Abstract
Objective: The extracellular matrix consists of critical components that affect fibroblast polarization and migration. The existence of both intrinsic and extrinsic electrical signals that play essential roles in the development, physiology, regeneration, and pathology of cells was discovered over a century ago. In this study, we study how the Bioelectric Field Enhancement (BEFE) device and its generated electromagnetic field (EMF) by continuous direct current (DC) significantly affect the membrane potential and cell migration of fibroblasts in vitro.
Approach: This is an experimental analysis of membrane potential and cell migration of murine fibroblasts when grown in treated media that has been reconstituted with an aqueous solution that has been exposed to an EMF, which is generated by this device versus fibroblasts grown in identically prepared control media that has not been exposed to the EMF.
Results: The growth of fibroblasts in the treated media shows a strong percent change in polarization of the plasma membrane and significant increase in cell migration compared to control groups.
Innovation: These experiments show the potential for an adjunct wound care therapy using a continuous DC EMF application through a medium of water.
Conclusion: Growth media that was reconstituted with an aqueous solution that had been exposed to this DC derived EMF shows significant changes in cell polarity and cell migration of fibroblasts in vitro. The BEFE device has shown enhanced chronic wound healing in anecdotal reports from patients globally for decades when used as a footbath/bath and could lead to a novel EMF application in wound healing.
Keywords: : bioelectrodynamics, membrane potential, cell migration, chronic wounds

Marcy C. Purnell, PhD, APN, FNP-C
Introduction
Electromagnetic fields (EMFs) activate multiple signaling pathways that are instrumental in cell polarization, migration, and wound healing.1–4 EMFs work in conjunction with and also override other signaling cues of cell migration such as: chemoattractant gradients, injury stimulation, contact inhibition, wound void, mechanical forces, and population pressure.1,5–7 Injured and inflamed cells are contained in a wound, and transmembrane potential differences have been found to exist in both injured and proliferating cells.8 Injured and proliferating cells have a measurable depolarized membrane potential of ∼<−30 mV, while healthy and nonproliferating cells have a resting potential of ∼>−70 mV.8,9 Cells respond to different EMFs/signals when undergoing mitosis, migration, and differentiation, and these magnetic fields also affect the changing gradients of Vmem that are produced and sensed by both excitable and nonexcitable cells in living organisms.6,8,10,11 Inadequate and unorganized cell migration is characteristic of chronic wounds and to heal, cells must migrate directionally and in an organized way for granulation tissues to begin to develop.2
EMF therapies in both in vitro and in vivo wound care research have been explored for decades and are currently being conducted with vibrating probe techniques that apply a flow of positive charge directed toward the wound using direct current (DC) or monophasic/biphasic pulsed electrical currents.5,12 Low frequency alternating current (AC) has not been used with much success since it appears to lack polarity.7 The extrapolation and translation of the use of these and other EMF applications appear to lack consensus in terms of standardization of parameters to facilitate protocol development for wound care.1,5
The Bioelectric Field Enhancement (BEFE) device is investigated in this body of work and has been used by people across the globe for over 20 years in holistic markets. Users of the BEFE device have reported pain relief, increased stamina, improvements in renal and hepatic function, support in cancer treatment recovery, decreased recovery time from illness and injury, enhanced wound healing etc., after immersing part or all of their body in the water while the 3 amperes of DC are flowing for 20–25 min every other day.13
Since fibroblasts synthesize the extracellular matrix (ECM), they are considered the structural framework for animal tissues and are known to play an important role in wound healing.1 We hypothesized that there would be a differential effect on cell migration and membrane potential between murine fibroblasts (L929 cells) grown in treated media that had been reconstituted with a hypotonic saline solution that had been exposed to the continuous DC driven EMF from the BEFE device compared to control media that is identically prepared without exposing the hypotonic saline solution to the EMF from the BEFE device. We have conducted and will discuss scratch and membrane potential assays that when coupled with anecdotal reports from across the globe offer potential for future translation of this device testing to in vivo models.
Clinical Problem Addressed
Wound healing has a major impact on both health and the economy. In the United States, 6.5 million patients have chronic wounds that cost 25 billion annually to treat and these costs are increasing rapidly due to the aging population coupled with the soaring rates of obesity and diabetes.14 Many of the biological mechanisms that both drive and prevent chronic wound healing are poorly understood and new and effective therapies are greatly needed to treat chronic wounds.1 Acute wounds are known to heal more quickly with electric stimulation such as is used in orthopedics and sports medicine where an electrical signal can lead to an upregulation of cartilage matrix protein production and nitric oxide–dependent chondrocyte proliferation from a bone growth stimulator.1,15 On the contrary, chronic nonhealing wounds that occur with diabetes, malnutrition, infection, chronic inflammation, and aging currently have no effective therapy available, and treatment for the biological impediments remains elusive.15 Thus, chronic wound care should continue to be a significant focus of our research endeavors.14
Materials and Methods
Cells and cell culture medias
L929 cells (mouse fibroblasts) (organism: Mus musculus; subcutaneous connective tissue/areolar and adipose tissue; age-100 days; gender-male; and strain-C3H/An) used in this research were obtained from ATCC. These fibroblasts (passage 4) were cultured in 1 × Dulbecco's modified Eagle's medium (No. D6429) with 10% fetal bovine serum (DMEM-10) from Atlanta Biologicals. To prepare 1 × DMEM, a 10 × DMEM (No. D2429) solution was diluted 9:1 with a hypotonic (3 mM) saline solution that had been treated for 30 min with a continuous 3.0 amperes DC driven EMF and filtered through Corning bottle top cellulose acetate membrane vacuum 0.22 micron filter (CLS430521; Sigma). The hypotonic saline solution was made using deionized water from the laboratory and a 5 M sodium chloride (Molecular Biology grade-No. V4221, Lot No. 18693201 DNase/RNase/Protease free) solution from Promega Corporation.
The medium was supplemented with the appropriate concentrations of folic acid, 0.004 gm/L (Sigma-Aldrich-F8758-5G, Lot No. SLBF 16021), glucose 4,000 mg/L (Sigma-Aldrich G7021-100G, Lot No. 071N01455), glutamine 0.584 gm/L (Sigma-Aldrich-G7513, Lot No. RNBC5892), and sodium bicarbonate 3.7 gm/L (BioWhittaker No. 15-6131) to obtain the exact concentrations of standard DMEM. Fetal bovine serum was added to the diluted DMEM to achieve a 10% concentration. Cells could not be directly treated with this device, which was originally built to be used by humans, and the size precludes it from being used in a petri dish.
Scratch assays
Murine fibroblasts (L929 cells), treated (5 × 105) (n = 18) and control (5 × 105) L929 (n = 18), were cultured in 60 mm petri dishes in standard DMEM-10 and were conducted with two repeats. The standard DMEM-10 that the cells were initially cultured in was aspirated and replaced with control and treated media in the respective plates. Then, a “scratch” in the cell monolayer was made with a p200 pipette tip that was attached to low suction. Three uniform scratches in parallel were made on each 60 mm dish along with two reference points that consisted of two perpendicular lines to the scratch lines made with a permanent marker on the bottom of the dishes. These reference points are critical to ensure consistent orientation to the same microscopic field when taking pictures and to insure accuracy of these measurements. The reference lines were framed in the picture for a reference point in each scratch to ensure we were capturing the same cell growth point of each photograph at each time interval.
Pictures were captured each scratch/wound at the time point zero of scratch and then at 3 h intervals until confluence in the treated group was reached at ∼12 h with a 10 × eye piece and 40 × objective utilizing AxioVision Imaging System. This time interval was calculated from the known doubling times of this cell line. The cells were placed in a 37°C/CO2 incubator between photographing time points.
Quantification of cell migration rate can be reported by the percentage change in area over time [Eq. (1)]:
 |
where A (0) is the initial area that is framed or enclosed by the population of the confluent cells, A (t) is the area enclosed by the population of confluent cells at time t, and M (t) is the percentage change in area at time t. Estimates of cell migration rates using this equation can be obtained by hand tracing that area enclosed by the leading edge of a spreading cell population and this can lead to errors and limitations due to subjectivity. To overcome this limitation, ImageJ, an automated image analysis software, was utilized to outline the leading edge of the cell migration. ImageJ is a public domain, Java-based image processing program developed at the National Institutes of Health. It performs standard image processing functions such as logical and arithmetical operations between images outlined by sharpening, smoothing, edge detection, and median filtering to develop geometric transformations and accurate measurement of the area of the wound. This leads to a standardization of and more accurate measurements of the wound areas that can then be used to calculate percentage change in areas of the in vitro wounds over time. After analysis with ImageJ software to obtain the percentage area of wound healed over time, we conducted Student's two-tailed t-tests between the percentage change in area at the time points of 0 and 9 h.
Membrane potential assays
Membrane potential analyses were performed on the fibroblasts using Five-Photon Membrane Potential Assay (Five-Photon Biochemical) with control (n = 9) and treated (n = 9) groups with three repeats being conducted. This assay utilizes a membrane permeant dye formulation of oxonol dyes for transmembrane potential measurement. Membrane potential assay dyes enter depolarized cells and bind to their intracellular membranes or proteins that lead to enhanced fluorescence. An increase in depolarization leads to an elevated influx of the voltage sensitive dye and an increase in fluorescence that can be measured by fluorescent microplate readers or flow cytometers. After washing twice with phosphate-buffered saline to remove serum factors, 60,000 cells were plated in the wells of a 96-well plate and placed in 100 μL of serum-free media. The wells were plated with both the treated and control groups of each cell line. The cells were exposed to the treated and control serum-free media for the time points of: 45 min, 12 h, and 24 h before the addition of 100 μL External Assay Buffer to make a 1 × dye solution, and 200 μL were added to each well. The cells were then incubated in the dark at 37°C in a CO2 incubator for 20 min to load the dye before placing in a fluorescent plate reader. The fluorescence was measured in the 530 excitation wavelength (nm) and 565 emission wavelength (nm) with a 550 emission cutoff (nm). The lipophilic anionic dye partitions across the plasma membrane of live cells and is dependent on the membrane potential across the membrane. When the cells are depolarized, more indicator dye enters the cells causing an increase in fluorescence signal. Analysis of data was conducted as percentage change in fluorescence between treated and control groups over time.
BEFE device
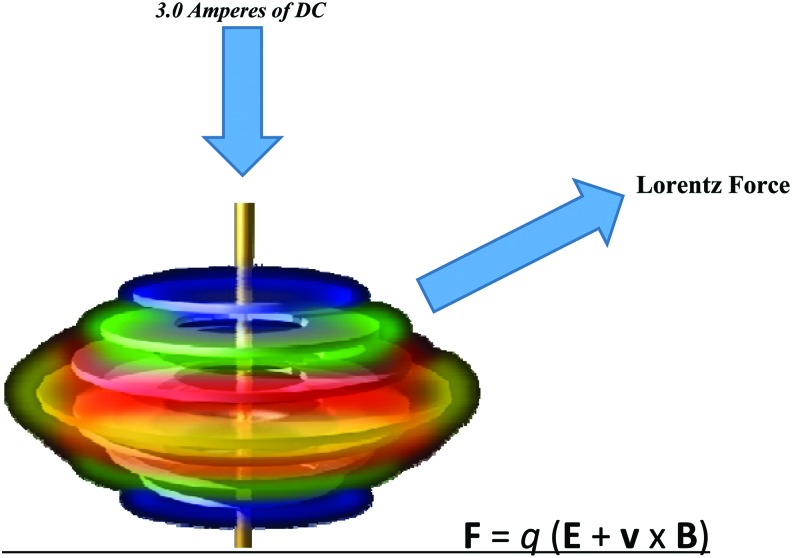
These experiments use an EMF that is generated by the BEFE device with 3 amperes of continuous DC. This EMF is applied through a Lorentz force that leads to dielectrophoretic dissociation and enhanced spin states of the metal ions in solution. These metal ions then continue to potentiate an EMF that affects cellular membrane function (Fig. 1). The array that is placed in the water consists of a series of conductive rings that contain ferromagnetic metals, which maintain their spin states after the active DC is removed. This ferromagnetism is responsible for the continued effects that remain in the saline solution and is then transferred to the growth media. The array design that is shown in Fig. 1 is powered by an external power supply that converts AC to DC.
Figure 1.
Bioelectric Field Enhancement (BEFE) Array. This array is placed in a hypotonic (3mM) saline solution and is powered by 3 amperes of direct current which is applied perpendicular to the conductive rings; thereby creating a Lorentz Force. This Lorentz force is exerted by a moving external electric field (E). The force (F) or direction of the spin will always occur perpendicular to both velocity (v) of the charge (q) and the magnetic field. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Results
Scratch assays
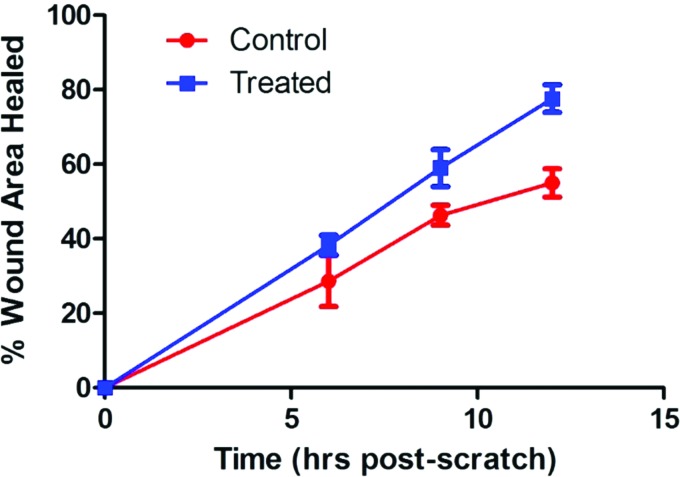
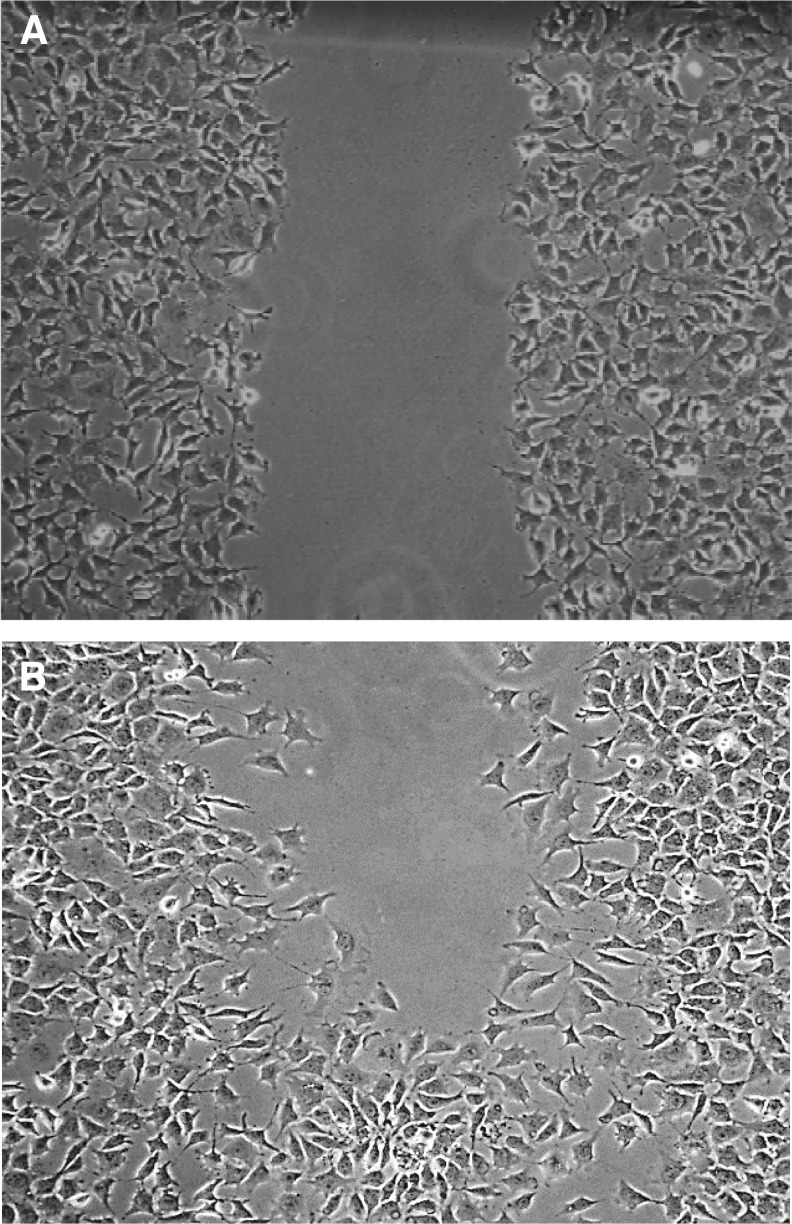
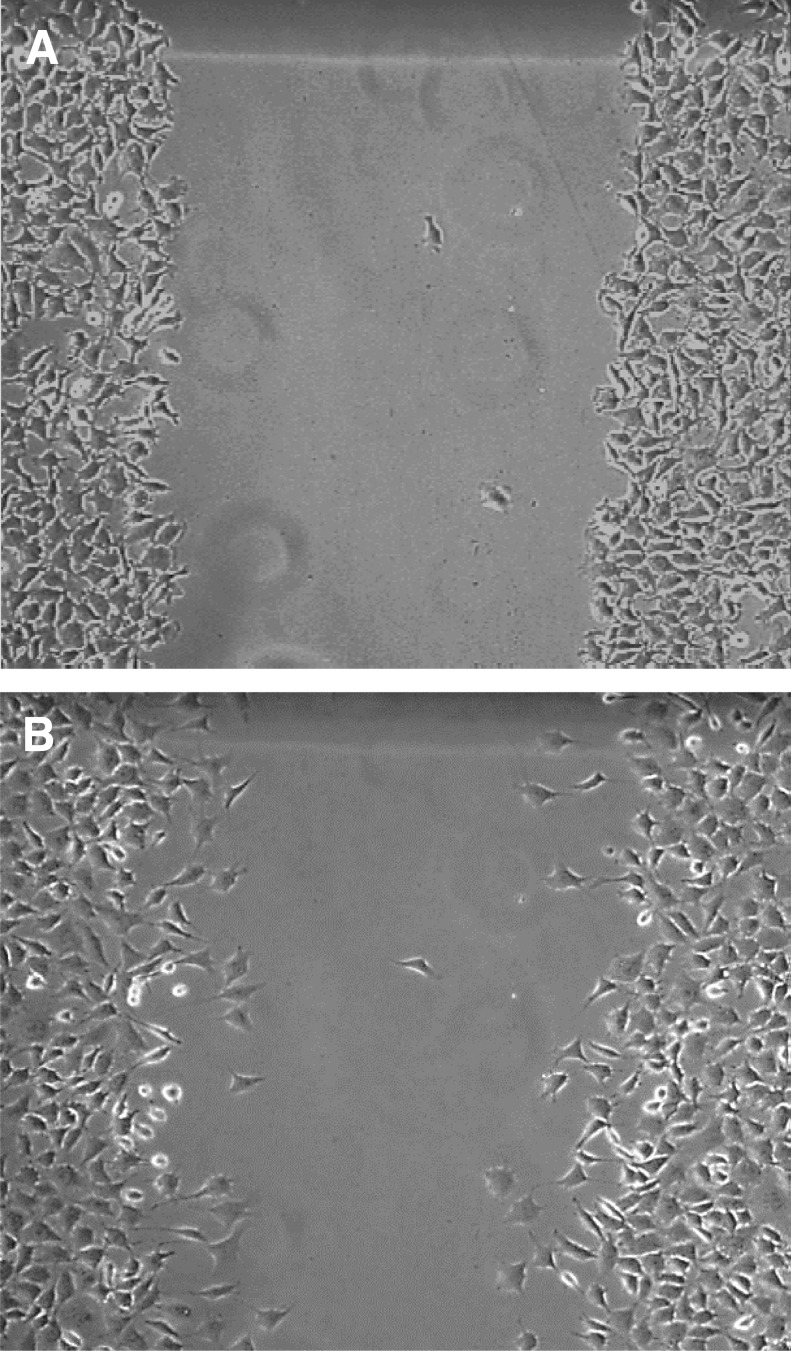
There was a significant difference in percentage area of wound healed over time in the treated versus the control groups (Fig. 2). On observation of the wound picture that was taken at the 9 h time point in the treated wound (Fig. 3B), migration appears to be in an organized directional movement. The control wound (Fig. 4B) appears to lack directional migration (9 h time point is the last interval measure before complete confluence in the treated wounds and thereby no measurements could continue to occur after this point) and has few cells that have migrated randomly into the wound.
Figure 2.
L292 Cells Scratch Assay-Percent Area Healed/Time. L929 cells Scratch Assay analyzed with student's unpaired t-tests; mean difference of two-point measurements at baseline/0 hours and 9 hours. Mean difference (control) 152057 +/− s.e.m; S.D. 38213; Mean difference (treated) 78198.22+/− s.e.m; S.D. 38213; df − 16; ts − 5.577858; n = 18, 18; p = 0.0000416. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Figure 3.
L292 Treated Cells Scratch Assay Phase Contrast Images with 4x objective. (A) Baseline/time 0 hours. (B) 9 hours post-scratch- notice polarized/directional movement of the fibroblasts. At 12 hours this wound was completely confluent/closed. Data were analyzed with student's unpaired t-tests; mean difference of two-point measurements at baseline/0 hours and 9 hours- (C) 152057 +/− s.e.m; S.D. 38213; mean difference (T) 78198.22 +/− s.e.m.; S.D 38213; df − 16; ts − 5.577858; n = 18, 18. p = 0.0000416.
Figure 4.
L292 Control Cells Scratch Assay Phase Contrast Images with 4x objective. (A) Baseline/time 0 hours (B) 9 Hours post-scratch. Data were analyzed with student's unpaired t-tests; mean difference of two-point measurements at baseline/0 hours and 9 hours. (C) 152057 +/− s.e.m.; S.D. 38213; mean difference (T) 78198.22 +/− s.e.m.; S.D. 38231; n = 18, 18; df − 16; ts − 5.577858. p = 0.0000416.
Membrane potential assays
Membrane potential was shown to have significant difference in percentage change over time in these treated and control groups when measured by the Five-Photon Membrane Potential Assay (Fig. 5). We see a 76% increase in Vmem in the fibroblasts in 45 min after the cells are placed in treated media compared to controls (Table 1). There is a narrowing of percentage change of treated and control groups in the fibroblast cells with a 32% difference in the treated and control groups at the 8 h time point with return to close to baseline Vmem at the 24 h time point (6% difference).
Figure. 5.
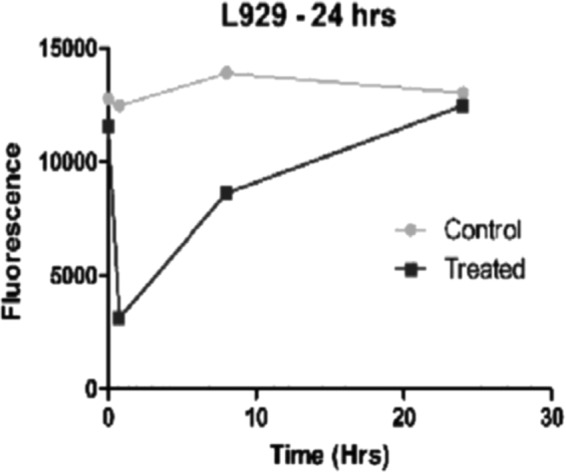
Membrane Potential Assay. The graphs show change in fluorescence over time. When a cell is depolarized the fluorescence increases and when it is hyperpolarized the fluorescence decreases. These dyes are permeant and work by a mechanism involving potential-dependent (follows cation flow) redistribution of the charged dye between the medium and the inside of the cell, organelle or vesicle. The dyes can show absorption and fluorescent changes as large as 80–90%. These data show an immediate strong increase in polarization of 76% (See Table 1) occurring in these fibroblasts in the first 45 minutes of exposure to the treated media versus controls. There is a 35% increase in polarization at the 8 hour time point and a gradual return to baseline (0.6%) after exposure for 24 hours to the treated media versus controls (n = 9, 9, 9).
Table 1.
Percentage change in membrane potential of fibroblasts
| Cell Line | 45 min in Treated Media Compared to Controls | 8 h in Treated Media Compared to Controls | 24 h in Treated Media Compared to Controls |
|---|---|---|---|
| L929 | ↑76% | ↑35% | ↑06% |
Statistical analyses
In our scratch assay analysis, we used standard two-point measurement of a start and end point to measure migration. The cell layer was first imaged at baseline (immediately after creating the scratch) and then at the 9 h measurement time, which was the last time point before the saturation phase of the migration curve since at the 12 h time point the treated cells reached 100% optical confluency. We then conducted Student's two-tailed t-test to determine if these two-time point measurements differed significantly. In our membrane potential assay, we calculated percent change in the measurements at the time points of 45 min, 8 h, and 24 h. These dyes are permeant and work by a mechanism involving potential-dependent redistribution of the charged dye between the medium and the inside of the cell, organelle, or vesicle. The dyes can show absorption and fluorescent changes as large as 80–90%. We show percentage change in Table 1 between the treated and control groups at the varying time points.
Discussion
An analysis of the membrane potential assay data shows that the biggest changes in the membrane potential occur in the 45 min to 8 h time points, which also correlates to the cell migration in the scratch assay from the baseline to 9 h time points. The hyperpolarization of the fibroblasts in the treated media appears to help the injured cells in the scratch to repair and begin migrating to heal the wound more quickly than the control (Fig. 2). The cells grown in the treated media also show enhanced wound healing in these fibroblasts through a more organized directional migration (Fig. 3B), whereas the murine fibroblasts grown in the control media are migrating sparsely and randomly (Fig. 4B).4 These data suggest that these injured fibroblasts in the scratch are responding to the initial hyperpolarization of the cell membrane with significantly enhanced and organized directional migration in the treated versus the control groups.
In the last decade, scientists have begun to see the comprehensive link between cell biology and bioelectricity.1,16,17 This application of DC differs from other current AC or DC probe applications since it is delivered through an aqueous solution. Changes in the magnetic moment and spin states of the dipole water molecule and metal aqueous ions may be interacting with the ECM leading to increased cell signaling, membrane polarity, proliferation, and migration of these fibroblasts. Studies previously conducted with the BEFE device in our laboratory experiments with an epithelial and a cancerous cell line show that the experimental effects in vitro are negated both by exposing the treated aqueous solution to high temperatures (120°C and 1.1 bar) for 40 min in an autoclave and also by exposure to a strong magnet for 24 h before reconstitution of the growth media. Since heat and magnets are known to change the properties of magnetism, this suggests that a magnetic moment effect could play a role in these observed experimental changes.18
Cells use a variety of mechanosensitive elements such as stretch-activated ion channels in the plasma membrane and conformational changes in proteins at the sites of focal adhesions and in the cytoskeleton to sense many different applied forces (magnetic moment changes etc.) and substrate viscosities and stiffness.19,20 Studies also suggest that the nucleus itself may act as a cellular mechanosensor, and therefore, these applied force changes can affect the expression of mechanosensitive genes. Cytoskeletal forces are required to position the nucleus during cell migration.19,20 Manipulation and augmentation of the ECM components in chronic wound healing therapies currently use biochemical products such as acellular dermal matrices, wound dressings, tissue scaffolds, and topical products that contain ECM proteins such as collagen, hyaluronan, or elastin.21 Hyperpolarization of the cell membrane in these experiments could be emitting a force that is affecting the poorly understood endogenous bioelectrical cues of the cell that ultimately lead to igniting mechanosensors in the cells causing changes in the cytoskeleton, which guide the directional migration seen in these fibroblasts.22,23 Could the addition of this bioelectric field (as a footbath/bath) to the current biochemical products used in chronic wound therapies offer more success in treating chronic wounds? These and other questions remain unanswered and will lead to future in vitro and in vivo testing with this technology.
Innovation
These data suggest that the BEFE device is the first to show significant membrane potential changes of fibroblasts in vitro. These changes in cell polarity could be a factor in enhanced cell migration of these fibroblasts and could offer the first application that utilizes an EMF that is applied through a footbath/bath. This application of DC through a medium of water may offer a new modality to the current continuous DC applications.
Key Findings.
• The BEFE appears to hyperpolarize the cell membranes of the L929 cells in vitro. Hyperpolarization of cell membranes has been a significant focus of research in recent years and is known to regulate the regeneration, migration, and differentiation of cells.
• This BEFE device is the first and only known device that has been used both in humans and that is known to hyperpolarize cell membranes.
• The hyperpolarization of the L929 plasma membranes could be playing a role in the significantly enhanced organized migration of these cells by scratch assay.
• Since this device has been used globally for 20 years and has had multiple anecdotal reports of improved chronic wound healing, this in vitro data could lay the foundation for future in vivo models and a novel bioelectric device application for wound care.
Abbreviations and Acronyms
- AC
alternating current
- ATCC
American Type Culture Collection
- BEFE
Bioelectric Field Enhancement Device
- DC
direct current
- DMEM
Dulbecco's modified Eagle's medium
- DNA
deoxyribonucleic acid
- ECM
extracellular matrix
- EMF
electromagnetic field
- L929
Clone 929 strain L (mouse fibroblasts)
- Vmem
transmembrane potential
Acknowledgments and Funding Sources
This work was supported by the Loewenberg College of Nursing, University of Memphis faculty grant awards; funds from the Department of Microbiology, Immunology and Biochemistry at the University of Tennessee Health Science Center; Southern Nursing Research Society/Council of Advancement for Nursing Science 2014–2015 Dissertation Award; 2014–2015; 2015–2016 Hal and Alma Reagan Cancer Fellowship, University of Tennessee Health Science Center, College of Graduate Health Sciences. Dr. Michael A. Whitt, PhD, Professor and Chair of the Department of Microbiology, Immunology and Biochemistry, UTHSC was my mentor and dissertation committee advisor through this entire project. The work was conducted in the laboratory of Michael A. Whitt, PhD and the Molecular Resource Center at the University of Tennessee Health Science Center.
Author Contributions
M.C.P. conceived the research project. T.J.S. designed and invented the device used in this research project. M.C.P. performed experiments and analyses. M.C.P. and T.J.S. wrote the article.
About the Authors
Marcy C. Purnell, PhD, APN, FNP-C, is an Assistant Professor at the Loewenberg College of Nursing, University of Memphis. She completed her BSN from the University of Tennessee Health Science Center, College of Nursing in 1986, MSN, FNP at the University of Memphis, Loewenberg College of Nursing in 2009, and her PhD in Nursing at the University of Tennessee Health Science Center, College of Graduate Health Sciences in 2016. She is currently conducting research with the Bioelectric Field Enhancement device in the area of cancer and wound care. Terence J. Skrinjar is the inventor of the Bioelectric Field Enhancement device. He is the CEO of Electroceutics and is also partnering in research with Dr. Purnell as a Biotechnology Consultant at the Loewenberg College of Nursing, University of Memphis.
Author Disclosure and Ghostwriting
T.J.S. holds original device patent-U.S. Patent No. 6,555,071 B2; M.C.P. PhD, APN, FNP-C and Michael A. Whitt hold methods/application patent: Declaration (37 CFR 1.63) for Utility or Design (35 USC 111 (a) “Bioelectrodynamics Modulation Method”); T.J.S. and M.C.P. are co-owners of Electroceutics LLC. The content of this article was expressly written by the author(s) listed. No ghostwriters were used to write this article.
References
- 1.Zhao M, Penninger J, Isseroff R. Electrical activation of wound-healing pathways. Adv Skin Wound Care 2010;1:567–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gurtner G, Werner S, Barrandon Y, Longaker M. Wound repair and regeneration. Nature 2008;453:314 [DOI] [PubMed] [Google Scholar]
- 3.Zhou SA, Uesaka M. Bioelectrodynamics in living organisms. Int J Eng Sci 2006;44:67–92 [Google Scholar]
- 4.Tseng A, Levin M. Cracking the bioelectric code: probing endogenous ionic controls of pattern formation. Commun Integr Biol 2013;6:e22595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isseroff RR, Dahle S. Electrical stimulation therapy and wound healing: where are we now? Adv Wound Care 2012;1:238–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCaig CD, Rajnicek AM, Song B, Zhao M. Controlling cell behavior electrically: current views and future potential. Physiol Rev 2005;85:943–978 [DOI] [PubMed] [Google Scholar]
- 7.Zhao M, Song B, Pu J, et al. Electrical signals control wound healing through phosphatidylylinositol-3-OH kinase-gamma and PTEN. Nature 2006;442:457 [DOI] [PubMed] [Google Scholar]
- 8.Levin M. Molecular bioelectricity: how endogenous voltage potential control cell behaviors and instruct pattern regulation in vivo. Mol Biol Cell 2014;25:3835–3850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmerman JW, Pennison MJ, Brezovich I, et al. Cancer cell proliferation is inhibited by specific modulation frequencies. Br J Cancer 2012;106:307–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Funk RH, Monsees T, Ozkucur N. Electromagnetic effects—from cell biology to medicine. Prog Histochem Cytochem 2009;43:177–264 [DOI] [PubMed] [Google Scholar]
- 11.Reddien PW, Sanchez Alvarado A. Fundamentals of planarian regeneration. Annu Rev Cell Dev Biol 2004;20:725–757 [DOI] [PubMed] [Google Scholar]
- 12.Kloth LC. Electrical stimulation technologies for wound healing. Adv Wound Care 2014;3:81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marsh R. A preliminary report on the new BEFE: Bioelectric Field Enhancement. Explore 2001;10 [Google Scholar]
- 14.Chandan K, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kantor J, Margolis DJ. Expected healing rates for chronic wounds. Wounds 2000;12:155 [Google Scholar]
- 16.Levin M. Large-scale biophysics: ion flows and regeneration. Trends Cell Biol 2007;17:261–270 [DOI] [PubMed] [Google Scholar]
- 17.Purnell M, Whitt M. Bioelectrodynamics: a new patient care strategy for nursing health and wellness. Holist Nurs Pract 2016;30:4–9 [DOI] [PubMed] [Google Scholar]
- 18.Sverjensky D, Shock E, Helgeson H. Prediction of the thermodynamic properties of aqueous metal complexes to 1000 degree Celsius and 5 kb. Geochim Cosmochim Acta 1997;61:1359–1412 [DOI] [PubMed] [Google Scholar]
- 19.Iserman P, Lammerding J. Nuclear mechanics and mechanotransduction in health and disease. Curr Biol 2013;23: R1113–R1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishiyama M, von Schimmelmann MJ, Togashi K, Findley WM, Hong K. Membrane potential shifts caused by diffusible guidance signals direct growth-cone turning. Nat Neurosci 2008;11:762–771 [DOI] [PubMed] [Google Scholar]
- 21.Tracy LE, Minasian RA, Caterson EJ. Extracellular matrix and dermal fibroblast function in the healing wound. Adv Wound Care 2016;5:119–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sundelacruz S, Levin M, Kaplan DL. Membrane potential controls adipogenic and osteogenic differentiation of mesenchymal stem cells. PLoS One 2008;3:e3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marder E. Electrical synapses: rectification demystified. Curr Biol 2009;19:R34–R35 [DOI] [PubMed] [Google Scholar]