Abstract
Prostate cancer (PCa) is one of the most prevalent malignant tumors. microRNAs (miRNAs) play an important role in cancer initiation, progression, and metastasis, and their roles in PCa are becoming more apparent. In this study, we found that microRNA-372 (miR-372) is downregulated in human PCa and inhibits the proliferation activity, migration, and invasion of DU145 cells. Subsequently, p65 is confirmed as a target of miR-372, and knockdown of p65 expression similarly resulted in decreased proliferation activity, migration, and invasion. CDK8, MMP-9, and prostate-specific antigen were involved in both these processes. Taken together, our results show evidence that miR-372 may function as a tumor suppressor gene by regulating p65 in PCa and may provide a strategy for blocking PCa metastasis.
Keywords: : miR-372, PCa, p65, migration, invasion
Introduction
Prostate cancer (PCa) is one of the most prevalent malignant tumors and the second major cause of cancer-related mortality in American men (Siegel et al., 2013). PCa-related death is mainly due to the high probability of its metastasis to bone and/or other organs (Mundy, 2002; Corey et al., 2005). Notably, bone metastasis is detected in ∼80% of advanced PCa patients (Sturge et al., 2011). Therefore, it would be best to diagnose the local tumor at the early stage, which needs better understanding of the molecular mechanisms underlying the incursive progression of PCa. As an androgen-regulated gene, prostate-specific antigen (PSA) is the most widely applied cancer biomarkers for prevention, diagnosis, and monitoring of PCa patients (Diamandis, 2010).
As a class of small noncoding RNAs, microRNAs (miRNAs) are unique in their ability to silence their targets by mRNA cleavage, translational repression, mRNA destabilization, or a combination of these mechanisms (Dykxhoorn, 2010). miRNAs play a key role in cell metastasis, likely owing to their posttranscriptional regulation of gene networks that are significant for cell motility, migration, and invasion (Dalmay and Edwards, 2006). Hence, miRNAs have become the focus of various research areas, especially in oncology. Several studies have noted the potential role of miRNAs in PCa. In addition, some studies have demonstrated that the expression of several miRNAs in body fluids could act as effective biomarkers for early cancer diagnosis (Cortez et al., 2011).
Nuclear factor-kappaB (NF-κB) is a transcription factor with pleiotropic activity due to its key roles in various biological processes (Ghosh and Karin, 2002; Chen and Greene, 2004). A critical link in NF-κB activation is phosphorylation of the p65 and p50 subunits. Aberrant NF-κB activation has been found in the progression of several human cancers, including PCa. Furthermore, some studies have suggested that the activation of the NF-κB pathway, defined by the nuclear translocation of the NF-κB p65 subunit, was related to lymph node metastasis and late bone metastasis of PCa (Lessard et al., 2003; Ismail et al., 2004). However, the molecular mechanisms underlying the regulation of the p65/NF-κB pathway by miRNAs in PCa remain unclear. This study was performed to confirm that the cascade of p65/NF-κB signaling may be used as a promising target in PCa.
Materials and Methods
Specimens
In total, 20 serum samples from patients with PCa and 20 matched samples from healthy control subjects were obtained from Xinhua Hospital, Shanghai Jiaotong University after receiving informed consent and ethical approval (time: 2013–2014). PSA tests confirmed the absence of PCa in the healthy control group. The blood samples were centrifuged at room temperature using the Capricorn CEP2000 for 20 min at 2200 rcf. Sera samples were stored at −80°C.
Cell culture and reagents
All cells were maintained in high-glucose Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand Island, NY), containing 10% fetal bovine serum (FBS; Gibco) and incubated in a humidified chamber with 5% CO2 at 37°C.
The miR-372 mimics (miR-372) and the miR-372 inhibitor (anti-miR-372) sequences were synthesized by Integrated Biotech Solutions Company (Shanghai, China), siR-p65 and siR-IKKβ were synthesized by GeneChem Company (Shanghai, China), and miR-NC and siRNA control were used as negative controls. The transfection of miRNA and siRNA was conducted with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) following the manufacturer's instructions.
Cell proliferation assays
DU145 cells were seeded onto a 96-well plate at 5000 cells per well and incubated for 72 h. Cell viability was determined at 24, 48, and 72 h after transfection using the Cell Counting Kit-8 (CCK-8) (Dojindo Laboratories, Kumamoto, Japan). The absorbance of each well was measured with a microplate reader set at 450 nM.
Cell cycle assay
For cell cycle analysis, 24 and 48 h after transfection, DU145 cells were collected and fixed with 70% ethanol at −20°C for 18 h; propidium iodide (BD Bioscience, Franklin) was then added to the cells. Samples were analyzed by flow cytometry on FACSCalibur (Becton Dickinson).
Colony formation assay
At 24 h after transfection, DU145 cells were seeded onto each well of six-well plates at the density of 3000 per well and cultured in DMEM containing 10% FBS at 37°C for 14 days. The colonies were washed with phosphate-buffered saline, fixed with 10% formaldehyde for 15 min, and stained with 1% crystal violet for 15 min. The colonies that had 50 cells or more per colony were counted.
Migration and invasion assay
Transwell chambers coated with or without 40 μL of 1 mg/mL Matrigel (BD Biosciences) were used to examine in vitro migration and invasion capability of the DU145 cells. After transfection, 5 × 104 cells were seeded onto the upper chamber containing 200 μL DMEM with 1% FBS, and the lower chamber was filled with 500 μL of DMEM with 20% FBS as a chemoattractant. Transwell chambers were then incubated at 37°C for 48 h. Cells adhering to the lower membrane of the inserts were counted as previously described (Ponti et al., 2005). Images of different fields were taken.
RNA extraction and quantitative reverse transcription-polymerase chain reaction
Total RNA was extracted from sera with TRIzol reagent according to the manufacturer's instructions (Invitrogen). The level of miR-372 was quantified by quantitative reverse transcription (RT)-polymerase chain reaction (PCR) using TaqMan MicroRNA Assay Kits (Applied Biosystems, Foster City, CA) with miR-1228 as an internal normalized reference as previously described (Hu et al., 2014). Their relative levels were measured in triplicate on a PRISM 7300 real-time PCR machine (Applied Biosystems). After transfection with miR-372, anti-miR-372, and NC, the expression levels of miR-372 mRNAs in DU145 cells were also quantified using quantitative RT-PCR (qRT-PCR).
Western blot
At 48 h after transfection, cells were lyzed at 4°C for 30 min using radio immunoprecipitation assay buffer. Total protein was quantified using bicinchoninic acid assay and separated by electrophoresis in sodium dodecyl sulfate–polyacrylamide gels before transferring to nitrocellulose membranes (Bio-Rad, Hercules). They were then blocked in 5% skim milk in Tris buffered saline with Tween 20. Immune complexes were formed by incubation of membranes with anti-p65 (1:1000) (Cell Signaling, Boston), anti-CDK8 (1:1000) (Cell Signaling), anti-IKKβ (1:1000) (Cell Signaling), anti-MMP-9 (1:1000) (Cell Signaling), anti-PSA (1:1000) (Cell Signaling), and anti-β-actin (1:1000) (Cell Signaling) antibodies overnight at 4°C. Blots were washed and incubated for 1 h with anti-rabbit secondary antibody. Immunoreactive protein bands were detected with an Odyssey Scanning system.
Luciferase reporter assay
The putative targets of miR-372 were predicted using the TargetScan, PicTar, and miRanda algorithms. The p65 3′-untranslated mRNA region (3′-UTR) containing the predicted miR-372-binding sites was cloned into the pMIR-REPORT vector (Ambion, Austin, TX) using PCR-generated fragments (WT-UTR). A mutant luciferase vector with miR-372 one pairing site deleted (DEL-UTR) was also constructed. When DU145 cells reached 60–70% confluence in 24-well plate, 100 ng of Luciferase plasmid were cotransfected with 50 ng of Renilla plasmid (Ambion) and 650 ng of miR-372 mimics, anti-miR-372 mimics, or NC using Lipofectamine 2000. After 48 h, luciferase activities were measured using a dual-luciferase reporter assay system (Promega, Madison) according to the manufacturer's instructions.
Statistical analyses
All experiments were done in triplicate. Continuous variables are expressed as mean ± standard deviation, *p < 0.05, **p < 0.01. The two-tailed t-test was used to draw a comparison between groups. All statistical analyses were performed using SPSS 19.0 (SPSS, Inc., Chicago, IL).
Results
miRNA-372 is downregulated in PCa
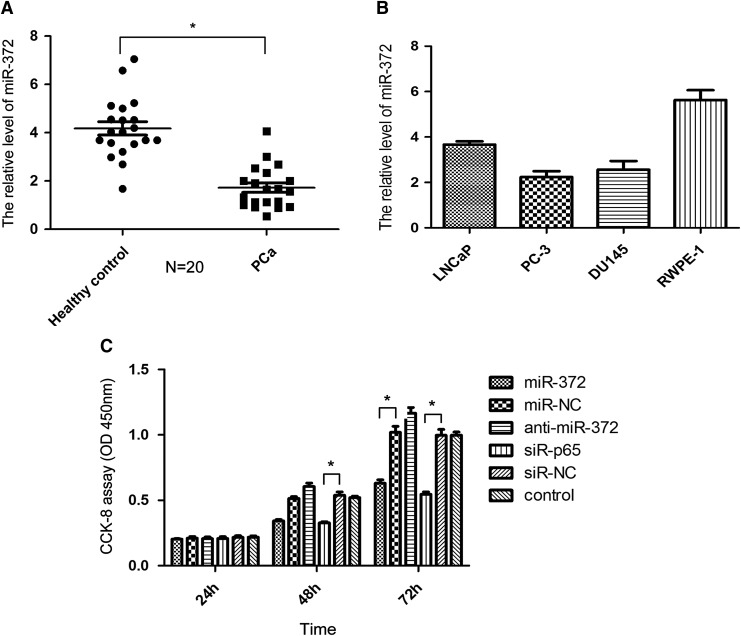
In this study, we selected miR-372 because of its wide range of functional regulation in many types of human cancers (Cho et al., 2009; Lai et al., 2012; Gu et al., 2013; Wu et al., 2015). In this study, 20 pairs of serum samples were collected from patients with PCa and healthy control subjects for further confirming the pattern of expression of miR-372 in PCa. qRT-PCR analysis exhibited that the expression level of miR-372 was downregulated in PCa serum samples, compared with samples from healthy control subjects (p < 0.05, Fig. 1A), suggesting that aberrant expression of miR-372 might be involved in the initiation and development of PCa. Figure 1B showed the expression level of miR-372 in a panel of human PCa cells and normal prostate epithelial cells RWPE-1 cell. The relationship between the clinicopathological parameters and miR-372 expression is shown in Table 1.
FIG. 1.
The expression of miR-372 in PCa and the proliferation assay in DU145 cells. (A) The relative expression of miR-372 in 20 pairs of serum samples from patients with PCa and healthy control subjects was analyzed by qRT-PCR. (B) The expression level of miR-372 in a panel of human prostate cancer cells LNCaP, PC-3, DU145 cell, and normal prostate epithelial cells RWPE-1 cell. (C) CCK-8 assay of DU145 cell transfection with miR-372, miR-NC, anti-miR-372, siR-P65, siR-NC, and control. (*p < 0.05). PCa, prostate cancer; qRT-PCR, quantitative reverse transcription-polymerase chain reaction.
Table 1.
Relationship Between Clinicopathological Parameters and miR-372 Expression in PCa
| miR-372 expression | ||||
|---|---|---|---|---|
| Clinicopathologic parameters | Number of cases | Low (%) | High (%) | p |
| Age (years) | ||||
| ≤60 | 13 | 6 (46.2) | 7 (53.8) | 0.189 |
| >60 | 7 | 5 (71.4) | 2 (28.6) | |
| Preoperative PSA (ng/mL) | ||||
| <10 | 6 | 2 (33.3) | 4 (66.7) | 0.013 |
| ≥10 | 14 | 8 (57.1) | 6 (42.9) | |
| Gleason score | ||||
| <7 | 11 | 6 (54.5) | 5 (45.5) | 0.234 |
| ≥7 | 9 | 4 (44.4) | 5 (55.6) | |
| Lymph node metastasis | ||||
| No | 7 | 3 (42.9) | 4 (57.1) | 0.003 |
| Yes | 13 | 9 (69.2) | 4 (30.8) | |
| Pathological stage | ||||
| I + II | 11 | 4 (36.4) | 7 (63.6) | 0.326 |
| III+IV | 9 | 5 (55.6) | 4 (44.4) | |
PCa, prostate cancer; PSA, prostate-specific antigen.
Overexpression of miRNA-372 in DU145 cells inhibits cell proliferation by inducing G0/G1 arrest
The CCK-8 assay showed that miR-372 mimics significantly inhibited proliferation of DU145 cells by 34.07% ± 1.93% and 44.65% ± 1.99% (p < 0.05) at 48 and 72 h, respectively. Conversely, anti-miR-372 transfection in DU145 cells could promote cell proliferation (Fig. 1C). Cell cycle analysis found that overexpression of miR-372 resulted in S and G0/G1 phase cell cycle arrest in DU145 cells after 24 and 72 h of exposure, respectively (Fig. 2A); on the contrary, anti-miR-372 transfection suppressed the effect. Colony formation assay was also performed to assess the long-term effects. After 2 weeks of selection, cells transfected with miR-372 formed significantly fewer colonies than the control cells and anti-miR-372-transfected cells (Fig. 2B), consistent with the results of CCK-8 assay.
FIG. 2.
(A) Cell cycle analysis showed that there was significant cell cycle transition in DU145 cells after 24 and 48 h of treatment with miR-372, NC, anti-miR-372, and siR-p65. (B) The colony formation assay was conducted to determine the colony-forming growth of cells, and the colonies were captured on the 14th day after seeding (*p < 0.05).
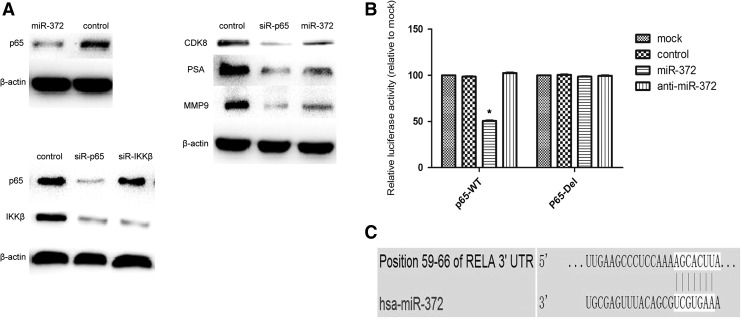
Western blot analysis revealed that overexpression of miR-372 significantly downregulated the protein levels of CDK8 (Fig. 4A). Taken together, these results indicated that miR-372 could transcriptionally regulate the expression of CDK8 and, therefore, suppress the proliferation of DU145 cells.
FIG. 4.
p65 is a target gene of miR-372 in DU145 cells. (A) Western blot analysis was used to detect the expression of p65, CDK8, PSA, and MMP9 in DU145 cells after treatment with miR-497, siR-p65, and control. The expression of p65 and IKKβ was also detected after transfection with siR-p65 and siR-IKKβ to clear the relationship between P65 and IKKβ in PCa. β-actin served as an internal control. (B) Relative luciferase activity of the p65 WT-UTR and the DEL-UTR luciferase constructs in DU145 cells transfected with miR-372 or control (*p < 0.05). (C) p65 was predicted to be a candidate target of miR-372 using the bioinformatics algorithm TargetScan. PSA, prostate-specific antigen.
Effects of miR-372 on DU145 cell migration and invasion of DU145 cells
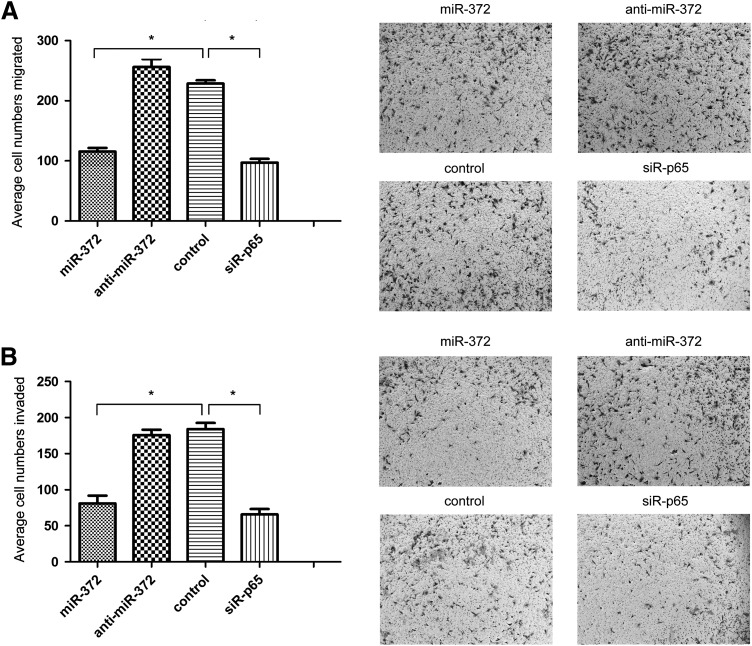
As shown in Figure 3A, Transwell assays without Matrigel illustrated the forced expression of miR-372 resulting in a 49.3% ± 6.5% decrease in the migratory ability of DU145 cells compared to the control cells (p < 0.05). Transwell assays with Matrigel showed that overexpression of miR-372 decreased the invasive ability of DU145 cells by 56.4% ± 7.2%, compared with the control cells (p < 0.05, Fig. 3B), while there was no significant statistical difference between anti-miR-372 transfection and control DU145 cells in both migration and invasion assays. All these results support the fact that overexpression of miR-372 inhibits the migration and invasion of DU145 cells.
FIG. 3.
miR-372 suppresses the migration and invasion of DU145 cells. (A) The migratory activity of DU145 cells was detected using a transwell migration assay treated with miR-372, anti-miR-372, control, and siR-p65. Representative images are shown on the right; the values shown are expressed as mean ± standard deviation. (B) The invasive activity of DU145 cells was detected using a transwell invasion assay. (*p < 0.05).
miR-372 directly targets p65
To explore the mechanism of miR-372 in PCa, three bioinformatic algorithms (TargetScan, PicTar, and miRanda) were used to identify putative targets. One binding site of miR-372 was predicted at positions 59–66 in the 3′-UTR of p65 (RELA) mRNA (Fig. 4C). To validate p65 as a genuine target of miR-372, we performed luciferase reporter assays to check for binding of miR-372 to the 3′-UTR of p65 mRNA. Luciferase activity was significantly inhibited by transfection with miR-372 and a vector carrying the wild-type 3′-UTR of p65 (p < 0.05, Fig. 4B), whereas transfection with deletion vectors blocked the inhibition of luciferase activity. These data suggested that miR-372 bound directly to a specific site in the 3′-UTR of p65 mRNA. We next determined whether endogenous p65 expression could be downregulated by miR-372 using western blot analysis to evaluate the expression of p65 protein after miR-372 mimics and NC transfection in DU145 cells. The results showed that miR-372 overexpression significantly decreased the expression of p65 protein (Fig. 4A). Collectively, these results showed that miR-372 acted as a negative regulator of p65 expression.
In addition, the levels of MMP-9 and PSA proteins decreased after overexpression of miR-372. These results indicated that miR-372 may regulate the migration and invasion of DU145 cells by targeting NF-κB/p65/MMP-9 signaling.
Knockdown of p65 suppresses cell proliferation, migration, and invasion in vitro
Expression of p65 was knocked down using siRNA in DU145 cells. Western blot analysis revealed that p65 expression in DU145 cells was significantly decreased after transfection with siR-p65 compared to NC (Fig. 4A). Knockdown of p65 significantly suppressed cell proliferation at 48 and 72 h in CCK-8 assays (p < 0.05, Fig. 1B), and significantly decreased the proportion of cells in G2/M phase, and increased cells in G1/G0 phase (Fig. 2), similar to that observed by the overexpression of miR-372. In the transwell assays, as shown in Figure 3A, B, knockdown of p65 markedly inhibited the migration and invasion of DU145 cells, compared to NC (p < 0.05). Furthermore, the levels of CDK8, MMP-9, and PSA proteins decreased by varying degrees after transfection with siR-p65 in DU145 cells.
IKKβ, one of the two catalytic subunits of the IKK complex (DiDonato et al., 1997), activates NF-κB through phosphorylation of inhibitors of NF-κB (IκBs), which leads to the translocation of cytoplasmic NF-κB into the nucleus (Ghosh and Karin, 2002; Chen and Greene, 2004; Annunziata et al., 2007). Surprisingly, the level of IKKβ protein decreased after transfection with siR-p65 in DU145 cells. The expression of IKKβ was also knocked down using siRNA in DU145 cells. However, there was no change in the level of p65 protein by western blot.
Discussion
Circulating miRNAs are abundant in various body fluids and provide valuable clinical perspectives due to their high stability and circulation. Although the significance of circulating miRNAs is not entirely clear, they have aroused interest as potential monitoring and diagnostic biomarkers for various diseases, especially cancer. In this study, we found that miR-372 was downregulated in PCa serum samples compared to healthy controls, which suggest that this miRNA could be a tumor suppressor. Until now, many reports have showed that there was significantly different expression pattern of the same kind of miRNA in different tumors. These results are not surprising considering the tumor specificity in the gene expression profile and the tumor context that is likely to strongly affect the miRNA alteration. Moreover, a single miRNA may regulate distinct downstream targets and signaling pathways in different cell types. Interestingly, we found that lower levels of miR-372 are associated with lymph node metastasis (p = 0.003) and preoperative PSA (p = 0.013), suggesting that miR-372 may be closely associated with PCa progression (Table. 1). Hence, miR-372 could be a potential diagnostic and prognostic biomarker for PCa. To verify this, we performed functional studies using the PCa cell line, DU145.
The aberrant expression of miRNAs is closely related to proliferation, cell cycle, apoptosis, differentiation, migration, metabolism, and prognosis of various cancers, including PCa (Xu et al., 2012; Zhu et al., 2013; Liu et al., 2014). Until now, more than 50 miRNAs have been found to be involved in PCa, but only few studies have successfully identified miRNA targets that are specifically modulated in PCa (Wu et al., 2012). Our study showed that overexpression of miR-372 inhibited proliferation by inducing G0/G1 cell cycle arrest in DU145 cells. We also found that miR-372 decreased the colony formation activity of DU145 cells. Obviously, uncontrolled proliferation due to aberrant regulation of cell cycle can result in the development of cancer. We discovered that CDK8, a colon cancer oncogene encoding the cyclin-dependent kinase (CDK) component of the mediator complex, was downregulated at the protein level by the transfection of miR-372. Furthermore, the migratory and invasive abilities were significantly decreased after transfection with miR-372.
PCa relapse and metastasis, as well as development of castration-resistant disease, remain the major causes of death (Siegel et al., 2013; Egan et al., 2014). Some PCa patients are prone to relapse and metastasis, which frequently happens even after surgery, and may lead to death. Improving our understanding of miRNA-mediated targets and signaling pathways would be useful for deciphering the regulatory mechanism of miRNAs in cancer metastasis and invasion. Based on bioinformatics analysis, we predicted p65, the upstream regulator of NF-κB, to be a theoretical target gene of miR-372. The luciferase activity assay illustrated that the p65 downregulation mediated by miR-372 was through the 3′-UTR of p65, and western blotting demonstrated that upregulation of miR-372 decreased p65 expression, which showed that p65 is a genuine target of miR-372. Furthermore, we found that the expression of MMP-9 and PSA decreased by varying degrees after transfection with miR-372 in DU145 cells. A single miRNA can modulate a signaling network by targeting multiple genes (Griffiths-Jones et al., 2006). The role of a miRNA strictly relies on its spatiotemporal expression pattern and target genes (Mestdagh et al., 2011). The activation of the NF-κB pathway, defined by the nuclear translocation of the p65 subunit, is indicator of lymph node invasion (Lessard et al., 2006) and associated with an increased risk of biochemical recurrence in PCa (Fradet et al. 2004; Koumakpayi et al., 2010). Multiple genes regulated by NF-κB, especially MMP-9, are involved in the metastasis of PCa (Aalinkeel et al., 2004; Uzzo et al., 2006). Therefore, miR-372 could regulate the migration and invasion of PCa by targeting NF-κB/p65/MMP-9.
Until now, p65 regulation by miRNA to affect proliferation, migration, and invasion in PCa is not reported. We have uncovered a novel relationship between miR-372 and p65 in PCa. To examine the role of p65 in PCa development and metastatic progression, the expression of p65 in DU145 cells was knocked down using siRNA. Similar to transfection with miR-372, the proliferation ability was suppressed by inducing G0/G1 cell cycle arrest, and the colony formation activity and CDK8 protein level also decreased. Therefore, we conclude that miR-372 may repress p65 expression to downregulate the transcriptional activity of CDK8, leading to conversion of DU145 cell cycle. In addition, knockdown of p65 significantly inhibited the migration and invasion of DU145 cells. The expression of MMP-9 and PSA also decreased during this process.
The expression of PSA significantly increased with overexpression of NF-κB in PCa LNCaP and DU145 cells, which was closely related to the progression of PCa (Chen and Sawyers, 2002). Given the considerable contribution of NF-κB in the development of PCa, regulation of miR-372/p65/NF-κB may have a therapeutic prospect in the control of PCa progression. Previous studies have shown that inhibition of NF-κB activity decreased the expression of CDK4/6 and cyclinD1 and D3, leading to cell cycle arrest and apoptosis (Ezhevsky et al., 1997; Liu and Greene, 2001). For the first time in this study, CDK8 was found to be involved in cell cycle transition through miR-372/p65/NF-κB pathway in PCa, although the specific mechanisms need further study. Previous studies have shown that PSA might affect the invasion of PCa cells by degrading the extracellular matrix components, fibronectin and laminin (Webber et al., 1995). The PSA gene promoter contains a κB site, and PSA is involved in prostate epithelial growth (Zhang et al., 2004), thus suggesting that p65 is associated with PSA expression. In this study, we found that miR-372 and siR-p65 decrease PSA protein expression, suggesting the potential involvement of miR-372/p65 in the metastasis of PCa through the PSA pathway.
Until now, the significance of PSA in PCa monitoring and prognosis is still being questioned, as population-based PSA screening can lead to overdiagnosis and overtreatment of PCa (Lilja et al., 2008; Lin et al., 2008; Schroder, 2009). Thus, more precise biomarkers are required. miRNAs may be potential biomarkers as their relatively small size protects them from RNase attack. Furthermore, the expression patterns of miRNA are more reliable and sensitive to alter in cell biology (Weber et al., 2010). In the present study, we conclude that miR-372 participates in the development and progression of human PCa by targeting the p65-mediated NF-κB/MMP-9/PSA signaling pathway. Of course, we have selected only one cell line to study, not yet fully applicable for PCa in general. So we also need more in-depth and comprehensive study. Taken together, these results suggest that targeting miR-372/p65 interaction or perturbing miR-372 expression could provide a new insight into prevention, diagnosis, and treatment of PCa patients.
Acknowledgments
This study was supported by grants from the National Natural Science Funds of China (no. 81070600 and 81570684) and the Shanghai Science and Technology Funds (no. 134119a0600, 09411950100, and 14430720800).
Disclosure Statement
No competing financial interests exist.
References
- Aalinkeel R., Nair M.P., Sufrin G., Mahajan S.D., Chadha K.C., Chawda R.P., and Schwartz S.A. (2004). Gene expression of angiogenic factors correlates with metastatic potential of prostate cancer cells. Cancer Res 64, 5311–5321 [DOI] [PubMed] [Google Scholar]
- Annunziata C.M., Davis R.E., Demchenko Y., Bellamy W., Gabrea A., Zhan F., Lenz G., Hanamura I., Wright G., Xiao W., Dave S., Hurt E.M., Tan B., Zhao H., Stephens O., Santra M., Williams D.R., Dang L., Barlogie B., Shaughnessy J.D., Jr., Kuehl W.M., and Staudt L.M. (2007). Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell 12, 115–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.D., and Sawyers C.L. (2002). NF-kappa B activates prostate-specific antigen expression and is upregulated in androgen-independent prostate cancer. Mol Cell Biol 22, 2862–2870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.F., and Greene W.C. (2004). Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol 5, 392–401 [DOI] [PubMed] [Google Scholar]
- Cho W.J., Shin J.M., Kim J.S., Lee M.R., Hong K.S., Lee J.H., et al. (2009). miR-372 regulates cell cycle and apoptosis of ags human gastric cancer cell line through direct regulation of LATS2. Mol Cells 28, 521–527 [DOI] [PubMed] [Google Scholar]
- Corey E., Brown L.G., Kiefer J.A., Quinn J.E., Pitts T.E., Blair J.M., and Vessella R.L. (2005). Osteoprotegerin in prostate cancer bone metastasis. Cancer Res 65, 1710–1718 [DOI] [PubMed] [Google Scholar]
- Cortez M.A., Bueso-Ramos C., Ferdin J., Lopez-Berestein G., Sood A.K., and Calin G.A. (2011). MicroRNAs in body fluids—the mix of hormones and biomarkers. Nat Rev Clin Oncol 8, 467–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmay T., and Edwards D.R. (2006). MicroRNAs and the hallmarks of cancer. Oncogene 25, 6170–6175 [DOI] [PubMed] [Google Scholar]
- Diamandis E.P. (2010). Prostate cancer screening with prostate-specific antigen testing: more answers or more confusion? Clin Chem 56, 345–351 [DOI] [PubMed] [Google Scholar]
- DiDonato J.A., Hayakawa M., Rothwarf D.M., Zandi E., and Karin M. (1997). A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature 388, 548–554 [DOI] [PubMed] [Google Scholar]
- Dykxhoorn D.M. (2010). MicroRNAs and metastasis: little RNAs go a long way. Cancer Res 70, 6401–6406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan A., Dong Y., Zhang H., Qi Y., Balk S.P., and Sartor O. (2014). Castration-resistant prostate cancer: adaptive responses in the androgen axis. Cancer Treat Rev 40, 426–433 [DOI] [PubMed] [Google Scholar]
- Ezhevsky S.A., Nagahara H., Vocero-Akbani A.M., Gius D.R., Wei M.C., and Dowdy S.F. (1997). Hypo-phosphorylation of the retinoblastoma protein (pRb) by cyclin D:Cdk4/6 complexes results in active pRb. Proc Natl Acad Sci U S A 94, 10699–10704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradet V., Lessard L., Begin L.R., Karakiewicz P., Masson A.M., and Saad F. (2004). Nuclear factor-kappaB nuclear localization is predictive of biochemical recurrence in patients with positive margin prostate cancer. Clin Cancer Res 10, 8460–8464 [DOI] [PubMed] [Google Scholar]
- Ghosh S., and Karin M. (2002). Missing pieces in the NF-kappaB puzzle. Cell 109 Suppl, S81–S96 [DOI] [PubMed] [Google Scholar]
- Griffiths-Jones S., Grocock R.J., van Dongen S., Bateman A., and Enright A.J. (2006). miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res 34, D140–D144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., Guo X., Zou L., Zhu H., and Zhang J. (2013). Upregulation of microRNA-372 associates with tumor progression and prognosis in hepatocellular carcinoma. Mol Cell Biochem 375, 23–30 [DOI] [PubMed] [Google Scholar]
- Hu J., Wang Z., Liao B.Y., Yu L., Gao X., Lu S., Wang S., Dai Z., Zhang X., Chen Q., Qiu S.J., Wu Y., Zhu H., Fan J., Zhou J., and Wang J. (2014). Human miR-1228 as a stable endogenous control for the quantification of circulating microRNAs in cancer patients. Int J Cancer 135, 1187–1194 [DOI] [PubMed] [Google Scholar]
- Ismail H.A., Lessard L., Mes-Masson A.M., and Saad F. (2004). Expression of NF-kappaB in prostate cancer lymph node metastases. Prostate 58, 308–313 [DOI] [PubMed] [Google Scholar]
- Koumakpayi I.H., Le Page C., Mes-Masson A.M., and Saad F. (2010). Hierarchical clustering of immunohistochemical analysis of the activated ErbB/PI3K/Akt/NF-kappaB signalling pathway and prognostic significance in prostate cancer. Br J Cancer 102, 1163–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J.H., She T.F., Juang Y.M., Tsay Y.G., Huang A.H., Yu S.L., et al. (2012). Comparative proteomicproling of human lung adenocarcinoma cells (CL1–0) expressing miR-372. Electrophoresis 33, 675–688 [DOI] [PubMed] [Google Scholar]
- Lessard L., Karakiewicz P.I., Bellon-Gagnon P., Alam-Fahmy M., Ismail H.A., Mes-Masson A.M., and Saad F. (2006). Nuclear localization of nuclear factor-kappaB p65 in primary prostate tumors is highly predictive of pelvic lymph node metastases. Clin Cancer Res 12, 5741–5745 [DOI] [PubMed] [Google Scholar]
- Lessard L., Mes-Masson A.M., Lamarre L., Wall L., Lattouf J.B., and Saad F. (2003). NF-kappa B nuclear localization and its prognostic significance in prostate cancer. BJU Int 91, 417–420 [DOI] [PubMed] [Google Scholar]
- Lilja H., Ulmert D., and Vickers A.J. (2008). Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nat Rev Cancer 8, 268–278 [DOI] [PubMed] [Google Scholar]
- Lin K., Lipsitz R., Miller T., Janakiraman S., U.S. Preventive Services Task Force. (2008). Benefits and harms of prostate-specific antigen screening for prostate cancer: an evidence update for the U.S. Preventive Services Task Force. Ann Intern Med 149, 192–199 [DOI] [PubMed] [Google Scholar]
- Liu D., Tao T., Xu B., Chen S., Liu C., Zhang L., Lu K., Huang Y., Jiang L., Zhang X., Huang X., Zhang L., Han C., and Chen M. (2014). MiR-361-5p acts as a tumor suppressor in prostate cancer by targeting signal transducer and activator of transcription-6(STAT6). Biochem Biophys Res Commun 445, 151–156 [DOI] [PubMed] [Google Scholar]
- Liu D.X., and Greene L.A. (2001). Neuronal apoptosis at the G1/S cell cycle checkpoint. Cell Tissue Res 305, 217–228 [DOI] [PubMed] [Google Scholar]
- Mestdagh P., Lefever S., Pattyn F., Ridzon D., Fredlund E., Fieuw A., Ongenaert M., Vermeulen J., De Paepe A., Wong L., Speleman F., Chen C., and Vandesompele J. (2011). The microRNA body map: dissecting microRNA function through integrative genomics. Nucleic Acids Res 39, e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy G.R. (2002). Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2, 584–593 [DOI] [PubMed] [Google Scholar]
- Ponti D., Costa A., Zaffaroni N., Pratesi G., Petrangolini G., Coradini D., Pilotti S., Pierotti M.A., and Daidone M.G. (2005). Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res 65, 5506–5511 [DOI] [PubMed] [Google Scholar]
- Schroder F.H. (2009). Review of diagnostic markers for prostate cancer. Recent Results Cancer Res 181, 173–182 [DOI] [PubMed] [Google Scholar]
- Siegel R., Naishadham D., and Jemal A. (2013). Cancer statistics, 2013. CA Cancer J Clin 63, 11–30 [DOI] [PubMed] [Google Scholar]
- Sturge J., Caley M.P., and Waxman J. (2011). Bone metastasis in prostate cancer: emerging therapeutic strategies. Nat Rev Clin Oncol 8, 357–368 [DOI] [PubMed] [Google Scholar]
- Uzzo R.G., Crispen P.L., Golovine K., Makhov P., Horwitz E.M., and Kolenko V.M. (2006). Diverse effects of zinc on NF-kappaB and AP-1 transcription factors: implications for prostate cancer progression. Carcinogenesis 27, 1980–1990 [DOI] [PubMed] [Google Scholar]
- Webber M.M., Waghray A., and Bello D. (1995). Prostate-specific antigen, a serine protease, facilitates human prostate cancer cell invasion. Clin Cancer Res 1, 1089–1094 [PubMed] [Google Scholar]
- Weber J.A., Baxter D.H., Zhang S., Huang D.Y., Huang K.H., Lee M.J., Galas D.J., and Wang K. (2010). The microRNA spectrum in 12 body fluids. Clin Chem 56, 1733–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Wang Y., Lu X., He H., Liu H., Meng X., Xia S., Zheng K., and Liu B. (2015). Low mir-372 expression correlates with poor prognosis and tumor metastasis in hepatocellular carcinoma. BMC Cancer 15, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Sun H., Zeng W., He J., and Mao X. (2012). Upregulation of MircoRNA-370 induces proliferation in human prostate cancer cells by downregulating the transcription factor FOXO1. PLoS One 7, e45825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B., Wang N., Wang X., Tong N., Shao N., Tao J., Li P., Niu X., Feng N., Zhang L., Hua L., Wang Z., and Chen M. (2012). MiR-146a suppresses tumor growth and progression by targeting EGFR pathway and in a p-ERK-dependent manner in castration-resistant prostate cancer. Prostate 72, 1171–1178 [DOI] [PubMed] [Google Scholar]
- Zhang L., Charron M., Wright W.W., Chatterjee B., Song C.S., Roy A.K., and Brown T.R. (2004). Nuclear factor-kappaB activates transcription of the androgen receptor gene in Sertoli cells isolated from testes of adult rats. Endocrinology 145, 781–789 [DOI] [PubMed] [Google Scholar]
- Zhu C., Li J., Ding Q., Cheng G., Zhou H., Tao L., Cai H., Li P., Cao Q., Ju X., Meng X., Qin C., Hua L., Shao P., and Yin C. (2013). miR-152 controls migration and invasive potential by targeting TGFalpha in prostate cancer cell lines. Prostate 73, 1082–1089 [DOI] [PubMed] [Google Scholar]