Abstract
Innovative treatments to cure type 1 diabetes are being actively researched. Among the different strategies, the replacement of β-cells has given promising results. Classically, islets from cadaveric donors are transplanted into diabetic patients, but recently phase I clinical trials that use stem cell-derived β-cells have been started. Such protocols require either an immunosuppressive treatment or the macroencapsulation of the β-cells. They involve cell aggregation and the exposure of the cells to hypoxia. Using an engineered human β-cell, we have addressed these two problems: a novel human β-cell line called EndoC-βH3 was cultured as single cells or aggregated clusters. EndoC-βH3 cells were also cultured at normal atmospheric oxygen tension (pO2 = 21%) or hypoxia (pO2 = 3%) in the presence or absence of modulators of the hypoxia-inducible factor 1α (HIF1α) pathway. Cell aggregation improved glucose-stimulated insulin secretion, demonstrating the benefit of cell–cell contacts. Low oxygen tension decreased β-cell viability and their sensitivity to glucose, but did not alter insulin production nor the insulin secretion capacity of the remaining cells. To investigate the role of HIF1α, we first used a HIF stabilizer at pO2 = 21%. This led to a mild decrease in cell viability, impaired glucose sensitivity, and altered insulin secretion. Finally, we used a HIF inhibitor on EndoC-βH3 pseudoislets exposed to hypoxia. Such treatment considerably decreased cell viability. In conclusion, aggregation of the EndoC-βH3 cells seems to be important to improve their function. A fraction of the EndoC-βH3 cells are resistant to hypoxia, depending on the level of activity of HIF1α. Thus, these cells represent a good human cell model for future investigations on islet cell transplantation analysis.
Key words: β-Cells, Hypoxia, Pseudoislets (PIs), HIF1α, Insulin secretion
INTRODUCTION
Over the last decade, considerable efforts have been made to improve methods to transplant islets from human donors into type 1 diabetic (T1D) patients. In such protocols, human islets can restore normoglycemia in T1D recipients1,2. However, two main parameters limit the feasibility of a large-scale application of this method. First, there is a low availability of transplantable human islets3. Second, studies in rodents and humans indicate that during isolation and transplantation, a significant percentage of islets are lost. One prominent explanation is that β-cells undergo severe cellular hypoxia during the procedure.
In order to better understand and improve long-term islet transplantation efficacy, new models that use human β-cells are necessary. In our laboratory, several genetically engineered human pancreatic β-cell lines have been produced that exhibit glucose-inducible insulin secretion4,5. More recently, we have generated a novel human β-cell line derived from EndoC-βH2 cells5 that contains floxed immortalizing transgenes and an integrated tamoxifen (TAM)-inducible form of CRE recombinase6. Such lines can be massively amplified and then have the immortalizing transgenes removed by simple addition of TAM, giving rise to nonproliferating functional human β-cells. These newly produced cells potentially represent a preclinical model for cell replacement therapy in diabetes. In comparison to stem cell-derived β-cells, or human and pig islets, our cells have the advantage of being efficiently amplified with a simple, direct, and fast protocol. Such a method allows for the generation of an unlimited quantity of homogenous human β-cells. Moreover, the use of EndoC-βH3 cells avoids some limitations associated with human islets. The advantages include a lower cost, a higher homogeneity and purity, and increased reproducibility in their quality. For these reasons, we have focused our work on these cells to determine their properties in an in vitro model that mimics the conditions of macroencapsulation and transplantation. The macroencapsulation process involves aggregation and hypoxia; so to analyze the effects of macroencapsulation on EndoC-βH3 cells, two main questions have to be addressed. First, it is important to delineate how cell aggregation and a resulting 3D environment will affect the function of β-cells and, second, the consequences of hypoxia on such cells.
Our previous experience with this new cell line was limited to morphological studies and functional analysis of single-cell structures6. Recently, several studies highlighted the importance of cell–cell contacts in rodent and human β-cells. Indeed, communication between β-cells suppressed basal insulin secretion but enhanced glucose-stimulated insulin secretion (GSIS), thereby facilitating the glucose homeostasis function of the islets7–10. Moreover, the size of rat islets was shown to be a determinant for the success rate of islets transplantation, as small rat islets were shown to be superior when compared to large islets during in vitro functional tests and in transplantation outcome studies11. Therefore, it is important to study first the in vitro consequence of EndoC-βH3 aggregation on the insulin content and secretion after a glucose challenge.
In the pancreas, the islets represent only 2% of the whole organ, but they use more than 10% of the oxygen supply, as they are normally highly vascularized. Within native pancreatic islets, the mean oxygen tension (pO2) is near 5% (37 mmHg)12. When they are transplanted in the liver, the pO2 drops to 0.6–1.3%. In a bioartificial pancreas, the pO2 in a macroencapsulation device is between 2% and 3%13,14. We have recently shown that the pO2 levels have an important role not only on rodent β-cell differentiation15 but also on β-cell function16–18. Thus investigating the effects of hypoxia in human β-cells is also a prerequisite for basic and preclinical studies. Therefore, the second objective of our research was to study the effect of decreased oxygen tension on insulin secretion and gene profile of our β-cell line organized in pseudoislets (PIs).
The cellular response to hypoxia is tightly controlled by the hypoxia-inducible factor (HIF) complex, which is regulated in an O2-dependent manner. In the presence of oxygen, HIF1α is degraded by the proteasome. On the other hand, during a hypoxic state, HIF1α is stabilized15. Genetic manipulations in mice have shown that HIF1α is involved in GSIS. Despite these recent progresses in rodent models, little is known about the role of the HIF pathway in human β-cells.
In this study, the EndoC-βH3 cells were first aggregated in clusters to favor cellular interactions and to mimic the islet architecture. Insulin expression and GSIS were increased in the aggregates versus isolated cells, demonstrating the positive effect of cell–cell contacts. Next we analyzed the effects of hypoxia (pO2 = 3%) compared with 21% ambient oxygen on such β-cell clusters. Cell survival was decreased during hypoxia, but the insulin content was unaltered. The sensitivity of the remaining β-cells to glucose was reduced, but surprisingly, their capacity to secrete insulin at high glucose levels was preserved. As expected, the HIF pathway was activated during hypoxic conditions. To delineate the importance of the HIF transcription factor in the EndoC-βH3 cell biology, we used both a HIF stabilizer at normal atmosphere and a HIF inhibitor during hypoxia. HIF stabilization induced by dimethyloxalylglycine (DMOG) decreased both insulin sensitivity and insulin secretion capacity. Finally, decreasing HIF during hypoxia considerably altered cell survival.
Altogether, we have shown that excised nonproliferating β-cells form aggregates and continue to produce insulin when cultured in a 3D structure. The cells that survive hypoxic conditions have a certain degree of resistance to low oxygen tension and maintain insulin production. With these important properties in mind, we believe that these cells offer an important preclinical model for β-cell transplantation.
MATERIALS AND METHODS
EndoC-βH3
This is a new conditionally immortalized human β-cell line, of male origin, in which the immortalizing transgene can be efficiently removed by simple addition of TAM (Sigma-Aldrich, Saint-Quentin-Fallavier, France)6.
Aggregates
EndoC-βH3 cells were seeded at 1.5 million/ml of complete medium6 in 2-ml Eppendorf tubes (Dutscher, Brumath, France). Cells were exposed to microgravity for 5–6 h at room temperature at 10−4 × g using a 2D clinostat (Dutscher)19 and then cultured overnight in 6-cm petri dishes (TPP, Trasadingen, Switzerland) at 37°C in the incubator (Thermo Fisher Scientific, Villebon sur Yvette, France). At day 1, aggregates were diluted (½) and cultured in 6-cm petri dishes (TPP) for 4 days.
Cell Culture and Hypoxia Treatment
EndoC-βH3 cells were cultivated as previously described6. Hypoxic cell cultures were performed in a hypoxia chamber (Cell Technology, Grenoble, France) at 1%, 3% O2, 5% CO2, 37°C. The HIF signaling inhibitor chetomin (Tocris, Lille, France) in dimethyl sulfoxide (DMSO) was added at 150 nM in the culture medium. DMOG (Interchim, Montluçon, France) was used at 1 mM. For the detection of hypoxia, pimonidazole (200 μM; Hypoxyprobe; NPI, Burlington, MA, USA) was added in the culture medium during the last 2 h of the culture period. Next, the pimonidazole staining was detected using a specific antibody (Hypoxyprobe; NPI).
RNA Isolation, Reverse Transcription, and RT-PCR
Total RNA was isolated from EndoC-βH3 monolayer cells or EndoC-βH3 PIs using the RNeasy Mini or Micro Kit (Qiagen, Courtaboeuf, France), as described previously15. First-strand cDNA was prepared using SuperScript II reagents (Invitrogen, Villebon sur Yvette, France), and quantitative RT-PCR was performed on a 7300 RT-PCR system (Applied Biosystems, Villebon sur Yvette, France) with a SYBR Green PCR master mix (Thermo Fisher Scientific), as described previously15. Relative changes in gene to cyclophilin or TATA-box-binding protein 1 (TBP) mRNA ratio between hypoxic and normoxic conditions were computed using the 2−DDCt method. The list of primers (Sigma-Aldrich) used is available upon request.
Real-Time PCR-Based Array Analysis
Differential gene expression was examined in PIs cultured under hypoxia and compared to control PIs cultured under normoxia. Following total RNA extraction, 84 key genes in the hypoxia pathway were examined by RT-PCR using RT2 Profiler PCR Array (SABioscience, Courtaboeuf, France). The qPCR was performed in Roche Real-Time PCR System (Roche, Boulogne Billancourt, France). Data analysis was done using the online software PCR Array Data Analysis Web Portal (SABioscience). Gene expression levels were normalized against four housekeeping genes [actin B (ACTB), β 2 microglobulin (B2M), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and ribosomal protein, large, P0 (RPLPO)] included in the array. Relative gene expression was calculated using the 2−ΔΔCt method.
Immunodetection of HIF1α
EndoC-βH3 PIs were fixed in 10% formalin (Sigma-Aldrich) and processed for immunohistochemistry, as described previously20. A rabbit anti-HIF1α antibody (a gift from Dr. Pouyssegur, Nice, France) was used15. Revelation was performed with a biotinylated anti-rabbit antibody (1:200; Vector, Les Ulis, France) and a tyramide signal amplification (TSA) Plus Cyanin 5 system (PerkinElmer, Villebon-sur-Yvette, France). Photographs were taken using a fluorescence microscope (Leitz DM4000; Leica Microsystems, Wetzlar, Germany) and a Hamamatsu C7780 cooled 3CCD camera (Hamamatsu Photonics, Hamamatsu, Japan). No signals were observed when the primary antibodies were omitted.
Insulin Secretion and Content
For glucose-stimulated insulin release assay, EndoC-βH3 monolayer cells were seeded onto Matrigel (Sigma-Aldrich)- and fibronectin (Sigma-Aldrich)-coated 96-well plates (TPP) at 7 × 104 cells/well, or groups of 50 day 3 or 6 PIs were handpicked up. Monolayer cells and PIs were incubated overnight in culture medium that contained 2.8 mM glucose (Sigma-Aldrich) and then in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)-buffered Krebs-Ringer buffer (KRB) [115 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L CaCl2, 1 mmol/L MgCl2, 24 mmol/L NaHCO3, 10 mmol/L HEPES, pH 7.4, and 0.2% bovine serum albumin (BSA)] (Sigma-Aldrich) that contained 0.5 mM glucose for 60 min. At the end of this incubation, stimulated insulin secretion of the cells was measured by static incubation in KRB that contained varying glucose concentrations (2.8 to 15 mM) for 60 min. Glucose stimulation was performed in the presence or absence of 500 μM 3-isobutyl-1-methylxanthine (IBMX; Sigma-Aldrich).
Insulin content was measured by acid ethanol extraction. Monolayer cells or PIs were lysed with Triton X-100 (1%) solution (Sigma-Aldrich) containing antiprotease tablet (Roche) and then subjected to two freeze–thaw cycles and stored at −20°C. The lysate was mixed with acid ethanol [0.18 M HCl (Sigma-Aldrich) in 96% ethanol (v/v)] (Carlo-Erba, Val de Reuil, France) in a 1:4 proportion for 48 h at 4°C until insulin determination was carried out by enzyme-linked immunosorbent assay (ELISA). Insulin secretion and intracellular content of the EndoC-βH3 cells were measured in duplicate by ELISA according to the manufacturer’s instructions using the human insulin kit (Mercodia, Uppsala, Sweden), which was chosen for the absence of cross-reactivity with proinsulin. To normalize the amount of insulin secretion, the total protein concentration of the cells in each condition was measured by the Bicinchoninic Acid (BCA) Protein Assay Kit (Pierce, Waltham, MA, USA).
Flow Cytometry
For the flow cytometry assays, monolayer cells and PIs were dispersed to a single cell suspension using trypsin solution of 0.05% (Life Technologies, Villebon sur Yvette, France) and 0.25% (Life Technologies), respectively. Cells were then inhibited by a fetal calf serum (FCS) 20% solution (Sigma-Aldrich), washed in phosphate-buffered saline (PBS; Sigma-Aldrich), passed through a 40-μm filter (EasyStreiner; Greiner Bio-one, Frickenhausen, Germany), and washed twice with PBS. All data were acquired on an LSRFortessa (BD Biosciences, Le Pont de Claix, France) and analyzed with fluorescence-activated cell sorting (FACS) Diva software (BD). A total of 50,000 events were collected for each sample.
Viability Assays
The Live/Dead Viability/Cytotoxicity Kit (Life Technologies) was used following the manufacturer’s protocol to determine viability in monolayer and PI cultures. Briefly, 1 × 106 of dissociated cells (adherent or PIs) were resuspended in 1 ml of culture medium. Two microliters of calcein AM (50 μM) dye and 4 μl of ethidium homodimer-1 (2 mM) were added to each sample, mixed, and then incubated in the dark for 15 min.
Cell Proliferation Assays
Cell proliferation analysis was conducted using Click-iT 5-ethynyl-2′-deoxyuridine (EdU) Alexa Fluor 647 Flow Cytometry Assay Kit (Thermo Fisher Scientific). Briefly, EdU was added into the cell culture medium to a final concentration of 10 μmol/L 1 h before the endpoint of the experiments. Adherent and aggregated cells were dissociated by trypsinization, and then washed once with 2 ml of 1% BSA in PBS, fixed using Click-iT fixative, and incubated for 15 min in saponin-based permeabilization solution. Cells were then treated with Click-iT reaction cocktail according to the manufacturer’s instructions for 30 min before flow cytometry analysis. Nuclear staining (1 μM) (FxCycle Violet Stain; Thermo Fisher Scientific) was added directly before FACS analysis.
Statistical Analysis
Statistical analysis was performed with a GraphPad 6 software (La Jolla, CA, USA) using the unpaired two-tailed Student’s t-test. Values of p < 0.05 were considered statistically significant. The presented results are all representative of at least two independent experiments with n > 3 samples. Data are expressed as average percentage ± SEM.
RESULTS
Aggregation of EndoC-βH3 Cells Improves Stimulated Insulin Secretion
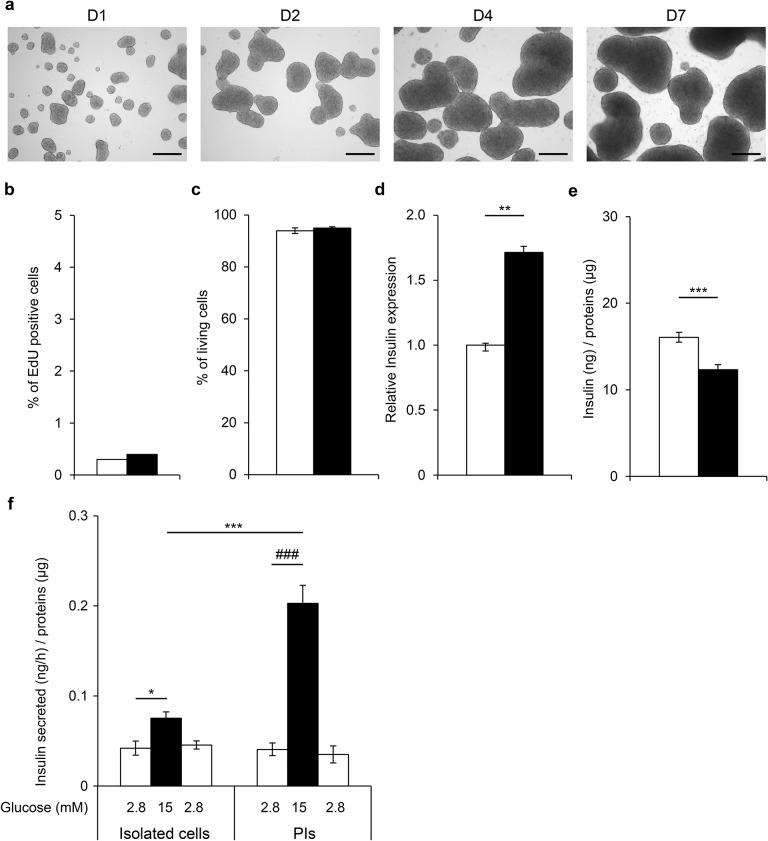
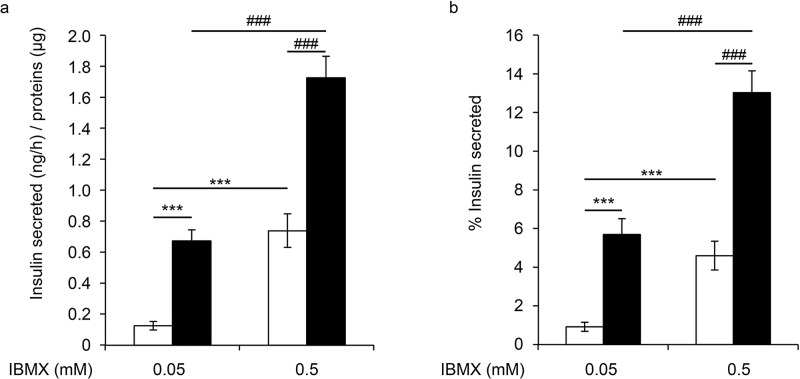
Recognizing the importance of cell–cell interactions, we first investigated the consequences of cell aggregation on β-cell function. All experiments were performed on nonproliferative excised cells. EndoC-βH3 cells were aggregated in spheroids called PIs. PIs had small masses from 20 to 800 μm with a mean size of 300 μm, similar to the size of a human islet (Fig. 1a). Within 7 days, their size increased due to the aggregation of several PIs together. Using EdU staining and cell sorting, we next compared β-cell proliferation between isolated EndoC-βH3 cells and PIs. In each experiment, the same number of cells was used to compare the isolated EndoC-βH3 cells and PIs. As expected for excised cells, proliferation was low and similar in cells studied in 2D culture and PIs (0.3% and 0.4%, respectively) (Fig. 1b). Moreover, the percentage of living cells was similar in isolated cells and PIs (94 ± 1.1% and 95 ± 0.43%, respectively) (Fig. 1c). To investigate the consequences of aggregation on EndoC-βH3 function, we next quantified insulin expression using RT-qPCR (Fig. 1d), insulin content (Fig. 1e), and GSIS (Fig. 1f). Insulin expression was increased by nearly twofold, and insulin content was slightly but significantly decreased. When isolated cells were exposed to glucose 15 versus 2.8 mM, insulin secretion was stimulated by 1.78-fold (p = 0.01), indicating that EndoC-βH3 is sensitive to glucose. This amplitude of insulin secretion was significantly improved in PIs (glucose stimulation index = 4.9, p = 0.018). The same observation was made when cells were stimulated in the presence of IBMX (Fig. 2). Accordingly, the percentage of secreted insulin was also enhanced in PIs compared to isolated cells (Fig. 2b). Finally, when glucose 2.8 mM was applied subsequently to glucose stimulation, basal insulin secretion was restored, demonstrating its reversibility (Fig. 1f). Together, these results show that when cells were aggregated in PIs, insulin secretion is preserved and even slightly improved.
Figure 1.
Improvement of glucose-stimulated insulin secretion (GSIS) in aggregated excised EndoC-βH3 cells. (a) Morphological images of EndoC-βH3 pseudoislets (PIs) over 7 days. Scale bars: 200 μm. Consequences of EndoC-βH3 cell aggregation on 5-ethynyl-2′-deoxyuridine (EdU) incorporation (b) and cell viability (c) at day 7. EndoC-βH3 isolated cells (white bars) and PIs (black bars) were cultured separately for 4 (e, f) or 7 days (b–d). Results are representative of two independent experiments performed in triplicates. Data are expressed as average percentage ± SEM. (d) Insulin (INS) gene expression was determined by quantitative RT-PCR (RT-qPCR) in isolated cells and PIs. Data are expressed as fold change of mRNA expression relative to TATA-box-binding protein 1 (TBP) ± SEM of two independent RNA preparations assayed in duplicates. **p < 0.01 versus isolated cells. (e) Insulin content in EndoC-βH3 isolated cells and in PIs. Data are expressed as insulin content in ng/μg of proteins ± SEM of 12 independent wells per condition assayed in duplicates by enzyme-linked immunosorbent assay (ELISA). ***p < 0.01 versus isolated cells. (f) GSIS on EndoC-βH3 isolated cells and PIs. Insulin secretion was first stimulated for 60 min with 15 mM glucose (black bars). This medium was then replaced with Krebs–Ringer buffer (KRB) that contained 2.8 mM glucose, and the cells were further incubated in this medium for 60 min (white bars). Data are expressed as nanogram of secreted insulin per hour per microgram of proteins ± SEM of four independent wells per condition assayed in duplicates by ELISA. *p < 0.05, ***p < 0.01, ###p < 0.01 PIs exposed to 15 mM glucose compared to 2.8 mM.
Figure 2.
GSIS on EndoC-βH3 isolated cells and PIs at day 4. Insulin secretion was first stimulated for 60 min with 15 mM glucose in the presence or absence of 3-isobutyl-1-methylxanthine (IBMX; black bars). The medium was then replaced with KRB that contained 2.8 mM glucose (white bars). (a) Data are expressed as secreted insulin in ng per hour/μg of proteins ± SEM of four independent wells per condition assayed in duplicates. (b) Results are expressed as the mean percentage ± SEM of the secreted insulin that was secreted in 1 h of four independent wells per condition assayed in duplicates by ELISA. ***p < 0.001, ###p < 0.001.
Altered Glucose Sensitivity But Preserved Insulin Production and Secretion by EndoC-βH3 Cells in Hypoxic Milieu
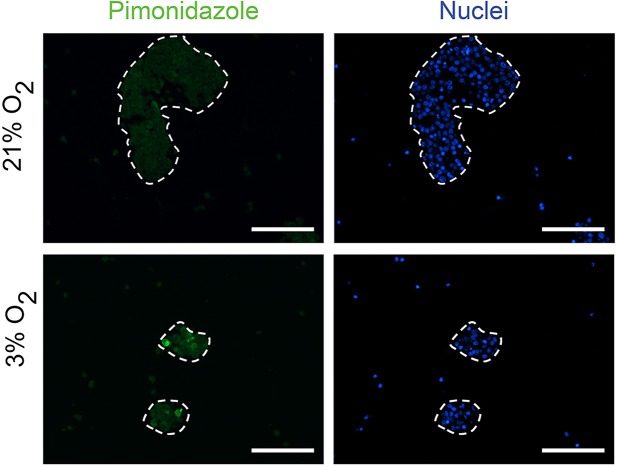
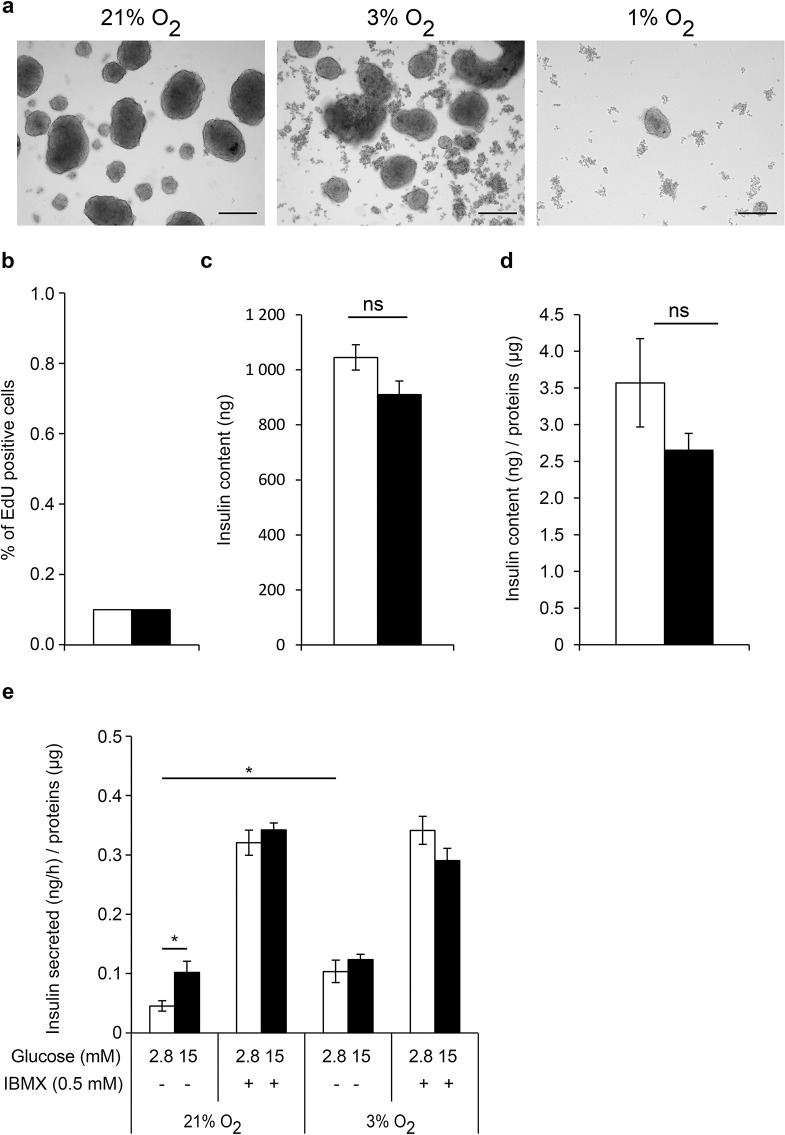
To evaluate the impact of hypoxia on EndoC-βH3 function, we compared PIs cultured at hypoxia (3% O2 or 1% O2) versus normal atmospheric pO2 (21%) for 6 days. Indeed, concentrations of O2 between 1% and 3% are generally considered as hypoxic conditions when compared to the pO2 around the pancreatic islets in vivo. The physioxia, that is, the physiological oxygen tension within the islets, is normally around 5% in the pancreas12. Moreover, the presence of the transcription factor HIF1α is detected only below 5% O2 21. We and other laboratories have previously shown that the atmospheric pO2 (21%) does not have adverse effects on the β-cell viability and function when compared to physioxia (5% O2)15,22. Thus, we used the atmospheric pO2 (21% O2) as a control, which we arbitrarily called normoxia. At 3% O2 and 1% O2, the majority of the cells within PIs were positive for pimonidazole staining, confirming their hypoxic state (Fig. 3). Pimonidazole stains the cells at pO2 < 1.3%. Few cells remained pimonidazole negative when 3% O2 was used. These data indicate that the pO2 within the aggregates was equal or below 1.3% O2 when PIs were exposed to this oxygen tension. By comparison, only very rare cells located at the center of PIs were positive with pimonidazole at normoxia. The morphology of aggregates cultivated in various oxygen concentrations is depicted in Figure 4a. In hypoxic conditions, the spheroids were smaller (Fig. 4a) and cellular debris was observed, increasing with the severity of hypoxia. In particular, only a few remaining cells were found at pO2 = 1% after 6 days. Thus, we focused on the milder condition (pO2 = 3%) to analyze the effect of the hypoxic milieu. The total number of cells was decreased by 62% at 3% O2 compared to 21% O2. This observation is in accordance with the increased number of cell debris seen in Figure 4a. The proliferation rate of β-cells was very low (0.1%) and was not affected by hypoxia (Fig. 4b). Moreover, insulin content was similar at hypoxia compared to normoxia, indicating a normal insulin production (Fig. 4c–d). Surprisingly, at a low glucose concentration (2.8 mM), the rate of insulin secretion was increased at hypoxia versus normoxia (Fig. 4e), reaching similar levels when a high glucose concentration (15 mM) was applied. These results show that the insulin secretion capacity is maintained in β-cells during hypoxia. With IBMX, the rates of insulin secretion reached the same levels in the hypoxic and normoxic conditions, confirming that hypoxia does not alter the secretion capacity of β-cells. Of note, after 6 days in culture, IBMX (0.5 mM) strongly activated insulin secretion at 2.8 mM glucose (Fig. 4e). This basal insulin secretion in the presence of IBMX reached the same value as the glucose-stimulated condition with IBMX and 15 mM glucose. This was not the case at culture day 4 (Fig. 2e). This observation suggests that glucose sensitivity of EndoC-βH3 is gradually lost with time when IBMX at a concentration of 0.5 M is used. Finally, the stimulation index of insulin secretion was only 1.22 at hypoxia compared to 2.57 at normoxia. Thus, this result indicates a decreased glucose sensitivity of the cells during hypoxia.
Figure 3.
Detection of hypoxia in the EndoC-βH3 aggregated cells when cultured under 3% O2. To detect hypoxic cells, pimonidazole (200 μmol/L; Hypoxyprobe; NPI) was added to the culture medium during the last hour of the culture period (6 days). PIs were analyzed with an anti-pimonidazole antibody (NPI). Control cells were cultured under normoxia.
Figure 4.
Effect of hypoxia on EndoC-βH3 viability, sensitivity to glucose, and insulin secretion capacity. EndoC-βH3 PIs were cultured under normoxia (21% O2, white bars) and hypoxia (3% O2, black bars) (b–e). Analyses were performed at day 6. (a) Morphological images of EndoC-βH3 PIs cultured under hypoxia (3% and 1% O2). Scale bars: 200 μm. Effect of hypoxia on EdU incorporation, insulin content, and GSIS in EndoC-βH3 PIs (b–e). (b) EdU was added for 1 h, and analysis was performed by fluorescence-activated cell sorting (FACS). Results are representative of two independent experiments performed in duplicates. Data are expressed as average ± SEM. (c, d) Insulin content in EndoC-βH3 PIs. Data are expressed as insulin content in nanogram of insulin (c) and in nanogram of insulin normalized per microgram of proteins (d) ± SEM of four independent wells per condition assayed in duplicates by ELISA. ns, nonsignificant. (e) GSIS on EndoC-βH3 PIs in the presence or absence of IBMX. Data are expressed as nanogram of secreted insulin per hour per microgram of proteins ± SEM of three independent wells per condition assayed in duplicates by ELISA. *p < 0.05.
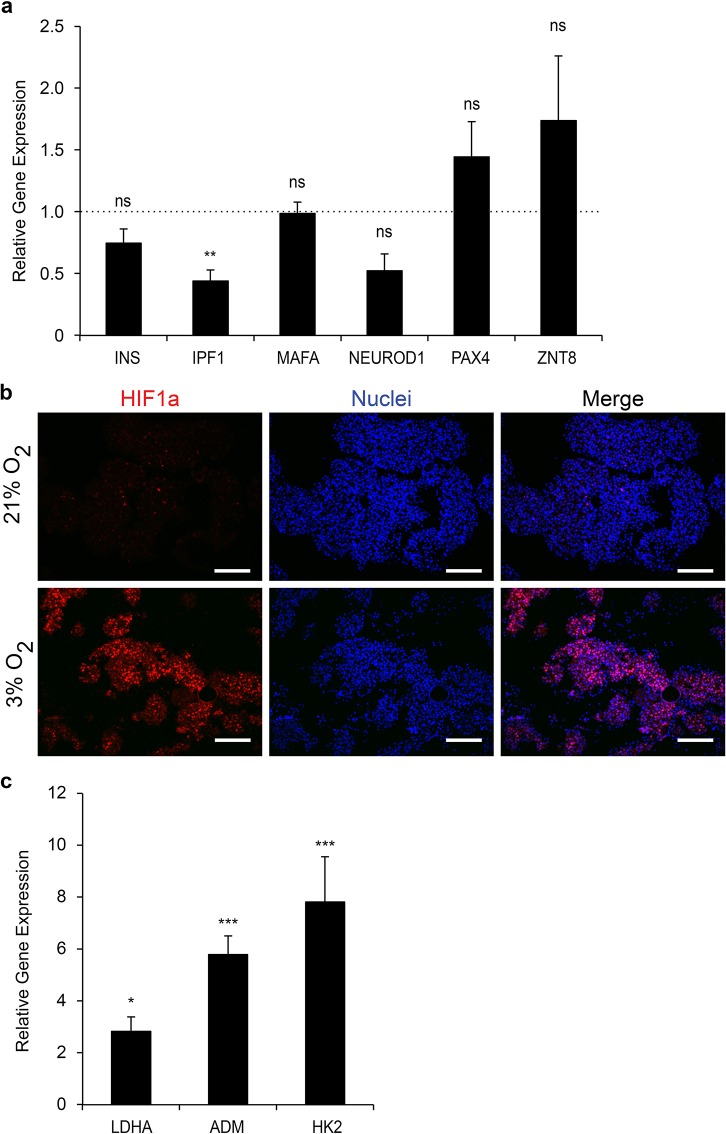
To determine the effects of hypoxia on the expression of β-cell markers, we first quantified the level of insulin (INS) mRNAs. Insulin expression was similar to controls in the hypoxic PIs despite a reduction in the level of insulin promoter factor 1 [IPF1; also known as pancreatic and duodenal homeobox 1 (PDX1)] mRNAs (Fig. 5a). Expression of other markers including v-maf musculoaponeurotic fibrosarcoma oncogene homolog A (MAFA), neurogenic differentiation factor 1 (NEUROD1), paired box protein (PAX4), and zinc transporter 8 (ZNT8) was not altered by hypoxia. Thus these results suggest that hypoxia (pO2 = 3%) probably does not alter the identity of the cells. Moreover, despite the negative effect of hypoxia on EndoC-βH3 survival, the remaining living cells are still functional as insulin secretion is preserved after 6 days in 3% O2.
Figure 5.
Molecular impact of hypoxia on the EndoC-βH3 PIs. (a) Expression of β-cell markers in EndoC-βH3 PIs cultured under hypoxia versus normoxia. RT-qPCR was normalized relative to cyclophilin A expression, and the relative expression of each β-cell marker was arbitrarily set to 1 for the control EndoC-βH3 PIs. Each point represents the mean ± SEM of two independent experiments performed in duplicates. **p < 0.01. INS, insulin; IPF1, insulin promotor factor 1 [also known as pancreatic and duodenal homeobox 1 (PDX1)]; MAFA, v-maf musculoaponeurotic fibrosarcoma oncogene homolog A; NEUROD1, neurogenic differentiation factor 1; PAX4, paired box protein 4; ZNT8, zinc transporter 8. (b) Detection by immunohistochemistry of hypoxia-inducible factor 1α (HIF1α) in EndoC-βH3 PIs cultured under hypoxia versus normoxia. Scale bars: 200 μm. (c) Expression of HIF1α target genes was analyzed by RT-qPCR. Each point represents the mean ± SEM of two independent experiments performed in duplicates. *p < 0.05, ***p < 0.001. LDHA, lactate dehydrogenase A; ADM, adenomedullin; HK2, hexokinase 2.
To analyze the effects of pO2 at the molecular level, we next analyzed the presence of HIF1α by immunohistochemistry. At normoxia (pO2 = 21%), only rare HIF1α+ cells were detected in PIs (Fig. 5b). As expected, numerous HIF+ cells were present at hypoxia. We also quantified the expression of the known HIF target genes using RT-qPCR15. Lactate dehydrogenase A (LDHA), adenomedullin (ADM), and hexokinase 2 (HK2) mRNA levels were increased by 2.8-, 5.8-, and 7.8-fold, respectively, in 3% O2 compared to 21% O2 (Fig. 5c). To further compare gene expression of the aggregates exposed to hypoxia versus normoxia, a transcriptomic analysis was performed (Fig. 6). We first confirmed that LDHA, ADM, and HK2 expression was upregulated by hypoxia (1.74-, 4.28-, and 5.64-fold change, respectively). Thus, these genes represent good markers to detect hypoxia in human β-cells. As expected, solute carrier family 2, facilitated glucose transporter member 1 [SCLC2A1; or glutamate transporter 1 (GLUT1)] gene expression was also induced (threefold induction). Surprisingly, the expression of genes generally used to detect hypoxia was not increased. This is indeed the case for vascular endothelial growth factor A (VEGF-A) and phosphoglycerate kinase 1 (PGK1). Expression of HIF1α was not modulated by hypoxia at the mRNA level (0.9-fold), indicating that its regulation is mainly posttranslational. Finally, this genetic profile also suggests an enhanced glycolytic activity.
Figure 6.
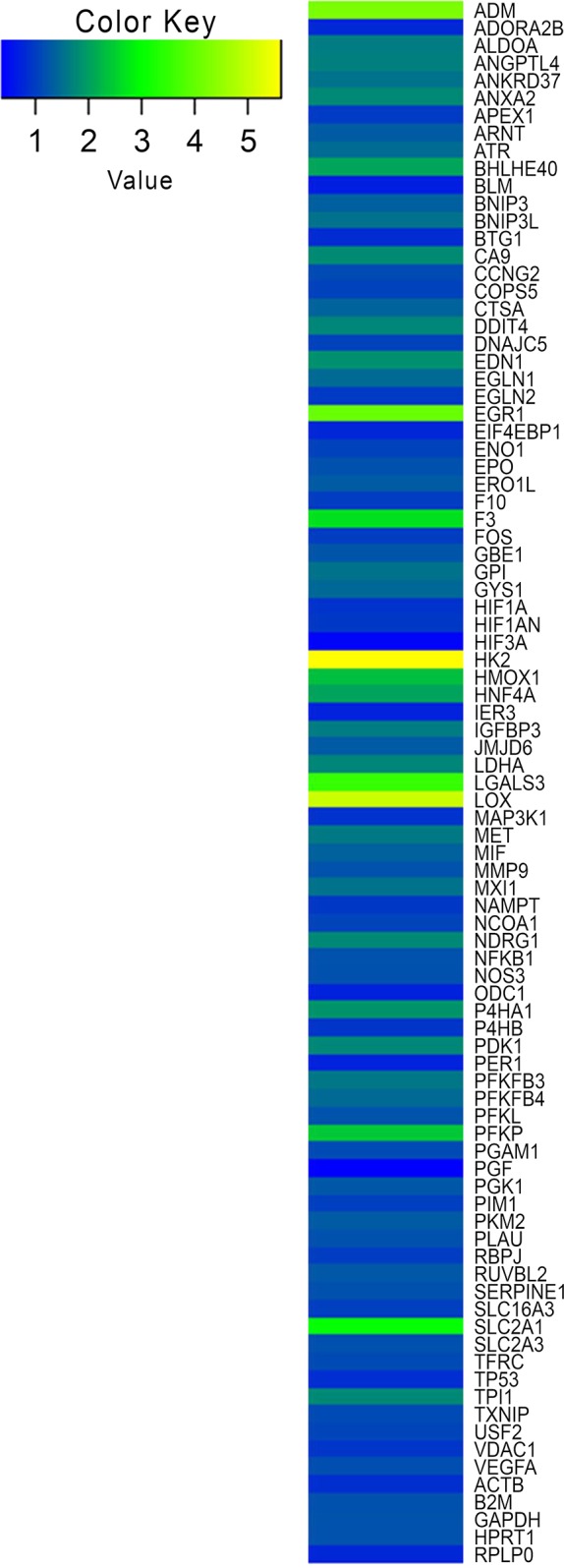
Heat map visualization of gene expression profiling of EndoC-βH3 PIs under hypoxic conditions. An RT2 Profiler PCR Array was used to screen a panel of 84 genes implicated in hypoxia signaling pathway in EndoC-βH3 under normal and hypoxic conditions. The blue color indicates low expression, and the yellow color indicates high expression.
Constitutive Stabilization of HIF1α at Normoxia Alters PIs Function
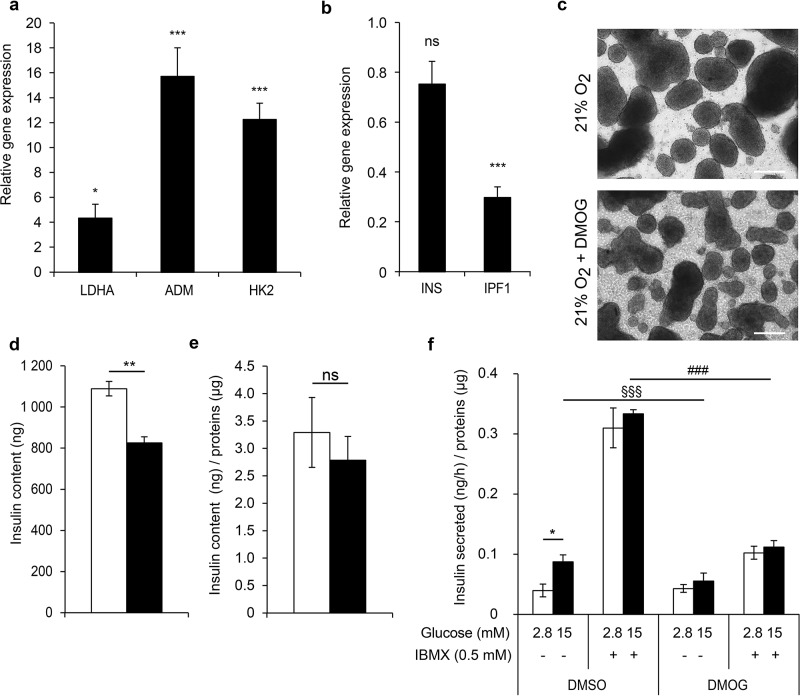
Recent studies in rodents have shown that the HIF1α transcription factor plays a crucial role in adult β-cell function16–18. Indeed, forced stabilization of HIF1α by von Hippel–Lindau (Vhl) deletion in β-cells leads to impaired GSIS in the mouse. Here we researched the consequences of HIF1α stabilization in the EndoC-βH3 PIs at normoxia. For this, we used a prolyl-hydroxylase inhibitor, DMOG. Expression of the HIF target genes LDHA, HK2, and ADM was increased by 4-, 12-, and 16-fold, respectively, in the DMOG samples, confirming the efficiency of this inhibitor on the activation of the HIF pathway (Fig. 7a). IPF1, but not INS, gene expression was significantly decreased with DMOG (inhibition of 70% and 25%, respectively) (Fig. 7b). Moreover, numerous cellular debris were detected in the DMOG-treated cultures (Fig. 7c). Consistently, when EndoC-βH3 PIs were treated with DMOG, the number of cells decreased by 30% compared to the nontreated cells, and the insulin content was reduced by 20% (Fig. 7d and e). Moreover, GSIS was dramatically reduced (Fig. 7f), indicating that the constitutive stabilization of the HIF1α protein has a deleterious repercussion on β-cell function. Of note, both glucose sensitivity and the insulin secretion capacity of EndoC-βH3 PIs were altered by DMOG.
Figure 7.
HIF1α stabilization affects insulin function in normoxic conditions. EndoC-βH3 PIs were cultured in normoxia and treated with dimethyloxalylglycine (DMOG) for 6 days. (a) Expression of HIF target genes in DMOG-treated PIs versus control PIs by qPCR. Each point represents the mean ± SEM of two independent experiments performed in duplicates. *p < 0.05, ***p < 0.001. (b) Insulin gene expression in DMOG-treated PIs versus control PIs by RT-qPCR. Each point represents the mean ± SEM of two independent experiments performed in duplicates. ***p < 0.001. (c) Morphological images of EndoC-βH3 PIs after a 6-day DMOG treatment. Scale bars: 300 μm. (d, e) Insulin content in EndoC-βH3 PIs treated with DMOG (black bars) versus untreated PIs (white bars). Data are expressed as insulin content in nanogram of insulin (d) and in nanogram of insulin normalized per microgram of proteins (e) ± SEM of four independent wells per condition assayed in duplicates by ELISA. **p < 0.01. (f) DMOG alters insulin secretion. Insulin secretion was stimulated for 60 min with 15 mM glucose in the presence or absence of IBMX on DMOG-treated (black bars) and nontreated (white bars) EndoC-βH3 PIs. GSIS data are expressed as nanogram of secreted insulin per hour per microgram of proteins ± SEM of four independent wells per condition assayed in duplicates by ELISA. *p < 0.05, §§§p < 0.001 PIs exposed to 15 mM glucose + DMOG when compared to 15 mM glucose alone, ###p < 0.001 PIs treated with DMOG and exposed to 15 mM glucose + IBMX when compared to controls.
Together, these results demonstrate that increasing the HIF pathway affects insulin production and β-cell function.
Inhibition of the HIF1α Pathway During Hypoxia Leads to Increased Cell Death
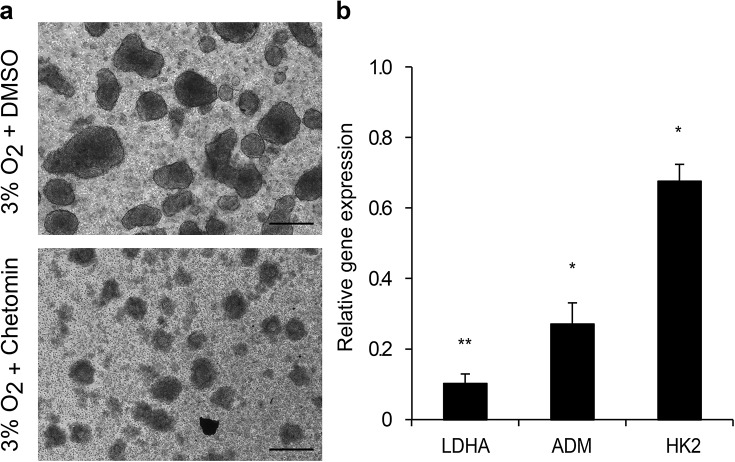
Some reports indicate that HIF1α stabilization predicts adverse transplantation outcomes. On the other hand, recent studies using transplanted human islets suggested that HIF1α might be protective during the transplantation process23. In order to evaluate whether HIF has a positive or negative role during hypoxia, we cultured EndoC-βH3 PIs with or without chetomin (CHT) (Fig. 8a), an inhibitor of the HIF pathway24. After CHT treatment, PIs disassembled. Expression of LDHA, ADM, and HK2 was induced during hypoxia (pO2 = 3%) by 3-, 13-, and 7-fold, respectively. In the presence of CHT, the induction of the HIF target genes was partially alleviated. Indeed, expression levels of LDHA, ADM, and HK2 were reduced by 90%, 70%, and 32%, respectively, compared to the hypoxic condition (Fig. 8b). Moreover, the number of cells was reduced by 96% compared to the controls. These results indicate that inhibition of the HIF1α pathway strongly affects the survival of β-cells. Altogether, inhibition and overexpression of the HIF pathway at hypoxia and normoxia, respectively, are both deleterious to the β-cell function.
Figure 8.
Inhibition of the HIF signaling pathway by chetomin alters the survival of EndoC-βH3 PIs at hypoxia. (a) Morphological images of EndoC-βH3 PIs after a 6-day chetomin treatment. Scale bars: 200 μm. (b) Expression of HIF1α target genes in chetomin-treated EndoC-βH3 PIs cultured under hypoxia versus control PIs was analyzed by qPCR. Each point represents the mean ± SEM of two independent experiments performed in duplicates. *p < 0.05, **p < 0.01. DMSO, dimethyl sulfoxide.
DISCUSSION
In the present study, we used the new cell line EndoC-βH3 to better understand which factors and mechanisms may influence the outcome of human islet transplantation. We focused on two parameters, cell–cell contacts and hypoxia. Our main results demonstrate that cell aggregation favors β-cell function, while HIF1α has protective effects against hypoxic stress. Moreover, the dosage of HIF1α seems to be a determinant for the β-cell outcome.
For several decades, interactions between rodent β-cells have been the subject of intense research. It was shown that adhesion between clusters of β-cells regulates both basal and glucose–insulin secretion25–28. Another possibility is that contacts between different cell types within the islets participate to the control of insulin secretion29. We did not exclude this hypothesis. However, taking advantage of the purity of our β-cell line, we focused our study on the role of the β-cell interactions. We show that clusters of EndoC-βH3 also have increased insulin mRNA levels and improved GSIS. This is consistent with other work using human β-cells. Indeed, it was shown that human β-cells are highly heterogeneous in terms of insulin secretion. In human islets, the homologous and heterologous intercellular contacts have a significant impact on insulin secretion, possibly due to the particular architecture of human islets29. The behavior of EndoC-βH3 seems to be similar to these islet cells. We thus think that using cell clusters of engineered β-cells, which can be produced on a large scale, should facilitate the studies of islet transplantation. Moreover, the mechanisms involved in the cell–cell contact-dependent GSIS are not completely understood. Recently, Geron et al. identified murine β-cell edges as surface microdomains of cell adhesion and signaling. Notably, the edge organization is E-cadherin contact dependent and correlates with insulin secretion capacity30. Further analysis will be necessary to characterize whether these signals are also involved in GSIS of EndoC-βH3 cells.
Recent work investigated whether prior exposure of mouse or human islets to hypoxia would improve or be deleterious for islet transplantation31. The murine islets exposed to hypoxia expressed higher levels of the hypoxic response genes, LDHA, SLC2A1, and PDK1, and glycolysis and lactate production were increased. On the other hand, the mRNA level for SLC2A2 was downregulated, and GSIS was impaired. In human isolated islets, expression of LDHA, HK1, and HK2 was increased 18-, 6-, and 3-fold, respectively. In the present work, the genetic profile of EndoC-βH3 resembles the one that was described in Cantley et al.31. Activation of LDHA, PDK1, and SLC2A1 is also detected, but insulin secretion is not impaired despite a lower glucose sensitivity. Thus, we can consider that EndoC-βH3 cells have a certain resistance to hypoxia. A difference between the study of Cantley et al. and ours is that a more severe hypoxia (pO2 = 1%) was used in Cantley et al.31, compared to pO2 = 3% in our present study. Moreover, other recent work showed that hypoxia causes a decrease in ZNT8 expression in pancreatic β-cells22. This result differs from our experiments. We think that this difference is also caused by the different values of pO2 that were used in each experiment. Thus, the intensity of the response to hypoxia, which depends on the cellular context, may be involved in the maintenance of the β-cell function.
Finally, we found that decreasing the HIF signaling pathway using CHT considerably decreases the cell viability. The effect of CHT was previously described in cancer studies where it inhibits HIF1α and consequently reduced the hypoxia-dependent transcription and radiosensitizes human fibrosarcoma cells24. Here we confirm this strong HIF inhibition by CHT in human β-cells. A number of studies suggested that HIF transcription predicts poor transplant success32,33. However, a more recent analysis demonstrated the need of HIF1α pathway activation for the β-cell survival after islet transplantation23. Indeed, when β-HIF1α-null islets were transplanted, the transplants had poor outcomes. Moreover, stabilization of HIF1α in mouse and human islets using desferioxamine improved islet transplantation by decreasing apoptosis and increasing the β-cell mass. In other cell types, for example, in neutrophils, the requirement of HIF1α for cell survival was also demonstrated34. Our data are consistent with such observations and suggest that the HIF1α activity is indispensable for the survival of the human engineered β-cells during exposure to hypoxia and probably for islet transplantation.
In conclusion, aggregation is beneficial for EndoC-βH3 cell biology. HIF1α seems to be necessary for the survival of EndoC-βH3 cells during hypoxia. However, increasing the HIF pathway modifies their genetic profile in terms of glycolysis but does not impair their insulin secretion capacity. Thus, this study suggests that EndoC-βH3 cells have a certain resistance to hypoxia and represent a good human cell model for future islet cell transplantation analysis.
ACKNOWLEDGMENTS
This work received financial support from the Cooperation Program of the European Community’s FP7 (Grant No. HEALTH-2012-2.4.3-1). The authors thank C. Blanc (Flow cytometry Platform, Pierre and Marie-Curie University) for technical assistance. The authors also thank Dr. P. Richards for his comments and editorial assistance in preparing the manuscript. R. Scharfmann, P. Czernichow, and P. Ravassard are shareholders and consultants for Endocells. B. Duvillié is a consultant for Endocells.
REFERENCES
- 1. Al-Adra DP, Gill RS, Imes S, O’Gorman D, Kin T, Axford SJ, Shi X, Senior PA, Shapiro AM. Single-donor islet transplantation and long-term insulin independence in select patients with type 1 diabetes mellitus. Transplantation 2014;98(9):1007–12. [DOI] [PubMed] [Google Scholar]
- 2. Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–8. [DOI] [PubMed] [Google Scholar]
- 3. Lechner A, Habener JF. Stem/progenitor cells derived from adult tissues: Potential for the treatment of diabetes mellitus. Am J Physiol Endocrinol Metab. 2003;284(2):E259–66. [DOI] [PubMed] [Google Scholar]
- 4. Ravassard P, Hazhouz Y, Pechberty S, Bricout-Neveu E, Armanet M, Czernichow P, Scharfmann R. A genetically engineered human pancreatic beta cell line exhibiting glucose-inducible insulin secretion. J Clin Invest. 2011;121(9):3589–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scharfmann R, Pechberty S, Hazhouz Y, von Bulow M, Bricout-Neveu E, Grenier-Godard M, Guez F, Rachdi L, Lohmann M, Czernichow P, Ravassard P. Development of a conditionally immortalized human pancreatic beta cell line. J Clin Invest. 2014;124(5):2087–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benzara B, Lecomte MJ, Colace C, Müller A, Machado C, Pechberty S, Bricout-Neveu E, Grenier-Godard M, Solimena M, Scharfmann R, Czernichow P, Ravassard P. A human beta cell line with drug inducible excision of immortalizing transgenes. Mol Metab. 2015;4(12):916–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guo-Parke H, McCluskey JT, Kelly C, Hamid M, McClenaghan NH, Flatt PR. Configuration of electrofusion-derived human insulin-secreting cell line as pseudoislets enhances functionality and therapeutic utility. J Endocrinol. 2012;214(3):257–65. [DOI] [PubMed] [Google Scholar]
- 8. Luther MJ, Hauge-Evans A, Souza KL, Jorns A, Lenzen S, Persaud SJ, Jones PM. MIN6 beta-cell-beta-cell interactions influence insulin secretory responses to nutrients and non-nutrients. Biochem Biophys Res Commun. 2006;343(1):99–104. [DOI] [PubMed] [Google Scholar]
- 9. Persaud SJ, Arden C, Bergsten P, Bone AJ, Brown J, Dunmore S, Harrison M, Hauge-Evans A, Kelly C, King A, Maffucci T, Marriott CE, McClenaghan N, Morgan NG, Reers C, Russell MA, Turner MD, Willoughby E, Younis MY, Zhi ZL, Jones PM. Pseudoislets as primary islet replacements for research: Report on a symposium at King’s College London, London UK. Islets 2010;2(4):236–9. [DOI] [PubMed] [Google Scholar]
- 10. Ravier MA, Guldenagel M, Charollais A, Gjinovci A, Caille D, Sohl G, Wollheim CB, Willecke K, Henquin JC, Meda P. Loss of connexin36 channels alters beta-cell coupling, islet synchronization of glucose-induced Ca2+ and insulin oscillations, and basal insulin release. Diabetes 2005;54(6):1798–807. [DOI] [PubMed] [Google Scholar]
- 11. MacGregor RR, Williams SJ, Tong PY, Kover K, Moore WV, Stehno-Bittel L. Small rat islets are superior to large islets in in vitro function and in transplantation outcomes. Am J Physiol Endocrinol Metab. 2006;290(5):E771–9. [DOI] [PubMed] [Google Scholar]
- 12. Carlsson PO, Liss P, Andersson A, Jansson L. Measurements of oxygen tension in native and transplanted rat pancreatic islets. Diabetes 1998;47(7):1027–32. [DOI] [PubMed] [Google Scholar]
- 13. Rodriguez-Brotons A, Bietiger W, Peronet C, Magisson J, Sookhareea C, Langlois A, Mura C, Jeandidier N, Pinget M, Sigrist S, Maillard E. Impact of pancreatic rat islet density on cell survival during hypoxia. J Diabetes Res. 2016;2016:3615286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fraker C, Timmins MR, Guarino RD, Haaland PD, Ichii H, Molano D, Pileggi A, Poggioli R, Presnell SC, Inverardi L, Zehtab M, Ricordi C. The use of the BD oxygen biosensor system to assess isolated human islets of langerhans: Oxygen consumption as a potential measure of islet potency. Cell Transplant. 2006;15(8–9):745–58. [DOI] [PubMed] [Google Scholar]
- 15. Heinis M, Simon MT, Ilc K, Mazure NM, Pouyssegur J, Scharfmann R, Duvillie B. Oxygen tension regulates pancreatic beta-cell differentiation through hypoxia-inducible factor 1alpha. Diabetes 2010;59(3):662–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cantley J, Selman C, Shukla D, Abramov AY, Forstreuter F, Esteban MA, Claret M, Lingard SJ, Clements M, Harten SK, Asare-Anane H, Batterham RL, Herrera PL, Persaud SJ, Duchen MR, Maxwell PH, Withers DJ. Deletion of the von Hippel-Lindau gene in pancreatic beta cells impairs glucose homeostasis in mice. J Clin Invest. 2009;119(1):125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Puri S, Cano DA, Hebrok M. A role for von Hippel-Lindau protein in pancreatic beta-cell function. Diabetes 2009;58(2):433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zehetner J, Danzer C, Collins S, Eckhardt K, Gerber PA, Ballschmieter P, Galvanovskis J, Shimomura K, Ashcroft FM, Thorens B, Rorsman P, Krek W. PVHL is a regulator of glucose metabolism and insulin secretion in pancreatic beta cells. Genes Dev. 2008;22(22):3135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tanaka H, Tanaka S, Sekine K, Kita S, Okamura A, Takebe T, Zheng YW, Ueno Y, Tanaka J, Taniguchi H. The generation of pancreatic beta-cell spheroids in a simulated microgravity culture system. Biomaterials 2013;34(23):5785–91. [DOI] [PubMed] [Google Scholar]
- 20. Attali M, Stetsyuk V, Basmaciogullari A, Aiello V, Zanta-Boussif MA, Duvillie B, Scharfmann R. Control of beta-cell differentiation by the pancreatic mesenchyme. Diabetes 2007;56(5):1248–58. [DOI] [PubMed] [Google Scholar]
- 21. Bracken CP, Fedele AO, Linke S, Balrak W, Lisy K, Whitelaw ML, Peet DJ. Cell-specific regulation of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha stabilization and transactivation in a graded oxygen environment. J Biol Chem. 2006;281(32):22575–85. [DOI] [PubMed] [Google Scholar]
- 22. Gerber PA, Bellomo EA, Hodson DJ, Meur G, Solomou A, Mitchell RK, Hollinshead M, Chimienti F, Bosco D, Hughes SJ, Johnson PR, Rutter GA. Hypoxia lowers SLC30A8/ZnT8 expression and free cytosolic Zn2+ in pancreatic beta cells. Diabetologia 2014;57(8):1635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stokes RA, Cheng K, Deters N, Lau SM, Hawthorne WJ, O’Connell PJ, Stolp J, Grey S, Loudovaris T, Kay TW, Thomas HE, Gonzalez FJ, Gunton JE. Hypoxia-inducible factor-1alpha (HIF-1alpha) potentiates beta-cell survival after islet transplantation of human and mouse islets. Cell Transplant. 2013;22(2):253–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Staab A, Loeffler J, Said HM, Diehlmann D, Katzer A, Beyer M, Fleischer M, Schwab F, Baier K, Einsele H, Flentje M, Vordermark D. Effects of HIF-1 inhibition by chetomin on hypoxia-related transcription and radiosensitivity in HT 1080 human fibrosarcoma cells. BMC Cancer 2007;7:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Halban PA, Wollheim CB, Blondel B, Meda P, Niesor EN, Mintz DH. The possible importance of contact between pancreatic islet cells for the control of insulin release. Endocrinology 1982;111(1):86–94. [DOI] [PubMed] [Google Scholar]
- 26. Hauge-Evans AC, Squires PE, Persaud SJ, Jones PM. Pancreatic beta-cell-to-beta-cell interactions are required for integrated responses to nutrient stimuli: Enhanced Ca2+ and insulin secretory responses of MIN6 pseudoislets. Diabetes 1999;48(7):1402–8. [DOI] [PubMed] [Google Scholar]
- 27. Konstantinova I, Lammert E. Microvascular development: Learning from pancreatic islets. BioEssays 2004;26(10):1069–75. [DOI] [PubMed] [Google Scholar]
- 28. Konstantinova I, Nikolova G, Ohara-Imaizumi M, Meda P, Kucera T, Zarbalis K, Wurst W, Nagamatsu S, Lammert E. EphA-Ephrin-A-mediated beta cell communication regulates insulin secretion from pancreatic islets. Cell 2007;129(2):359–70. [DOI] [PubMed] [Google Scholar]
- 29. Wojtusciszyn A, Armanet M, Morel P, Berney T, Bosco D. Insulin secretion from human beta cells is heterogeneous and dependent on cell-to-cell contacts. Diabetologia 2008;51(10):1843–52. [DOI] [PubMed] [Google Scholar]
- 30. Geron E, Boura-Halfon S, Schejter ED, Shilo BZ. The edges of pancreatic islet beta cells constitute adhesive and signaling microdomains. Cell Rep. 2015;10(3):317–25. [DOI] [PubMed] [Google Scholar]
- 31. Cantley J, Walters SN, Jung MH, Weinberg A, Cowley MJ, Whitworth TP, Kaplan W, Hawthorne WJ, O’Connell PJ, Weir G, Grey ST. A preexistent hypoxic gene signature predicts impaired islet graft function and glucose homeostasis. Cell Transplant. 2013;22(11):2147–59. [DOI] [PubMed] [Google Scholar]
- 32. Miao G, Ostrowski RP, Mace J, Hough J, Hopper A, Peverini R, Chinnock R, Zhang J, Hathout E. Dynamic production of hypoxia-inducible factor-1alpha in early transplanted islets. Am J Transplant. 2006;6(11):2636–43. [DOI] [PubMed] [Google Scholar]
- 33. Moritz W, Meier F, Stroka DM, Giuliani M, Kugelmeier P, Nett PC, Lehmann R, Candinas D, Gassmann M, Weber M. Apoptosis in hypoxic human pancreatic islets correlates with HIF-1alpha expression. FASEB J. 2002;16(7):745–7. [DOI] [PubMed] [Google Scholar]
- 34. Walmsley SR, Print C, Farahi N, Peyssonnaux C, Johnson RS, Cramer T, Sobolewski A, Condliffe AM, Cowburn AS, Johnson N, Chilvers ER. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J Exp Med. 2005;201(1):105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]