Abstract
Employing in vitro selection techniques, we have generated biostable RNA-based compounds, so-called Spiegelmers, that specifically bind n-octanoyl ghrelin, the recently discovered endogenous ligand for the type 1a growth hormone secretagogue (GHS) receptor. Ghrelin is a potent stimulant of growth hormone release, food intake, and adiposity. We demonstrate that our lead compound, l-NOX-B11, binds ghrelin with low-nanomolar affinity and inhibits ghrelin-mediated GHS-receptor activation in cell culture with an IC50 of 5 nM. l-NOX-B11 is highly specific for the bioactive, n-octanoylated form of ghrelin. Like the GHS receptor, it does not recognize the inactive unmodified peptide and requires only the N-terminal five amino acids for the interaction. The i.v. administration of polyethylene glycol modified l-NOX-B11 efficiently suppresses ghrelin-induced growth hormone release in rats. These results demonstrate that the neutralization of circulating bioactive ghrelin leads to inhibition of ghrelin's secretory effects in the CNS.
Spiegelmers (German Spiegel = mirror) are l-oligonucleotides with specific binding activity toward a given target molecule (1, 2). They differ from aptamers in their sugar moiety, which consists of l-ribose, rather than the d-ribose found in naturally occurring nucleic acids. The presence of l-ribose makes Spiegelmers highly resistant to nucleases, resulting in stability of >60 h in biological fluids (1). Spiegelmers and aptamers have been shown to work as antagonists in vivo (3–6).
Generally, the generation of Spiegelmers involves three steps: (i) the synthesis of the enantiomer of the naturally occurring target molecule (e.g., the synthesis of a peptide consisting of d-amino acids instead of the naturally occurring l-amino acids); (ii) the screening of a large combinatorial nucleic acid library employing the SELEX process (Systematic Evolution of Ligands by Exponential) enrichment (7, 8) to identify an aptamer that binds to the nonnatural enantiomer; and (iii) the synthesis of the l-enantiomer (Spiegelmer) of the selected aptamer. We have identified Spiegelmers that bind to the peptide hormone ghrelin. Ghrelin is an endogenous ligand for the growth hormone secretagogue receptor 1a (GHS-R1a), a G-protein-coupled, seven-transmembrane-spanning receptor predominantly expressed in the hypothalamus and pituitary (9, 10). The peptide was discovered in 1999 when Kangawa and coworkers isolated the acylated 28-aa hormone from stomach extract capable of activating Ca2+ influx in a recombinant cell line expressing GHS-R1a (9). Receptor binding and activation requires the presence of a posttranslational octanoic acid (C8:0), decanoic acid (C10:0), or decenoic acid (C10:1) modification at serine-3. Although several C-terminal amino acid residues of ghrelin can be deleted without loosing receptor binding in vitro, the specific acylation is essential for bioactivity, a feature unique in mammalian biology (11–13). Ghrelin is the only known endogenous agonist for the GHS-R1a to date. Like its receptor, it is highly conserved across vertebrates, indicating an important biological role (14).
As had originally been shown for synthetic ligands of the GHS-R, growth hormone secretagogues (GHS) (15), administration of ghrelin triggers the release of growth hormone in rats (9, 16) and humans (17, 18). This effect is mediated through GSH-R1a activation at the level of the pituitary as well as the hypothalamus (19, 20). In addition to its stimulation of GH release, ghrelin acts as an important regulator of energy balance (21–23). Systemic, as well as central, administrations were shown to increase food intake in rodents (22, 24). In humans, i.v. injection of ghrelin increases sensations of hunger and leads to the consumption of larger quantities of food (25). In addition to the orexigenic effects, ghrelin was shown to induce adiposity and decrease fat utilization after repeated administrations in mice (21). The importance of ghrelin in obesity has recently been challenged by the finding that mice carrying a deletion in the ghrelin gene exhibit no abnormalities in food intake, weight development, or appetite (26). The discrepancy reflects the difficulty to correlate pharmacological and genetic data. However, a separate deletion study, although unable to detect a change in food intake, discovered a preference for fat utilization (27).
In the bloodstream, ghrelin circulates freely or is bound to carrier proteins such as high-density lipoproteins. Its predominant site of synthesis is in oxcyntic cells of the gastrointestinal tract (28), but several other tissues, including the hypothalamus, produce the hormone as well (29, 30). Nevertheless, the stomach is primarily responsible for the blood ghrelin levels.
With ghrelin being the only peripherally circulating orexigenic agent known to date, antagonism of the ghrelin–GHS receptor system has become the subject of interest in the treatment of obesity (31, 32). The validity of this approach gained support recently when it was shown that GHS-R1a antagonism with [d-Lys-3]GHRP-6, a known receptor antagonist (33), as well as transgenic expression of antisense GHS-R1a mRNA, reduced food intake in rats (34).
Here we report the generation of a synthetic compound capable of specific high-affinity binding to bioactive ghrelin. Using SELEX, we first isolated an aptamer that binds to d-ghrelin, the enantiomer of the naturally occurring l-ghrelin. We show that the corresponding Spiegelmer binds the bioactive l-ghrelin with nanomolar affinity in vitro and inhibits ghrelin-mediated increase of intracellular Ca2+ levels in cells expressing GHS-R1a. The Spiegelmer differentiates between the octanoylated and desoctanoylated forms of ghrelin and requires only the highly preserved N-terminal five amino acids of bioactive ghrelin for binding. Moreover, we demonstrate that systemic administration of ghrelin-binding Spiegelmer to male Sprague–Dawley rats specifically suppresses ghrelin-induced GH release in a dose-dependent fashion.
Materials and Methods
Peptides and Nucleic Acids. All-d-ghrelin was custom synthesized with an octanoyl residue at Ser-3, and a biotin group linked by d-lysine and two amino-ethyloxy-ethyloxy-acetyl groups (ghrelin-d-Lys-AEEAc-AEEAc-biotinyl-OH) at the C terminus by Bachem. l-ghrelin and desoctanoyl l-ghrelin were from Bachem and l-ghrelin-(1–5) and desoctanoyl l-ghrelin-(1–5) were from Phoenix Pharmaceuticals (Belmont, CA). d- and l-RNA were synthesized in-house with standard phosphoramidite chemistry. l-amidites were obtained from Chem-Genes (Wilmington, MA). The RNA starting pool had the sequence 5′-GGAGCUCAGACUUCACUCGUG-N40-CACGUACCACUGUCGGUUCCAC-3′. For amplification we used primers 5′-TCTAATACGACTCACTATAGGAGCTCAGACTTCACTCG-3′ (forward) and 5′-GTGGAACCGACAGTGGTACG-3′ (reverse). The control l-RNA had the sequence 5′-UA AGGA A ACUCGGUCUGAUGCGGUAGCGCUGUGCAGAGCU-3′. For studies in animals l-NOX-B11 and the control Spiegelmer were modified with a 40-kDa polyethylene glycol moiety as described in ref. 6.
Selection. The starting pool was synthesized as single-stranded DNA and amplified by PCR. The forward primer introduced the T7 promoter for in vitro transcription of the DNA pool with T7 RNA polymerase (Stratagene). Transcripts were purified on denaturing 8% polyacrylamide gels. RNA (100–1,000 pmol) was denatured, reannealed, and incubated with biotinylated rat d-ghrelin in physiological selection buffer [20 mM Tris·HCl (pH 7.4)/150 mM NaCl/5mMKCl/1 mM MgCl2/1 mM CaCl2/0.1% Tween 20] at 37°C for 1–2 h (35). Ghrelin-bound RNA was separated from nonbinding RNA by capturing the biotinylated target with streptavidin or neutravidin beads (Pierce) and subsequent washing with selection buffer at 37°C. Bound RNA was eluted at 95°C for 3 min, except for the first two rounds, where denaturation with 4 M guanidinium thiocyanate at 37°C was used. The stringency of the selection was increased by lowering the peptide concentration from 1 μM in round 1 to 6.2 nM in round 17. DNA obtained from round 17 was cloned and sequenced (GATC Biotech, Konstanz, Germany).
Binding Studies. Dissociation constants were determined by surface plasmon resonance (SPR) real-time kinetic analysis and/or pull-down assays with radioactively labeled RNA and biotinylated ghrelin. SPR data were obtained at 37°C on a Biacore 2000 with biaevaluation 3.0 software (Biacore, Uppsala). Fixed amounts of biotinylated peptide corresponding to 100 and 300 response units on Flowcell 1 and 2, respectively, were immobilized on a streptavidin-conjugated sensor chip (Biacore, Freiburg, Germany). RNA samples were injected at concentrations from 0.1 to 1 μM with the Kinject command defining an association time of 300 s and a dissociation time of 300 s. Flowcell 3 was used as buffer control, Flowcell 4 was used as dextran matrix control (Biacore SA-Chip surface). For data analysis we used the Langmuir 1:1 stoichiometric fitting algorithm.
Radioactive RNA for pull-down assays was obtained through in vitro transcription in the presence [α-32P]GTP/ATP or labeling with T4 polynucleotide kinase (Invitrogen) by using [γ-32P]ATP (Hartmann Analytic, Braunschweig, Germany) as described by the manufacturer. Labeled RNA was purified on a denaturing 10% polyacrylamide gel, and 0.5–5 pmol was incubated for 2 h at 37°C with biotinylated d-ghrelin ranging from 1 nM to 3 μM. A constant amount of streptavidin-conjugated UltraLink matrix was added, and the bound ghrelin–RNA complexes were washed and radioactivity was measured in a Beckman Coulter LS 6500 scintillation counter. The results were plotted as a percentage of total binding over the peptide concentration. Dissociation constants were obtained by using the software grafit 4.0 (Erithacus Software, Surrey, U.K.). Values are the means of at least three independent experiments.
Inhibition of GHS-R1a Activation in Cell Culture. Stably transfected Chinese hamster ovary (CHO) cells expressing the human ghrelin receptor GHS-R1a (Euroscreen, Gosselies, Belgium) were seeded with 5–7 × 104 cells per well in a black 96-well plate with clear bottom (Greiner, Frickenhausen, Germany) and grown overnight at 37°C with 5% CO2 in UltraCHO medium (Cambrex, Verviers, Belgium) containing 100 units/ml penicillin, 100 μg/ml streptomycin, 400 μg/ml geneticin, and 2.5 μg/ml Fungizone. Spiegelmer was incubated for 15–60 min with bioactive human or rat ghrelin, ghrelin-(1–5), or the corresponding desoctanoyl forms in UltraCHO medium containing 5 mM probenecid and 20 mM Hepes (CHO-U+) at 24°C in a 0.2-ml low-profile 96-well plate. Cells were washed once with 200 μl of CHO-U+, loaded with 0.08% pluronic 127, 50 μl of 10 μM fluo-4 indicator dye solution (Molecular Probes) in CHO-U+, and incubated for 60 min at 37°C. Thereafter, cells were washed three times with 180 μl of CHO-U+. Ninety microliters of CHO-U+ was added per well, and cells were stimulated with 10 μl of the preincubated Spiegelmer–ghrelin mixture. Fluorescence was measured at an excitation wavelength of 485 nm and an emission wavelength of 520 nm in a Fluostar Optima multi-detection plate reader (BMG, Offenburg, Germany). For each well the difference between the maximum fluorescence and the baseline value was determined and plotted against ghrelin concentration or, in Spiegelmer inhibition experiments, against concentration of Spiegelmer. The EC50 or IC50 was read from the graphs.
Inhibition of Ghrelin-Induced GH Release. Male Sprague–Dawley rats weighing ≈150 g were obtained from Charles River Laboratories. The animals were anesthetized with an i.p. injection of ketamine/xylazine and received an i.v. injection of either PBS or Spiegelmer at the doses indicated in the figures. Rats were rested for 15 min, and the first blood sample was taken from the orbital sinus immediately before the i.v. injection of 10 μg of bioactive rat ghrelin in PBS (time point 0). Additional blood samples were obtained from the orbital sinus at the time points indicated in Fig. 4. EDTA plasma was prepared and immediately frozen at –70°C. The plasma GH content was determined with the Biotrak GH enzyme immunoassay kit (Amersham Pharmacia), according to the manufacturer's instructions.
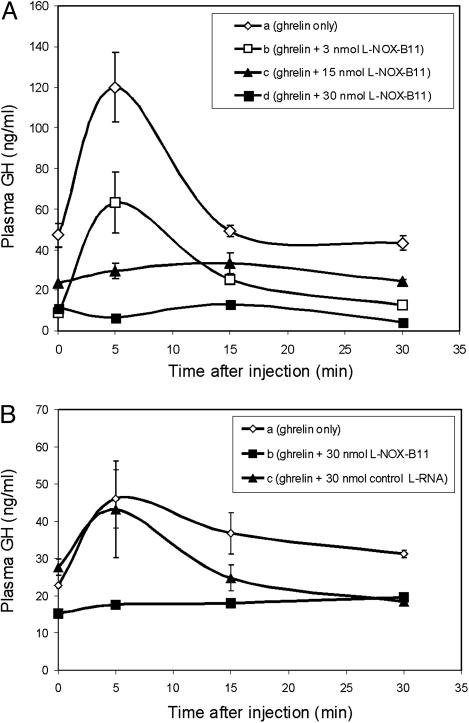
Fig. 4.
Ghrelin-mediated GH release is suppressed by prior i.v. administration of Spiegelmer l-NOX-B11. (A) Animals were injected i.v. with either PBS (curve a) or the indicated doses of Spiegelmer (curves b–d) 15 min before stimulation with 3 nmol of ghrelin. Plasma GH levels were determined at the indicated time points. (B) The ghrelin-induced stimulation of GH release is not inhibited by a random Spiegelmer control sequence.
Results
Generation of Aptamers Binding to d-Ghrelin. The starting pool of RNA oligonucleotides for SELEX had a complexity of ≈2 × 1015 different molecules. After 17 selection rounds with biotinylated rat d-ghrelin, the selected d-RNA exhibited an enrichment in binding sequences. We determined the affinity to d-ghrelin after round 17 to be ≈50 nM (data not shown). Because additional selection rounds did not improve this value we used double-stranded DNA from round 17 for cloning and sequencing. A sample of 87 sequences returned a single family in which sequence B11 was the most frequent representative at a rate of 75% (Table 1). The majority of the other sequences differed from B11 by point mutations and occurred once or twice in the sample. A subgroup comprising 14 sequences contained insertions of 1–12 adenosines.
Table 1. d-ghrelin-binding sequences identified by in vitro selection and sequencing.
| Clone | Selected sequences, variable region | Frequency | Kd, nM |
|---|---|---|---|
| B11 | UG---AGGCAAU------------AAAACU--UAAGUCCGAAGGUAACCAAUCCUA | 65× | 44.4 |
| G2 | UG---AGGCAGU------------AAAACU--UAAGUCCGAAGGUAACCAAUCCUA | 1× | |
| E12 | UG---AGGCAAU------------AAAACU--UAAGUCCGAAGGUAACCAAUCCUG | 1× | |
| B7 | UG---AGGCAAU------------AAAACA--UAAGUCCGAAGGUAACCAAUCCUA | 2× | |
| A8 | UG---AGGCAAU------------AAAACG--UAAGUCCGAAGGUAACCAAUCCUA | 2× | |
| B12 | UG---AGGCAAU------------AAAACUUGUAAGUCCGAAGGUAACCAAUCCUA | 1× | |
| E3 | UG---AGGCAAUA-----------AAAACU--UAAGUCCGAAGGUAACCAAUCCUA | 5× | 89.9 |
| C11 | UG---AGGUAGUAAA---------AAAACG--UAAAUCCGAAGGUAACCAAUCCUA | 2× | |
| A3 | UG---AGGUAGUAAAAAA------AAAACG--UAAAUCCGAAGGUAACCAAUCCUA | 2× | |
| F5 | UG---AGGUAGUAAAAAAA-----AAAACG--UAAAUCCGAAGGUAACCAGUCCUA | 1× | |
| A12 | UG---AGGUAGUAAAAAAAA----AAAACG--UAAAUCCGAAGGUAACCAAUCCUA | 1× | |
| F12 | UG---AGGUAGUAAAAAAAAA---AAAACG--UAAAUCCGAAGGUAACCAAUCCUA | 2× | 87 |
| G5 | UG---AGGUAGUAAAAAAAAAAAAAAAACA--UAAAUCCGAAGGUAACCAAUCCUA | 1× | |
| C12 | UGGUGAGGCA--------------AAAACG--UAAGACCGAAGGUAACCAUUCCUA | 1× |
After 17 selection rounds, 87 full-length clones were sequenced. All sequences belonged to a single family, with sequence B11 being the most common representative. Other sequences differed by point mutations or insertions (in bold). Binding to biotinylated d-ghrelin was determined for the three most frequent sequences by using pull-down assays.
The two most frequent sequences, B11 and E3, as well as sequence F12 containing nine adenosine insertions, were obtained through in vitro transcription. Their dissociation constants Kd for d-ghrelin were determined in pull-down assays with neutravidin-coated beads at 37°C and calculated to be between 45 and 90 nM (Table 1). We proceeded with the most potent binder B11 for a more detailed characterization.
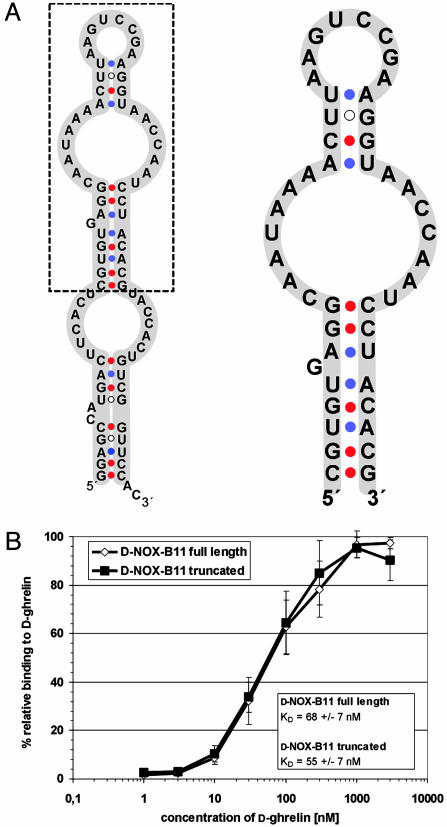
Secondary structure predictions of B11 by using the Zuker algorithm for thermodynamic folding (36) suggested a structure in which the central portion covering mainly the variable region (Fig. 1A, inside box) is stabilized by a stem and bulge formed by the 5′ and 3′ termini (Fig. 1 A, outside box). On the basis of this structure prediction we synthesized B11 without this stem and bulge. The resulting 47-mer, comprising nucleotides 18–64 (d-NOX-B11), was found to bind d-ghrelin with essentially the same affinity as the full-length sequence (Fig. 1B). Binding was dependent on the presence of physiological concentrations of Ca2+ and Mg2+ and was stable over a pH range of 6.2–8.2. d-NOX-B11 also bound human d-ghrelin, which differs from rat ghrelin by two conservative amino acid changes, without loss of affinity (data not shown).
Fig. 1.
The central 47 nucleotides of RNA B11 are sufficient for binding to d-ghrelin. (A) Secondary structure predictions for full-length (Left) and truncated (Right) RNA B11. (B) On the basis of the predicted secondary structure the 81-mer B11 was truncated to 47 nt and the Kd for ghrelin of the resulting RNA NOX-B11 was determined. No loss in affinity was associated with the truncation.
Spiegelmer Binding to l-Ghrelin. After having generated aptamer d-NOX-B11, we next synthesized the corresponding enantiomer in the l-configuration. The resulting Spiegelmer l-NOX-B11 was found to bind biologically active l-ghrelin with a Kd of 35 nM by Biacore surface plasmon resonance analysis, essentially the same affinity exhibited by d-NOX-B11 to d-ghrelin. The binding of l-NOX-B11 was specific for the l-enantiomer of ghrelin, because no binding to d-ghrelin was observed at a concentration of 5 μM (data not shown). Thus, binding is not the result of any unspecific peptide–nucleic acid interactions.
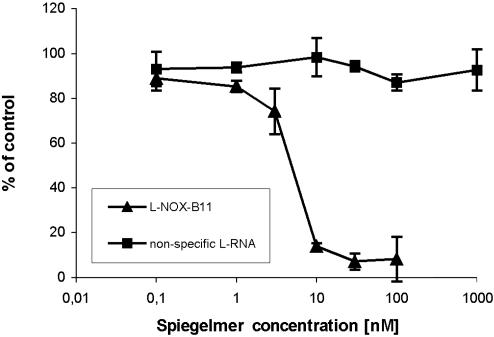
Inhibition of GHS-R1a Signaling in the Presence of Spiegelmer l-NOX-B11. Binding of ghrelin to GHS-R1a triggers an increase in intracellular Ca2+. We therefore examined whether l-NOX-B11 can interfere with ghrelin's function as a ligand to GHS-R1a. For this purpose, CHO cells stably expressing the human GHS-R1a were stimulated with ghrelin at a concentration of 5 nM, the concentration found to be the EC50 (data not shown). We then measured Ca2+-dependent fluorescence in the presence of different concentrations of Spiegelmer. l-NOX-B11 suppressed the ghrelin-induced increase in intracellular Ca2+ with an IC50 of 5 nM (Fig. 2). The specificity of this effect was verified by including a nonfunctional control l-RNA, which had no effect on the receptor–ghrelin interaction over a concentration range of 10–1,000 nM (Fig. 2). Moreover, the stimulation by growth hormone releasing peptide-6 (GHRP-6), a synthetic receptor agonist with no homology to ghrelin, could not be antagonized with l-NOX-B11 (data not shown). Hence, l-NOX-B11 specifically binds l-ghrelin and, thereby, antagonizes the activation of GHS-R1a in cell culture.
Fig. 2.
Ghrelin-mediated activation of GHS-R1a is inhibited in the presence of Spiegelmer l-NOX-B11. GHS-R1a-expressing CHO cells were stimulated with ghrelin at 5 nM in the presence of the indicated concentrations of Spiegelmer l-NOX-B11, and the resulting Ca2+-associated fluorescence was measured. The response to ghrelin binding to the receptor is suppressed by l-NOX-B11 in a dose-dependent manner, whereas a nonspecific l-RNA sequence does not affect receptor activation.
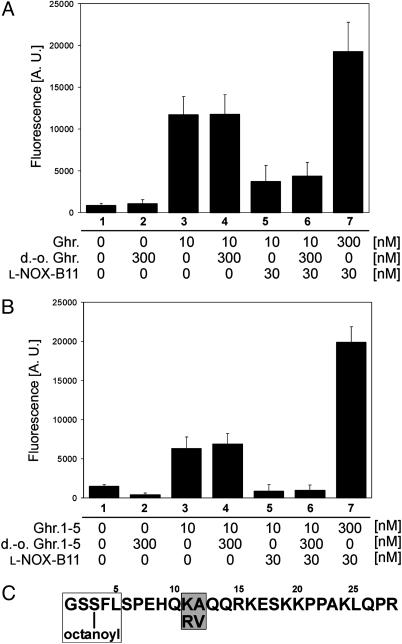
Spiegelmer l-NOX-B11 Binds the n-Octanoylated N Terminus of Ghrelin. Ghrelin's stimulatory effect on GHS-R1a depends on its modification with octanoic acid at Ser-3. To determine whether this modification influences the association of ghrelin with l-NOX-B11 we carried out cell culture assays with modified and unmodified peptides (Fig. 3A). We observed a 12-fold stimulation of Ca2+-mediated fluorescence with 10 nM ghrelin (Fig. 3A, compare columns 1 and 3). No stimulation was observed with 300 nM desoctanoyl ghrelin alone (Fig. 3A, column 2), nor was the activation by 10 nM ghrelin affected in the presence of a 30-fold excess of desoctanoyl ghrelin (Fig. 3A, column 4). These findings confirm that octanoylation at Ser-3 is essential for receptor activation. In contrast, ghrelin-mediated activation was clearly reduced in the presence of 30 nM l-NOX-B11 (Fig. 3A, column 5), and this signal reduction could not be blocked by competition with a concentration of 300 nM desoctanoyl ghrelin (Fig. 3A, column 6). These results demonstrate that l-NOX-B11, like the receptor, requires the presence of ghrelin's octanoyl side chain for binding. As a control we also raised the ghrelin concentration to 300 nM, 10-fold higher than the concentration of l-NOX-B11 (Fig. 3A, column 7). This concentration of ghrelin was sufficient to saturate the receptor as well as the Spiegelmer, leading to a higher response than stimulation with 10 nM ghrelin alone.
Fig. 3.
Spiegelmer l-NOX-B11 binds the n-octanoylated N terminus of ghrelin. l-NOX-B11 requires the octanoylated N terminus of ghrelin for binding and inhibition. GHS-R1a-expressing CHO cells were stimulated with 10 nM ghrelin (A) or ghrelin-(1–5) (B) in the presence of 30 nM l-NOX-B11, 300 nM desoctanoyl ghrelin (d.-o. Ghr.), or both. Ca2+-associated fluorescence triggered by ghrelin's binding to the receptor was measured. (C) The sequence of rat ghrelin, showing the minimal binding motif for l-NOX-B11 (boxed) and the differing amino acids in the human sequence (shaded). See Results for details.
It had been shown that octanoylated ghrelin fragments as short as the N-terminal five amino acids bind and stimulate GHS-R1A (11). Replacing ghrelin with ghrelin-(1–5), we used the same experimental setup to test the amino acid requirements for ghrelin binding by l-NOX-B11 (Fig. 3B). The results were essentially the same as with the full-length peptide: No activation of the receptor could be observed with desoctanoyl ghrelin-(1–5) at 300 nM (Fig. 3B, column 2), whereas ghrelin-(1–5) stimulated Ca2+ influx (Fig. 3B, column 3). The activation of the receptor by ghrelin-(1–5) persisted in the presence of an excess of 300 nM desoctanoyl ghrelin-(1–5) (Fig. 3B, column 4) but could be inhibited by l-NOX-B11 at 30 nM (column 5). The inhibition of ghrelin-(1–5) by l-NOX-B11 remained unaffected in the presence of an excess of desoctanoyl ghrelin-(1–5) (Fig. 3B, column 6), whereas the same excess of ghrelin-(1–5) resulted in full receptor activation. Taken together, these findings indicate that the octanoyl side chain at Ser-3 is required for binding of ghrelin by l-NOX-B11. In contrast, the 23 C-terminal amino acids are dispensable. Because rat and human ghrelin differ only in amino acids 11 and 12 (Fig. 3C), it is unsurprising that l-NOX-B11 binds to both.
Spiegelmer l-NOX-B11 Inhibits Ghrelin-Induced GH Release in Rats. Systemic administration of ghrelin is known to increase the circulating plasma levels of GH in rats. We therefore examined whether the ghrelin-binding Spiegelmer interferes with the reported stimulation of GH release by ghrelin. We used a version of Spiegelmer l-NOX-B11 with polyethylene glycol (PEG) at the 5′ end for these studies because the PEG moiety substantially increases the half-life of Spiegelmers in the bloodstream (6). An i.v. injection of 3 nmol of ghrelin triggered a burst of GH release comparable with the original report by Kojima et al. (9) (Fig. 4A, curve a). In the presence of an equimolar amount of Spiegelmer l-NOX-B11, the stimulation of GH release persisted (Fig. 4A, curve b). However, at Spiegelmer doses of 15 (Fig. 4A, curve c) and 30 nmol (Fig. 4A, curve d), 5 and 10 times the dose of ghrelin, respectively, no increase of GH in plasma after the administration of ghrelin was observed. The GH baselines for each group naturally differ before ghrelin injection (time 0) because of the individual patterns of GH release throughout the day. Importantly, however, 30 min after the injection of ghrelin, the average plasma GH concentration of each study group returned to its respective baseline value.
To exclude a nonspecific effect of Spiegelmer we added a nonfunctional control Spiegelmer with a random sequence to this assay (Fig. 4B). In rats that had received 30 nmol of control Spiegelmer, GH increased after ghrelin administration (curve c) similar to animals who had received ghrelin only (Fig. 4B, curve a). In contrast, in rats that had received l-NOX-B11, no GH response was observed (Fig. 4B, curve b). Thus, Spiegelmer l-NOX-B11 specifically inhibits ghrelin-mediated release of GH in anesthetized male rats.
Discussion
Employing an in vitro selection approach, we have identified l-RNA oligonucleotides (Spiegelmers) capable of specifically binding n-octanoyl-l-ghrelin with low nanomolar affinity. The tight complex formation between Spiegelmer l-NOX-B11 and l-ghrelin interferes with the peptide's binding to GHS-R1a, thereby preventing receptor activation as well as the ensuing signaling cascade. Most importantly, Spiegelmer l-NOX-B11 is capable of neutralizing circulating bioactive ghrelin in vivo, making it a candidate for therapeutic development. This finding is promising given the recent results with Macugen, a modified aptamer tested successfully for the treatment of wet macular degeneration in phase II clinical trials (37).
Our data demonstrate that binding of ghrelin by l-NOX-B11 is dependent on the presence of the n-octanoyl group at Ser-3, indicating an essential role for the fatty acid in the molecular interaction. The fatty acid is achiral and, therefore, structurally the same in d- and l-ghrelin. Because the Spiegelmer distinguishes between the d- and l-forms of ghrelin, the octanoyl group, though necessary for the interaction, is not likely to be the sole binding site. An octanoylated ghrelin fragment consisting of the N-terminal five amino acids is sufficient for in vitro receptor activation (11). This fragment is also bound by l-NOX-B11, suggesting that the Spiegelmer targets the peptide's GHS-R1a binding site. Given the hydrophilic nature of ghrelin's positively charged C terminus, it was surprising that the negatively charged oligonucleotide binds to the peptide's more hydrophobic N terminus. The result may best be explained by a model in which the n-octanoyl group creates the appropriate 3D context for binding, presenting the N terminus of ghrelin to allow for recognition of Spiegelmer l-NOX-B11 or the receptor. The observation that other bulky, hydrophobic groups at Ser-3 can functionally replace octanoic acid (11) supports this hypothesis. Alternatively, contacts between l-NOX-B11 and ghrelin may not be limited to the N-terminal five amino acids but include the hydrophobic interactions with the octanoyl group for efficient complex assembly.
Several in vivo studies have aimed at correlating acute and chronic states of positive energy balance with circulating plasma ghrelin levels (38–41). However, up to 90% of the circulating ghrelin levels are not octanoylated (31) and, therefore, unable to activate GHS-R1a. The vast majority of studies examining ghrelin secretion have not been able to differentiate between relative concentrations of active versus inactive peptide. The conclusions drawn from such experiments may therefore need reconsideration. A Spiegelmer with the ability to differentiate between ghrelin and desoctanoyl ghrelin offers the opportunity to develop an assay system to examine these important issues.
Systemically administered Spiegelmer l-NOX-B11 specifically inhibited ghrelin-induced GH release in male rats, demonstrating that Spiegelmer binding of ghrelin antagonizes the ghrelin–GHS-receptor system in vivo. This finding confirms the utility of Spiegelmers as a potential therapeutic in the living organism. Tannenbaum et al. (20) have shown that the GH response to exogenous ghrelin depends on the presence of a functional growth hormone-releasing hormone system, implying ghrelin's transfer across the blood–brain barrier to reach the hypothalamus. However, the natural amplitudes of GH pulsatility are not evident under anesthesia, an observation thought to be due to the anesthetics' inhibitory effects on growth hormone-releasing hormone and somatostatin pathways (42). Therefore, the GH-releasing effects measured in our assay are almost certainly the result of ghrelin's direct stimulation of the pituitary gland. Consequently, the neutralization of ghrelin with l-NOX-B11 most probably occurs in the periphery without the antagonist crossing into hypothalamic areas. This result is consistent with previous tissue distribution analyses in our laboratories in which we were unable to detect Spiegelmer in the brain (data not shown).
Despite the powerful effect on GH release, ghrelin's most important physiological role may be in the regulation of energy homeostasis. Ghrelin and its receptor have attracted substantial attention as potential targets in the treatment of obesity (31). Recent experiments with [d-Lys-3]GHRP-6, a known GHS receptor antagonist, resulted in reduced food intake in mice (33), and intracerebroventricular administration of polyclonal antighrelin antibodies decreased food intake in rats (24). These results indicate that the antagonism of the ghrelin–GHS-receptor system in the CNS may represent a viable pharmacological approach to reduce food intake. The observation that gastric bypass surgery leads to extremely low plasma ghrelin levels and diminished sensations of hunger suggests that the suppression of peripheral ghrelin can also cause a decrease in food intake (39, 44). However, this claim so far has not been substantiated experimentally.
Our results indicate that treatment with a ghrelin-binding Spiegelmer provides a feasible approach to block the effect of circulating ghrelin on GH release. Spiegelmer l-NOX-B11 may lead the way in an approach to the treatment of obesity and obesity-related diseases through blocking of peripheral ghrelin. These findings could be especially relevant in diseases that are associated with high levels of circulating ghrelin, like the Prader–Willi Syndrome (44, 45).
Acknowledgments
We dedicate this article to our colleague and collaborator Dr. Stefan Rosewicz, who passed away in May of this year. We thank the following members of the NOXXON team for their contributions to the synthesis of all oligonucleotides used in this work: S. Grbic, G. Anlauf, S. Hoffmann, K. Gottsche, and I. Röhl. We also thank C. Bohnes and S. Schülzchen for their work on the in vitro selection; R. Bleise and F. Kleinjung for Biacore measurements; A. Dahlke, K. Schindle, N. Sayed Suleiman, and M. Bell for their excellent cell culture work; C. Kaduk for help with the growth hormone study; and D. Tornus and M. Courtney for helpful discussion.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CHO, Chinese hamster ovary; GH, growth hormone; GHS, GH secretagogues; GHS-R1a, GHS receptor 1a.
References
- 1.Klussmann, S., Nolte, A., Bald, R., Erdmann, V. A. & Furste, J. P. (1996) Nat. Biotechnol. 14, 1112–1115. [DOI] [PubMed] [Google Scholar]
- 2.Vater, A. & Klussmann, S. (2003) Curr. Opin. Drug Discov. Dev. 6, 253–261. [PubMed] [Google Scholar]
- 3.Hicke, B. J., Watson, S. R., Koenig, A., Lynott, C. K., Bargatze, R. F., Chang, Y. F., Ringquist, S., Moon-McDermott, L., Jennings, S., Fitzwater, T., et al. (1996) J. Clin. Invest. 98, 2688–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Floege, J., Ostendorf, T., Janssen, U., Burg, M., Radeke, H. H., Vargeese, C., Gill, S. C., Green, L. S. & Janjic, N. (1999) Am. J. Pathol. 154, 169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostendorf, T., Kunter, U., Eitner, F., Loos, A., Regele, H., Kerjaschki, D., Henninger, D. D., Janjic, N. & Floege, J. (1999) J. Clin. Invest. 104, 913–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wlotzka, B., Leva, S., Eschgfäller, B., Burmeister, J., Kleinjung, F., Kaduk, C., Muhn, P., Hess-Stumpp, H. & Klussmann, S. (2002) Proc. Natl. Acad. Sci. USA 99, 8898–8902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellington, A. D. & Szostak, J. W. (1990) Nature 346, 818–822. [DOI] [PubMed] [Google Scholar]
- 8.Tuerk, C. & Gold, L. (1990) Science 249, 505–510. [DOI] [PubMed] [Google Scholar]
- 9.Kojima, M., Hosoda, H., Date, Y., Nakazato, M., Matsuo, H. & Kangawa, K. (1999) Nature 402, 656–660. [DOI] [PubMed] [Google Scholar]
- 10.Howard, A. D., Feighner, S. D., Cully, D. F., Arena, J. P., Liberator, P. A., Rosenblum, C. I., Hamelin, M., Hreniuk, D. L., Palyha, O. C., Anderson, J., et al. (1996) Science 273, 974–977. [DOI] [PubMed] [Google Scholar]
- 11.Bednarek, M. A., Feighner, S. D., Pong, S. S., McKee, K. K., Hreniuk, D. L., Silva, M. V., Warren, V. A., Howard, A. D., Van Der Ploeg, L. H. & Heck, J. V. (2000) J. Med. Chem. 43, 4370–4376. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto, M., Hosoda, H., Kitajima, Y., Morozumi, N., Minamitake, Y., Tanaka, S., Matsuo, H., Kojima, M., Hayashi, Y. & Kangawa, K. (2001) Biochem. Biophys. Res. Commun. 287, 142–146. [DOI] [PubMed] [Google Scholar]
- 13.Torsello, A., Ghe, C., Bresciani, E., Catapano, F., Ghigo, E., Deghenghi, R., Locatelli, V. & Muccioli, G. (2002) Endocrinology 143, 1968–1971. [DOI] [PubMed] [Google Scholar]
- 14.Smith, R. G., Leonard, R., Bailey, A. R., Palyha, O., Feighner, S., Tan, C., McKee, K. K., Pong, S. S., Griffin, P. & Howard, A. (2001) Endocrine 14, 9–14. [DOI] [PubMed] [Google Scholar]
- 15.Momany F. A., Powers, C. Y., Reynolds G. A., Chang, D., Hong, A. & Newlander, K. (1981) Endocrinology 108, 31–39. [DOI] [PubMed] [Google Scholar]
- 16.Tolle, V., Zizzari, P., Tomasetto, C., Rio, M. C., Epelbaum, J. & Bluet-Pajot, M. T. (2001) Neuroendocrinology 73, 54–61. [DOI] [PubMed] [Google Scholar]
- 17.Takaya, K., Ariyasu, H., Kanamoto, N., Iwakura, H., Yoshimoto, A., Harada, M., Mori, K., Komatsu, Y., Usui, T., Shimatsu, A., et al. (2000) J. Clin. Endocrinol. Metab. 85, 4908–4911. [DOI] [PubMed] [Google Scholar]
- 18.Peino, R., Baldelli, R., Rodriguez-Garcia, J., Rodriguez-Segade, S., Kojima, M., Kangawa, K., Arvat, E., Ghigo, E., Dieguez, C. & Casanueva, F. F. (2000) Eur. J. Endocrinol. 143, R11–R14. [DOI] [PubMed] [Google Scholar]
- 19.Seoane, L. M., Tovar, S., Baldelli, R., Arvat, E., Ghigo, E., Casanueva, F. F. & Dieguez, C. (2000) Eur. J. Endocrinol. 143, R7–R9. [DOI] [PubMed] [Google Scholar]
- 20.Tannenbaum, G. S., Epelbaum, J. & Bowers, C. Y. (2003) Endocrinology 144, 967–974. [DOI] [PubMed] [Google Scholar]
- 21.Tschop, M., Smiley, D. L. & Heiman, M. L. (2000) Nature 407, 908–913. [DOI] [PubMed] [Google Scholar]
- 22.Wren, A. M., Small, C. J., Abbott, C. R., Dhillo, W. S., Seal, L. J., Cohen, M. A., Batterham, R. L., Taheri, S., Stanley, S. A., Ghatei, M. A. & Bloom, S. R. (2001) Diabetes 50, 2540–2547. [DOI] [PubMed] [Google Scholar]
- 23.Horvath, T. L., Diano, S. & Tschop, M. (2003) Curr. Top. Med. Chem. 3, 921–927. [DOI] [PubMed] [Google Scholar]
- 24.Nakazato, M., Murakami, N., Date, Y., Kojima, M., Matsuo, H., Kangawa, K. & Matsukura, S. (2001) Nature 409, 194–198. [DOI] [PubMed] [Google Scholar]
- 25.Wren, A. M., Seal, L. J., Cohen, M. A., Brynes, A. E., Frost, G. S., Murphy, K. G., Dhillo, W. S., Ghatei, M. A. & Bloom, S. R. (2001) J. Clin. Endocrinol. Metab. 86, 5992. [DOI] [PubMed] [Google Scholar]
- 26.Sun, Y., Ahmed, S. & Smith, R. G. (2003) Mol. Cell. Biol. 23, 7973–7981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wortley, K. E., Anderson, K., Garcia, K., Murray, J., Malinova, L., Liu, R., Moncrieffe, M., Thabet, K., Cox, H., Yancopoulos, G. D., et al. (2004) Proc. Natl. Acad. Sci. USA 101, 8227–8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Date, Y., Kojima, M., Hosoda, H., Sawaguchi, A., Mondal, M. S., Suganuma, T., Matsukura, S., Kangawa, K. & Nakazato, M. (2000) Endocrinology 141, 4255–4261. [DOI] [PubMed] [Google Scholar]
- 29.Ariyasu, H., Takaya, K., Tagami, T., Ogawa, Y., Hosoda, K., Akamizu, T., Suda, M., Koh, T., Natsui, K., Toyooka, S., et al. (2001) J. Clin. Endocrinol. Metab. 86, 4753–4758. [DOI] [PubMed] [Google Scholar]
- 30.Cowley, M. A., Smith, R. G., Diano, S., Tschop, M., Pronchuk, N., Grove, K. L., Strasburger, C. J., Bidlingmaier, M., Esterman, M., Heiman, M. L., et al. (2003) Neuron 37, 649–661. [DOI] [PubMed] [Google Scholar]
- 31.Horvath, T. L., Castaneda, T., Tang-Christensen, M., Pagotto, U. & Tschop, M. H. (2003) Curr. Pharm. Des. 9, 1383–1395. [DOI] [PubMed] [Google Scholar]
- 32.Petersenn, S. (2002) Horm. Res. 58, Suppl. 1, 56–61. [DOI] [PubMed] [Google Scholar]
- 33.Asakawa, A., Inui, A., Kaga, T., Katsuura, G., Fujimiya, M., Fujino, M. A. & Kasuga, M. (2003) Gut 52, 947–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shuto, Y., Shibasaki, T., Otagiri, A., Kuriyama, H., Ohata, H., Tamura, H., Kamegai, J., Sugihara, H., Oikawa, S. & Wakabayashi, I. (2002) J. Clin. Invest. 109, 1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vater, A., Jarosch, F., Buchner, K. & Klussmann, S. (2003) Nucleic Acids Res. 31, e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuker, M. (2003) Nucleic Acids Res. 31, 3406–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eyetech Study Group (2003) Ophthalmology 110, 979–986. [DOI] [PubMed] [Google Scholar]
- 38.Tschop, M., Weyer, C., Tataranni, P. A., Devanarayan, V., Ravussin, E. & Heiman, M. L. (2001) Diabetes 50, 707–709. [DOI] [PubMed] [Google Scholar]
- 39.Cummings, D. E., Weigle, D. S., Frayo, R. S., Breen, P. A., Ma, M. K., Dellinger, E. P. & Purnell, J. Q. (2002) N. Engl. J. Med. 346, 1623–1630. [DOI] [PubMed] [Google Scholar]
- 40.Tolle, V., Bassant, M. H., Zizzari, P., Poindessous-Jazat, F., Tomasetto, C., Epelbaum, J. & Bluet-Pajot, M. T. (2002) Endocrinology 143, 1353–1361. [DOI] [PubMed] [Google Scholar]
- 41.Cummings, D. E., Purnell, J. Q., Frayo, R. S., Schmidova, K., Wisse, B. E. & Weigle, D. S. (2001) Diabetes 50, 1714–1719. [DOI] [PubMed] [Google Scholar]
- 42.Giustina, A. & Veldhuis, J. D. (1998) Endocr. Rev. 19, 717–797. [DOI] [PubMed] [Google Scholar]
- 43.Cummings, D. E. & Shannon, M. H. (2003) J. Clin. Endocrinol. Metab. 88, 2999–3002. [DOI] [PubMed] [Google Scholar]
- 44.Cummings, D. E., Clement, K., Purnell, J. Q., Vaisse, C., Foster, K. E., Frayo, R. S., Schwartz, M. W., Basdevant, A. & Weigle, D. S. (2002) Nat. Med. 8, 643–644. [DOI] [PubMed] [Google Scholar]
- 45.DelParigi, A., Tschop, M., Heiman, M. L., Salbe, A. D., Vozarova, B., Sell, S. M., Bunt, J. C. & Tataranni, P. A. (2002) J. Clin. Endocrinol. Metab. 87, 5461–5464. [DOI] [PubMed] [Google Scholar]