Abstract
Objectives
Chest pain is a common complaint in the emergency department, and a small but important minority represents an acute coronary syndrome (ACS). Variation in diagnostic workup, risk stratification, and management may result in underuse, misuse, and/or overuse of resources.
Methods
From July to October 2014, we conducted a prospective cohort study in an academic medical center by implementing a Standardized Clinical Assessment and Management Plan (SCAMP) for chest pain based on the HEART score. In addition to capturing adherence to the SCAMP algorithm and reasons for any deviations, we measured troponin sample timing; rates of stress test utilization; length of stay (LOS); and 30-day rates of revascularization, ACS, and death.
Results
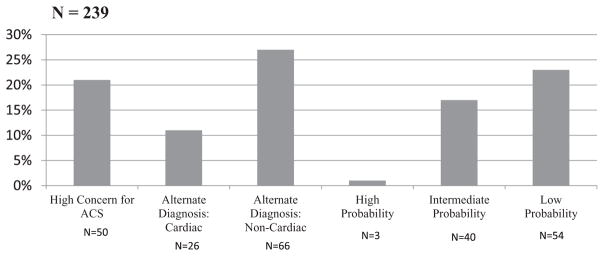
We identified 239 patients during the enrollment period who were eligible to enter the SCAMP, of whom 97 patients were entered into the pathway. Patients were risk stratified into one of 3 risk tiers: high (n = 3), intermediate (n = 40), and low (n = 54). Among low-risk patients, recommendations for troponin testing were not followed in 56%, and 11% received stress tests contrary to the SCAMP recommendation. None of the low-risk patients had elevated troponin measurements, and none had an abnormal stress test. Mean LOS in low-risk patients managed with discordant plans was 22:26 h/min, compared with 9:13 h/min in concordant patients (P < 0.001). Mean LOS in intermediate-risk patients with stress testing was 25:53 h/min, compared with 7:55 h/min for those without (P < 0.001). At 30 days, 10% of intermediate-risk patients and 0% of low-risk patients experienced an ACS event (risk difference 10% [0.7%–19%]); none experienced revascularization or death. The most frequently cited reason for deviation from the SCAMP was lack of confidence in the tool.
Conclusions
Compliance with SCAMP recommendations for low- and intermediate-risk patients was poor, largely due to lack of confidence in the tool. However, in our study population, outcomes suggest that deviation from the SCAMP yielded no additional clinical benefit while significantly prolonging emergency department LOS.
More than 8 million people per year visit emergency departments(EDs) in the United States with a chief complaint of chest pain, which constitutes 5% to 7% of all visits.1,2 The variation and subtlety in acute coronary syndrome (ACS) presentations creates a challenging task for emergency physicians to manage these patients when they present to the ED; for example, a presenting complaint of dyspnea without chest pain could represent ACS.1 As a result, missed myocardial infarction continues to be one of the most common reasons behind malpractice lawsuits filed against emergency physicians today, representing about 20% of all claims.3 Emergency physicians have to use their clinical judgment to piece together several elements of a patient’s presentation to determine the appropriate disposition: history, physical exam, electrocardiogram (EKG), and cardiac biomarkers all inform this judgment, but none is sufficiently sensitive and specific enough to rely on in isolation to adequately exclude the presence of ACS in the relatively short amount of time permitted in an ED visit.1
Patients with chest pain present to the ED across a wide spectrum of acuity, from unstable patients in distress to patients with minor and chronic discomfort. Protocols are used to rapidly identify and disposition patients who would benefit from emergent coronary revascularization. For example, triage nurses typically use standing orders to obtain an EKG on any patient presenting with chest pain, with an arrival to interpretation goal within 10 minutes to screen for ST-segment elevation myocardial infarction and door to balloon time goal of 90 minutes or less.2,4 Patients without evidence of ST-segment elevation myocardial infarction remain in the ED for further evaluation. Typically, this evaluation includes a focused history and physical exam, and for patients where ACS is suspected, additional information such as serial EKGs, troponin testing, and discussions with outpatient cardiologists are added. Patients are risk stratified, either informally via attending physician gestalt or by a validated decision tool such as the HEART score (Table 1).5 Frequently, high-risk patients are admitted to the cardiology or medicine service, those at intermediate risk can be admitted to an inpatient service or managed in an observation unit, and those at low risk are managed in an observation unit or sent home.
TABLE 1.
HEART Score5
| Factor | Value | Points* |
|---|---|---|
| History | Highly suspicious (2) Moderately suspicious (1) Slightly suspicious (0) |
0–2 |
| EKG | Significant ST-depression (2) Nonspecific repolarization disturbance (1) Normal (0) |
0–2 |
| Age | ≥65 years (2) 45–64 years (1) <45 years (0) |
0–2 |
| Risk factors | ≥3 risk factors or history of atherosclerotic disease (2) 1–2 risk factors (1) No risk factors known (0) Hypercholesterolemia Hypertension Diabetes mellitus Cigarette smoking Positive family history Obesity |
0–2 |
| Troponin | >3× normal limit (2) 1–3× normal limit (1) <normal limit (0) |
0–2 |
0–3 = low risk; 4–6 = intermediate risk; 7–10 = high risk.
Our objective was to develop and implement a consensus risk stratification and management pathway for patients presenting to the ED with chest pain or anginal equivalents, such as dyspnea. The main goals of the chest pain pathway were to explicitly define the strategy for serial troponin and stress test ordering, provide clarity for resident and nursing staff, and reduce variability between providers. We also hypothesized that using a common risk stratification tool and management pathway as the vehicle for this process would reduce the ED length of stay (LOS) without missing clinically-relevant 30-day outcomes in our cohort.
METHODS
Study Design
We conducted a prospective cohort quality improvement project of patients arriving to the ED at Brigham and Women’s Hospital with a chief complaint concerning for ACS. Trained research assistants prospectively screened patients over approximately 4 months between July and October of 2014 via an electronic tracking board and routine rounds with the clinical staff. In addition to chest pain, we included other complaints that are common anginal equivalents. In addition, they were alerted via page whenever a troponin test was resulted for an ED patient.
This project was part of our hospital’s Standardized Clinical Assessment and Management Plan (SCAMP) intervention. Originally developed at Boston Children’s Hospital, SCAMPs are a quality improvement approach that aim to provide insight into undefined areas of care and optimize outcomes through iterative implementation and refinement of a standardized care pathway.6 With a goal of developing guidelines for standardizing and improving care in areas where there is variation in clinical practice, SCAMPs facilitate the collection and analysis of clinician decision data. Physicians are free to deviate from the guideline at any point, but are asked to explain why. The Institute for Relevant Clinical Data Analytics (IRCDA), a nonprofit, tax-exempt organization that provides the education and resources for the development, implementation, and analysis of SCAMPs at its member institutions, provided oversight of guideline development.7 This project was exempt from institutional review board review at our hospital based upon the institutional review board’s guidelines for quality improvement projects.
Study Setting and Population
Brigham and Women’s Hospital is an urban, tertiary-care teaching hospital with 60,050 adult ED visits in 2014. All ED patients are evaluated by a board-certified or board-eligible emergency physician. The department contains 39 acute-care beds, with additional capacity for up to 22 additional hallway patients and 20 dedicated observation unit beds, with emergency medicine oversight. All adult patients (≥18 years of age) presenting to the ED during hours of research assistant screening (8 AM–10 PM daily) with clinical concern for ACS during the project were considered eligible for inclusion in the SCAMP algorithm.
Design of SCAMP Decision Making Algorithm for Chest Pain
We developed the SCAMP algorithm (Fig. 1) via an iterative process with a multidisciplinary team including both emergency medicine and cardiology. First, we performed a structured literature review of chest pain pathways and risk stratification tools. We identified the HEART score as an intuitive, validated tool designed to be used with undifferentiated ED patients and most likely to gain widespread acceptance by our ED providers.5,8 Next, we formed a working group and held biweekly meetings and calls, developing the SCAMP documents (algorithm, data collection forms) collaboratively with the hospital’s SCAMP team, who had SCAMP implementation experience. When a final set of documents was created, clinical champions introduced the project and gave in-service educational sessions at staff meetings before implementation.
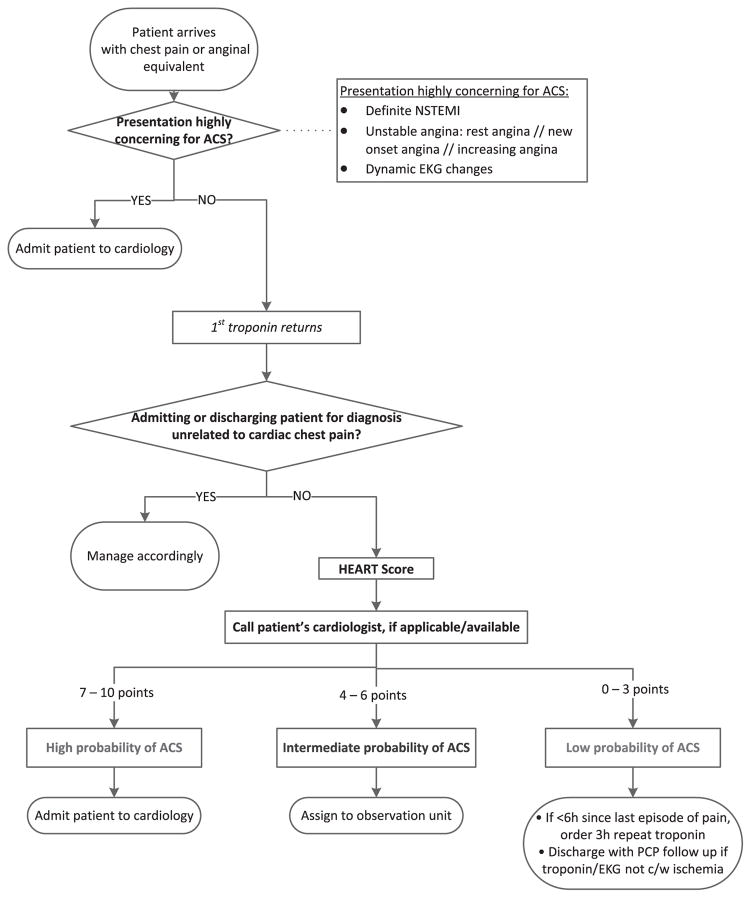
FIGURE 1.
SCAMP algorithm.
Patients were eligible to be risk stratified via the HEART score if they did not have a presentation highly concerning for ACS or an alternative non-ACS diagnosis confirmed after the initial ED evaluation (Fig. 1). Those who remained had an initial troponin resulted, which informed their HEART score. The recommendation for low-risk patients (HEART score 0–3) was a 3-hour repeat troponin and EKG if they presented to the ED within 6 hours of onset of pain or a single initial troponin for patients arriving with >6 hours of pain and no stress testing, provocative cardiac testing, or anatomical investigations (eg, coronary computed tomography angiography). We recommended intermediate-risk patients (HEART score 4–6) receive a repeat troponin and EKG at 6 hours and no default stress testing if the remainder of their visit was unremarkable. We recommended inpatient cardiology admission for high-risk patients (HEART score 7–10). Our ED uses the Roche fourth-generation troponin T assay, which is considered a contemporary assay.
Data Sources and Collection
Project staff abstracted clinical data, demographics, comorbidities, and laboratory results from ED visit notes in our electronic medical record. Vital signs were obtained from the patients’ paper nursing records. Data about provider adherence and reasons for deviation from the proposed SCAMP algorithm, and prospective HEART score calculation by the attending emergency physician were obtained from the SCAMP forms. Provider compliance with completing SCAMP forms was monitored by a data coordinator, who followed up with providers daily to ensure form completion; 100% of SCAMPs forms were completed for the initial ED assessment.
Outcome Measures
Our main outcome measure was compliance with SCAMP management recommendations, based on risk category. Recommendations were focused on troponin sampling timing, use of stress testing, cardiology consultation, and disposition. We permitted attending emergency physicians to manage patients discordant to pathway recommendations, but required an explanation for doing so, and cataloged their responses. We considered a troponin strategy to be compliant if providers ordered a troponin approximate to the recommendations within 1.5 hours, allowing for variation in sample acquisition, lab transport, and lab processing time.
Our secondary outcomes were LOS and 30-day major adverse cardiovascular events (eg, death, ACS events, and revascularization). Trained research assistants performed 30-day chart reviews of the electronic medical record, which includes both inpatient and outpatient records for our entire integrated health system, to determine the presence of a secondary outcome event. Potential ACS events were secondarily reviewed by a cardiologist blinded to SCAMP compliance to determine the most accurate final diagnosis.
Data Analysis
Patient demographic and clinical characteristics are reported as counts and percentages. Wilcoxon rank sum tests were used to compare mean LOS (observation and hospital) between those who adhered to SCAMP recommendations and those who did not. Chi square and Fisher exact tests were used to evaluate differences in incidence of abnormal stress tests, ACS, 30-day revascularization, and 30-day mortality between low- and intermediate-risk patients. All analyses were performed using R 3.2.2 (The R Foundation for Statistical Computing).9
RESULTS
During the study period, 239 patients were included in the SCAMP pathway. Of these, 50 patients were highly concerning for ACS during the initial ED evaluation and were admitted to the cardiology service without further testing or risk stratification. Additionally, 92 patients were found to have an alternative non-ACS diagnosis explaining their symptoms during their ED visit. The HEART score was used for risk stratification on the remaining 97 patients (Fig. 2). Table 2 describes the patient characteristics informing the HEART score points awarded for each of the 3 risk tiers: high, intermediate, and low.
FIGURE 2.
Patient risk stratification distribution.
TABLE 2.
Patient Characteristics of SCAMP Patients Who Underwent HEART Score Risk Stratification
| Criteria | High Risk of ACS (7–10 Points) (N = 3) | Intermediate Risk of ACS (4–6 Points) (N = 40) | Low Risk of ACS (0–3 Points) (N = 54) |
|---|---|---|---|
| History | |||
| Highly suspicious (2 points) | 2 (67%) | 7 (18%) | 1 (2%) |
| Moderately suspicious (1 point) | 1 (33%) | 23 (57%) | 18 (33%) |
| Slightly or nonsuspicious (0 point) | — | 10 (25%) | 35 (65%) |
| EKG | |||
| Significant ST-depression (2 points) | 1 (2%) | ||
| Nonspecific repolarization disturbance (1 point) | 1 (33%) | 18 (45%) | 10 (18%) |
| Normal (0 points) | 2 (67%) | 22 (55%) | 43 (80%) |
| Age | |||
| ≥65 years (2 points) | 2 (67%) | 25 (63%) | 11 (20%) |
| 46–64 years (1 point) | 1 (33%) | 13 (33%) | 26 (48%) |
| ≤45 years (0 points) | - | 2 (4%) | 17 (32%) |
| Risk factors (diabetes mellitus, current smoker, hypertension, hypercholesterolemia, family history of CAD) | |||
| ≥3 risk factors, or history of atherosclerotic disease (2 points) | 3 (100%) | 24 (60%) | 2 (4%) |
| 1 or 2 risk factors (1 point) | — | 14 (35%) | 35 (65%) |
| No risk factors known (2 points) | — | 2 (5%) | 17 (31%) |
| Troponin | |||
| ≥0.03 ng/mL (2 points) | 2 (67%) | — | — |
| ≥0.01 to <0.03 ng/mL (1 point) | 1 (33%) | 2 (5%) | — |
| <assay (0 points) | — | 38 (95%) | 54 (100%) |
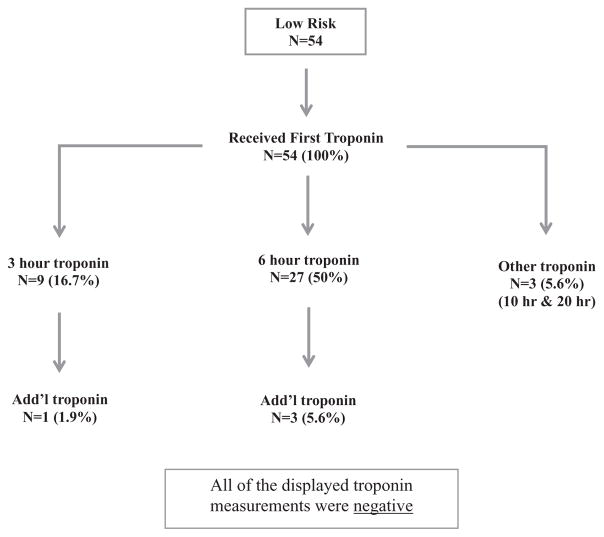
Figure 3 describes the troponin sampling that occurred in low-risk patients. Seventy-two percent (39/54) of these patients subsequently received additional troponin measurements, all yielding negative results as well. Overall, the troponin timing recommendation was not followed in 56% of low-risk patients (ie, additional troponin assays were ordered beyond 3 hours). Stress testing occurred in 11% of low-probability patients and 38% of intermediate-risk patients. Stress results were abnormal in none of the low-risk patients and abnormal in 5 (33%) of the intermediate-risk patients (risk difference 33.3% [9%–57%]). Stress test resulted in changes in management in no low-risk patients and 3 of 15 (20%) intermediate-risk patients.
FIGURE 3.
Troponin sampling in low-risk patients (HEART 0–3).
The mean LOS in low-risk patients with stress testing was 22:26 h/min, compared with 9:13 h/min for those without (P < 0.001). Mean LOS in intermediate-risk patients with stress testing was 25:53 h/min, compared with 7:55 h/min for those without (P < 0.001; Table 3). As illustrated in Table 4, 10% of intermediate-risk patients and 0% of low-risk patients experienced an ACS event (risk difference 10% [0.7%–19%]) and none experienced revascularization or death within 30 days of discharge. Table 5 describes the most common reasons for deviation from the SCAMP pathway recommendations for low-risk patients.
TABLE 3.
Length of Stay for Low-Risk and Intermediate-Risk Patients: Impact of Stress Testing
| N | Mean LOS* (hour:min) | P | |
|---|---|---|---|
| Low risk (n = 54) | |||
| Deviated from SCAMP: ordered stress test | 6 | 22:26 | <0.001 |
| Followed SCAMP: did not order stress test | 48 | 9:13 | |
| Intermediate risk (n = 40) | |||
| Deviated from SCAMP: ordered stress test | 15 | 25:53 | <0.001 |
| Followed SCAMP: did not order stress test | 25 | 7:55 | |
Time from arrival to departure for emergency department plus observation unit stay (if used).
TABLE 4.
30-Day Outcomes for Low- and Intermediate-Risk Patients
| Low Risk (N = 54) | Intermediate Risk (N = 40) | Risk Difference (95% CI) | |
|---|---|---|---|
| ACS | 0 | 4 | 10% (0.7%–19%) |
| Revascularization | 0 | 0 | N/A |
| Death within 30 days | 0 | 0 | N/A |
N/A, not applicable.
TABLE 5.
Reasons for Deviation in Low-Risk Patients
| Low Risk: Plan of Care | N (%) |
|---|---|
| Order 3-hour troponin in ED (recommendation) | 9 (17) |
| Discharge home | 8 (15) |
| Reasons | |
| Initial troponin to rule out myocarditis | 1 |
| Chest pain resolved with ibuprofen | 1 |
| Patient left against medical advice before determining plan of care | 1 |
| Recommendation of cardiologist | 1 |
| Not recorded | 4 |
| Order 6-hour troponin in ED | 2 (4) |
| Reasons | |
| Outside hospital transfer first troponin 6 hours ago | 1 |
| Previous attending ordered it | 1 |
| Assign to ED observation | 32 (58) |
| Reasons | |
| Disagree with SCAMP pathway | 7 |
| 6-hour troponin | 6 |
| To obtain stress test | 3 |
| Second troponin and stress test | 2 |
| Patient does not have cardiologist | 2 |
| *illegible* | 1 |
| Second troponin (probably peripheral vertigo) | 1 |
| 6-hour troponin and neurology consult | 1 |
| MRI/physical therapy evaluation | 1 |
| Observation for GI workup | 1 |
| V/Q scan for possible pulmonary embolism | 1 |
| Telemetry for arrhythmia check, rule out pulmonary embolism | 1 |
| 6-hour troponin, likely gastritis | 1 |
| Low suspicion cardiac chest pain | 1 |
| Patient has atrial fibrillation | 1 |
| Severity of pain | 1 |
| Unreliable patient | 1 |
| Admit to cardiology | 1 (2) |
| Reason | |
| Tetralogy of fallot, unusual chest pain patient | 1 |
| Admit to medicine/other | 2 (4) |
| Reasons | |
| Atrial fibrillation, came back and was symptomatic so was admitted to heart failure service for further evaluation | 1 |
| Pulmonary embolism diagnosis | 1 |
MRI, magnetic resonance imaging; GI, gastroenterology; V/Q, ventilation/perfusion.
DISCUSSION
In this single-center prospective cohort, implementation of an accelerated diagnostic protocol featuring the HEART score helped to identify and expedite the ED evaluation of low-risk chest pain patients. However, clinicians often deviated from pathway recommendations by extending the interval between troponin assay measurements and adding stress testing in a minority of patients—all without any additional diagnostic benefit. Many other risk stratification tools have been previously developed, validated, and published, such as the Goldman rule, Acute Cardiac Ischemia-Time Insensitive Predictive Instrument (ACI-TIPI), and Thrombolysis in Myocardial Infarction risk score.10–12 However, in routine clinical practice in the ED, it is unusual to see one of these tools formally applied to guide patient management. Local factors, such as the institutional standard of care, provider-specific experience, knowledge and risk tolerance, unique patient characteristics, and expectations and resource availability often influence the plan of care, resulting in variable testing and dispositions. No single risk stratification tool is universally recognized as the gold standard by all providers. As a result, there is an opportunity to develop and implement an institution-specific risk stratification tool tied to specific testing and disposition recommendations that takes local factors into account.13
The risk stratification tool at the center of our SCAMP intervention was the HEART score. First introduced in 2008 and further validated several times, there is increasing awareness and acceptance of this tool among emergency physicians.5,8 Prior studies with prospective use of this tool have shown an extremely low 30-day outcome rate in low-risk patients without the benefit of stress testing or coronary computed tomography angiography.14 However, there has been a paucity of prospective US-based studies featuring the HEART score among undifferentiated ED patients. Our study further shows that although many low-risk patients did undergo further risk stratification at the request of their treating physician, none of those were abnormal—further adding to the evidence against routine testing in this population. Our data also illustrate the significant LOS cost of lengthening troponin testing intervals and adding stress testing in patients unlikely to benefit from additional testing. Our nonadherence rate was even higher than those reported in other recent investigations, which also show providers tend to be more conservative with diagnostic ordering without clinical benefit in the low-risk cohort as defined by the HEART score.15
Health systems and hospitals are under increasing pressure to reduce use of hospitalization and accurately classify hospitalized patients as inpatient or observation status. As a result, the number of observation patients has increased and many ED leaders have been asked by hospital leadership to expand ED observation capacity.16 Patients eligible for outpatient care can be assigned to an inpatient team for any one of several factors: lack of observation unit capacity (or access to one), inadequate observation unit nursing resources, outpatient cardiologist request for inpatient management, inadequate access to diagnostic resources (ie, access to a stress test over a holiday weekend), representation with the same complaint, lack of access to cardiology consultation, and lack of access to expedited outpatient follow up, among others. Some of the above factors can be mitigated (ie, increase access to expedited cardiology clinic follow-up), and others are much more difficult to address. Obtaining real-time logic for the decision to assign a low-risk chest pain patient to an inpatient service provides a critical insight into why some patients are diverted to inpatient care. Our findings suggest that resistance to the pathway itself was the most common reason for deviation, as most providers elected a more conservative approach. Practice inertia around longer troponin sampling intervals and routine stress testing should be expected when clinical leaders attempt to implement new care recommendations that are seen as less conservative, even if based on the most recent literature. One reason for this practice is the longstanding American Heart Association and American College of Cardiology recommendations that stress testing within 72 hours “is reasonable” for the low-risk patient.17 Advocates for less testing in low-risk population continue to add to the evidence base supporting this practice and lobby specialty societies to adjust their guidance accordingly.14,18,19
A favorable stress test result is well correlated to a very low risk of serious cardiac events over the short and intermediate term.17,20 As a result, clinicians and patients perform stress testing to provide a measure of reassurance, especially when a cardiac origin of chest pain is suspected. However, in low-risk patients, stress testing, especially exercise tolerance tests, has poor test characteristics: a sensitivity of 78% and a specificity of 70%.21 They have a good negative predictive value because the population being tested has essentially no risk of serious events. Clinicians and researchers are questioning the need for routine stress testing in patients presenting with low-risk chest pain to the ED.22 This also includes coronary computed tomography angiography, which, while typically adding less time to an evaluation, also exposes the patient to potential harms of radiation and contrast media.23 As a result, there is a call for a large trial to provide the clinical evidence for stress testing in this population; however, due to the low rate of major adverse clinical events in these patients, and the bias that an abnormal stress test may lead to coronary revascularization, regardless of relevance to the patient’s presenting symptoms, it is unlikely that such a trial will be successfully conducted in the near future.
As highly sensitive troponin assays make their way to the US market, the importance of advance guidance around sampling timing and interpretation of results will increase. Recent evidence supports decreasing the time between serial troponin measurements using contemporary troponin assays (available in the United States) among low-risk patients; as a result, more patients are being managed entirely in the ED with serial troponin testing alone, with a disposition to home if testing is normal.14,24 Historically, these patients have either remained in the ED or remained in the hospital in a dedicated observation unit or an inpatient area to undergo further risk stratification via stress testing as part of a “rule out myocardial infarction” evaluation. The need for stress testing in those patients deemed low risk by a validated tool such as the HEART score has also recently been questioned.22,23 New “accelerated diagnostic protocols,” performed both with highly sensitive and contemporary troponin assays, have shown favorable sensitivity in a low-risk population when shortening the troponin interval to as little as 2 hours without additional stress testing.14,24–26
LIMITATIONS
Our study has several limitations. First, the study involved a single academic medical center; therefore, generalizability to other settings (ie, community hospitals, centers without access to an observation unit) may be limited. Clinical decision making can vary based on site of practice, which further limits the generalizability of this study. On the basis of these factors, we believe an expansion of our study to multiple, diverse clinical sites is warranted. A potential confounding factor is that providers who choose to adhere to the SCAMP recommendation may have been inherently “better providers” and, therefore, had better outcomes compared with those that did not adhere to the SCAMP. We feel that asking providers to explain reasons for deviation potentially mitigates this concern as all providers had to justify, and therefore carefully consider, nonadherence to the SCAMP. This study was not powered to validate the HEART score’s ability to exclude 30-day adverse clinical outcomes in low-risk patients. Finally, implementation of the SCAMP itself required careful tracking of patients and reminders to providers to utilize the decision-making algorithm, which calls into question feasibility of implementation outside of a quality improvement setting. Careful consideration of implementation is necessary, and potential options include incorporation of the SCAMP algorithm into a daily progress note or design of an electronic medical record prompt that meets criteria.
CONCLUSIONS
In this prospective cohort quality improvement project, low-risk patients identified via the HEART score who had longer troponin sampling intervals or stress testing did not have abnormal results. These patients also did not have any adverse 30-day clinical outcomes. Compliance with management recommendations for low-risk patients was poor and additional testing yielded no additional clinical benefit while significantly prolonging ED LOS. This study supports an accelerated diagnostic protocol for low-risk patients identified via the HEART score with shorted troponin cycling times and avoidance of stress testing during or immediately after the index ED visit. Future research is needed to identify strategies to improve provider compliance with accelerated diagnostic protocols for chest pain patients in the ED.
Footnotes
C.W.B., J.O.G., and B.W.S. conceived the study and C.W.B. served as principal investigator. R.G., S.P., and C.C. provided statistical advice on study design. G.R.C. performed the statistical analysis of main results. C.W.B. drafted the manuscript, and all authors contributed substantially to its revision. C.W.B. takes responsibility for the paper as a whole.
DISCLOSURES
C.W.B. is an advisory board member and paid speaker for Roche Diagnostics, and also is an advisory board member and consultant for Janssen Pharmaceuticals. J.M.K. is a member of a Clinical Endpoints Committee for a trial sponsored by Roche Diagnostics.
References
- 1.Chandra A, Rudraiah L, Zalenski RJ. Stress testing for risk stratification of patients with low to moderate probability of acute cardiac ischemia. Emerg Med Clin North Am. 2001;19:87–103. doi: 10.1016/s0733-8627(05)70169-3. [DOI] [PubMed] [Google Scholar]
- 2.Amsterdam EA, Kirk JD, Bluemke DA, et al. American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology, Council on Cardiovascular Nursing, and Interdisciplinary Council on Quality of Care and Outcomes Research. Testing of low-risk patients presenting to the emergency department with chest pain: a scientific statement from the American Heart Association. Circulation. 2010;122:1756–1776. doi: 10.1161/CIR.0b013e3181ec61df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta RH, Eagle KA. Missed diagnoses of acute coronary syndromes in the emergency room–continuing challenges. N Engl J Med. 2000;342:1207–1210. doi: 10.1056/NEJM200004203421610. [DOI] [PubMed] [Google Scholar]
- 4.O’Gara PT, Kushner FG, Ascheim DD, et al. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–e425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 5.Backus BE, Six AJ, Kelder JC, et al. Chest pain in the emergency room: a multicenter validation of the HEART Score. Crit Pathw Cardiol. 2010;9:164–169. doi: 10.1097/HPC.0b013e3181ec36d8. [DOI] [PubMed] [Google Scholar]
- 6.Farias M, Jenkins K, Lock J, et al. Standardized clinical assessment and management plans (SCAMPs) provide a better alternative to clinical practice guidelines. Health Aff (Millwood) 2013;32:911–920. doi: 10.1377/hlthaff.2012.0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Institute for Relevant Clinical Data Analytics. [Accessed June 2016];Adult SCAMPs. Available at: http://www.scamps.org.
- 8.Six AJ, Cullen L, Backus BE, et al. The HEART score for the assessment of patients with chest pain in the emergency department: a multinational validation study. Crit Pathw Cardiol. 2013;12:121–126. doi: 10.1097/HPC.0b013e31828b327e. [DOI] [PubMed] [Google Scholar]
- 9.Team RC. [Accessed June 2016];R: A language and environment for statistical computing. Available at: https://www.r-project.org.
- 10.Goldman L, Cook EF, Brand DA, et al. A computer protocol to predict myocardial infarction in emergency department patients with chest pain. N Engl J Med. 1988;318:797–803. doi: 10.1056/NEJM198803313181301. [DOI] [PubMed] [Google Scholar]
- 11.Selker HP, Beshansky JR, Griffith JL, et al. Use of the acute cardiac ischemia time-insensitive predictive instrument (ACI-TIPI) to assist with triage of patients with chest pain or other symptoms suggestive of acute cardiac ischemia. A multicenter, controlled clinical trial. Ann Intern Med. 1998;129:845–855. doi: 10.7326/0003-4819-129-11_part_1-199812010-00002. [DOI] [PubMed] [Google Scholar]
- 12.Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284:835–842. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 13.Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8:421–427. doi: 10.1002/jhm.2054. [DOI] [PubMed] [Google Scholar]
- 14.Mahler SA, Riley RF, Hiestand BC, et al. The HEART Pathway randomized trial: identifying emergency department patients with acute chest pain for early discharge. Circ Cardiovasc Qual Outcomes. 2015;8:195–203. doi: 10.1161/CIRCOUTCOMES.114.001384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahler SA, Riley RF, Russell GB, et al. Adherence to an accelerated diagnostic protocol for chest pain: secondary analysis of the HEART pathway randomized trial. Acad Emerg Med. 2016;23:70–77. doi: 10.1111/acem.12835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schrager J, Wheatley M, Georgiopoulou V, et al. Favorable bed utilization and readmission rates for emergency department observation unit heart failure patients. Acad Emerg Med. 2013;20:554–561. doi: 10.1111/acem.12147. [DOI] [PubMed] [Google Scholar]
- 17.Amsterdam EA, Wenger NK, Brindis RG, et al. ACC/AHA Task Force Members; Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2354–2394. doi: 10.1161/CIR.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 18.Choosing Wisely. [Accessed June 2016];Stress Tests for Chest Pain: When You Need an Imaging Test–And When you Don’t. Available at: http://www.choosingwisely.org/patient-resources/stress-tests-for-chest-pain.
- 19.Hess EP, Brison RJ, Perry JJ, et al. Development of a clinical prediction rule for 30-day cardiac events in emergency department patients with chest pain and possible acute coronary syndrome. Ann Emerg Med. 2012;59:115–25. e1. doi: 10.1016/j.annemergmed.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 20.Manini AF, McAfee AT, Noble VE, Bohan JS. Prognostic value of the Duke treadmill score for emergency department patients with chest pain. J Emerg Med. 2010;39:135–143. doi: 10.1016/j.jemermed.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 21.Hill J, Timmis A. Exercise tolerance testing. BMJ. 2002;324:1084–1087. doi: 10.1136/bmj.324.7345.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prasad V, Cheung M, Cifu A. Chest pain in the emergency department: the case against our current practice of routine noninvasive testing. Arch Intern Med. 2012;172:1506–1509. doi: 10.1001/archinternmed.2012.4037. [DOI] [PubMed] [Google Scholar]
- 23.Redberg RF. Coronary CT angiography for acute chest pain. N Engl J Med. 2012;367:375–376. doi: 10.1056/NEJMe1206040. [DOI] [PubMed] [Google Scholar]
- 24.Than M, Cullen L, Aldous S, et al. 2-Hour accelerated diagnostic protocol to assess patients with chest pain symptoms using contemporary troponins as the only biomarker: the ADAPT trial. J Am Coll Cardiol. 2012;59:2091–2098. doi: 10.1016/j.jacc.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 25.Keller T, Zeller T, Ojeda F, et al. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA. 2011;306:2684–2693. doi: 10.1001/jama.2011.1896. [DOI] [PubMed] [Google Scholar]
- 26.Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med. 2012;172:1211–1218. doi: 10.1001/archinternmed.2012.3698. [DOI] [PubMed] [Google Scholar]