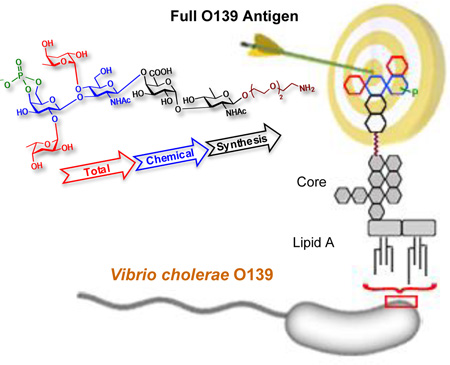
Abstract
The first chemical synthesis of the complete protective O-antigen of a human disease-causing pathogenic bacterium is described. The synthesis involved a protecting group strategy which facilitated regioselectivity of the key transformations, stereoselective glycosylations, and allowed one-step global deprotection of the completely assembled, fully protected, phosphorylated hexasaccharide by hydrogenation/hydrogenolysis. The final, amino group-functionalized, linker-equipped antigen was obtained in the form ready for conjugation to suitable carriers, e.g. proteins, to yield immunogens.
Keywords: Oligosaccharides, O-Specific Antigen, Glycosylations, Stereoselectivity, Vibrio cholerae O139
Graphical abstract
Cholera in humans is caused by two strains of Vibrio cholerae O1 and Vibrio cholerae O139.[1–4] The disease is endemic in over 50 countries; it affects 3 to 5 million individuals each year, resulting in the deaths of over 100,000 annually. Programs aimed at reducing the global burden of cholera by providing adequate sanitation and safe water have been unsuccessful. The field has realized that development and deployment of an improved cholera vaccine will be a critical component in cholera control programs, until adequate sanitation and safe water are a reality for the most impoverished individuals on the planet.
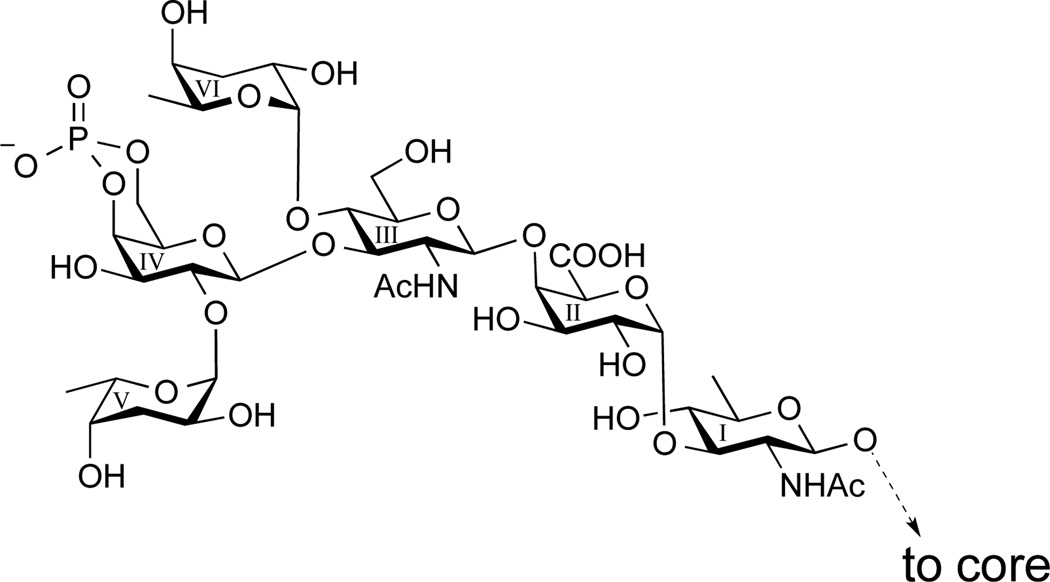
We have been involved in developing a glycoconjugate vaccine for cholera from synthetic carbohydrates that mimic structure of the O-specific polysaccharides (O-SP, O-antigen) of bacterial pathogens for a number of years.[5–7] Generally, the prerequisite for developing such a vaccine is the availability of a large fragment of the O-specific polysaccharide characteristic of the bacterium because O-SPs are the protective antigens.[8] Vibrio cholerae O139 is unique among bacteria that cause disease in humans in that the complete protective antigen is a single phosphorylated hexasaccharide consisting of five different monosaccharides (α-Colp-(1→2)-4,6-P-β-d-Galp-(1→3)-[α-Colp-(1→4)]-β-d-GlcpNAc-(1→4)-α-d-GalpA-(1→3)-β-d-QuipNAc-(1→, Figure 1)[9,10] and not a chain of oligosaccharide repeating units, which is the usual scenario within the Gram-negative bacteria.[11] In the case of Vibrio cholerae O139, the work towards a synthetic vaccine has been hampered because the critical hexasaccharide has not been synthesized, although the structure was elucidated two decades ago. The lack of such synthesis lies undoubtedly in the difficulties involved in the synthesis, isolation and purification of the charged substance.
Figure 1.
Structure of the O-Antigen of Vibrio cholerae O139.
Having first verified the methodology that would be involved in the synthesis of the title antigen,[12,13] we now report on the first total synthesis of the complete protective O-antigen of Vibrio cholerae O139 in the form ready for conjugation.
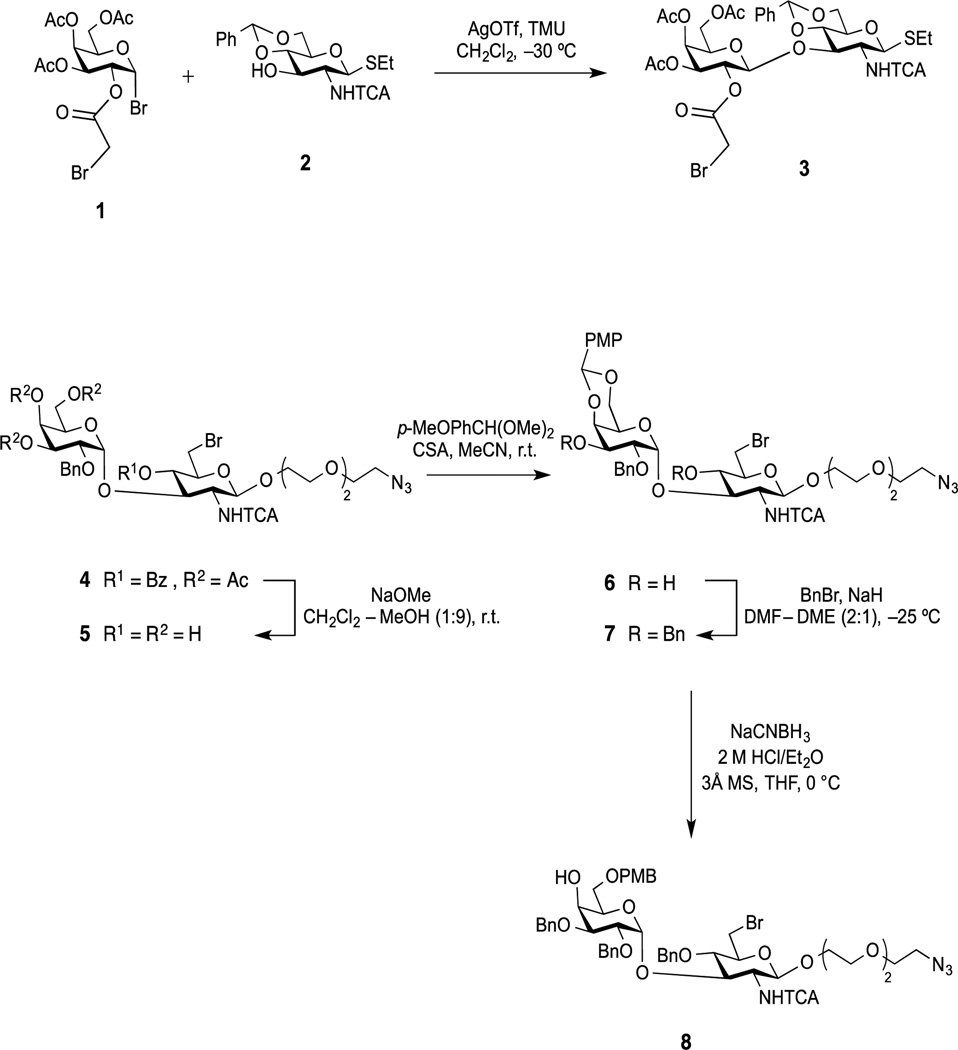
The synthesis started with the preparation of the key disaccharide building blocks 3 and 8 (Scheme 1). Glycosidation of the α-glycosyl bromide 1[13] with the 4,6-benzylidene acceptor 2[14] was successfully carried out in the presence of AgOTf promoter using our improved protocol,[15] which avoids the use of molecular sieves, to afford exclusively the β-(1→3)-linked disaccharide 3 in excellent yield (90%). The configuration of the β-interglycosidic linkage was confirmed by both 1H NMR (δH 4.75, d, J1,2 = 8.1 Hz, H-1II) and 13C NMR (δC 99.4, 1JC-1,H-1 = 165.2 Hz, C-1II) spectra.
Scheme 1.
Synthesis of the Key Disaccharide Building Blocks 3 and 8. (TCA = trichloroacetyl, AgOTf = silver trifluoromethanesulfonate = silver triflate, TMU = 1,1,3,3-tetramethylurea, PMP = p-methoxyphenyl, PMB = p-methoxybenzyl, CSA = 10-camphorsulfonic acid, MS = molecular sieves, DMF = N,N-dimethylformamide, DME = 1,2-dimethoxyethane, THF = tetrahydrofuran).
Zemplén de-O-acylation of the α-(1→3)-linked disaccharide 4[12] gave tetraol 5 in virtually quantitative yield, and subsequent p-methoxybenzylidenation of 5 gave selectively the 4II,6II-acetal derivative 6 (92%). Controlled benzylation of 6 using benzyl bromide and sodium hydride in DMF-DME at low temperature (6 to 7, 86%), to minimize elimination at C-6I in the presence of strong base, followed by regioselective reductive opening of the alkylidene ring in 7, afforded the linker-equipped disaccharide acceptor 8 in 89% yield. Compared to the 13C NMR spectrum of 7, the signal for C-4II in 8 (δC 68.2) shifted upfield (by ~6 ppm), which confirmed that the reductive opening of the p-methoxybenzylidene acetal led to the HO-4II-free, 6II-p-methoxybenzyl ether.
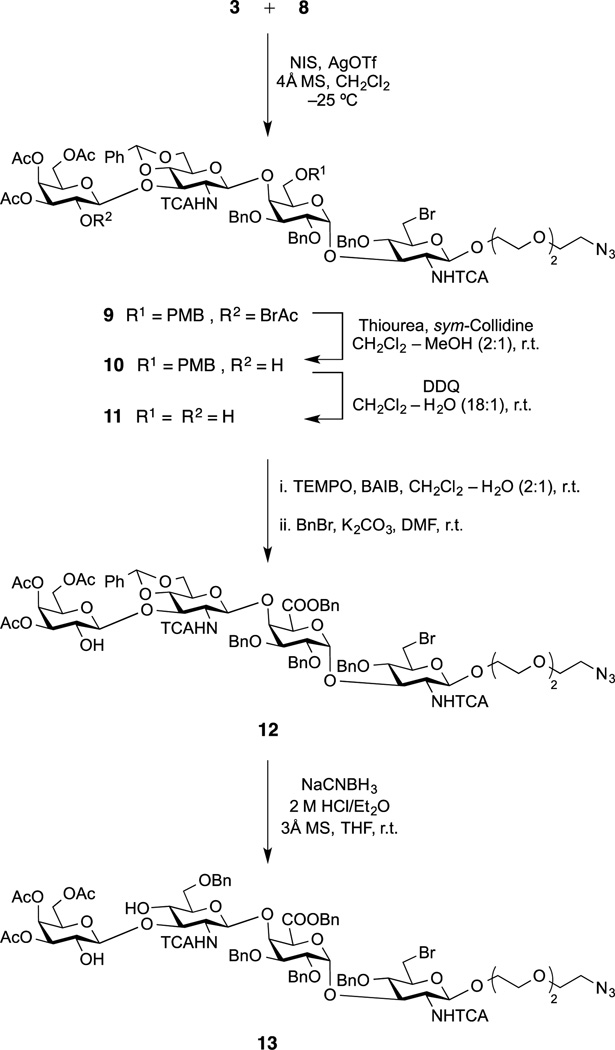
With the two building blocks 3 and 8 at hand, we focused on the 2+2 coupling. Accordingly (Scheme 2), NIS/AgOTf-promoted glycosylation of the spacer-equipped disaccharide acceptor 8 with the β-thioglycoside disaccharide donor 3 at −25°C proceeded stereoselectively to afford the desired linear tetrasaccharide 9 (84%). The acidity of the reaction medium was optimized by using excess AgOTf to minimize the conversion of the donor into the corresponding stable oxazoline.[16] Structural identification of the tetrasaccharide product 9 was provided by its 1H and 13C NMR spectra, which showed signals characteristic of both the acceptor and the donor moieties.
Scheme 2.
Synthesis of the Linear Tetrasaccharide Diol Acceptor 13. (NIS = N-iodosuccinimide, DDQ = 2,3-dichloro-5,6-dicyano-1,4-benzoquinone, TEMPO = 2,2,6,6-tetramethyl-1-piperidinyloxy free radical, BAIB = [bis(acetoxy)iodo] benzene).
Selective removal of the bromoacetyl ester in 9 by treatment with thiourea and sym-collidine[17] (9 to 10, 95%), and subsequent oxidative removal of the 6II-O-p-methoxybenzyl group using DDQ in CH2Cl2-H2O gave diol 11 (88%). Regioselective oxidation of the primary hydroxyl group in 11 with a combination of a catalytic amount of TEMPO free radical and a slight excess of BAIB in a diphasic CH2Cl2-H2O solvent system, followed by benzylation (BnBr/K2CO3 in DMF) of the formed carboxylic acid furnished uronate 12 (89% over two steps). Reductive ring opening of the 4II-6II-O-benzylidene acetal in 12 using sodium cyanoborohydride and 2M HCl-Et2O in THF at room temperature gave, with complete regioselectivity, the tetrasaccharide diol acceptor 13 (85%).
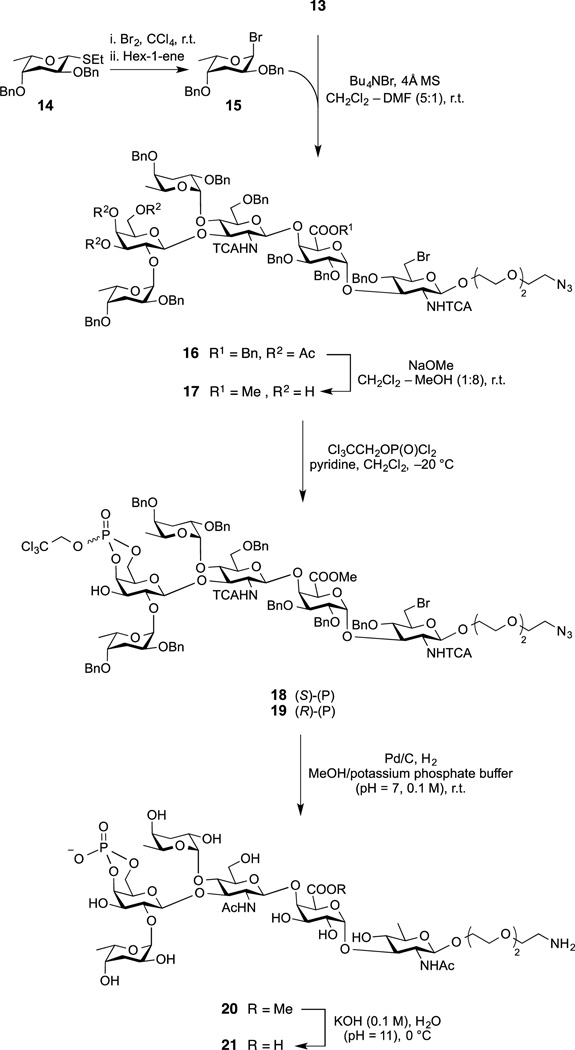
The stereoselective installation of the two colitose residues at positions 2IV and 4III in diol 13 was first attempted by activation of ethyl 2,4-di-O-benzyl-3,6-dideoxy-1-thio-β-l-xylo-hexopyranoside (14)[18] with CuBr2/Bu4NBr.[19,20] However the reaction was largely incomplete. The activation of the freshly prepared α-colitosyl bromide 15 at halide-assisted 1,2-cis glycosylation conditions,[21] was successful (Scheme 3), and afforded the desired hexasaccharide 16 as the major product (66%) along with two isomeric pentasaccharides (~23% combined yield), which were readily separable by chromatography. The two pentasaccharides can be either colitosylated, to give more of the desired hexasaccharide 16 or they can be deprotected, to arrive at fragments of the O-antigen, and used in Vibrio cholerae O139-related antigenicity studies. The 13C NMR spectra for 16 showed the expected downfield shift of the signal for C-4III and C-2IV as a result of colitosylation at these positions. In addition, signals for the two anomeric protons of the colitose moieties appeared as doublets (1H NMR) at δH 5.24 and 5.03 ppm (J = 3.2 and 3.7 Hz, respectively), which confirmed the formation of the desired α-glycosidic linkages.
Scheme 3.
Synthesis of the Spacer-Equipped, Phosphorylated Hexasaccharide 21.
Subsequent de-O-acetylation (Zemplén) of 16, followed by selective phosphorylation with 2,2,2-trichloroethyl phosphorodichloridate[22] at −20°C gave predominantly the (S)-(P)-4IV,6IV-cyclic 2,2,2-trichloroethyl phosphate 18 (S/R = 9:1, 31P NMR, ~91% combined yield). Global deprotection of 18 (by transforming 13 functional groups in one-pot reaction) was successfully carried out by catalytic hydrogenation/hydrogenolysis (Pd/C, H2, 1 atm.) at pH=7 (0.1 M potassium phosphate buffer, to neutralize HCl formed). Compound 20 was obtained (87%) in pure state (TLC, NMR[23]) by HPLC.
Performing the phosphorylation at a very late stage of the overall synthesis is an important feature in the design of this synthetic sequence. Although the two isomeric (S,R) cyclic phosphates can be separated by chromatography, a mixture of the isomeric phosphates formed can be used directly for the reductive deprotection step. Because the phosphorus atom is no longer asymmetric after removal of the trichloroethyl group,[24,25] the same product is formed from S and R isomers, and the amount of the product can be increased. Accordingly, similar treatment of a mixture of the two isomeric cyclic phosphates 18 and 19 gave, as expected, the desired compound 20 in comparable yield.
Saponification (0.1 M KOH in H2O at pH=11) of the methyl ester 20 was followed by HPLC purification to give pure spacer-equipped, phosphorylated hexasaccharide 21 (83% yield), and its structure was confirmed by NMR and HRMS data.[26]
In conclusion, we have achieved the first synthesis of the full O-antigen of a human disease-causing pathogen (Vibrio cholerae O139, which is a complex, branched hexasaccharide consisting of five different monosaccharides and a cyclic phosphate, Figure 1). The highlights of the synthesis include: regio- and stereoselective transformations, and the protecting group strategy that allows global deprotection. The final hexasaccharide is equipped with a linker, which is functionalized for conjugation to yield a vaccine. Both conjugation and related antigenicity studies with the hexasaccharide antigen and a wide spectrum of fragments thereof are in progress.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health (NIH) and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
References
- 1.Kaper JB, Morris JG, Levine MM. Clin. Microbiol. Rev. 1995;8:48–86. doi: 10.1128/cmr.8.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramamurthy T, Garg S, Sharma R, Bhattacharya SK, Nair GB, Shimada T, Takeda T, Karasawa T, Kurazano H, Pal A, Takeda Y. Lancet. 1993;341:703–704. doi: 10.1016/0140-6736(93)90480-5. [DOI] [PubMed] [Google Scholar]
- 3.Albert MJ, Siddique AK, Islam MS, Faruque ASG, Ansaruzzaman M, Faruque SM, Sack RB. Lancet. 1993;341:704. doi: 10.1016/0140-6736(93)90481-u. [DOI] [PubMed] [Google Scholar]
- 4.Albert MJ, Ansaruzzaman M, Bardhan PK, Faruque ASG, Faruque SM, Islam MS, Mahalanabis D, Sack RB, Salam MA, Siddique AK, Yunus MD, Zaman K. Lancet. 1993;342:387–390. [Google Scholar]
- 5.Kováč P. In: Protein-Carbohydrate Interactions in Infectious Diseases. Bewley C, editor. London: Royal Society of Chemistry; 2006. pp. 175–220. [Google Scholar]
- 6.Chernyak A, Kondo S, Wade TK, Meeks MD, Alzari PM, Fournier JM, Taylor RK, Kováč P, Wade WF. J. Infect. Dis. 2002;185:950–962. doi: 10.1086/339583. [DOI] [PubMed] [Google Scholar]
- 7.Tarique AA, Kalsy A, Arifuzzaman M, Rollins SM, Charles RC, Leung DT, Harris JB, LaRocque RC, Sheikh A, Bhuiyan MS, Saksena R, Clements JD, Calderwood SB, Qadri F, Kováč P, Ryan ET. Clin. Vaccine Immunol. 2012;19:594–602. doi: 10.1128/CVI.05689-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robbins JB, Schneerson R, Szu SC, Pozsgay V. Pure Appl. Chem. 1999;71:745–754. [Google Scholar]
- 9.Cox AD, Brisson JR, Varma V, Perry MB. Carbohydr. Res. 1996;290:43–58. doi: 10.1016/0008-6215(96)00135-8. [DOI] [PubMed] [Google Scholar]
- 10.Cox AD, Perry MB. Carbohydr. Res. 1996;290:59–65. doi: 10.1016/0008-6215(96)00131-0. [DOI] [PubMed] [Google Scholar]
- 11.Knirel YA. In: Microbial Glycobiology: Structures, Relevance and Applications. Moran A, Brennan P, Holst O, von Itzstein M, editors. Amsterdam: Elsevier; 2009. pp. 57–73. [Google Scholar]
- 12.Soliman SE, Kováč P. J. Org. Chem. 2015;80:4851–4860. doi: 10.1021/acs.joc.5b00562. [DOI] [PubMed] [Google Scholar]
- 13.Soliman SE, Kováč P. J. Org. Chem. 2015;80:11227–11232. doi: 10.1021/acs.joc.5b02105. [DOI] [PubMed] [Google Scholar]
- 14.Sherman AA, Yudina ON, Mironov YV, Sukhova EV, Shashkov AS, Menshov VM, Nifantiev NE. Carbohydr. Res. 2001;336:13–46. doi: 10.1016/s0008-6215(01)00213-0. [DOI] [PubMed] [Google Scholar]
- 15.Soliman SE, Kováč P. Synthesis. 2014;46:748–751. [Google Scholar]
- 16.Banoub J, Boullanger P, Lafont D. Chem. Rev. 1992;92:1167–1195. [Google Scholar]
- 17.Sym-collidine, as a weak non-nucleophilic organic base, was used to prevent any acyl group migration during the selective O-debromoacetylation reaction.
- 18.Ruttens B, Kováč P. Synthesis. 2004:2505–2508. [Google Scholar]
- 19.Sato S, Mori M, Ito Y, Ogawa T. Carbohydr. Res. 1986;155:C6–C10. doi: 10.1016/s0008-6215(00)90162-9. [DOI] [PubMed] [Google Scholar]
- 20.Soliman SE, Bassily RW, El-Sokkary RI, Banoub J, Nashed MA. Carbohydr. Res. 2009;344:395–399. doi: 10.1016/j.carres.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 21.Lemieux RU, Hendriks KB, Stick RV, James K. J. Am. Chem. Soc. 1975;97:4056–4062. [Google Scholar]
- 22.Grześkowiak K. Synthesis. 1980:831–833. [Google Scholar]
- 23.Data for compound 20: 31P NMR (162 MHz, D2O): δ= −3.73 (3JP,H = 21.9 Hz); 1H NMR (600 MHz, D2O): δ= 5.23 (d, J1,2 = 3.8 Hz, H-1II), 4.96 (d, J1,2 = 3.4 Hz, H-1V), 4.88 (d, J1,2 = 3.3 Hz, H-1VI), 4.66 (d, J1,2 = 8.2 Hz, H-1IV), 4.48 (d, J1,2 = 8.3 Hz, H-1III), 4.46 (d, J1,2 = 8.5 Hz, H-1I); 13C NMR (150 MHz, D2O): δ = 102.95 (C-1III), 101.17 (C-1I), 101.12 (C-1IV), 100.88 (C-1II), 99.56 (C-1V) 97.76 (C-1VI); HRMS (ESI-TOF): m/z [M – H]− calcd for C47H79N3O31P: 1212.4435; found: 1212.4446.
- 24.Grześkowiak K, Adamiak RW, Wiewiorowski M. Nucleic Acids Res. 1980;8:1097–1105. doi: 10.1093/nar/8.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paquet A. Int. J. Peptide Protein Res. 1992;39:82–86. doi: 10.1111/j.1399-3011.1992.tb01559.x. [DOI] [PubMed] [Google Scholar]
- 26.Data for compound 21: 31P NMR (162 MHz, D2O): δ = −3.72 (3JP,H = 21.4 Hz); 1H NMR (600 MHz, D2O): δ = 5.26 (d, J1,2 = 3.9 Hz, H-1II), 4.96 (d, J1,2 = 3.7 Hz, H-1V), 4.77 (d, overlapped, H-1VI), 4.67 (d, J1,2 = 8.1 Hz, H-1IV), 4.47 (J1,2 = 8.7 Hz, H-1I), 4.45 (J1,2 = 8.5 Hz, H-1III); 13C NMR (150 MHz, D2O): δ = 102.79 (C-1III), 101.31 (C-1I), 101.17 (C-1IV), 100.22 (C-1II), 99.45 (C-1V) 97.94 (C-1VI); HRMS (ESI-TOF): m/z [M – H]− calcd for C46H77N3O31P: 1198.4279; found: 1198.4282.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.