Abstract
Objective: Burn injuries remain a large financial burden on the healthcare system. According to CDC statistics (2010), nonfatal and hospitalized burns in the U.S. cost $1.8 billion for an annual incidence of ∼486,000 cases. To date, no technique proves to be the ideal therapy of deep partial-thickness burns. In this study, we review a trial usage of ACell (ACell, Inc.) wound matrix on deep partial-thickness burns.
Approach: Burn patients were admitted through the Vanderbilt Emergency Department. Three were consented to receive ACell therapy. Each patient suffered extremity burns, to which ACell MatriStem matrix was applied. Time to epithelialization and healing was monitored up to 1 month postintervention.
Results: ACell MatriStem matrix use in deep partial-thickness burns enabled healing by 29 days on average without requiring autografts. The average total body surface area (TBSA) of injury was 7.2% with average TBSA treated with ACell equal to 2.5%. All burn sites underwent re-epithelialization after 5.6 days on average (range 4–7 days). Average length of stay after ACell placement totaled 2 days. All patients fully healed without the need for subsequent grafting or contracture development. No postoperative complications were noted.
Innovation: To the extent of our knowledge, this is one of the first reported series to utilize ACell MatriStem product in deep partial-thickness extremity burns.
Conclusion: Despite numerous products currently available for burn reconstruction, no one product embodies all the characteristics of an ideal graft. ACell biological extracellular matrix scaffolding appears promising, allowing for healing without use of an autograft.
Keywords: : partial thickness burn, urinary bladder matrix, skin substitute, extracellular matrix scaffold

Wesley P. Thayer, PhD, MD
Introduction
Patients who sustain burn injuries are some of the most complex patients to care for, often requiring a multidisciplinary team of providers specialized in burn pathophysiology. While many facets of burn care must be accounted for, including fluid management, nutrition, respiratory status, immunologic and cardiovascular function, the use of biological dressings after surgical management for deep partial-thickness burns is of paramount importance.
Burn injuries continue to be a significant financial burden on the U.S. healthcare system. The 2015 National Burn Registry—accounting for 9 years of cumulative data from 99 U.S. Burn Centers/hospitals—presents 203,422 cases. Approximately 70% of the patients were male; 73% of cases occurred at home, classified as accidental and nonwork related. The mean age for all cases was 32 years. Of the reported total burn sizes, more than 75% were less than 10% total body surface area (TBSA) and the mortality rate was equal to 0.6%, compared to 3.2% and 5.7% for fire/flame injuries. Over the 10-year period, improvements in care decreased the average length of stay for both genders from 9 to 8 days. Additionally, mortality rates decreased from 3.4% to 2.7% in males and 4.6% to 3.3% in females. However, the costs of care remained elevated. The cost per case for each death is estimated to be three times greater than that of a survivor ($309,733 vs. $93,167).1
In the acute setting, management of deep partial-thickness burns involves the standard approach to any trauma patient—securing the airway, establishing adequate pulmonary function and cardiovascular support, appropriate resuscitation, and debridement of nonviable tissue.2 Currently, the gold standard for full-thickness burn coverage is the autograft, or a graft from the patient's own body, which undergoes imbibition, vascularization, and remodeling after placement.3 Coverage of the wound bed decreases potential wound contamination and infection by offering a barrier to bacteria, limiting fluid loss, preventing desiccation, accelerating granulation and epithelialization, and decreasing pain. Ideally, coverage should also conform to irregularly contoured surfaces. It should remain minimally immunogenic, flexible, durable, inexpensive, and require minimal preparation.4,5 Additionally, the treatment should not incite an inflammatory response. It should, however, facilitate growth, maintain its structure, and resist degradation until the host has had sufficient time to initiate angiogenesis, cell proliferation, migration, and differentiation. Current methods for coverage include nonadherent dressings, which require daily changes that are painful, labor intensive, and expensive.
A second method, the allograft is a graft from the same species as the recipient. One example is a temporary cadaveric graft, which possesses several attributes of the ideal graft. Products like cadaveric AlloDerm (LifeCell Corporation) have been used in conjunction with meshed autografts to enhance prevention of fluid loss and decrease metabolic stress. Other allograft acellular matrix products on the market include DermaMatrix (Synthes), AlloMax (Bard), and FlexHD (Ethicon, Inc.). Despite the numerous options, allografts are temporary, lasting only weeks until immunologic rejection by the recipient, expensive, and have not been shown to have definitive advantages over other products.6
A third method, the xenograft is a graft donor from a different species than the recipient. Most commonly derived from porcine skin, they have been used as a temporizing measure either preceding autografting or in conjunction with an autograft to promote wound healing. The xenograft, unlike the autograft, does not undergo vascularization, capillary ingrowth, or vessel-to-vessel connection. Also over time, avascular necrosis occurs as the nutrition supply ceases. Although there is no evidence of immunological rejection, the graft is functionally rejected as host epithelium grows underneath it. Porcine skin grafts are currently used for partial-thickness burns (most common use), split skin graft donor sites, and exfoliative conditions, including Stevens–Johnson syndrome and toxic epidermal necrolysis. In terms of use, the graft is typically applied after exudation is decreased, enabling better adherence. The graft can either be removed after epithelialization or changed at regular intervals as appropriate with dirty wounds.7 Studies have shown that porcine skin grafts reduce pain, decrease heat, protein, and electrolyte losses, offer physical protection, and decrease risk of infection.7–9
ACell (ACell, Inc.) is one of the newest xenograft biological dermal substitutes composed of extracellular matrix (ECM) derived from porcine urothelium that is lyophilized and dehydrated. Products maintain their natural collagen structure—the intact epithelial basement membrane enabling natural healing and tissue remodeling—and are subsequently resorbed by the body. ACell MatriStem possesses several of the ideal graft attributes including barrier protection, pliability, and minimal preparation. Furthermore, it has not been identified as a vector for infectious agents. ACell has several forms, including singular and multi ply sheets, in addition to moralized granules. Few rigorous clinical studies have been performed using this method, with the majority of the literature originating from materials science papers. This study aims to review a trial usage of ACell for early application on deep partial-thickness burns. Extremity deep partial-thickness burns, in particular, were evaluated to allow assessment of functional outcomes.
Clinical Problem Addressed
To date, no one technique proves to be the ideal therapy of deep partial-thickness burns. In this study, we review current products as well as a trial usage of ACell (ACell, Inc.) wound matrix on deep partial-thickness burns.
Materials and Methods
Deep partial-thickness burn patients (n = 3), determined clinically, were admitted through the Vanderbilt Emergency Department. Exclusion criteria included full-thickness burns that clinically required skin grafting. Before application of ACell therapy for deep partial-thickness burns, informed consent for the procedure was obtained from all patients. No IRB approval was required for this intervention. At the time of initial debridement, we discussed treatment options with the patient, offering standard treatment versus ACell therapy. Procedures were done under deep sedation. The wound bed was cleaned with removal of necrotic and scar tissue, then rinsed with normal saline solution. Excessive exudate was irrigated and hemostasis achieved. ACell MatriStem burn matrix (shown in Fig. 1)10 fenestrated sheet xenograft was used in all cases. The xenoderm was prepared in normal saline solution and affixed with standard application of skin adhesive. Petroleum gauze dressing was applied with the graft, followed by a circumferential woven gauze wrap and elastic bandage. Each patient had a postoperative wound check within 7 days. Patients were followed for 1 month and evaluated for time to epithelialization, time to heal, contracture percentage, and postoperative complications.
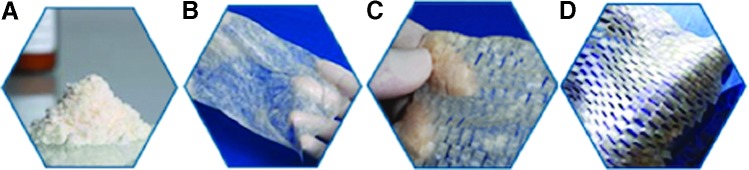
FIG. 1.
ACell MatriStem product illustrations; (A) MicroMatrix, (B) Wound Matrix, (C) Multilayer Wound Matrix, (D) Burn Matrix.
Results
ACell has received FDA clearance for use in several wound types including partial- and full-thickness wounds.11 For this retrospective study, IRB approval was submitted to review the deidentified data. A total of three patients with deep partial-thickness burns on the dorsum of the hand, dorsum of the foot, or volar wrist received ACell MatriStem matrix. Two of the patients were male and one female with an average age of 39 years (range of 21–59 years). Two of the patients were smokers with an average of 12.5 pack years and none of the patients had any other known comorbidities (Table 1). The average time from burn to treatment with ACell was equal to 2 days (range 1–4 days). An average of 166 cm2 of ACell xenograft was used (range of 150–200 cm) with an average of TBSA burned equal to 7.1% (range of 2.5–10%). The average area treated with ACell was 2.5%. No patient experienced any complications from the ACell therapy. All patients demonstrated evidence of re-epithelialization on their postoperative wound check at an average of 5.6 days (range of 4–7 days). The average length of stay after ACell placement totaled 2 days; one patient was admitted, underwent treatment, and discharged on the same day. Furthermore, all patients fully healed by an average of 29 days without the need for subsequent grafting or development of contracture (Figs. 2–4). There were no postoperative complications noted.
Table 1.
Patient Demographics
| Patient | Age, Years | Gender | Smoking (Pack Years) | Burn Location | % Total Body Surface Area |
|---|---|---|---|---|---|
| 1 | 39 | Male | 20 | Face and bilateral hands | 3 |
| 2 | 59 | Male | 0 | Face, wrists, right hand, right leg | 2.5 |
| 3 | 21 | Female | 5 | Face, right hand, left arm, right foot | 2 |
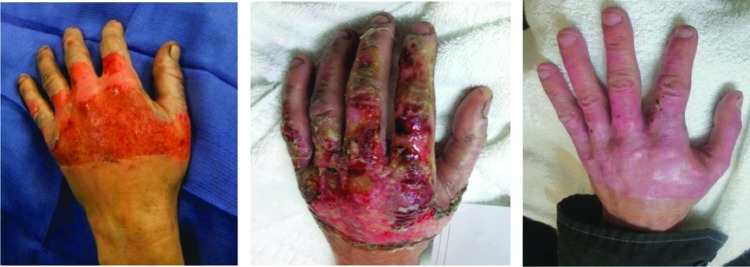
FIG. 2.
A 39-year-old male with furnace flash burn of the left hand. Images from left to right: initial injury, 7 days post-ACell, and 21 days post-ACell.
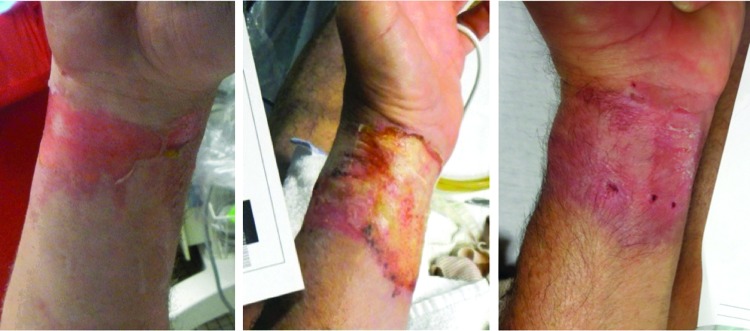
FIG. 3.
A 59-year-old male with flash burn from diesel garbage. Images from left to right: initial injury, 4 days post-ACell, and 50 days post-ACell.
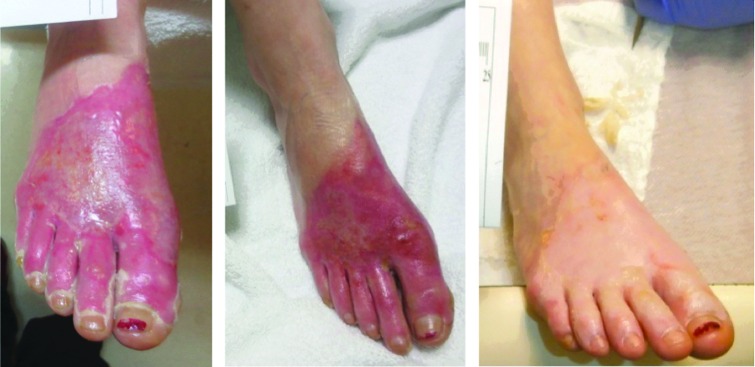
FIG. 4.
A 21-year-old female with a grease burn. Images from left to right: initial injury, 14 days post-ACell, and 28 days post-ACell.
Discussion
To date, only small clinical case series have reported favorable outcomes using ACell for recalcitrant radiation wounds, chronic wounds, open complex pilonidal excisional wounds, and a mixture of chronic peripheral vascular disease.12–15 The ACell product has shown promising results in the setting of both acute and chronic wounds. Moreover, these wounds have been in various anatomical locations and secondary to different pathologies. Due to its biological profile, ease of use, and versatility, ACell matrix and its application in burn wounds should be further investigated. Data from this retrospective study demonstrate promise for patients with deep partial-thickness injuries. In all three cases, healing occurred in a timely fashion with epithelialization evident as early as postoperative day 4. None of the cases required additional grafting and no complications from ACell therapy were observed. Furthermore, no contracture development was observed in these patients, a significant finding in the setting of extremity burn injuries. Additionally, the patients in this case series were discharged quickly after ACell application. One patient was admitted, operated, and discharged on the same day. The data from this study demonstrate the successful utilization of ACell in deep partial-thickness burns.
Nonetheless, there are several alternative skin substitute therapies currently available on the market. Classified by Kumar, these alternatives include class I (temporary impervious dressings), class II (single-layer durable skin substitutes), and class III (composite skin substitutes) products.16 Class I comprises substances that do not have an epidermal component, thus, serving as a mechanical barrier. Examples include potato peels, Tegaderm (3M Healthcare), and TransCyte (Advanced Tissue Sciences). The last is composed of a polymer membrane and newborn human fibroblast cells, which are cultured on a nylon mesh that enables proliferation. The fibroblasts secrete human dermal collagen, matrix proteins, and growth factors. These factors allow the patient's epithelial cells to migrate quickly, increasing the rate of healing. TransCyte is primarily used for excised burns before autografting and has great use in facial burn cases due to its flexibility. Kumar et al. (2004) determined that TransCyte increases the rate of re-epithelialization and decreases the amount of dressings required compared to Biobrane and Silvazine cream in children with partial-thickness burns.17 Similarly, Lukish et al. (2001) established that TransCyte in children with burns greater than 7% TBSA led to reduced length of stay and served as a safe and effective treatment method.18 Another study by Noordenbos et al. (1999) found that TransCyte led to wound healing with less hypertrophic scarring compared to wounds treated with silver sulfadiazine.19 Several other studies support the successful use of Transcyte in partial-thickness burns regarding wound infections, healing time, wound closure, and safety.20,21 However, its use with deep partial- and full-thickness burns is less well studied.
Class II substitutes include cultured epithelial autograft, bovine collagen sheet, porcine collagen sheet, or Permacol (Tissue Science Laboratories PLC), bovine dermal matrix or Matriderm (Skin & Health Care AG), and human dermal matrix or AlloDerm (LifeCell Corp.). AlloDerm, specifically, is an acellular dermal matrix that consists of fibrillar collagen, collagen VI, elastin, hyaluronan, proteoglycans, fibronectin, and vascular channels.16 These components enable tissue regeneration. The matrix is, however, derived from donated human skin. Therefore, restrictions in use involve limited quantity and costliness compared to other skin substitutes. Nevertheless, Yim et al. demonstrated that early intervention with AlloDerm could prevent scar formation and joint contracture in patients with greater than 20% TBSA burns.22 A study by Callcut et al. showed AlloDerm to be a method successful in reducing donor site morbidity, enhancing cosmetic as well as functional outcomes, and eradicating the need for follow-up procedures in acute thermal burn injuries.23 Published data on AlloDerm in patients with deep partial-thickness burns, however, continue to be limited.
The third class of skin substitutes includes allografts, xenografts, and tissue-engineered skin such as Integra (Integra LifeSciences Corp.) and Biobrane (Smith & Nephew). Developed in the 1980s, Integra contains three layers—an outer temporary silicone epidermal layer, a dermal layer, and a matrix. The matrix is composed of bovine collagen and glycosaminoglycans, and incorporates the patient's cells. Regeneration of the dermis occurs and the silicone epidermal layer can be removed. This skin substitute is indicated for both full-thickness and deep partial-thickness burns. Branski et al. determined that Integra led to improved scarring with regard to height, thickness, vascularity, and pigmentation. This therapy, however, did not significantly affect mortality or length of stay for pediatric patients with 58–88% TBSA burns.24 Pham et al. (2007) conducted a systematic review of the literature on bioengineered skin substitutes. In terms of graft take and infection rates, data showed that Integra was not as efficient compared to autograft, allograft, and Biobrane although it was proven to decrease time to healing.25,26 Moreover, Integra is costly and requires ∼2 weeks to mature, at which point a split-thickness skin graft can be applied for definitive wound closure. Biobrane xenograft is a second example. It also has an outer silicone layer as well as an inner knitted nylon mesh layer that is coated with porcine collagen. It serves as a temporary cover for clean, non-full-thickness burns, donor sites, and meshed autografts. Again, Pham et al. found that Biobrane did not significantly affect the number of dressing changes or need for pain medications when compared to silver sulfadiazine and TransCyte.17,27–29 Specific studies on the use of Biobrane in deep partial-thickness burns are limited.
In general, animal studies have shown that the ECM scaffolding retains basement membrane morphology as well as collagen IV, laminin, and collagen VII. The content of these scaffolds exceeds those of porcine small intestine and liver-based ECM.30 Although the clinical implications have not been tested, there is potential for improvement in the scaffolding geometry and three-dimensional molecular architecture for regeneration of the dermis. Furthermore, in vitro studies have suggested an antimicrobial property of urothelium against Staphylococcus aureus and Escherichia coli.31 Although there is a variety of products available on the market used to mitigate deleterious physiological effects of burn reconstruction, no one product has embodied all the characteristics of an ideal graft. The ACell biological ECM scaffolding is promising as it allows for healing without use of autografting. There are limitations to this study including the small sample size and length of follow-up. Future studies may extend the type of burn patient population to those with similar injuries as well as increase the period for follow-up. Further study is necessary to demonstrate superior efficacy and to determine the safety profile. A more complete assessment of the cost profile for treatment of deep partial-thickness wounds is similarly needed.
Innovation
There is a great variety of burn injury reconstruction therapies on the market today. Skin substitutes, including ACell MatriStem, are one class of therapy proven to be effective. However, no one product demonstrates comprehensive properties of the ideal treatment for deep partial-thickness burns. The study results reveal ACell to be promising in this regard. Data showed quick time to re-epithelialization and healing, no contracture development or postoperative complications, and eliminated the need for subsequent autografting. To the extent of our knowledge, this is the first reported series to utilize ACell MatriStem product in deep partial-thickness burns.
Key Findings.
• ACell enables rapid healing with all patients demonstrating evidence of re-epithelialization by an average of 5.6 days.
• Patients treated with ACell did not require an extensive hospital stay. The average length of stay after ACell placement totaled 2 days while one patient was admitted, underwent treatment, and discharged on the same day.
• All ACell recipients were fully healed by an average of 29 days without need for subsequent grafting or development of contracture.
• Although additional studies are necessary to determine the safety profile, there were no postoperative complications noted for any patient in this study.
Abbreviations and Acronyms
- ECM
extracellular matrix
- TBSA
total body surface area
Author Discolure and Ghostwriting
Justine S. Kim has no disclosures. Alexander J. Kaminsky has no disclosures. J. Blair Summitt is a consultant for ACell, Inc, Colombia, MD. Wesley P. Thayer is a consultant for ACell, Inc., Colombia, MD. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article. Adherence to the Declaration of Helsinki was maintained.
About the Authors
Justine S. Kim, BS, is a third year medical student at Vanderbilt University School of Medicine, Nashville, TN. Alexander J. Kaminsky, MD, MPH, is a fourth year General Surgery resident at Inova Fairfax Hospital, Falls Church, VA with a strong interest in plastic and reconstructive surgery. J. Blair Summitt, MD, is an Assistant Professor of Plastic Surgery and the Director of the Burn Center at Vanderbilt University, Nashville, TN. Wesley P. Thayer, PhD, MD, is an Assistant Professor of Plastic Surgery at Vanderbilt University, Nashville, TN; Chief of Plastic Surgery at Nashville Veteran's Hospital; Wound Care Director at Kindred Hospital, Nashville, TN.
References
- 1.The American Burn Association. 2015 NBR report. www.ameriburn.org/NBR.php (last accessed October6, 2015)
- 2.Barret JP, Herndon DN, eds. Principles and Practice of Burn Surgery. New York, NY: Marcel Dekker, 2005 [Google Scholar]
- 3.Andreassi A, Bilenchi R, Biagioli M, D'Aniello C. Classification and pathophysiology of skin grafts. Clin Dermatol 2005;23:332–337 [DOI] [PubMed] [Google Scholar]
- 4.Herndon DN, ed. Total Burn Care, 4th ed. Edinburgh: Saunders Elsevier, 2012 [Google Scholar]
- 5.Sood R, Achauer BM, eds. Achauer and Sood's Burn Surgery: Reconstruction and Rehabilitation. Philadelphia, PA: Saunders Elsevier, 2006 [Google Scholar]
- 6.Sheridan RL, ed. Burns: A Practical Approach to Immediate Treatment and Long-Term Care. London: Manson, 2012 [Google Scholar]
- 7.Chiu T, Burd A. Xenograft dressing in the treatment of burns. Clin Dermatol 2005;23:419–423 [DOI] [PubMed] [Google Scholar]
- 8.Pruitt BA, Levine NS. Characteristics and uses of biologic dressings and skin substitutes. Arch Surg 1984;119:312–322 [DOI] [PubMed] [Google Scholar]
- 9.Bromberg BE, Song IC, Mohn MP. The use of pigskin as a temporary biological dressing. Plast Reconstr Surg 1965;36:80–90 [DOI] [PubMed] [Google Scholar]
- 10.Wound Care Products. Acell.com [website]. Columbia, MD: ACel, Inc., 2001 [Google Scholar]
- 11.Department of Health & Human Services. 510(k) summary for ACell MatriStem Wound Sheet. www.accessdata.fda.gov/cdrh_docs/pdf9/K092926.pdf (last accessed July15, 2014)
- 12.Rommer EA, Peric M, Wong A. Urinary bladder matrix for the treatment of recalcitrant nonhealing radiation wounds. Adv Skin Wound Care 2013;26:450–455 [DOI] [PubMed] [Google Scholar]
- 13.Kimmel H, Rahn M, Gilbert TW. The clinical effectiveness in wound healing with extracellular matrix derived from porcine urinary bladder matrix: a case series on severe chronic wounds. J Am Col Certif Wound Spec 2010;30:55–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sasse KC, Brandt J, Lim DC, Ackerman E. Accelerated healing of complex open pilonidal wounds using MatriStem extracellular matrix xenograft: nine cases. J Surg Case Rep 2013;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lecheminant J, Field C. Porcine urinary bladder matrix: a retrospective study and establishment of protocol. J Wound Care 2012;21. [DOI] [PubMed] [Google Scholar]
- 16.Kumar P. Classification of skin substitutes. Burns 2008;34:148–149 [DOI] [PubMed] [Google Scholar]
- 17.Kumar RJ, Kimble RM, Boots R, Pegg SP. Treatment of partial-thickness burns: a prospective, randomized trial using Transcyte (TM). ANZ J Surg 2004;74:622–626 [DOI] [PubMed] [Google Scholar]
- 18.Lukish JR, Eichelberger MR, Newman KD, et al. The use of a bioactive skin substitute decreases length of stay for pediatric burn patients. J Pediatr Surg 2001;36:1118–1121 [DOI] [PubMed] [Google Scholar]
- 19.Noordenbos J, Doré C, Hansbrough JF. Safety and efficacy of TransCyte for the treatment of partial-thickness burns. J Burn Care Rehabil 1999;20:275–281 [DOI] [PubMed] [Google Scholar]
- 20.Demling RH, DeSanti L. Management of partial thickness facial burns (comparison of topical antibiotics and bioengineered skin substitutes). Burns 1999;25:256–261 [DOI] [PubMed] [Google Scholar]
- 21.Demling RH, DeSanti L. Closure of partial-thickness facial burns with a bioactive skin substitute in the major burn population decreases the cost of care and improves outcome. Wounds 2002;14:230–234 [Google Scholar]
- 22.Yim H, Cho YS, Seo CH, et al. The use of AlloDerm on major burn patients: AlloDerm prevents post-burn joint contracture. Burns 2010;36:322–328 [DOI] [PubMed] [Google Scholar]
- 23.Callcut RA, Schurr MJ, Sloam M, Faucher LD. Clinical experience with Alloderm: A one-staged composite dermal/epidermal replacement utilizing processed cadaver dermis and thin autografts. Burns 2006;32:583–588 [DOI] [PubMed] [Google Scholar]
- 24.Branski LK, Herndon DN, Pereira C, et al. Longitudinal assessment of Ingetra in primary burn management: a randomized pediatric clinical trial. Crit Care Med 2007;35:2615–2623 [DOI] [PubMed] [Google Scholar]
- 25.Pham C, Greenwood J, Cleland H, Woodruff P, Maddern G. Bioengineered skin substitutes for the management of burns: a systematic review. Burns 2007;33:946–957 [DOI] [PubMed] [Google Scholar]
- 26.Heimbach D, Luterman A, Burke J, et al. Artificial dermis for major burns: A multi center randomized clinical trial. Ann Surg 1988;208:313–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cassidy C, St Peter SD, Lacey S, et al. Biobrane versus duoderm for the treatment of intermediate thickness burns in children: a prospective, randomized trial. Burns 2005;31:890–893 [DOI] [PubMed] [Google Scholar]
- 28.Lal S, Barrow RE, Wolf SE, et al. Biobrane improves wound healing in burned children without increased risk of infection. Shock 2000;14:314–318 [DOI] [PubMed] [Google Scholar]
- 29.Barret JP, Dziewulski P, Ramzy PI, Wolf SE, Desai MH, Herndon DN. Biobrane versus 1% silver sulfadiazine in second-degree pediatric burns. Plast Reconstr Surg 2000;105:62–65 [DOI] [PubMed] [Google Scholar]
- 30.Brown B, Lindberg K, Reing J, Stolz DB, Badylak SF. The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Eng 2006;12:519–526 [DOI] [PubMed] [Google Scholar]
- 31.Brennan EP, Reing J, Chew D, Myers-Irvin JM, Young EJ, Badylak SF. Antibacterial activity within degradation products of biological scaffolds composed of extracellular matrix. Tissue Eng 2006;12:2949–2955 [DOI] [PMC free article] [PubMed] [Google Scholar]