Abstract
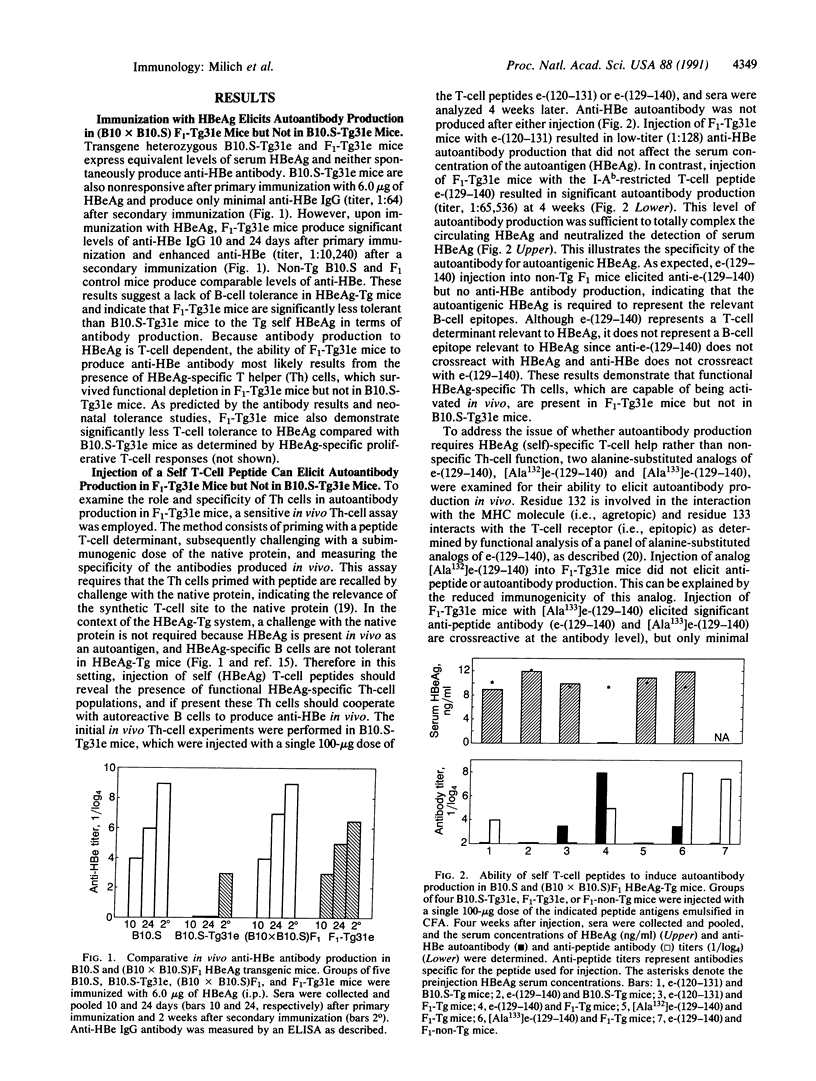
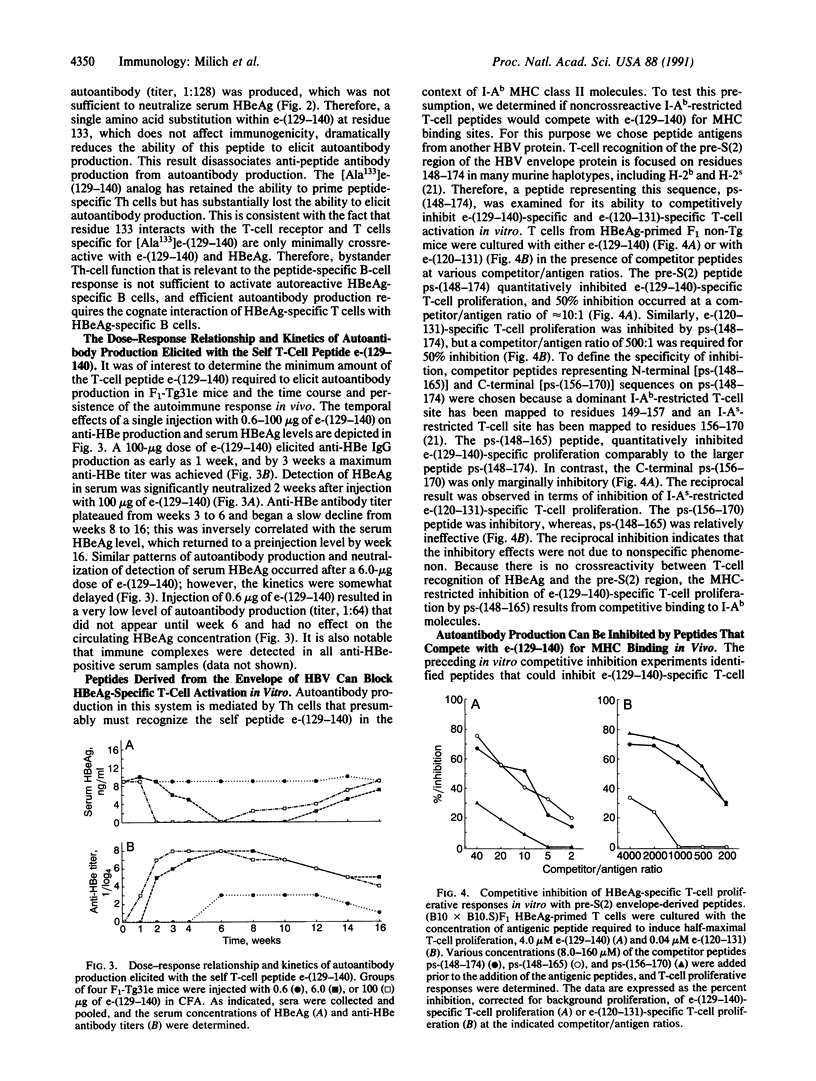
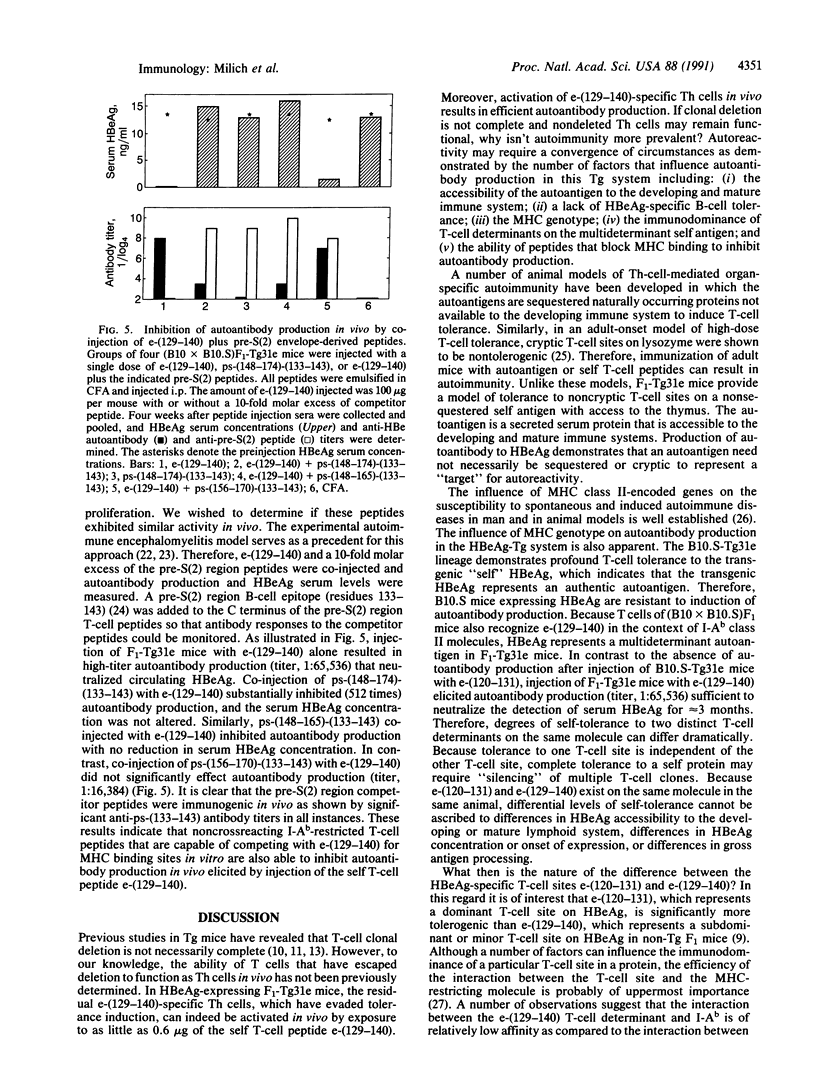
Studies in hepatitis B e antigen (HBeAg)-expressing transgenic mice indicate that self tolerance to two T-cell determinants on the same transgenic self molecule can differ markedly. The dominant T-cell site on HBeAg is tolerogenic, whereas a proportion of T cells recognizing a second T-cell site evade tolerance induction, persist in the periphery, and can be activated in vivo by a single injection of a 12-residue T-cell self peptide. The self-reactive T cells mediate in vivo autoantibody production sufficient to neutralize detection of the autoantigen in serum. Furthermore, autoantibody production can be inhibited by nonself peptides that compete with the self peptide for binding to major histocompatibility complex molecules. This model illustrates that T cells specific for an immunogenic T-cell site on a nonsequestered autoantigen can escape tolerance induction and, more importantly, can mediate autoreactivity in vivo. Furthermore, these results suggest that synthetic T-cell sites may be useful as immunotherapeutic agents for the purpose of circumventing nonresponse to HBeAg during persistent hepatitis B virus infection.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adorini L., Appella E., Doria G., Nagy Z. A. Mechanisms influencing the immunodominance of T cell determinants. J Exp Med. 1988 Dec 1;168(6):2091–2104. doi: 10.1084/jem.168.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman M. A., Burgert H. G., Gerhard-Burgert H., Woodland D. L., Palmer E., Kappler J. W., Marrack P. A role for clonal inactivation in T cell tolerance to Mls-1a. Nature. 1990 Jun 7;345(6275):540–542. doi: 10.1038/345540a0. [DOI] [PubMed] [Google Scholar]
- Burkly L. C., Lo D., Kanagawa O., Brinster R. L., Flavell R. A. T-cell tolerance by clonal anergy in transgenic mice with nonlymphoid expression of MHC class II I-E. Nature. 1989 Nov 30;342(6249):564–566. doi: 10.1038/342564a0. [DOI] [PubMed] [Google Scholar]
- Gammon G., Sercarz E. How some T cells escape tolerance induction. Nature. 1989 Nov 9;342(6246):183–185. doi: 10.1038/342183a0. [DOI] [PubMed] [Google Scholar]
- Houghten R. A. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5131–5135. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J. W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Staerz U., White J., Marrack P. C. Self-tolerance eliminates T cells specific for Mls-modified products of the major histocompatibility complex. Nature. 1988 Mar 3;332(6159):35–40. doi: 10.1038/332035a0. [DOI] [PubMed] [Google Scholar]
- Lakey E. K., Margoliash E., Flouret G., Pierce S. K. Peptides related to the antigenic determinant block T cell recognition of the native protein as processed by antigen-presenting cells. Eur J Immunol. 1986 Jul;16(7):721–727. doi: 10.1002/eji.1830160702. [DOI] [PubMed] [Google Scholar]
- Milich D. R., Hughes J. L., Houghten R., McLachlan A., Jones J. E. Functional identification of agretopic and epitopic residues within an HBcAg T cell determinant. J Immunol. 1989 Nov 15;143(10):3141–3147. [PubMed] [Google Scholar]
- Milich D. R., Hughes J. L., McLachlan A., Langley K. E., Thornton G. B., Jones J. E. Importance of subtype in the immune response to the pre-S(2) region of the hepatitis B surface antigen. I. T cell fine specificity. J Immunol. 1990 May 1;144(9):3535–3543. [PubMed] [Google Scholar]
- Milich D. R., Hughes J. L., McLachlan A., Thornton G. B., Moriarty A. Hepatitis B synthetic immunogen comprised of nucleocapsid T-cell sites and an envelope B-cell epitope. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1610–1614. doi: 10.1073/pnas.85.5.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milich D. R., Jones J. E., Hughes J. L., Price J., Raney A. K., McLachlan A. Is a function of the secreted hepatitis B e antigen to induce immunologic tolerance in utero? Proc Natl Acad Sci U S A. 1990 Sep;87(17):6599–6603. doi: 10.1073/pnas.87.17.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milich D. R., Jones J. E., McLachlan A., Houghten R., Thornton G. B., Hughes J. L. Distinction between immunogenicity and tolerogenicity among HBcAg T cell determinants. Influence of peptide-MHC interaction. J Immunol. 1989 Nov 15;143(10):3148–3156. [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Chisari F. V., Nakamura T., Thornton G. B. Two distinct but overlapping antibody binding sites in the pre-S(2) region of HBsAg localized within 11 continuous residues. J Immunol. 1986 Oct 15;137(8):2703–2710. [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Chisari F. V., Thornton G. B. Nonoverlapping T and B cell determinants on an hepatitis B surface antigen pre-S(2) region synthetic peptide. J Exp Med. 1986 Aug 1;164(2):532–547. doi: 10.1084/jem.164.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Stahl S., Wingfield P., Thornton G. B., Hughes J. L., Jones J. E. Comparative immunogenicity of hepatitis B virus core and E antigens. J Immunol. 1988 Nov 15;141(10):3617–3624. [PubMed] [Google Scholar]
- Mondelli M., Vergani G. M., Alberti A., Vergani D., Portmann B., Eddleston A. L., Williams R. Specificity of T lymphocyte cytotoxicity to autologous hepatocytes in chronic hepatitis B virus infection: evidence that T cells are directed against HBV core antigen expressed on hepatocytes. J Immunol. 1982 Dec;129(6):2773–2778. [PubMed] [Google Scholar]
- Morahan G., Allison J., Miller J. F. Tolerance of class I histocompatibility antigens expressed extrathymically. Nature. 1989 Jun 22;339(6226):622–624. doi: 10.1038/339622a0. [DOI] [PubMed] [Google Scholar]
- Murphy K. M., Weaver C. T., Elish M., Allen P. M., Loh D. Y. Peripheral tolerance to allogeneic class II histocompatibility antigens expressed in transgenic mice: evidence against a clonal-deletion mechanism. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10034–10038. doi: 10.1073/pnas.86.24.10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B. Molecular mimicry and autoimmune disease. Cell. 1987 Sep 11;50(6):819–820. doi: 10.1016/0092-8674(87)90507-1. [DOI] [PubMed] [Google Scholar]
- Sakai K., Zamvil S. S., Mitchell D. J., Hodgkinson S., Rothbard J. B., Steinman L. Prevention of experimental encephalomyelitis with peptides that block interaction of T cells with major histocompatibility complex proteins. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9470–9474. doi: 10.1073/pnas.86.23.9470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J., Gao E. K., Webb S. R. T cell reactivity to MHC molecules: immunity versus tolerance. Science. 1990 Jun 15;248(4961):1357–1363. doi: 10.1126/science.1694041. [DOI] [PubMed] [Google Scholar]
- Stahl S., MacKay P., Magazin M., Bruce S. A., Murray K. Hepatitis B virus core antigen: synthesis in Escherichia coli and application in diagnosis. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1606–1610. doi: 10.1073/pnas.79.5.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh H. S., Kishi H., Scott B., Von Boehmer H. Deletion of autospecific T cells in T cell receptor (TCR) transgenic mice spares cells with normal TCR levels and low levels of CD8 molecules. J Exp Med. 1989 Mar 1;169(3):795–806. doi: 10.1084/jem.169.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd J. A., Acha-Orbea H., Bell J. I., Chao N., Fronek Z., Jacob C. O., McDermott M., Sinha A. A., Timmerman L., Steinman L. A molecular basis for MHC class II--associated autoimmunity. Science. 1988 May 20;240(4855):1003–1009. doi: 10.1126/science.3368786. [DOI] [PubMed] [Google Scholar]
- Whiteley P. J., Poindexter N. J., Landon C., Kapp J. A. A peripheral mechanism preserves self-tolerance to a secreted protein in transgenic mice. J Immunol. 1990 Sep 1;145(5):1376–1381. [PubMed] [Google Scholar]
- Wood P. J., Socarras S., Streilein J. W. Modification of the cytotoxic T cell repertoire in neonatal tolerance. Evidence for preferential survival of cells with low avidity for tolerogen. J Immunol. 1987 Nov 15;139(10):3236–3244. [PubMed] [Google Scholar]
- Wraith D. C., Smilek D. E., Mitchell D. J., Steinman L., McDevitt H. O. Antigen recognition in autoimmune encephalomyelitis and the potential for peptide-mediated immunotherapy. Cell. 1989 Oct 20;59(2):247–255. doi: 10.1016/0092-8674(89)90287-0. [DOI] [PubMed] [Google Scholar]
- Yui K., Komori S., Katsumata M., Siegel R. M., Greene M. I. Self-reactive T cells can escape clonal deletion in T-cell receptor V beta 8.1 transgenic mice. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7135–7139. doi: 10.1073/pnas.87.18.7135. [DOI] [PMC free article] [PubMed] [Google Scholar]



