell GC, Bandara P, Byth K, Kench JG, McCaughan GW, George J. Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression [corrected] Gastroenterology. 2003;125:1695–1704. doi: 10.1053/j.gastro.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 4.Negro F. Abnormalities of lipid metabolism in hepatitis C virus infection. Gut. 2010;59:1279–1287. doi: 10.1136/gut.2009.192732. [DOI] [PubMed] [Google Scholar]
- 5.Mehta SH, Brancati FL, Strathdee SA, Pankow JS, Netski D, Coresh J, Szklo M, Thomas DL. Hepatitis C virus infection and incident type 2 diabetes. Hepatology. 2003;38:50–56. doi: 10.1053/jhep.2003.50291. [DOI] [PubMed] [Google Scholar]
- 6.Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med. 2000;133:592–599. doi: 10.7326/0003-4819-133-8-200010170-00009. [DOI] [PubMed] [Google Scholar]
- 7.Huang JF, Yu ML, Dai CY, Hsieh MY, Hwang SJ, Hsiao PJ, Lee LP, Lin ZY, Chen SC, Hsieh MY, et al. Reappraisal of the characteristics of glucose abnormalities in patients with chronic hepatitis C infection. Am J Gastroenterol. 2008;103:1933–1940. doi: 10.1111/j.1572-0241.2008.01996.x. [DOI] [PubMed] [Google Scholar]
- 8.Nelson DR, Lim HL, Marousis CG, Fang JW, Davis GL, Shen L, Urdea MS, Kolberg JA, Lau JY. Activation of tumor necrosis factor-alpha system in chronic hepatitis C virus infection. Dig Dis Sci. 1997;42:2487–2494. doi: 10.1023/a:1018804426724. [DOI] [PubMed] [Google Scholar]
- 9.Kawaguchi T, Yoshida T, Harada M, Hisamoto T, Nagao Y, Ide T, Taniguchi E, Kumemura H, Hanada S, Maeyama M, et al. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004;165:1499–1508. doi: 10.1016/S0002-9440(10)63408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pazienza V, Clément S, Pugnale P, Conzelman S, Foti M, Mangia A, Negro F. The hepatitis C virus core protein of genotypes 3a and 1b downregulates insulin receptor substrate 1 through genotype-specific mechanisms. Hepatology. 2007;45:1164–1171. doi: 10.1002/hep.21634. [DOI] [PubMed] [Google Scholar]
- 11.Miyamoto H, Moriishi K, Moriya K, Murata S, Tanaka K, Suzuki T, Miyamura T, Koike K, Matsuura Y. Involvement of the PA28gamma-dependent pathway in insulin resistance induced by hepatitis C virus core protein. J Virol. 2007;81:1727–1735. doi: 10.1128/JVI.01683-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernsmeier C, Duong FH, Christen V, Pugnale P, Negro F, Terracciano L, Heim MH. Virus-induced over-expression of protein phosphatase 2A inhibits insulin signalling in chronic hepatitis C. J Hepatol. 2008;49:429–440. doi: 10.1016/j.jhep.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Dai CY, Huang JF, Hsieh MY, Hou NJ, Lin ZY, Chen SC, Hsieh MY, Wang LY, Chang WY, Chuang WL, et al. Insulin resistance predicts response to peginterferon-alpha/ribavirin combination therapy in chronic hepatitis C patients. J Hepatol. 2009;50:712–718. doi: 10.1016/j.jhep.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Kawaguchi T, Ide T, Taniguchi E, Hirano E, Itou M, Sumie S, Nagao Y, Yanagimoto C, Hanada S, Koga H, et al. Clearance of HCV improves insulin resistance, beta-cell function, and hepatic expression of insulin receptor substrate 1 and 2. Am J Gastroenterol. 2007;102:570–576. doi: 10.1111/j.1572-0241.2006.01038.x. [DOI] [PubMed] [Google Scholar]
- 15.Delgado-Borrego A, Jordan SH, Negre B, Healey D, Lin W, Kamegaya Y, Christofi M, Ludwig DA, Lok AS, Chung RT. Reduction of insulin resistance with effective clearance of hepatitis C infection: results from the HALT-C trial. Clin Gastroenterol Hepatol. 2010;8:458–462. doi: 10.1016/j.cgh.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang JF, Yu ML, Huang CF, Juo SH, Dai CY, Hsieh MY, Hou NJ, Yeh ML, Hsieh MH, Yang JF, et al. The outcomes of glucose abnormalities in pre-diabetic chronic hepatitis C patients receiving peginterferon plus ribavirin therapy. Liver Int. 2012;32:962–969. doi: 10.1111/j.1478-3231.2012.02771.x. [DOI] [PubMed] [Google Scholar]
- 17.Thompson AJ, Patel K, Chuang WL, Lawitz EJ, Rodriguez-Torres M, Rustgi VK, Flisiak R, Pianko S, Diago M, Arora S, et al. Viral clearance is associated with improved insulin resistance in genotype 1 chronic hepatitis C but not genotype 2/3. Gut. 2012;61:128–134. doi: 10.1136/gut.2010.236158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358–1364. doi: 10.1053/jhep.2001.24432. [DOI] [PubMed] [Google Scholar]
- 19.Perlemuter G, Sabile A, Letteron P, Vona G, Topilco A, Chrétien Y, Koike K, Pessayre D, Chapman J, Barba G, et al. Hepatitis C virus core protein inhibits microsomal triglyceride transfer protein activity and very low density lipoprotein secretion: a model of viral-related steatosis. FASEB J. 2002;16:185–194. doi: 10.1096/fj.01-0396com. [DOI] [PubMed] [Google Scholar]
- 20.Syed GH, Amako Y, Siddiqui A. Hepatitis C virus hijacks host lipid metabolism. Trends Endocrinol Metab. 2010;21:33–40. doi: 10.1016/j.tem.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simon TG, Butt AA. Lipid dysregulation in hepatitis C virus, and impact of statin therapy upon clinical outcomes. World J Gastroenterol. 2015;21:8293–8303. doi: 10.3748/wjg.v21.i27.8293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang ML, Tsou YK, Hu TH, Lin CH, Lin WR, Sung CM, Chen TH, Cheng ML, Chang KC, Chiu CT, et al. Distinct patterns of the lipid alterations between genotype 1 and 2 chronic hepatitis C patients after viral clearance. PLoS One. 2014;9:e104783. doi: 10.1371/journal.pone.0104783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuo YH, Chuang TW, Hung CH, Chen CH, Wang JH, Hu TH, Lu SN, Lee CM. Reversal of hypolipidemia in chronic hepatitis C patients after successful antiviral therapy. J Formos Med Assoc. 2011;110:363–371. doi: 10.1016/S0929-6646(11)60054-5. [DOI] [PubMed] [Google Scholar]
- 24.Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, Zarski JP, Agarwal K, Buggisch P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889–1898. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 25.Lawitz E, Poordad FF, Pang PS, Hyland RH, Ding X, Mo H, Symonds WT, McHutchison JG, Membreno FE. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet. 2014;383:515–523. doi: 10.1016/S0140-6736(13)62121-2. [DOI] [PubMed] [Google Scholar]
- 26.Gane EJ, Stedman CA, Hyland RH, Ding X, Svarovskaia E, Subramanian GM, Symonds WT, McHutchison JG, Pang PS. Efficacy of nucleotide polymerase inhibitor sofosbuvir plus the NS5A inhibitor ledipasvir or the NS5B non-nucleoside inhibitor GS-9669 against HCV genotype 1 infection. Gastroenterology. 2014;146:736–743.e1. doi: 10.1053/j.gastro.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Lawitz E, Sulkowski MS, Ghalib R, Rodriguez-Torres M, Younossi ZM, Corregidor A, DeJesus E, Pearlman B, Rabinovitz M, Gitlin N, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384:1756–1765. doi: 10.1016/S0140-6736(14)61036-9. [DOI] [PubMed] [Google Scholar]
- 28.Lawitz E, Gane EJ. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;369:678–679. doi: 10.1056/NEJMc1307641. [DOI] [PubMed] [Google Scholar]
- 29.Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 30.Arase Y, Suzuki F, Suzuki Y, Akuta N, Kobayashi M, Kawamura Y, Yatsuji H, Sezaki H, Hosaka T, Hirakawa M, et al. Sustained virological response reduces incidence of onset of type 2 diabetes in chronic hepatitis C. Hepatology. 2009;49:739–744. doi: 10.1002/hep.22703. [DOI] [PubMed] [Google Scholar]
- 31.Meissner EG, Lee YJ, Osinusi A, Sims Z, Qin J, Sturdevant D, McHutchison J, Subramanian M, Sampson M, Naggie S, et al. Effect of sofosbuvir and ribavirin treatment on peripheral and hepatic lipid metabolism in chronic hepatitis C virus, genotype 1-infected patients. Hepatology. 2015;61:790–801. doi: 10.1002/hep.27424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adinolfi LE, Zampino R, Restivo L, Lonardo A, Guerrera B, Marrone A, Nascimbeni F, Florio A, Loria P. Chronic hepatitis C virus infection and atherosclerosis: clinical impact and mechanisms. World J Gastroenterol. 2014;20:3410–3417. doi: 10.3748/wjg.v20.i13.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romero-Gómez M, Fernández-Rodríguez CM, Andrade RJ, Diago M, Alonso S, Planas R, Solá R, Pons JA, Salmerón J, Barcena R, et al. Effect of sustained virological response to treatment on the incidence of abnormal glucose values in chronic hepatitis C. J Hepatol. 2008;48:721–727. doi: 10.1016/j.jhep.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 34.Ampuero J, Romero-Gómez M. Assessing cardiovascular risk in hepatitis C: An unmet need. World J Hepatol. 2015;7:2214–2219. doi: 10.4254/wjh.v7.i19.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chien CH, Lin CL, Hu CC, Chang JJ, Chien RN. Clearance of Hepatitis C Virus Improves Insulin Resistance During and After Peginterferon and Ribavirin Therapy. J Interferon Cytokine Res. 2015;35:981–989. doi: 10.1089/jir.2014.0200. [DOI] [PubMed] [Google Scholar]
- 36.Huang YW, Yang SS, Fu SC, Wang TC, Hsu CK, Chen DS, Hu JT, Kao JH. Increased risk of cirrhosis and its decompensation in chronic hepatitis C patients with new-onset diabetes: a nationwide cohort study. Hepatology. 2014;60:807–814. doi: 10.1002/hep.27212. [DOI] [PubMed] [Google Scholar]
- 37.Everhart JE, Lok AS, Kim HY, Morgan TR, Lindsay KL, Chung RT, Bonkovsky HL, Ghany MG. Weight-related effects on disease progression in the hepatitis C antiviral long-term treatment against cirrhosis trial. Gastroenterology. 2009;137:549–557. doi: 10.1053/j.gastro.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dai CY, Chuang WL, Ho CK, Hsieh MY, Huang JF, Lee LP, Hou NJ, Lin ZY, Chen SC, Hsieh MY, et al. Associations between hepatitis C viremia and low serum triglyceride and cholesterol levels: a community-based study. J Hepatol. 2008;49:9–16. doi: 10.1016/j.jhep.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 39.Serfaty L, Andreani T, Giral P, Carbonell N, Chazouillères O, Poupon R. Hepatitis C virus induced hypobetalipoproteinemia: a possible mechanism for steatosis in chronic hepatitis C. J Hepatol. 2001;34:428–434. doi: 10.1016/s0168-8278(00)00036-2. [DOI] [PubMed] [Google Scholar]


