Abstract
Keratoconjunctivitis sicca (KCS) is a dysfunction in tear production associated with clinical signs, which include conjunctival hyperemia, ocular discharge, discomfort, pain, and, eventually, corneal vascularization and pigmentation. Immunosuppressive drugs are routinely administrated for long periods to treat KCS but with side effects and limited results. Evaluation of the clinical benefits of intralacrimal transplantation of allogeneic mesenchymal stem cells (MSCs) in dogs with mild–moderate and severe KCS was done. A total of 24 eyes with KCS from 15 dogs of different breeds were enrolled in the present study. A single transplantation of MSCs (1 × 106) directly into lacrimal glands (dorsal and third eyelid) was performed. The Schirmer tear tests (STTs) and ocular surface improvements were used to assess short- and long-term effects of these cells. The STTs were carried out on day 0 (before MSCs transplantation) and on days 7, 14, 21, and 28, as well as 6 and 12 months after MSC transplantation. Our data demonstrate that allogeneic MSC transplantation in KCS dogs is safe since no adverse effects were observed immediately after transplantation and in short- and long-term follow-ups. A statistically significant increase in the STT and ocular surface improvements was found in all eyes studied. In all the eyes with mild–moderate KCS, STT values reverted to those of healthy eyes, while in eyes with severe KCS, although complete reversion was not found, there was improvement in tear production and in other clinical signs. Our study shows that a single dose of a low number of MSCs can be used to treat KCS in dogs. In contrast to immunosuppressive drug use, MSC transplantation has an effect over a long period (up to 12 months), even after a single administration, and does not require daily drug administration.
Key words: Allogeneic mesenchymal stem cell transplantation, Keratoconjunctivitis sicca (KCS), Dry eye syndrome, Schirmer tear test (STT), Dogs
INTRODUCTION
Keratoconjunctivitis sicca (KCS), also known as “dry eye syndrome,” is a common ocular disease in dogs resulting from lacrimal gland (LG) inflammation and decreased tear production. KCS can occur either as a quantitative deficiency in the aqueous component of tears or as a qualitative deficiency in the lipid or mucin layers of the tear film, causing tear film instability, with potential damage to the ocular surface1. This damage is characterized by the presence of mucoid ocular discharge, conjunctival hyperemia, blepharospasm, recurrent corneal ulceration, corneal vascularization, fibrosis, and, eventually, corneal pigmentation. In severe cases, dense corneal opacification (clouding) or corneal perforation secondary to deep ulceration can lead to blindness or even loss of the eye2–4. The diagnosis of quantitative KCS is based on typical ocular surface changes, as well as on dysfunction in tear production, which is evaluated by biomicroscopy of the anterior segment and by the Schirmer tear test (STT), respectively. The STT determines whether the eye produces enough tears to keep it moist and ranges from normal (15–25 mm/min), mild (9–14 mm/min), moderate (>4 to 8 mm/min), to severe (≤4 mm/min)3.
Any condition that impairs the ability to produce adequate amounts of tear film can result in KCS2. Local immune-mediated disease is the most widely accepted cause of KCS based on histopathology of tear-producing glands and on the clinical response to immunomodulators2,5,6. However, other systemic diseases may also be associated with KCS, such as infection with canine distemper virus, hypothyroidism, diabetes mellitus, and Cushing’s disease7. In addition, systemic administration of pharmaceutical agents for long periods and at high doses has also been reported to cause dry eye8. The most common treatment for KCS is the prescription of immunosuppressive drugs, such as cyclosporine and tacrolimus, which may need to be used indefinitely9. Furthermore, some authors believe a small number of dogs are resistant to the action of cyclosporine10. It is important to explain to the owner that the dog with KCS needs constant care, such as removal of secretions from around the eyes many times a day to minimize irritation of the eyelids, conjunctiva, and cornea. Thus, efforts are being made to develop alternative therapies to inhibit the immune response and inflammatory processes in order to reduce the suffering of animals with KCS and the need for their constant care.
It is known that mesenchymal stem cells (MSCs) are powerful regulators of the immune response and that they have been shown to be effective in treating various immune disorders in human and animal models11–19. Previous studies have already demonstrated safety aspects of MSC transplantation into the LG and tear production improvement after MSC transplantation in dogs with KCS20–22. However, it remains unclear whether MSC transplantation is efficient and leads to a good prognosis—tear production levels reverting to normal—in cases of severe KCS, especially in the long term. We thereby carried out the present study to evaluate the effects of MSC transplantation into LGs on tear production and clinical signs in dogs with mild–moderate versus severe KCS.
Veterinary patients, such as dogs, are increasingly recognized as critical translational models of human diseases because the etiopathogenesis of canine diseases is similar to that of humans23, particlularly regarding Sjögren’s syndrome24,25. Sjögren’s syndrome is a systemic autoimmune disease diagnosed by its two most common symptoms—dry eyes and dry mouth24. For this reason, canine KCS studies may aid in the development of therapeutic interventions that can benefit humans. Over the last few years, there has been an increase in the demand for sophisticated therapies, such as the use of stem cells, in animal companion care, which has led to a surge in stem cell studies using dogs26. These studies should provide a unique opportunity for assessing both efficacy and safety of human adult stem cell therapies that can be translated to human medicine.
MATERIALS AND METHODS
Animals
This study comprises a series of dogs with the diagnosis of KCS that were enrolled at Campinas, São Paulo, SP, Brazil, from January 2014 to March 2015 (presented in Table 1). The animal owners signed informed consent forms. All practices adhered to the standards for the care and use of laboratory animals established by the Universidade Estadual de Campinas (UNICAMP), Brazil, and were approved by the Institutional Animal Care and Use Committee (Protocol No. 3096-1).
Table 1.
Dog Description, Including Medication Use
| Dog | Breed | Sex | Eye(s) Affected | Age (Years) | Treatment BF MSC Immunosuppressive Drugs | Treatment With AT |
|---|---|---|---|---|---|---|
| 1 | Shitzu | M | L | 3 | No | Yes |
| 2 | Mongrel | F | R | 4 | No | No |
| 3 | Great Dane | F | R | 8 | No | No |
| 4 | Bulldog | F | R and L | 4 | No | Yes |
| 5 | Ihasa Apso | M | R and L | 11 | Tacrolimus | No |
| 6 | Poodle | F | R and L | 6 | Tacrolimus | Yes |
| 7 | Cocker | M | R and L | 10 | No | No |
| 8 | ShihTzu | F | R | 3 | No | No |
| 9 | Beagle | M | R | 5 | Tacrolimus | Yes |
| 10 | Pit Bull | F | R | 11 | Ciclosporine | Yes |
| 11 | Ihasa Apso | F | R and L | 9 | Tacrolimus | No |
| 12 | Lhasa | F | R and L | 5 | Tacrolimus | No |
| 13 | Cocker | F | R and L | 12 | Tacrolimus | No |
| 14 | Lhasa | F | R and L | 8 | Tacrolimus | No |
| 15 | Golden Retriever | F | R and L | 9 | Tacrolimus | Yes |
Artificial treatment (0.2% sodium hyaluronate) was allowed to continue throughout the study. Use of immunosuppresants was discontinued 1 month before transplantation and throughout the study. R, right eye; L, left eye; BF, before; AT, artificial tears; F, female; M, male.
Inclusion criteria adopted were STT value lower than 15 mm/min for at least 1 year in at least one eye and the presence of at least one of the following symptoms: presence of mucoid ocular discharge, conjunctival hyperemia, blepharospasm, corneal vascularization, or corneal opacity (Tables 2–5). Animals also had to be regularly vaccinated to be included in the study. Exclusion criteria were presence of corneal ulceration, infection processes, and other ocular or systemic diseases, including the presence of tumors. STT values (presented in Table 6) indicate whether the eye produces enough tears to keep it moist and are used to classify disease severity as follows: normal values (15–25 mm/min), mild (9–14 mm/min), moderate (>4–8 mm/min), to severe (≤4 mm/min)3. Sixteen eyes were classified as having mild to moderate KCS (group 1), and nine were classified as being severely affected (group 2) with this disease.
Table 2.
Scores of Short- and Long-Term Ocular Health: Ocular Discharge
| Animal/Eye(s) | Ocular Discharge BL | Ocular Discharge 28 Days AF MSC Transplantation | Ocular Discharge 12 Months AF MSC Transplantation |
|---|---|---|---|
| 1L | +++ | + | − |
| 2R* | +++ | + | − |
| 3R | ++ | + | − |
| 4R | + | − | − |
| 4L | ++ | − | − |
| 5R* | +++ | + | − |
| 5L* | +++ | + | − |
| 6R | + | + | − |
| 6L | ++ | + | − |
| 7R | +++ | − | − |
| 7L* | +++ | ++ | − |
| 8R | +++ | + | + |
| 8L | ++ | ++ | − |
| 9R | + | − | N/A |
| 10R* | +++ | + | N/A |
| 11R | +++ | ++ | N/A |
| 12R* | ++ | − | N/A |
| 12L | + | − | N/A |
| 13R* | ++ | − | N/A |
| 13L | ++ | − | N/A |
| 14R* | ++ | ++ | N/A |
| 14L* | +++ | ++ | N/A |
| 15R | ++ | ++ | N/A |
| 15L | ++ | ++ | N/A |
Ocular discharge was graded as absent (−), mild (+), moderate (++), or severe (+++). The eye data were collected at BL and 28 days and 12 months AF MSC transplantation. BL, baseline; AF, after; N/A, not evaluated.
Eyes with severe KCS.
Table 5.
Scores of Short- and Long-Term Ocular Health: Vascularization
| Animal/Eye(s) | Vascularization BL | Vascularization 28 Days AF MSC Transplantation | Vascularization12 Months AF MSC Transplantation |
|---|---|---|---|
| 1L | − | − | − |
| 2R* | ++ | + | − |
| 3R | ++ | ++ | − |
| 4R | + | + | − |
| 4L | − | − | − |
| 5R* | − | − | − |
| 5L* | − | − | − |
| 6R | − | − | − |
| 6L | − | − | − |
| 7R | − | − | − |
| 7L* | + | + | − |
| 8R | ++ | ++ | ++ |
| 8L | ++ | ++ | + |
| 9R | − | − | N/A |
| 10R* | ++ | + | N/A |
| 11R | ++ | ++ | N/A |
| 12R* | + | + | N/A |
| 12L | − | − | N/A |
| 13R* | + | − | N/A |
| 13L | + | − | N/A |
| 14R* | − | − | N/A |
| 14L* | + | + | N/A |
| 15R | + | + | N/A |
| 15L | + | + | N/A |
Vascularization was graded as absent (−), mild (+), moderate (++), or severe (+++). The eye data were collected at BL and 28 days and 12 months AF MSC transplantation. BL, baseline; AF, after; N/A, not evaluated.
Eyes with severe KCS.
Table 6.
Effect of MSC Transplantation on Short-Term Tear Production
| Animal/Eye(s) | BL | STT AF MSC7 Days | STT AF MSC 14 Days | STT AF MSC 21 Days | STT AF MSC 28 Days |
|---|---|---|---|---|---|
| 1L | 6 | 13 | 14 | 18 | 23 |
| 2R* | 2 | 7 | 10 | 12 | 16 |
| 3R | 11 | 6 | 12 | 15 | 15 |
| 4R | 7 | 20 | 25 | 25 | 27 |
| 4L | 13 | 25 | 30 | 30 | 30 |
| 5R* | 2 | 9 | 9 | 9 | 8 |
| 5L* | 4 | 13 | 13 | 12 | 12 |
| 6R | 8 | 22 | 22 | 22 | 24 |
| 6L | 13 | 13 | 14 | 14 | 19 |
| 7R | 14 | 25 | 25 | 25 | 27 |
| 7L* | 3 | 8 | 8 | 10 | 8 |
| 8R | 12 | 15 | 14 | 14 | 15 |
| 8L | 9 | 14 | 12 | 13 | 15 |
| 9R | 13 | 18 | 17 | 18 | 20 |
| 10R* | 2 | 8 | 8 | 8 | 7 |
| 11R | 12 | 14 | 15 | 18 | 18 |
| 12R* | 0 | 0 | 2 | 0 | 0 |
| 12L | 12 | 14 | 15 | 20 | 20 |
| 13R* | 3 | 9 | 10 | 14 | 15 |
| 13L | 5 | 8 | 10 | 18 | 18 |
| 14R* | 3 | 3 | 14 | 12 | 4 |
| 14L* | 3 | 3 | 7 | 7 | 3 |
| 15R | 13 | 13 | 15 | 20 | 5 |
| 15L | 7 | 7 | 10 | 19 | 5 |
Schirmer tear test (STT; mm/min) was determined at BL and 7, 14, 21, and 28 days AF MSC transplantation. BL, baseline; AF, after.
Eyes with severe KCS; all other eyes had mild/moderate KCS.
A total of 24 eyes from 15 adult dogs of different sexes, mongrel, or mixed breeds, aged between 3 and 12 years, participated in this study (Table 1). Nine dogs (5, 6, and 9–15) were under conventional immunosuppressive treatment upon recruitment and underwent a washout period of 1 month before transplantation. Six dogs (1, 4, 6, 9, 10, and 15) were receiving artificial tears (sodium hyaluronate 0.2%; Pfizer, São Paulo, SP, Brazil) at the time of transplantation and were allowed to continue treatment.
Clinical Evaluation
Prior to enrollment, dogs received all essential clinical evaluations: physical and imaging evaluations (abdominal ultrasound and thorax X-ray radiology), and hematocrit and biochemical analyses, which were performed in order to exclude other systemic diseases. The STT values were recorded using commercial sterile test strips (Schering-Plough Animal Health, Kennilworth, NJ, USA) placed in the lower conjunctival fornix of each eye and maintained there for 1 min before readout. The presence of corneal ulcers was excluded using fluorescein staining (Fluoresceína Strips Ophthalmos, São Paulo, SP, Brazil).
Furthermore, ocular structures were evaluated by biomicroscopy (Reichert PSL portable slit lamp; Reichert Inc., Buffalo, NY, USA), indirect ophthalmoscopy (VOLK Panretinal lens; VolkOptical Inc., Mentor, OH, USA), and direct ophthalmoscopy (Panoptic 11820 Ophthalmoscope with Cobalt Blue Filter and Corneal Viewing Lens; Welch Allyn Inc., Skaneateles Falls, NY, USA).
A comprehensive physical and ophthalmologic evaluation with photographic documentation was performed before and after the implementation of MSCs. All examinations and data acquisition were executed by the same researcher.
We developed a clinical scoring system with respect to ocular symptoms. The symptoms evaluated were conjunctival hyperemia, ocular discharge, corneal pigmentation, and corneal vascularization, which were classified as normal (−), mildly affected (+), moderately affected (++), or severely affected (+++). These data are presented in Tables 2–5.
Adipose Tissue-Derived MSCs
A total of three female 6- to 12-month-old (two animals of 6 months and one of 12 months) healthy mongrel dogs were used to isolate adipose MSCs. Visceral (ovary fat) fat samples were collected during elective surgeries (surgery independent of the study). Before enrolment, dogs underwent routine clinical examination, hematologic evaluation (plasma proteins, red blood cells count, white blood cells count, platelet number and hemoglobin concentration), and viral screening.
After collection, fat fragments were transported in a cooler box, under strict control of temperature, in transport medium composed of Dulbecco’s modified Eagle’s medium high glucose (DMEM-H) and 500 U/ml streptomycin and 500 U/ml penicillin (Thermo Fisher Scientific Waltham, MA, USA)—the samples were processed within 2 h. Adipose tissue cells were isolated using a standard protocol based on fragmentation followed by collagenase IV digestion, following procedures described in Mambelli and coauthors27. The isolated cells were plated at 1 × 105 on 36-mm dishes (TPP, Trasadingen, Switzerland) with DMEM-H supplemented with 15% HyClone fetal bovine serum (Catalog No. SH30070-03; Logan, UT, USA), 100 U/ml streptomycin and 100 U/ml penicillin, 2 mM l-glutamine, and 1% nonessential amino acids (all Thermo Fisher Scientific), which is here designated as basal culture medium. The cultures were incubated at 37°C in a humidified atmosphere containing 5% CO2. After 4 to 7 days, cells were washed twice in phosphate-buffered saline (PBS; Gibco, Gaithersburg, MD, USA), dissociated with 0.25% trypsin (Thermo Fisher Scientific), and expanded in 75-cm2 culture flasks (TPP).
The stem cells isolated from each animal did not present any differences in MSC markers and differentiation potential. All lineages express the principal MSC markers as defined by the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy, such as cluster of differentiation 44 (CD44), CD73, CD90, CD105, vimentin, and nestin, and are negative for CD34, CD45, CD31, and Kruppel-like factor 4 (KLF4) based on immunofluorescence analysis (data not shown)27,28. The ability of the cells to differentiate into osteoblasts, adipocytes, and chondrocytes was also confirmed following the protocols of Dominici et al.28 (data not shown). The cells were screened for pathogens and contaminants (e.g., bacteria, fungi, virus, mycoplasma, and endotoxins), and no contamination was detected (data not shown). After characterization, cells were cryopreserved at 2 × 106 cells/ml in cryogenic medium [10% dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA), 40% fetal bovine serum, and DMEM-H] and maintained in liquid nitrogen. Three batches of adipose canine MSCs were generated for clinical use, and no differences were detected among the three MSC lineages; thus the lineages were used interchangeably.
Allogeneic MSC Transplantation in Dogs With KCS
Prior to MSC transplantation, animals were anesthetized with propofol (6 mg/kg; Cristália, São Paulo, SP, Brazil), followed by topical eye anesthesia using proxymetacaine hydrochloride drops (0.5%; Anestalcon; Alcon, São Paulo, SP, Brazil), and then both eyes and the surrounding skin were aseptically prepared. Cryopreserved MSCs were rapidly thawed (<2 min) in a 37°C water bath and washed with 5 ml of basal culture medium followed by centrifugation at 300 × g for 5 min. Afterward, cells were washed twice with 4 ml of PBS. One million MSCs were resuspended in 0.5 ml of 0.9% NaCl (Eurofarma, São Paulo, SP, Brazil), and this suspension was partially injected into the anatomic region of the dorsal LG (0.3 ml) using a 1-ml syringe with a 25-mm × 7-mm-gauge needle (Becton Dickinson, São Paulo, SP, Brazil). To access the dorsal LGs, the syringe was inserted through the conjunctival fornix of the superior eyelid, into the dorsolateral region of the ocular bulb, below the orbital ligament. The remaining 0.2 ml of cell suspension was injected into the third eyelid LG, which was accessed through the bulbar face of the third eyelid. LG inoculations followed procedures as described in Cabral et al.29 and Zwingenberger et al.30.
The only treatment allowed for animals in this study, besides the MSC transplantation itself, was the use of artificial tears (sodium hyaluronate 0.2%) in the cases of severe KCS, which is used in order to maintain animal comfort. This type of lubricant has topical and immediate action and does not interfere with tear production. The administration of the artificial tear occurred three times per day over the first 30 days after MSC transplantation.
Statistics
A total of 15 dogs and 24 eyes were used in this study. All dogs were evaluated up to 28 days (short-term evaluation), and a subset of 13 eyes (eight dogs) were evaluated at 6 and 12 months (long-term evaluation). Each eye was considered as an independent sample. No separate control group was used in this study. We used the initial [baseline (BL)] diseased parameter (clinical signs and STT values) of each eye as a matched control.
Statistical analyses regarding STT data included repeated-measures analysis of variance (ANOVA) (p < 0.05) test followed by comparisons with control (BL) using the Dunnett’s multiple comparison test (p < 0.05). All analyses were carried out using the Prism 7.0 software (GraphPad, San Diego, CA, USA).
Clinical data classified in ranks were analyzed by the nonparametric paired-group Wilcoxon signed-rank test using the “Social Science Statistics” calculator (http://www.socscistatistics.com/tests/signedranks/Default2.aspx, accessed on July 27 2016). Tests applied Z values when the result of the number of samples minus the number of ties was greater than 10; below this number of samples (as with corneal opacity), the W value was considered. Tests were performed for p < 0.01 (two tailed) for ocular discharge, hyperemia, and corneal opacity, and p < 0.05 (n = 6, this value is too low for calculations for p < 0.01) for vascularization.
RESULTS
Safety of Allogeneic MSC Transplantation
No side effects, such as ocular pain, inflammation, blepharospasm, photophobia, blinking, or epiphora, were observed with the eyes following MSC transplantation in the short term (7–28 days) or long term (6 and 12 months). No changes were detected with respect to appetite, fecal output, weight, or body temperature, and no allergic reaction was noticed.
Ocular Surface Changes
Prior to MSC transplantation, dogs presented ocular discharge, hyperemia, corneal opacity, and vascularization with varying scores (Figs. 1–3 and Tables 2–5). All eyes had some level of ocular discharge and hyperemia at BL, while some eyes presented vascularization (14 eyes) or corneal opacity (18 eyes).
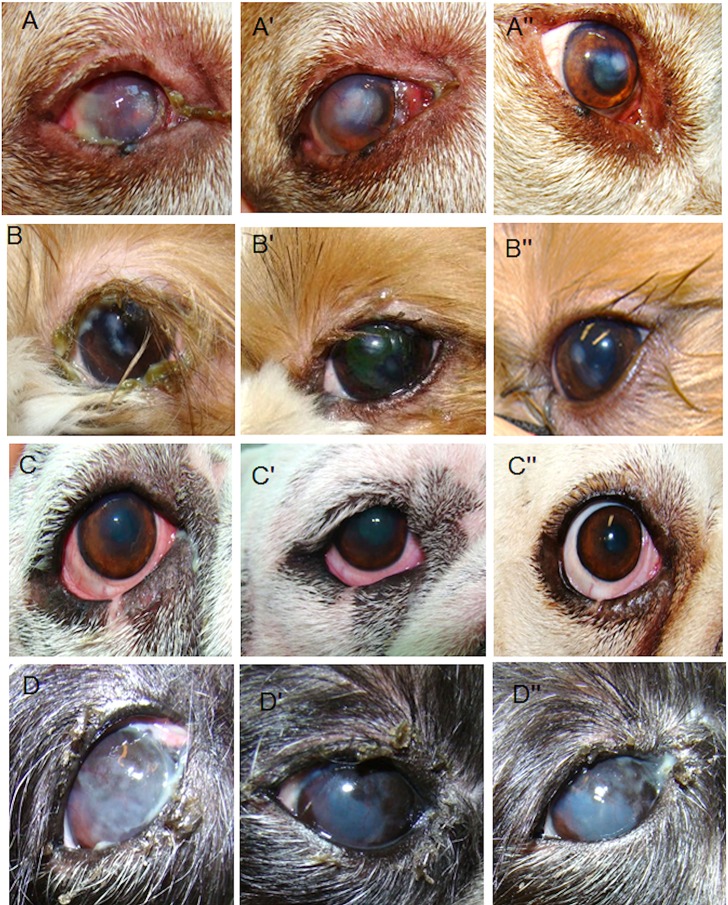
Figure 1.
Evaluation of the clinical signs at different times: baseline (A–D), short term (A′–D′; 28 days), and long-term (A″–D″; 12 months) after allogeneic mesenchymal stem cell (MSC) transplantation. Right eye dog number 2 (A, A″): clinical signs before MSC transplantation [baseline (BL)]: eye with severe keratoconjunctivitis sicca (KCS) and Schirmer tear tests (STTs) of 2 mm/min, severe ocular discharge (+++), hyperemia (+++), corneal opacity (++), and vascularization (++) (A). Twenty-eight days after MSC transplantation, an improvement was observed on ocular discharge (+), hyperemia (++), corneal opacity (+), and vascularization (+) (A′). The improvement was maintained for up to 12 months, with absence of ocular discharge (−), hyperemia (−), and corneal vascularization (−), although central corneal opacity was still present (+) (A″). Left eye dog number 1 (B, B″): clinical signs before MSC transplantation (BL): mild–moderate KCS and STT of 6 mm/min, severe ocular discharge (+++), moderate hyperemia (++), mild corneal opacity (+) (B), and absence of corneal vascularization (−). Same animal 28 days after MSC transplantation presented mild ocular discharge (+) and absence of hyperemia, although corneal opacity was still present (+) (B′). The improvement was maintained for up to 12 months after MSC transplantation, and absence of ocular discharge and hyperemia was reported, although corneal opacity was still present (+) (B″). Right eye dog number 4 (C, C″): at BL, the eye presented mild–moderate KCS and STT of 7 mm/min, ocular discharge (+), hyperemia (+++), corneal opacity (+), and vascularization (+) (C). Twenty-eight days after MSC transplantation, the eye had no ocular discharge (−); however, the other signs are still present (C′). A greater improvement was observed 12 months after MSC transplantation, when there was absence of all clinical signs (C″). Right eye dog number 8 (D, D″): clinical signs before MSC transplantation (BL): moderate KCS and STT of 12 mm/min, severe ocular discharge (+++), mild hyperemia (+), severe corneal opacity (+++), and moderate vascularization (++) (D). Twenty-eight days after MSC transplantation, there was an improvement in ocular discharge (+), hyperemia (−), and corneal opacity (++). However, vascularization did not improve (++) (D′). At the 12-month follow-up, improvement was maintained, with the absence of hyperemia (−), but ocular discharge (+), corneal opacity (++), and vascularization (++) still being present (D′).
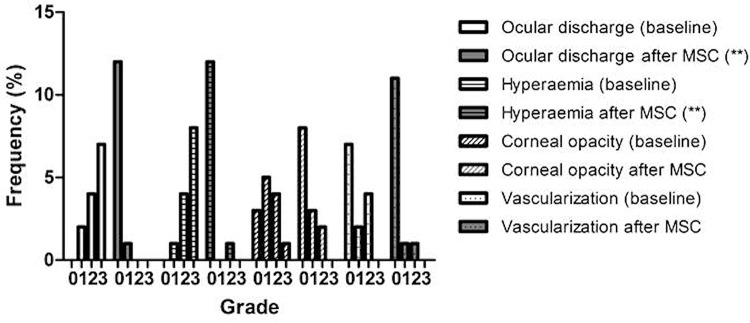
Figure 3.
Frequency of ocular health scores—data at BL and 1 year post-MSC transplantation. Ocular discharge, hyperemia, corneal opacity, and vascularization were graded as absent (0), mild (1), moderate (2), or severe (3). The data presented correspond to 11 animals that at BL were classified with mild–moderate KCS and 1 animal with severe KCS. The Wilcoxon matched pairs test was used to compare scores at BL and after treatment with MSCs; **p < 0.01. Although lower grade frequency increased overall with treatment and there were no regression or worsening, statistically significant improvements were only observed regarding ocular discharge and hyperemia. There was not enough statistical power to test improvements in corneal opacity and vascularization, though results show a trend toward improvement. @Insufficient data for statistical evaluation. BL, baseline; MSC, mesenchymal stem cell.
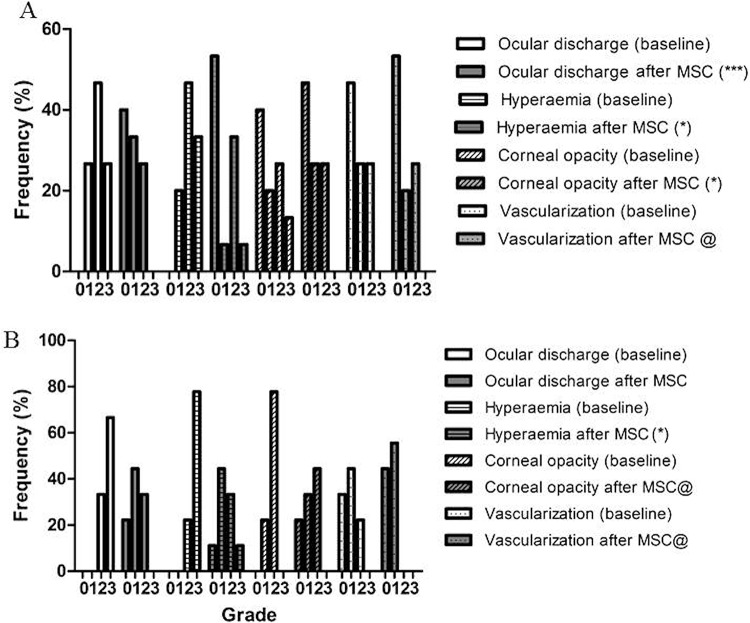
After allogeneic MSC transplantation, improvements were observed by day 28, as shown by the reduction in ocular discharge for all treated animals, compared to controls, and for group 1 (no statistical difference for group 2) (Figs. 1A′–D′ and 2 and Table 2), hyperemia (statistically different for groups 1 and 2) (Figs. 1A′, B′, and D′, and 2 and Table 3), and corneal opacity (statistically different when all treated animals were compared to controls) (Figs. 1A′–C′ and 2 and Table 4). There is not enough statistical power to determine the difference for groups 1 and 2 compared with controls. There were too many tied values to carry out a statistical test for corneal vascularization, but a trend toward improvement can be seen 28 days after transplantation (Figs. 1A′ and D′ and 2 and Table 5). A follow-up was carried out with 13 animals after 12 months, when it was observed that improvement was maintained, being significantly different, compared with BL values, regarding ocular discharge (Figs. 1A″–C″ and 3 and Table 2) and hyperemia (Figs. 1A″–C″ and 3 and Table 3). The statistical analysis was carried out with groups 1 and 2 combined. However, corneal opacity (Figs. 1A″, B″, and D″ and 3 and Table 4) and vascularization (Figs. 1A″, C″, and D″ and 3 and Table 5) were still present in the majority of animals, and there was not enough statistical power to carry out a test. A trend for improvement, however, can be observed for these two clinical symptoms.
Figure 2.
Frequency of ocular health scores—data at BL and a short time (28 days) post-MSC transplantation. Ocular discharge, hyperemia, corneal opacity, and vascularization were graded as absent (0), mild (1), moderate (2), or severe (3). Eyes were classified according to the severity of KCS at presentation into mild–moderate (A; n = 15, STT >4 mm/min) or severe (B; n = 9, STT ≤4 mm/min). The Wilcoxon matched pairs test was used to compare scores at BL and after treatment with MSCs; *p < 0.05 or **p < 0.01. Significant improvements were observed after MSC transplantation, especially regarding ocular discharge, hyperemia, and corneal opacity. @Insufficient data for statistical evaluation. BL, baseline; MSC, mesenchymal stem cell.
Table 3.
Scores of Short- and Long-Term Ocular Health: Hyperemia
| Animal/Eye(s) | Hyperemia BL | Hyperemia 28 Days AF MSC Transplantation | Hyperemia 12 Months AF MSC Transplantation |
|---|---|---|---|
| 1L | ++ | − | − |
| 2R* | +++ | ++ | − |
| 3R | +++ | ++ | − |
| 4R | +++ | +++ | − |
| 4L | ++ | + | − |
| 5R* | +++ | + | − |
| 5L* | +++ | + | − |
| 6R | ++ | − | − |
| 6L | ++ | − | − |
| 7R | +++ | − | − |
| 7L* | +++ | − | − |
| 8R | + | − | − |
| 8L | +++ | ++ | ++ |
| 9R | + | − | N/A |
| 10R* | +++ | + | N/A |
| 11R | ++ | ++ | N/A |
| 12R* | +++ | +++ | N/A |
| 12L | + | − | N/A |
| 13R* | ++ | + | N/A |
| 13L | +++ | − | N/A |
| 14R* | ++ | ++ | N/A |
| 14L* | +++ | ++ | N/A |
| 15R | ++ | ++ | N/A |
| 15L | ++ | ++ | N/A |
Hyperemia was graded as absent (−), mild (+), moderate (++), or severe (+++). The eye data were collected at BL and 28 days and 12 months AF MSC transplantation. BL, baseline; AF, after; N/A, not evaluated.
Eyes with severe KCS.
Table 4.
Scores of Short- and Long-Term Ocular Health: Corneal Opacity
| Animal/Eye(s) | Corneal Opacity BL | Corneal Opacity 28 Days AF MSC Transplantation | Corneal Opacity 12 Months AF MSC Transplantation |
|---|---|---|---|
| 1L | + | + | + |
| 2R* | ++ | + | + |
| 3R | ++ | + | + |
| 4R | + | + | − |
| 4L | + | − | − |
| 5R* | + | − | − |
| 5L* | + | − | − |
| 6R | − | − | − |
| 6L | − | − | − |
| 7R | − | − | − |
| 7L* | ++ | ++ | − |
| 8R | +++ | ++ | ++ |
| 8L | ++ | ++ | ++ |
| 9R | − | − | N/A |
| 10R* | ++ | + | N/A |
| 11R | +++ | ++ | N/A |
| 12R* | ++ | ++ | N/A |
| 12L | − | − | N/A |
| 13R* | ++ | ++ | N/A |
| 13L | − | − | N/A |
| 14R* | ++ | ++ | N/A |
| 14L* | ++ | + | N/A |
| 15R | ++ | ++ | N/A |
| 15L | ++ | + | N/A |
Corneal opacity was graded as absent (−), mild (+), moderate (++), or severe (+++). The eye data were collected at BL and 28 days and 12 months AF MSC transplantation. BL, baseline; AF, after; N/A, not evaluated.
Eyes with severe KCS.
STT Values Before and After MSC Transplantation
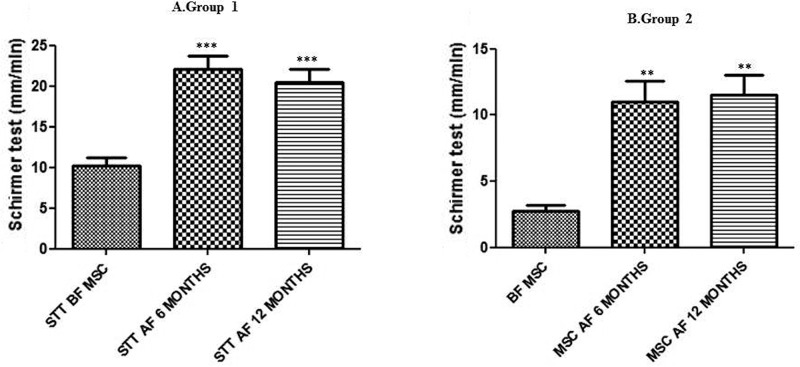
All eyes used in this study before MSC transplantation presented STT values lower than 15 mm/min (individual details presented in Table 6). The eyes were divided into two groups according to BL STT: group 1, mildly to moderately affected, and group 2, severely affected (Tables 6 and 7). Statistically significant increases in STT values were observed in both groups after MSC transplantation at 28 days (Fig. 4 and Table 6) and 6 and 12 months (Fig. 5 and Table 7).
Table 7.
Effect of MSC Transplantation on Long-Term Tear Production
| Animal/Eye(s) | STT BL | STT 6 Months AF MSC | STT 12 Months AF MSC |
|---|---|---|---|
| 1L | 6 | 25 | 23 |
| 2R* | 2 | 15 | 15 |
| 3R | 11 | 15 | 17 |
| 4R | 7 | 26 | 27 |
| 4L | 13 | 27 | 25 |
| 5R* | 2 | 9 | 10 |
| 5L* | 4 | 12 | 13 |
| 6R | 8 | 24 | 25 |
| 6L | 13 | 25 | 14 |
| 7R | 14 | 24 | 20 |
| 7L* | 3 | 8 | 8 |
| 8R | 12 | 15 | 15 |
| 8L | 9 | 18 | 18 |
Schirmer tear test (STT; mm/min) was determined at BL and 6 and 12 months AF MSC transplantation. BL, baseline; AF, after.
Eyes with severe KCS; all other eyes had mild/moderate KCS.
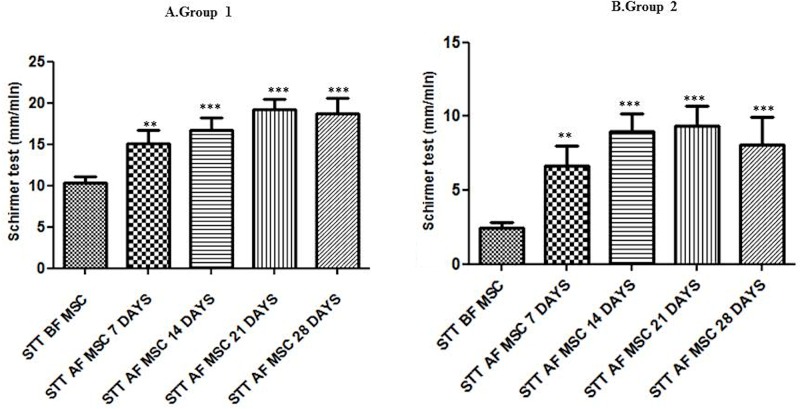
Figure 4.
Short-term effect of MSC transplantation on tear production (STT; mean ± standard error of the mean). STT was measured at BL and at different time points after MSC administration to dogs with mild–moderate KCS (group 1, n = 15) (A) and severe KCS (group 2, n = 9) (B). A marked increase in tear volume was observed after MSC administration in both groups. Asterisks indicate statistically significant (ANOVA) differences between BL (SST BF MSC) and post-MSC transplantation. **p < 0.01, ***p < 0.001. Both groups showed an improvement in tear production after MSC transplantation in all short-term follow-up evaluations. BL, baseline; MSC, mesenchymal stem cell; BF, before; AF, after.
Figure 5.
Long-term effect of MSC transplantation on tear production (STT; mean ± standard error of the mean). STT was determined 6 and 12 months after MSC administration to dogs with mild–moderate KCS (group 1, n = 9) (A) and severe KCS (group 2, n = 4) (B). A marked increase in tear volume was observed after MSC administration in both groups. Asterisks indicate statistically significant (ANOVA) differences between BL (SST BF MSC) and post-MSC transplantation. **p < 0.01, ***p < 0.001. Both groups showed an improvement in tear production 6 and 12 months after MSC transplantation, and in the case of group 1, normal STT levels were achieved with treatment. BL, baseline; MSC, mesenchymal stem cell; BF, before; AF, after.
At BL, groups 1 and 2 presented STT values (mean ± standard error of the mean in mm/min) of 10.33 ± 0.78 and 2.44 ± 0.38, respectively. One week after MSC transplantation, a significant improvement in STT values, compared to STT values before MSC transplantation, could be detected [group 1: 15.1 ± 1.5 (p < 0.01); group 2: 6.7 ± 1.3 (p < 0.01)]. Fourteen days after MSC transplantation, tear production increased in both groups, compared with controls [group 1: 16.67 ± 1.55 (p < 0.001); group 2: 9.00 ± 1.16 (p < 0.001)]. The improvement in STT values persisted until day 21 [group 1: 19.27 ± 1.21 (p < 0.001); group 2: 9.33 ± 1.38 (p < 0.001)]. After 28 days, a slight alteration in STT values was observed in both groups [group 1: 18.73 ± 1.87 (p < 0.001); group 2: 8.11 ± 1.80 (p < 0.001)]. The STT values were maintained in evaluations carried out 6 and 12 months after MSC transplantation. Group 1 STT levels at 6 months was 22.11 ± 1.58 mm/min and at 12 months was 20.44 ± 1.58 mm/min (p < 0.001) (Table 7 and Fig. 5)—both higher than 15 mm/min and therefore in the normal range. Regarding group 2, however, in spite of a significant improvement, the final STT values were still below 15 mm/min, reaching 11.00 ± 1.58 mm/min at 6 months and 11.50 ± 1.55 mm/min at 12 months (p < 0.01 for both) (Table 7 and Fig. 5).
DISCUSSION
This study was carried out in an effort to verify the short- and long-term clinical benefits of allogeneic MSC intralacrimal transplantation in dogs with unilateral or bilateral KCS, as well as to compare the outcome of transplantation on eyes with mild–moderate versus severe KCS, using a total of 24 eyes from 15 adult dogs. The study demonstrates that the allogeneic MSC transplantation procedure is well tolerated by dogs.
A 1-year follow-up did not reveal the occurrence of any type of pathology associated with abnormal tissue formation, as well as no tumor incidence or tissue rejection. Indeed, allogeneic MSC transplantation has been proven to be safe, regarding rejection, by many previous studies, not requiring the use of immunosuppressant drugs20,31–33. This is advantageous, since it is possible to produce large batches of standardized cells from animal donors, as described here, and transplant them into patients without having to check for compatibility. Thus, our data suggest that MSC treatment of KSC in dogs is a safe procedure and free of adverse effects.
A statistically significant increase in tear production, which reached normal STT values, was detected in the eyes of dogs with mild–moderate KCS after MSC transplantation, during a short period (7 days) of follow-up. Such effect improved over time (14 to 28 days, 6 and 12 months). In severely affected dogs, although STT values demonstrated increased tear production after MSC transplantation, on average, normal STT values were not achieved in either the short or long term. However, the majority of the eyes originally with severe KCS showed constant improvement even at long-term follow-ups, and in one case, the STT reached normal (>15 mm/min) levels. In contrast, one severely affected eye did not respond to the single MSC transplantation, probably due to the fact that the LG could have been fibrotic at the time of transplantation and not have enough viable cells and tissue for recovery.
Because of safety and financial implications, it is desirable that the minimal number of applications, as well as the lowest quantity of MSCs, be used for treatment. Some inconvenience may also occur, on the other hand, with the use of high doses of MSCs. Previously, it has been reported that a high dose of MSCs may be associated with cell clumping forming aggregates, especially when passed through a narrow needle, and these aggregates can cause pulmonary emboli or infarctions after the systemic application of MSCs34,35. Furthermore, it has been demonstrated that multiple administrations of high doses of allogeneic MSCs affect alloreactive immune responses in recipient baboons36. For these reasons, we decided to use a low dose of MSCs (1 × 106). We showed that clinical amelioration occurs after a single transplantation of low quantities (1 × 106) of MSCs both in dogs with mild–moderate and severe KCS. In a previously published study, 12 dogs (24 eyes) were used, among which 10 eyes presented severe KCS. Notably, three eyes did not present a significant increase in STT values even after transplantation of a high amount of allogeneic MSCs (1 × 108), which corresponds to two orders of magnitude higher than the dose we used in the present study20. Based on our results that showed an improvement, but not total reversal, of severe KCS upon a single-dose transplantation, we believe that the prescription of multiple doses of MSCs could help these animals even further. However, it must be pointed out that mild congestion may occur after multiple MSC intralacrimal injections, even at low cell doses (2 × 106) in normal dogs21.
KCS, especially severe, besides being associated with a decrease in STT values, also presents signs of keratitis (including infiltration of inflammatory cells, vascularization, pigmentation, and corneal thickening) and intense mucoid to mucopurulent discharge2,37,38. Following allogeneic MSC transplantation, clinical improvements in conjunctivitis and ocular discharge were registered in the majority of eyes. However, the improvements related to corneal transparency and decreased corneal vascularization were less evident. Such symptoms are related with disease severity, and their improvement is expected to require more time10,39. There is evidence, however, that reversal of corneal transparency is possible upon stem cell transplantation. For instance, in contrast, human immature dental pulp stem cell transplantation was capable of restoring corneal transparency in a rabbit model of total limbal stem cell deficiency40.
One of the major issues in MSC therapy is the choice of the administration route41. Previously, MSCs were transplanted using periocular or subconjunctival routes in dogs and mice with KCS20–22,42,43. The periocular route is associated with low numbers or an absence of MSCs engrafted into LGs22,42,43, as well as mild transient conjunctival congestion after MSC transplantation21. In our study, we performed direct transplantation of MSCs into LGs of dogs with KCS, a procedure that was shown to be safe and effective, despite its apparently more invasive character.
The exact etiology of KCS is unknown, but it is believed to be multifactorial44. KCS is usually treated with immunosuppressive drugs, such as cyclosporine or tacrolimus10,45,46. Satisfactory results after topical application of these drugs have been found, especially for severe KCS. However, normal amounts of tear production were not achieved in these studies, and such posttreatment tear levels were inferior to those observed after MSC transplantation, as previously reported20 and as reported in the present work. Immunosuppressive medication must be administered two to four times per day in the long term, while MSCs present superior effects after a unique transplantation, for long periods, as shown by their effect at the 1-year follow-up47,48. It is noteworthy that the clinical improvements observed in the present study can be attributed to the MSC transplantation alone, given that the administration of immunosuppressive drugs was suspended in all dogs 1 month before transplantation. Similar to immunosuppressive drugs, MSCs act on inflammation and on immune-mediated local responses. MSCs are also used in human clinical trials for the treatment of inflammatory conditions49. These cells modulate inflammation by decreasing immune cell number and products of the inflammatory response16,50–53. Additionally, they are able to remodel tissue damage induced by excessive inflammation, acting through multiple trophic mechanisms12,41,42,54,55. The dry eye syndrome model has a similar disease manifestation as KCS. In this disease, which has a pathogenesis associated with the presence of T cells (CD4+)1,12, it has been shown that MSC transplantation decreases the number of interferon-γ (IFN-γ)-secreting CD4+ cells in vivo and suppresses CD4+ cell proliferation and IFN-γ+CD4+ cell differentiation in vitro56. The mechanism by way of which MSCs inhibit T cells is uncertain, but there are data that show that MSCs inhibit T cells by inducing regulatory T cells or by inhibiting tryptophan metabolism via indoleamine 2,3-dioxygenase57,58. We believe that the same immunomodulatory and immunosuppressive mechanism observed in the dry eye syndrome may explain how MSC transplantation improves KCS as found in our study.
The present study was carried out with dog patients taken for treatment to a standard ophthalmologic veterinary clinic. This sampling method, in opposition to studies designed with animal facility-derived animals and standardized-induced diseases, includes the variability and heterogeneity of “real-world” dogs with KCS. The fact that we have found improvements upon treatment, in spite of the use of animals of different ages, genders, races, and disease etiologies, is a strong indication that the protocol will be successful in other settings. However, some limitations were found during the course of the study, such as the fact that owners may have submitted animals to medical treatment to reduce pain and suffering, and the difficulty following up all dogs over long periods (6–12 months), which depended on the owner’s cooperation. Moreover, this study did not include a group of nontreated or placebo-treated animals, as it was not considered an ethical procedure. However, inclusion criteria encompassed the need for animals to have had KCS for at least 1 year, and statistics were done using the initial diseased eyes as a matched control. KCS is a chronic condition that rarely reverts spontaneously. Most studies with dogs have no untreated control groups2,10,20,46,48,59 and mouse and rabbit models, which do include nontreated or vehicle-only treated controls, show that the condition does not resolve itself spontaneously either regarding STT or other clinical symptoms60–63.
Overall, our study shows that MSC transplantation is a safe and effective treatment, especially for mild–moderate KCS in dogs, and does not require lifelong medical care or diligent attention and monitoring. We believe that the costs of this treatment will be lower for dog owners, compared to immunosuppressive treatment, since the MSC effect is maintained for at least 1 year. We show that MSC transplantation can also be used to treat dogs with mild and severe KCS, although the dogs with severe KCS had only partial improvement in STT levels. Further studies using serial MSC transplantations may prove to be more successful for the treatment of these severe cases of KCS. In addition, we believe that future studies should be evaluated over longer periods than the 1 year described here, since even after receiving a single MSC administration, the beneficial effects on tear production may take longer to be observed. We believe that although this study was carried out in dogs, it will prove to be useful in the development of treatments in human dry eye conditions, especially since the dogs studied came from a varied background, with KCS having developed spontaneously, representing real clinical conditions. Additionally, these results provide information that can be applied to human dry eye studies. Dry eye syndrome requires a multipronged approach including tear conservation and tear replacement through methods such as the punctal plug procedure, use of anti-inflammatory drugs, and surgery. The advantage of the use of MSC transplantation in KCS, as shown in this study, is the requirement of a single intervention with results lasting long periods (at least 1 year), which leads to a higher patient quality of life and also reduces treatment cost.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
REFERENCES
- 1. Williams DL. Immunopathogenesis of keratoconjunctivitis sicca in the dog. Vet Clin North Am Small Anim Pract. 2008;38(2):251–68. [DOI] [PubMed] [Google Scholar]
- 2. Moore C. Diseases and surgery of the lacrimal secretory system. In: Gelatt K, editor. Veterinary ophthalmology, 3rd ed. Philadelphia (PA): Lippincott Williams & Wilkins; 1999. p. 583–608. [Google Scholar]
- 3. Giuliano EA, Moore CP. Disease and surgery of the lacrimal secretory system. In: Gelatt KN, editor. Veterinary ophthalmology, 4th ed. Ames (IA): Wiley-Blackwell; 2007. p. 633–61. [Google Scholar]
- 4. Aguirre GD, Rubin LF, Harvey CE. Keratoconjunctivitis sicca in dogs. J Am Vet Med Assoc. 1971;158(9):1566–79. [PubMed] [Google Scholar]
- 5. Fullard RJ, Kaswan RM, Bounous DI, Hirsh SG. Tear protein profiles vs. clinical characteristics of untreated and cyclosporine-treated canine KCS. J Am Optom Assoc. 1995;66(7):397–404. [PubMed] [Google Scholar]
- 6. Barabino S, Chen Y, Chauhan S, Dana R. Ocular surface immunity: Homeostatic mechanisms and their disruption in dry eye disease. Prog Retin Eye Res. 2012;31(3):271–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cullen CL, Ihle SL, Webb AA, McCarville C. Keratoconjunctival effects of diabetes mellitus in dogs. Vet Ophthalmol. 2005;8(4):215–24. [DOI] [PubMed] [Google Scholar]
- 8. Berger SL, Scagliotti RH, Lund EM. A quantitative study of the effects of Tribrissen on canine tear production. J Am Anim Hosp Assoc. 1995;31(3):236–41. [DOI] [PubMed] [Google Scholar]
- 9. Carter R, Colitz CMH. The causes, diagnosis, and treatment of canine keratoconjunctivitis sicca. Vet Med. 2002;97(9):683–94. [Google Scholar]
- 10. Hendrix DV, Adkins EA, Ward DA, Stuffle J, Skorobohach B. An investigation comparing the efficacy of topical ocular application of tacrolimus and cyclosporine in dogs. Vet Med Int. 2011;2011:487592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beyer Nardi N, da Silva Meirelles L. Mesenchymal stem cells: Isolation, in vitro expansion and characterization. Handb Exp Pharmacol. 2006(174):249–82. [PubMed] [Google Scholar]
- 12. Stern ME, Schaumburg CS, Pflugfelder SC. Dry eye as a mucosal autoimmune disease. Int Rev Immunol. 2013;32(1):19–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gonzalez MA, Gonzalez-Rey E, Rico L, Buscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology 2009;136(3):978–89. [DOI] [PubMed] [Google Scholar]
- 14. Rafei M, Campeau PM, Aguilar-Mahecha A, Buchanan M, Williams P, Birman E, Yuan S, Young YK, Boivin MN, Forner K, Basik M, Galipeau J. Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner. J Immunol. 2009;182(10):5994–6002. [DOI] [PubMed] [Google Scholar]
- 15. Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood 2005;106(5):1755–61. [DOI] [PubMed] [Google Scholar]
- 16. Hoogduijn MJ, Popp F, Verbeek R, Masoodi M, Nicolaou A, Baan C, Dahlke MH. The immunomodulatory properties of mesenchymal stem cells and their use for immunotherapy. Int Immunopharmacol. 2010;10(12):1496–500. [DOI] [PubMed] [Google Scholar]
- 17. Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30(1):42–8. [DOI] [PubMed] [Google Scholar]
- 18. Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm. 2005;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Villatoro AJ, Fernandez V, Claros S, Rico-Llanos GA, Becerra J, Andrades JA. Use of adipose-derived mesenchymal stem cells in keratoconjunctivitis sicca in a canine model. Biomed Res Int. 2015;2015:527926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Park SA, Reilly CM, Wood JA, Chung DJ, Carrade DD, Deremer SL, Seraphin RL, Clark KC, Zwingenberger AL, Borjesson DL, Hayashi K, Russell P, Murphy CJ. Safety and immunomodulatory effects of allogeneic canine adipose-derived mesenchymal stromal cells transplanted into the region of the lacrimal gland, the gland of the third eyelid and the knee joint. Cytotherapy 2013;15(12):1498–510. [DOI] [PubMed] [Google Scholar]
- 22. Wood JA, Chung DJ, Park SA, Zwingenberger AL, Reilly CM, Ly I, Walker NJ, Vernau W, Hayashi K, Wisner ER, Cannon MS, Kass PH, Cherry SR, Borjesson DL, Russell P, Murphy CJ. Periocular and intra-articular injection of canine adipose-derived mesenchymal stem cells: An in vivo imaging and migration study. J Ocul Pharmacol Ther. 2012;28(3):307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brown DC. Control of selection bias in parallel-group controlled clinical trials in dogs and cats: 97 trials (2000–2005). J Am Vet Med Assoc. 2006;229(6):990–3. [DOI] [PubMed] [Google Scholar]
- 24. Snyder PW. Síndrome de Sjögren. In: Tilley LP, Smith FWK, editors. Consulta veterinária em 5 minutos. São Paulo, Brazil: Manole; 2003. p. 1198–9. [Google Scholar]
- 25. Smith RE. The tear film complex: Pathogenesis and emerging therapies for dry eyes. Cornea 2005;24(1):1–7. [DOI] [PubMed] [Google Scholar]
- 26. Volk SW, Theoret C. Translating stem cell therapies: The role of companion animals in regenerative medicine. Wound Repair Regen. 2013;21(3):382–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mambelli LI, Santos EJ, Frazão PJ, Chaparro MB, Kerkis A, Zoppa AL, Kerkis I. Characterization of equine adipose tissue-derived progenitor cells before and after cryopreservation. Tissue Eng Part C Methods 2009;15(1):87–94. [DOI] [PubMed] [Google Scholar]
- 28. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8(4):315–7. [DOI] [PubMed] [Google Scholar]
- 29. Cabral VP, Laus JL, Dagli MLZ, Pereira GT, Talieri IC, Monteiro ER, Mamede FV. Canine lacrimal and third eyelid superficial glands’ macroscopic and morphometric characteristics Aspectos macroscópicos e morfométricos das glândulas lacrimal e superficial da terceira pálpebra de cães (Canis familiares; LINNAEUS, 1758). Ciência Rural 2005;35(2):391–7. [Google Scholar]
- 30. Zwingenberger AL, Park SA, Murphy CJ. Computed tomographic imaging characteristics of the normal canine lacrimal glands. BMC Vet Res. 2014;10:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reinders ME, Dreyer GJ, Bank JR, Roelofs H, Heidt S, Roelen DL, Zandvliet ML, Huurman VA, Fibbe WE, van Kooten C, Claas FH, Rabelink TJ, de Fijter JW. Safety of allogeneic bone marrow derived mesenchymal stromal cell therapy in renal transplant recipients: The neptune study. J Transl Med. 2015;13:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Togel F, Cohen A, Zhang P, Yang Y, Hu Z, Westenfelder C. Autologous and allogeneic marrow stromal cells are safe and effective for the treatment of acute kidney injury. Stem Cells Dev. 2009;18(3):475–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005;105(4):1815–22. [DOI] [PubMed] [Google Scholar]
- 34. Prockop DJ, Olson SD. Clinical trials with adult stem/progenitor cells for tissue repair: Let’s not overlook some essential precautions. Blood 2007;109(8):3147–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grigg A, Gibson R, Bardy P, Szer J. Acute portal vein thrombosis after autologous stem cell transplantation. Bone Marrow Transplant. 1996;18(5):949–53. [PubMed] [Google Scholar]
- 36. Beggs KJ, Lyubimov A, Borneman JN, Bartholomew A, Moseley A, Dodds R, Archambault MP, Smith AK, McIntosh KR. Immunologic consequences of multiple, high-dose administration of allogeneic mesenchymal stem cells to baboons. Cell Transplant. 2006;15(8–9):711–21. [DOI] [PubMed] [Google Scholar]
- 37. Moore PA. Diagnosis and management of chronic corneal epithelial defects (indolent corneal ulcerations). Clin Tech Small Anim Pract. 2003;18(3):168–77. [DOI] [PubMed] [Google Scholar]
- 38. Montes-Mico R, Caliz A, Alio JL. Changes in ocular aberrations after instillation of artificial tears in dry-eye patients. J Cataract Refract Surg. 2004;30(8):1649–52. [DOI] [PubMed] [Google Scholar]
- 39. Quimby JM, Webb TL, Habenicht LM, Dow SW. Safety and efficacy of intravenous infusion of allogeneic cryopreserved mesenchymal stem cells for treatment of chronic kidney disease in cats: Results of three sequential pilot studies. Stem Cell Res Ther. 2013;4(2):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gomes JA, Geraldes Monteiro B, Melo GB, Smith RL, Cavenaghi Pereira da Silva M, Lizier NF, Kerkis A, Cerruti H, Kerkis I. Corneal reconstruction with tissue-engineered cell sheets composed of human immature dental pulp stem cells. Invest Ophthalmol Vis Sci. 2010;51(3):1408–14. [DOI] [PubMed] [Google Scholar]
- 41. Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103(11):1204–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Beyazyildiz E, Pinarli FA, Beyazyildiz O, Hekimoglu ER, Acar U, Demir MN, Albayrak A, Kaymaz F, Sobaci G, Delibasi T. Efficacy of topical mesenchymal stem cell therapy in the treatment of experimental dry eye syndrome model. Stem Cells Int. 2014;2014:250230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee HK, Finniss S, Cazacu S, Xiang C, Brodie C. Mesenchymal stem cells deliver exogenous miRNAs to neural cells and induce their differentiation and glutamate transporter expression. Stem Cells Dev. 2014;23(23):2851–61. [DOI] [PubMed] [Google Scholar]
- 44. Gao J, Schwalb TA, Addeo JV, Ghosn CR, Stern ME. The role of apoptosis in the pathogenesis of canine keratoconjunctivitis sicca: The effect of topical cyclosporin A therapy. Cornea 1998;17(6):654–63. [DOI] [PubMed] [Google Scholar]
- 45. Kaswan RL, Salisbury MA. A new perspective on canine keratoconjunctivitis sicca—Treatment with ophthalmic cyclosporine. Vet Clin North Am Small Anim Pract. 1990;20(3):583–613. [DOI] [PubMed] [Google Scholar]
- 46. Ofri R, Lambrou GN, Allgoewer I, Graenitz U, Pena TM, Spiess BM, Latour E. Clinical evaluation of pimecrolimus eye drops for treatment of canine keratoconjunctivitis sicca: A comparison with cyclosporine A. Vet J. 2009;179(1):70–7. [DOI] [PubMed] [Google Scholar]
- 47. Zhou XQ, Wei RL. Topical cyclosporine A in the treatment of dry eye: A systematic review and meta-analysis. Cornea 2014;33(7):760–7. [DOI] [PubMed] [Google Scholar]
- 48. Barachetti L, Rampazzo A, Mortellaro CM, Scevola S, Gilger BC. Use of episcleral cyclosporine implants in dogs with keratoconjunctivitis sicca: Pilot study. Vet Ophthalmol. 2015;18(3):234–41. [DOI] [PubMed] [Google Scholar]
- 49. Newman RE, Yoo D, LeRoux MA, Danilkovitch-Miagkova A. Treatment of inflammatory diseases with mesenchymal stem cells. Inflamm Allergy Drug Targets 2009;8(2):110–23. [DOI] [PubMed] [Google Scholar]
- 50. Le Blanc K, Ringden O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262(5):509–25. [DOI] [PubMed] [Google Scholar]
- 51. Uccelli A, Pistoia V, Moretta L. Mesenchymal stem cells: A new strategy for immunosuppression? Trends Immunol. 2007;28(5):219–26. [DOI] [PubMed] [Google Scholar]
- 52. Glenn JD, Whartenby KA. Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World J Stem Cells 2014;6(5):526–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Griffin MD, Ritter T, Mahon BP. Immunological aspects of allogeneic mesenchymal stem cell therapies. Hum Gene Ther. 2010;21(12):1641–55. [DOI] [PubMed] [Google Scholar]
- 54. Prasad VK, Lucas KG, Kleiner GI, Talano JA, Jacobsohn D, Broadwater G, Monroy R, Kurtzberg J. Efficacy and safety of ex vivo cultured adult human mesenchymal stem cells (Prochymal) in pediatric patients with severe refractory acute graft-versus-host disease in a compassionate use study. Biol Blood Marrow Transplant. 2011;17(4):534–41. [DOI] [PubMed] [Google Scholar]
- 55. Zoukhri D. Mechanisms involved in injury and repair of the murine lacrimal gland: Role of programmed cell death and mesenchymal stem cells. Ocul Surf. 2010;8(2):60–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Weng J, He C, Lai P, Luo C, Guo R, Wu S, Geng S, Xiangpeng A, Liu X, Du X. Mesenchymal stromal cells treatment attenuates dry eye in patients with chronic graft-versus-host disease. Mol Ther. 2012;20(12):2347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Prevosto C, Zancolli M, Canevali P, Zocchi MR, Poggi A. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica 2007;92(7):881–8. [DOI] [PubMed] [Google Scholar]
- 58. Ren G, Su J, Zhang L, Zhao X, Ling W, L’Huillie A, Zhang J, Lu Y, Roberts AI, Ji W, Zhang H, Rabson AB, Shi Y. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells 2009;27(8):1954–62. [DOI] [PubMed] [Google Scholar]
- 59. Nell B, Walde I, Billich A, Vit P, Meingassner JG. The effect of topical pimecrolimus on keratoconjunctivitis sicca and chronic superficial keratitis in dogs: Results from an exploratory study. Vet Ophthalmol. 2005;8(1):39–46. [DOI] [PubMed] [Google Scholar]
- 60. Vijmasi T, Chen FY, Chen YT, Gallup M, McNamara N. Topical administration of interleukin-1 receptor antagonist as a therapy for aqueous-deficient dry eye in autoimmune disease. Mol Vis. 2013;19:1957–65. [PMC free article] [PubMed] [Google Scholar]
- 61. Quinto GG, Castro-Combs J, Li L, Gupta N, Campos M, Behrens A. Outcomes of different concentrations of human amniotic fluid in a keratoconjunctivitis sicca-induced mouse model. Int Ophthalmol. 2016;36(5):643–50. [DOI] [PubMed] [Google Scholar]
- 62. Gilbard JP. Treatment of keratoconjunctivitis sicca in rabbits with 3-isobutyl-1-methylxanthine. Arch Ophthalmol. 1994;112(12):1614–6. [DOI] [PubMed] [Google Scholar]
- 63. Yingfang F, Zhuang B, Wang C, Xu X, Xu W, Lv Z. Pimecrolimus micelle exhibits excellent therapeutic effect for keratoconjunctivitis sicca. Colloids Surf B Biointerfaces 2016;140:1–10. [DOI] [PubMed] [Google Scholar]