Abstract
Purpose: Medical treatment of glaucoma relies on intraocular pressure (IOP)-lowering medications, typically administered daily by the patient. While these medications are effective when applied correctly, patient adherence is a major obstacle in glaucoma treatment. We have developed a sustained-release formulation of timolol maleate that can be injected subconjunctivally to avoid patient noncompliance.
Methods: A biodegradable microsphere formulation for timolol maleate was injected subconjunctivally in normal rabbits. We measured timolol levels in tears, aqueous humor, vitreous humor, and serum of study rabbits. Furthermore, IOP profiles were recorded longitudinally. Tissue compatibility and side effects were evaluated using histochemistry.
Results: The microsphere formulation led to measureable amounts of timolol in the aqueous humor and the tear film for up to 90 days. Timolol was not detectable in the serum at any time. A significant reduction of IOP was observed in treated eyes. Clinically, the subconjunctival administration of the microspheres was well tolerated with no signs of inflammation or infection. The absence of local inflammation was confirmed by histology.
Conclusions: A single subconjunctival administration of timolol microspheres achieved delivery and IOP reduction in rabbits for up to 90 days without local or systemic inflammation or toxicity. This approach has the potential to improve the management of glaucoma in patient populations, who are challenged to adhere to a regimen of daily eye drops.
Keywords: : glaucoma pharmacology, timolol maleate, sustained delivery, rabbit
Introduction
There are ∼3 million people in the United States with glaucoma and 67 million people worldwide.1–3 Glaucoma is an optic neuropathy in which the retinal ganglion cells (RGC) degenerate, leading to progressively increasing vision loss.4,5 The majority of cases of glaucoma are associated with elevated intraocular pressure (IOP). Decreasing the pressure significantly reduces the rate of RGC degeneration, including in cases of normal tension glaucoma, and is the current standard of care.6,7
One of the most commonly used drugs for glaucoma is timolol maleate (Timoptic®; Merck & Co., Inc.). Timolol was approved by the Food and Drug Administration (FDA) in 1979 for ophthalmic applications, and it has become the FDA's “gold standard” drug for IOP reduction.8 It is a beta-adrenergic receptor antagonist that works as a topical drop. It is believed to function by decreasing the production of aqueous humor, which is made in the epithelium of the ciliary processes.9 The use of eye drops leads to systemic absorption of the administered drug and side effects of timolol can include cardiac and pulmonary suppression.8,10 However, when applied correctly, timolol is a very effective drug and IOP is lowered within 30 min of administration. Drops are typically needed once or twice daily to maintain a reduction in IOP. Unfortunately, patient compliance with daily administration of eye drops for IOP control is often very poor. In one study, nearly 1 half of the individuals who had filled a glaucoma prescription discontinued all therapy within 6 months.11 Other investigators found that 51% of patients had insufficient drops dispensed to comply with treatment as prescribed and 24% admitted to omitting eye drops either occasionally or frequently.12 Furthermore, involuntary noncompliance is caused by the inability of the patient to place drops in the eye appropriately, leading to insufficient delivery of drug in as many as 20% of patients.13 Compliance appears to be lowest in older patients who are also at the greatest risk of developing glaucoma.14 Thus, patient compliance is a significant challenge in the management of this chronic condition. Delivery of IOP-lowering drugs through sustained-release formulations, which are applied by the physician at the time of routine office visits, would solve the issue of patient compliance and likely promote preservation of vision.
One approach to improve compliance is through the use of a sustained-release formulation of the drug, which can be administered by the prescribing physician. We have previously described timolol maleate encapsulated in poly(D,L-lactic-co-glycolic acid)/poly(lactic acid) (PLGA/PLA) formulation with in vitro release over a 107-day period in vitro.15 We now report the effect of a sustained-release formulation of biodegradable timolol microsphere in vivo.
Methods
Fabrication of microspheres
Timolol microspheres were fabricated using a double emulsion technique of a blend of 50/50 (w/w) poly(lactic-co-glycolic acid) and poly(d,l-lactic acid) as described previously.10 The size distribution of the microspheres from each batch was characterized using a Beckman Coulter Multisizer 3 apparatus with a 100-μm diameter aperture, and the volume-weighted mean diameter is reported. Blank microspheres, without timolol, were prepared and characterized in the same manner.
In vitro release study
Microspheres were prepared by adding 1 mL of phosphate-buffered saline (PBS) to 10 mg of microspheres. The mixtures were then incubated at 37°C on a rotating Labquake shaker (Barnstead/Thermolyne, Dubuque, IA). At specific time points (2 h, 8 h, 1 day, 3 days, 5 days, and so on, up to 107 days or when no spheres can be seen following pelleting), the mixture was centrifuged, and the supernatant was removed. Fresh PBS was added and the microspheres were returned to the shaker. The supernatant for each of the sets of microspheres was frozen and stored at −80°C for subsequent analysis using ultraviolet (UV) spectroscopy at 293 nm. All experiments were carried out in triplicate.
In vivo studies
Normal, 3-month old, male New Zealand white rabbits were used for these studies (N = 15/group). All animals were treated in accordance with the statements of the Association for Research in Vision and Ophthalmology (ARVO) and experimental procedures were approved by the University of Iowa Animal Care and Use Committee. All rabbits were monitored for clinical signs of infection, inflammation, or irritation by a specialty trained ophthalmologist (A.V.D.).
Injection of spheres
Microspheres were brought to room temperature and resuspended in sterile PBS at a concentration of 25 mg/mL. Each eye to be injected received 2 drops of proparacaine hydrochloride ophthalmic solution. Microspheres were delivered subconjunctivally by a single injection into the superior quadrant of both eyes of rabbits. Each injection contained 250 μL of timolol microsphere solution. Injections were carried out using a 25-gauge needle and a 1-cc syringe. Rabbits having received injections of microspheres containing no timolol (vehicle only) were used as controls.
Measurement of IOP
IOP measurements in rabbit were taken using a Tonopen XL tonometer (Reichert Technologies, Depew, NY). A series of at least 3 measurements was taken in awake animals after application of a topical anesthetic (proparacaine, 0.5% solution). All IOP measurements were obtained between 10AM and 12PM.
Sample collection
Aqueous humor was collected weekly during the entire duration of the trial. Following application of 2 drops of proparacaine hydrochloride ophthalmic solution, ∼200 μL of aqueous humor was evacuated from the anterior chamber using a 30-gauge needle and 1-cc syringe. Only 1 quarter of all eyes was sampled each week on a rotating schedule, allowing each eye to recover for 4 weeks before it was used again for collection. Samples obtained were stored in individual vials at −80°C until use.
Serum samples were obtained from blood collected from the ear vein. Approximately, 2 mL serum was collected into uncoated Vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ) and allowed to coagulate at room temperature overnight. Serum was then obtained by centrifugation and frozen at −80°C until use. Serum samples were collected on the same schedule as the aqueous samples.
Extraction of timolol from tear samples
The tear samples were collected by adsorption into a circle of Whatman filter paper (5 mm diameter) placed in the lower fornix of the eye. Before analysis, the drug was released by soaking the paper in 500 μL of an ethanol/1 N HCl solution (4:1) overnight.
Extraction of timolol from aqueous humor, vitreous humor, and serum
The aqueous humor, vitreous humor, and serum were all prepared in the same manner. Eighty milliliters of cold ethanol with 1 μg/mL of propranolol as an internal standard was added to 20 μL of the sample. The solution was then vortexed and subsequently centrifuged for 15 min at 13,000 rpm to remove protein components. The supernatant was then quantified using high-performance liquid chromatography (HPLC) or HPLC-MS (mass spectrometry).
HPLC method
The solution was then spiked with a known concentration of propanolol and analyzed using HPLC (Shimadzu Instruments) equipped with an LC-18 column (Intersil ODS-3) and an aqueous/acetonitrile mobile phase following the protocol of Fatouros and Bouwstra.16 The HPLC system included the SIL-20AC UFLC autosampler, SPD-20AV UV-Vis detector, and an LC-20AB HPLC pump (all Shimadzu components). The flow rate was 1 mL/min. Measurements were made at 294 nm and compared to a standards curve generated under the same conditions. The limit of detection was found to be 0.02 μg/mL. The calibration curve was linear (R2 = 0.999) from 0.06 to 1 μg/mL. Propranolol was used as an internal standard for all samples, and blank samples as well as samples of known timolol concentration were used throughout the runs to validate the HPLC analysis.
Serum samples were also measured using HPLC MS to obtain more sensitive measurements following Ref.16 The samples were prepared in the same manner as above and run on a Shimadzu reverse-phase HPLC system with a tandem mass spec attachment.
To validate our HPLC techniques, timolol drops (timolol maleate ophthalmic solution 0.5%; Falcon Pharmaceuticals) were administered to rabbits twice daily. Thirty minutes after the second administration, the aqueous humor was aspirated and analyzed for timolol content as described above.
Histology
Eyes were fixed in 4% paraformaldehyde for 4–6 h immediately upon euthanasia. Eyes were then dissected and embedded in sucrose, as described previously.4,17 Sagittal sections of the anterior segment, conjunctiva, and eyelids were cut on a Microm HM505E cryostat and stained using hematoxylin/eosin or were used for immunohistochemical detection of tissue inflammation (CD3 and CD45; Santa Cruz Biotech, Santa Cruz, CA). Sections were viewed using an Olympus BX41 microscope equipped with a SPOT-RT digital camera.
Statistical analyses
IOP data were analyzed using Student's t-test. Differences are considered significant if the P value is <0.05.
Results
Release of timolol from PLGA/PLA microspheres
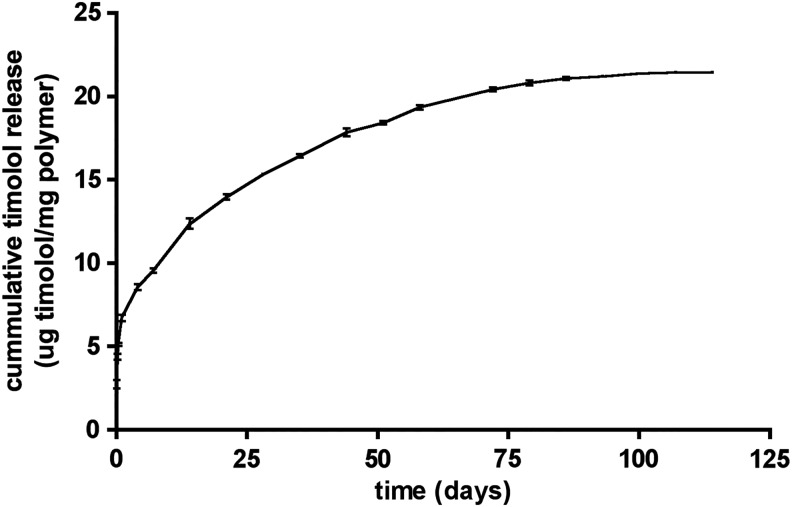
The average diameter of the timolol spheres and blanks used in this study was 14 ± 4 μm. The in vitro release curve for these spheres, designated PLGA 502H/P(dI)LA, indicates that, following an initial release burst (∼25% over the first day), timolol is continuously released for ∼90 days (Fig. 1). This release curve is almost identical to those obtained for previously fabricated batches, demonstrating that encapsulation of timolol by this method is a readily reproducible process.15 Timolol loading capability varied <5% when several batches are compared to one another.
FIG. 1.
Release curve for timolol maleate microspheres PLGA 502H/P(dI)LA in vitro in an infinite sink system. PLGA, poly(D,L-lactic-co-glycolic acid).
Subconjunctival injection of timolol spheres
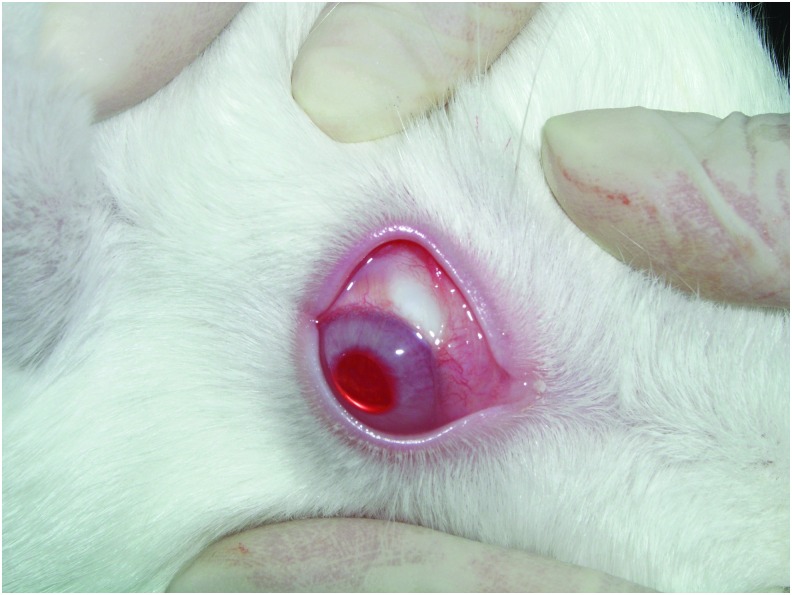
Spheres were resuspended immediately before injection in sterile PBS at a concentration of 25 mg/mL and 250 μL of the solution was injected subconjunctivally (superior bulbar area) in both eyes of normal New Zealand rabbits (N = 15). The microspheres contain ∼21.8 μg/mg timolol15 and consequently each eye received 136 μg timolol maleate. Injection results in the formation of a bleb (Fig. 2) that typically completely resorbs within 24 h. Forty-eight hours after injection, all eyes appeared grossly similar to preinjected or uninjected eyes without visible signs of spheres, which have a white color similar to the sclera. The rabbits tolerated the procedure well, showing no signs of distress or pain during or immediately following administration. No clinical signs of irritation, inflammation, or infections were observed in any injected eye throughout the study. At the end of the study period, the residual spheres were no longer observed and it is assumed that the spheres have dissolved completely, as they do in vitro.
FIG. 2.
Rabbit eye showing the bleb induced by microsphere administration immediately after injection. The bleb is resorbed over the first 24 h.
Effect on IOP
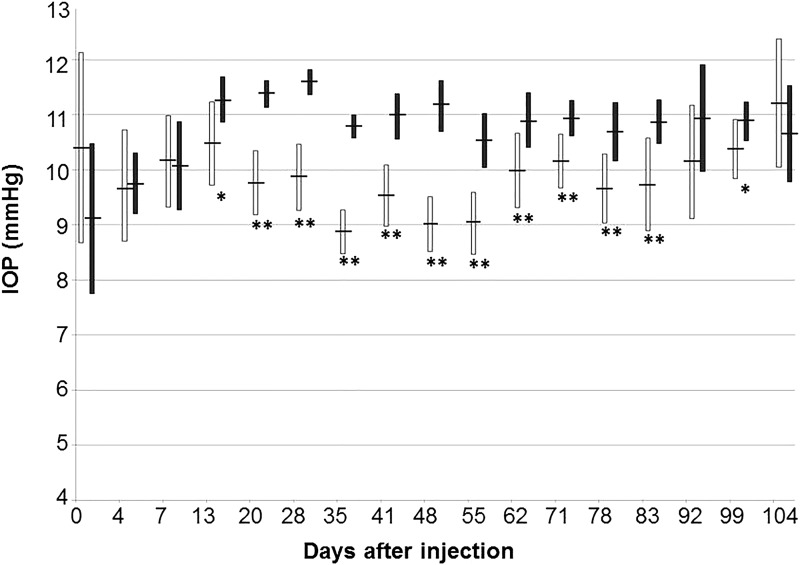
As previously reported by others,18 we observed an age-dependent increase of IOP in our control cohort. Measured IOP increased by ∼2 mmHg during the first month of the study, but remained stable once the animals reached ∼4 months of age. Timolol has relatively modest IOP-reducing effects in rabbits when compared to those observed in humans. However, we observed a statistically significant decrease in IOP following the administration of the timolol spheres (Fig. 3). Fourteen days after administration, the IOP in eyes having received timolol spheres was 0.9 mmHg lower than that measured in control eyes having received blank spheres (P = 0.004). The IOP-lowering effect was highly significant from 21 days (1.6 mmHg reduction, P = 4 × 10−9) to 56 days (1.5 mmHg reduction, P = 9 × 10−7) postadministration. The average IOP in treated eyes slowly began to approach that observed in control eyes, but remained statistically lower in treated than in control eyes. While some individual treated eyes still exhibited markedly decreased IOPs, an overall statistically significant difference was no longer observed 93 days after injection (0.8 mmHg reduction, P = 0.1). These data correlate well with the timolol delivery rate observed in vitro (Fig. 1).
FIG. 3.
IOP profile of rabbits having received subconjunctival injections of timolol (white bars, N = 10) or empty (black bars, N = 5) microspheres. Bars indicate average IOP ± standard deviation. *P < 0.05; **P < 0.01. IOP, intraocular pressure.
Quantification of timolol in the eye
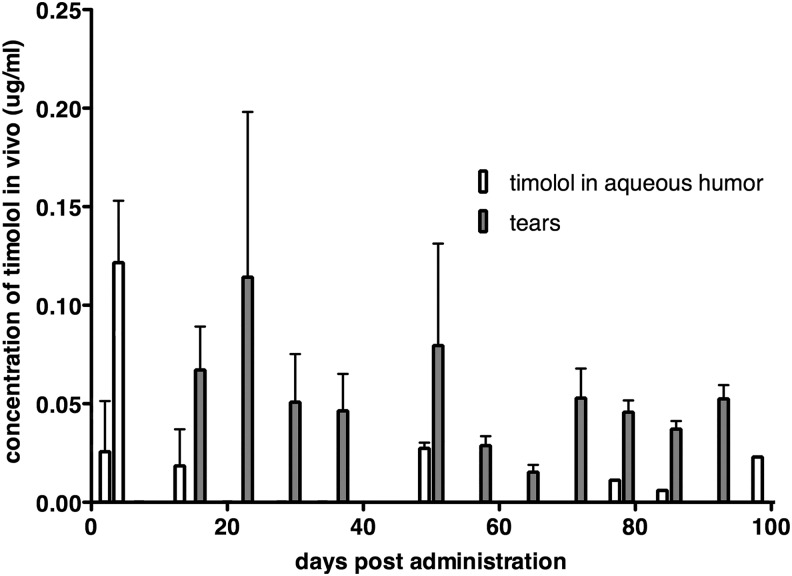
HPLC analysis of aqueous humor samples obtained 30 min after application of timolol drops (positive control) showed that aqueous humor levels of the drug reach 0.5 μg/mL and demonstrate the validity of the analytic approach. In aqueous humor obtained from eyes treated with timolol spheres, the drug was detected 2, 4, 13, 49, 77, 84, and 98 days after injection (Fig. 4 and Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/jop). Timolol was not detectable in samples collected on days 7, 20, 28, and 34. This was unexpected as some of the same animals tested positive for the drug at later time points. We suspect that these samples did contain low amounts of timolol, but presumably below the sensitivity of this HPLC method (limit of detection = 0.02 μg/mL). Furthermore, it must be cautioned that the recovery of timolol from the aqueous humor is estimated to be around 90%.19 Therefore it is possible that some samples contained timolol that was not recovered.
FIG. 4.
Detection of timolol in the aqueous humor and tear film of rabbits after injection of timolol microspheres. The drug was detected in both aqueous humor and tears over 90 days after injection. Open bars: Concentration in aqueous humor samples, shaded bars: concentration in tear samples. Bars indicate average concentrations of all samples ± standard deviation.
We also determined the presence of timolol in the tear film collected by using adsorptive paper (Fig. 4). Care was taken to harvest approximately equal volumes of tears. The process is influenced by a number of variables, such as enhanced tear production as a result of ocular irritation, which may affect both concentration of the drug and tear volume.20 Reproducible findings can be obtained with this approach, the data are qualitative in nature, and drug concentrations cannot be strictly compared to those reported elsewhere in this article. Nevertheless, samples were positive for timolol on all days for which samples were collected (days 14, 21, 28, 35, 49, 56, 63, 70, 77, 84, and 91).
Finally, we quantified timolol in the vitreous humor of 2 samples obtained from animals sacrificed on day 34 after injection. Both of these samples were positive for timolol. Taken together, these data demonstrate that the microbeads used herein released the drug throughout the study period.
Quantification of timolol in the serum
Serum levels of timolol were examined as an important parameter to assess the potential for systemic side effects. Measurements were carried out using a highly sensitive tandem MS HPLC system. Timolol was undetectable in all serum samples at all time points using this approach. The sensitivity of the mass spec approach is 0.05 ng/mL of timolol in the serum and consequently these data strongly suggest that systemic levels of the drug following the use of timolol microspheres are very low.
Histology of injected rabbit eyes
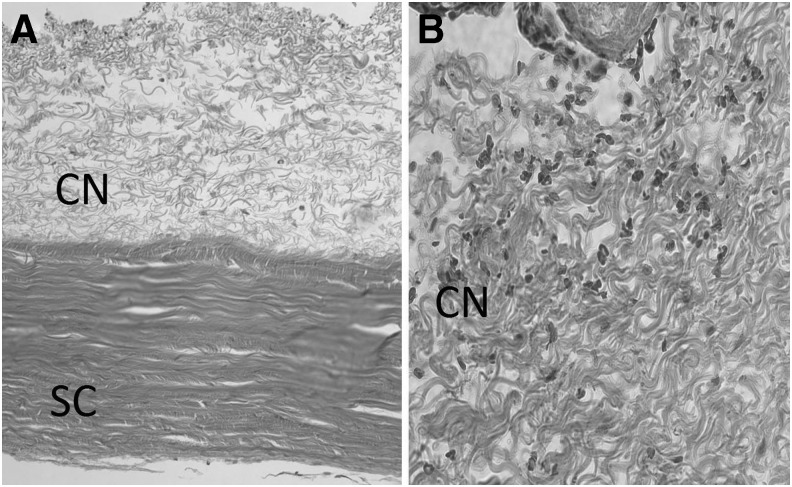
While inflammation reactions were not apparent on clinical examination of injected eyes, we wanted to further investigate the eyes for possible cellular evidence of inflammation. Toward this end, we carried out a histological examination of tissue harvested 21 days after the injection of microspheres. Particular attention was directed toward the sclera and conjunctival tissue surrounding the injection site. The tissue appeared normal and we did not detect any signs of inflammation such as accumulation of macrophages, lymphocytic infiltrate, or evidence of giant cells (Fig. 5). In addition, immunohistochemical investigation failed to indicate the presence of CD3- or CD45-positive cells (data not shown). These findings confirm the excellent tissue compatibility and safety of the microspheres.
FIG. 5.
Histology of the injection site. (A) Appearance of the sclera (SC) and conjunctiva (CN) at 100 × magnification. (B) Histology of conjunctival tissue at 400 × magnification. An infiltration of inflammatory cells is not observed. PLGA microspheres are lost during tissue sectioning and accordingly are not detected.
Discussion
Timolol maleate is a highly water-soluble drug, which makes it challenging to develop a formulation that will deliver the molecule for extended periods of time. However, through a blend formulation, we were able to develop a microsphere delivery system that released timolol for over 90 days in vitro. As we have previously shown, the particles are designed to degrade over a period of several weeks, leading to release of timolol.15 These microspheres are small enough to allow injection in buffer solutions and, as we show herein, are capable of lowering IOP for over 3 months when placed subconjunctivally.
Timolol was detected throughout the study in the aqueous humor and, more readily, in the tear film of treated eyes. It should be noted that the measured concentration values from these 2 types of samples are not directly comparable. The extraction of timolol from aqueous humor is not complete: a fraction of the drug is not recovered, which artificially decreases concentration estimates.19 Furthermore, the collection of tear samples causes a temporary increase in tear production, which can alter transport and result in the enrichment of molecules in the tear film respective to the steady-state concentration.20 Certainly, the presence of timolol in both ocular compartments is related, as modeling of timolol transport suggests that the presence of the drug in the subconjunctival space results in delivery to the aqueous humor.21
As expected, the timolol concentration in the aqueous humor was modest. The correlation between therapeutic doses of drops and sustained delivery approaches is not straightforward and is further complicated by the absence of steady-state levels for topical delivery. Timolol concentration rises sharply within the first hour after topical administration, but subsequently declines rapidly and already 4 h after application, the concentration in the aqueous humor is at a very low level.22 High doses followed by rapid clearance are typical of drop therapies and often such high doses are needed to elicit a therapeutic response. In contrast, the consistent delivery of the active compound by sustained delivery vehicles often achieves results at much lower concentrations than those required using sporadic delivery.23–25
A significant advantage of sustained delivery systems is that systemic side effects are frequently much less pronounced than when oral or topical formulations of the same drug are used. With eye drops, the overwhelming majority of active compounds do not reach the target tissue and studies have indicated that only 1%–2% of timolol delivered by drops becomes available within the aqueous humor.26,27 Thus, despite the considerably lower amount of timolol contained within the microspheres delivered here, when compared to that contained in a 90-day supply of drops twice daily, the amount of timolol that becomes available to the eye over this period is similar between the 2 delivery methods.
A second advantage of sustained delivery systems is that undesirable side effects are often much less pronounced than with topical doses. Previous studies have shown that >80% of the drug contained in eye drops is absorbed by the vasculature and becomes systemically available.28,29 Furthermore, the high dosing spike associated with drops at the time of application further increases the possibility of systemic side effects. By creating a sustained release system that delivers timolol at a low yet consistent rate, the risk due to both these factors was reduced. While our data consistently indicate the presence of timolol in the aqueous and vitreous humor, as well as in the tear samples, it was not detectable in the serum at any time, indicating very low systemic loading. Indeed systemic side effects were not noted in any of the animals used.
In this study, the observed decrease in IOP is, while statistically highly significant, modest, but similar to that reported by other using normotensive rabbits.30–32 In this proof of principle study, we did not intend to determine the amount of microbeads required to achieve, for example, a reduction of IOP by 5 mmHg since the pharmacokinetics of timolol differ between humans and rabbits. Indeed, previous studies have demonstrated that timolol is less effective in rabbits than humans,33–37 suggesting that the use of timolol spheres may have a more marked effect in humans, and particularly in eyes with elevated IOP. Our data do demonstrate that sustained delivery of timolol is possible using this vehicle. Additional studies designed to determine the most effective dose or how well the IOP-reducing effect compares to topical drops will have to be carried out.
Both timolol and PLGA have been used in clinical practice and are generally quite safe. In our study, the microspheres were well tolerated by all animals. We did not observe any indication of inflammation or toxicity associated with the administration or presence of the microspheres. Subsequent histology of the conjunctiva and adjacent sclera showed no signs of inflammatory cells. Thus our data suggest that timolol microspheres are highly compatible with the ocular tissues into which they were injected.
Previous studies used a variety of approaches to extend the delivery of timolol beyond that achieved by eye drops. A 0.1% timolol hydrogel once daily was as effective in lowering the IOP as 0.5% timolol eye drops twice daily, with fewer cardiac side effects.29 Timoptic XE (Merck, Inc., West Point, PA) was introduced as a gellan gum formulation that forms a gel when applied to ocular surface to increase the bioavailability and decrease dosing frequency. Other reported sustained-release formulations of timolol include poly(D,L-lactic-co-glycolic acid) (PLGA) films,38 chitosan-treated alginate bead,39 soft contact lenses,40–44 hydrogels,45,46 and implants.27,47 These formulations show significant promise, but either did not achieve sustained delivery for longer than 14 days in vivo or relied on implantation of larger objects. Additional strategies for sustained delivery exist, including ocular inserts and bioadhesive matrix polymers (recently reviewed in Knight and Lawrence48). However, these have not been tested using timolol and differences in the physical and chemical properties of glaucoma drugs are likely to influence results. Our current formulation of timolol maleate delivers the drug from biodegradable microspheres for over 90 days in vitro, a period sufficiently long enough for eventual clinical application.
Another commonly prescribed glaucoma drug is latanoprost (Xalatan®; Pfizer, Inc.).10 It is part of the family of prostaglandin analogues.10 These are lipophilic prodrugs that are enzymatically cleaved to their active forms, thus minimizing systemic side effects. Several formulations of latanoprost, including biodegradable nanosheets with sustained delivery up to 1 month, have been reported.49–51 Recently, a subconjunctival injection of latanoprost-loaded egg phosphatidylcholine liposomes showed sustained IOP reduction for 120 days in a primate model of glaucoma.52 A pilot study of this approach involving 6 human subjects has recently been conducted in Singapore.53
There are several shortcomings of this approach and study design. (1) Once injected, the microspheres cannot be easily removed. As timolol is occasionally poorly tolerated by some patients, it would be advisable to inject microspheres only in those patients with a demonstrated ability to tolerate the (topical) drug. (2) Timolol drops have been known to produce tachyphylaxis or become less effective when used for many years. The same limitation may also apply to the sustained-release formulations. (3) The observed IOP-lowering effect was modest. This may be related to the use of normotensive rabbits, which do not respond to timolol as well as glaucoma models.30,54,55 However, it is also possible that a higher rate of timolol delivery would have yielded larger IOP changes. This could be addressed, in part, through delivery of additional timolol microspheres. (4) We studied the effect of a single subconjunctival injection. While PLGA microspheres degrade completely, the benefits or side effects of repeated subconjunctival injections in the eye are unknown.
In conclusion, we show that a single subconjunctival injection of microspheres can effectively deliver timolol for over 90 days in rabbit eyes. This approach has the potential to address patient noncompliance and improve glaucoma treatment.
Supplementary Material
Acknowledgments
This work was funded by the Marlene S. and Leonard A. Hadley Glaucoma Research Fund, the Coulter Foundation, and NIH Director's New Innovator Award Grant, DP20D007338.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Quigley H.A., and Broman A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 90:262–267, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliver J.E., Hattenhauer M.G., Herman D., Hodge D.O., Kennedy R., Fang-Yen M., and Johnson D.H. Blindness and glaucoma: a comparison of patients progressing to blindness from glaucoma with patients maintaining vision. Am. J. Ophthalmol. 133:764–772, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Munier A., Gunning T., Kenny D., and O'Keefe M. Causes of blindness in the adult population of the republic of Ireland. Br. J. Ophthalmol. 82:630–633, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuehn M.H., Fingert J.H., and Kwon Y.H. Retinal ganglion cell death in glaucoma: mechanisms and neuroprotective strategies. Ophthalmol. Clin. North Am. 18:383–395, vi, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Kwon Y.H., Fingert J.H., Kuehn M.H., and Alward W.L. Primary open-angle glaucoma. N. Engl. J. Med. 360:1113–1124, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alward W.L., Feldman F., Cashwell L.F., Wilensky J., Geijssen H.C., Greeve E., Quigley H., Skuta G., Lichter P.R., Blondeau P., Anderson D.R., Grajewski A., Balazsi G., Amyot M., Levene R.Z., Minckler D., Heuer D., Drance S.M., Mikelberg F., Douglas G., Johnstone M., Trope G., Hoskins H.D., Pollack I.P., Mills R., Kasner O.P., Schwartz A., Liebmann L., Ritch R., Cohen J., Tuulonen A., Airaksinen P.J.; Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am. J. Ophthalmol. 126:498–505, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Alward W.L.M. Medical management of glaucoma. N. Engl. J. Med. 339:1298–1307, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Marquis R.E., and Whitson J.T. Management of glaucoma: focus on pharmacological therapy. Drugs Aging. 22:1–21, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Johnson T.V., Fan S., Zhan G., Camras C.B., and Toris C.B. Efficacy and mechanisms of intraocular pressure reduction with latanoprost and timolol in participants with ocular hypertension: a comparison of 1 and 6 weeks of treatment. J. Glaucoma. 19:356–364, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Schuman J.S. Antiglaucoma medications: a review of safety and tolerability issues related to their use. Clin. Ther. 22:167–208, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Nordstrom B.L., Friedman D.S., Mozaffari E., Quigley H.A., and Walker A.M. Persistence and adherence with topical glaucoma therapy. Am. J. Ophthalmol. 140:598–606, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Rotchford A.P., and Murphy K.M. Compliance with timolol treatment in glaucoma. Eye (Lond.). 12(Pt 2):234–236, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Brown M.M., Brown G.C., and Spaeth G.L. Improper topical self-administration of ocular medication among patients with glaucoma. Can. J. Ophthalmol. 19:2–5, 1984 [PubMed] [Google Scholar]
- 14.Gurwitz J.H., Glynn R.J., Monane M., Everitt D.E., Gilden D., Smith N., and Avorn J. Treatment for glaucoma: adherence by the elderly. Am. J. Public Health. 83:711–716, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertram J.P., Saluja S.S., McKain J., and Lavik E.B. Sustained delivery of timolol maleate from poly(lactic-co-glycolic acid)/poly(lactic acid) microspheres for over 3 months. J. Microencapsul. 26:18–26, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Fatouros D.G., and Bouwstra J.A. Iontophoretic enhancement of timolol across human dermatomed skin in vitro. J. Drug Target. 12:19–24, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Kuehn M.H., Kim C.Y., Jiang B., Dumitrescu A.V., and Kwon Y.H. Disruption of the complement cascade delays retinal ganglion cell death following retinal ischemia-reperfusion. Exp. Eye Res. 87:89–95, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Zhang H., Yang D., Ross C.M., Wigg J.P., Pandav S., and Crowston J.G. Validation of rebound tonometry for intraocular pressure measurement in the rabbit. Exp. Eye Res. 121:86–93, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Nasir F., Iqbal Z., Khan A., Ahmad L., Shah Y., Khan A.Z., Khan J.A., and Khan S. Simultaneous determination of timolol maleate, rosuvastatin calcium and diclofenac sodium in pharmaceuticals and physiological fluids using HPLC-UV. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 879:3434–3443, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Stuchell R.N., Feldman J.J., Farris R.L., and Mandel I.D. The effect of collection technique on tear composition. Invest. Ophthalmol. Vis. Sci. 25:374–377, 1984 [PubMed] [Google Scholar]
- 21.Sakanaka K., Kawazu K., Tomonari M., Kitahara T., Nakashima M., Nishida K., Nakamura J., Sasaki H., and Higuchi S. Ocular pharmacokinetic/pharmacodynamic modeling for timolol in rabbits using a telemetry system. Biol. Pharm. Bull. 31:970–975, 2008 [DOI] [PubMed] [Google Scholar]
- 22.El-Kamel A.H. In vitro and in vivo evaluation of pluronic f127-based ocular delivery system for timolol maleate. Int. J. Pharm. 241:47–55, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Chou S.F., Carson D., and Woodrow K.A. Current strategies for sustaining drug release from electrospun nanofibers. J. Control. Release. 220:584–591, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang S.J., Durairaj C., Kompella U.B., O'Brien J.M., and Grossniklaus H.E. Subconjunctival nanoparticle carboplatin in the treatment of murine retinoblastoma. Arch. Ophthalmol. 127:1043–1047, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amrite A.C., Edelhauser H.F., and Kompella U.B. Modeling of corneal and retinal pharmacokinetics after periocular drug administration. Invest. Ophthalmol. Vis. Sci. 49:320–332, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higashiyama M., Inada K., Ohtori A., and Tojo K. Improvement of the ocular bioavailability of timolol by sorbic acid. Int. J. Pharm. 272:91–98, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Jung H.J., and Chauhan A. Extended release of timolol from nanoparticle-loaded fornix insert for glaucoma therapy. J. Ocul. Pharmacol. Ther. 29:229–235, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nomoto H., Shiraga F., Kuno N., Kimura E., Fujii S., Shinomiya K., Nugent A.K., Hirooka K., and Baba T. Pharmacokinetics of bevacizumab after topical, subconjunctival, and intravitreal administration in rabbits. Invest. Ophthalmol. Vis. Sci. 50:4807–4813, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Uusitalo H., Kahonen M., Ropo A., Maenpaa J., Bjarnhall G., Hedenstrom H., and Turjanmaa V. Improved systemic safety and risk-benefit ratio of topical 0.1% timolol hydrogel compared with 0.5% timolol aqueous solution in the treatment of glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 244:1491–1496, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Chiang C.H., Ho J.I., and Chen J.L. Pharmacokinetics and intraocular pressure lowering effect of timolol preparations in rabbit eyes. J. Ocul. Pharmacol. Ther. 12:471–480, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Watanabe K., and Chiou G.C. Action mechanism of timolol to lower the intraocular pressure in rabbits. Ophthalmic Res. 15:160–167, 1983 [DOI] [PubMed] [Google Scholar]
- 32.Vareilles P., Silverstone D., Plazonnet B., Le Douarec J.C., Sears M.L., and Stone C.A. Comparison of the effects of timolol and other adrenergic agents on intraocular pressure in the rabbit. Invest. Ophthalmol. Vis. Sci. 16:987–996, 1977 [PubMed] [Google Scholar]
- 33.Andres-Guerrero V., Vicario-de-la-Torre M., Molina-Martinez I.T., Benitez-del-Castillo J.M., Garcia-Feijoo J., and Herrero-Vanrell R. Comparison of the in vitro tolerance and in vivo efficacy of traditional timolol maleate eye drops versus new formulations with bioadhesive polymers. Invest. Ophthalmol. Vis. Sci. 52:3548–3556, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Fulgencio Gde O., Viana F.A., Ribeiro R.R., Yoshida M.I., Faraco A.G., and Cunha-Junior Ada S. New mucoadhesive chitosan film for ophthalmic drug delivery of timolol maleate: in vivo evaluation. J. Ocul. Pharmacol. Ther. 28:350–358, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Martinez-Aguila A., Fonseca B., Bergua A., and Pintor J. Melatonin analogue agomelatine reduces rabbit's intraocular pressure in normotensive and hypertensive conditions. Eur. J. Pharmacol. 701:213–217, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Shayegan M.R., Boloorian A.A., and Kianoush S. Comparative study of topical application of timolol and verapamil in patients with glaucoma within 6 months. J. Ocul. Pharmacol. Ther. 25:551–553, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Zhang H.H., Luo Q.H., Yang Z.J., Pan W.S., and Nie S.F. Novel ophthalmic timolol meleate liposomal-hydrogel and its improved local glaucomatous therapeutic effect in vivo. Drug Deliv. 18:502–510, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Huang S.F., Chen J.L., Yeh M.K., and Chiang C.H. Physicochemical properties and in vivo assessment of timolol-loaded poly(d,l-lactide-co-glycolide) films for long-term intraocular pressure lowering effects. J. Ocul. Pharmacol. Ther. 21:445–453, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Sezer A.D., and Akbuga J. Release characteristics of chitosan treated alginate beads: II. Sustained release of a low molecular drug from chitosan treated alginate beads. J. Microencapsul. 16:687–696, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Alverez-Lorenzo C., Hiratani H., Gomez-Amoza J.L., Martinez-Pacheco R., Souto C., and Concheiro A. Soft contact lenses capable of sustained delivery of timolol. J. Pharm. Sci. 91:2182–2192, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Briuglia M.L., Urquhart A.J., and Lamprou D.A. Sustained and controlled release of lipophilic drugs from a self-assembling amphiphilic peptide hydrogel. Int. J. Pharm. 474:103–111, 2014 [DOI] [PubMed] [Google Scholar]
- 42.Guidi G., Korogiannaki M., and Sheardown H. Modification of timolol release from silicone hydrogel model contact lens materials using hyaluronic acid. Eye Contact Lens. 40:269–276, 2014 [DOI] [PubMed] [Google Scholar]
- 43.Jung H.J., Abou-Jaoude M., Carbia B.E., Plummer C., and Chauhan A. Glaucoma therapy by extended release of timolol from nanoparticle loaded silicone-hydrogel contact lenses. J. Control. Release. 165:82–89, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Peng C.C., Ben-Shlomo A., Mackay E.O., Plummer C.E., and Chauhan A. Drug delivery by contact lens in spontaneously glaucomatous dogs. Curr. Eye Res. 37:204–211, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Dubey A., and Prabhu P. Formulation and evaluation of stimuli-sensitive hydrogels of timolol maleate and brimonidine tartrate for the treatment of glaucoma. Int. J. Pharm. Investig. 4:112–118, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maulvi F.A., Lakdawala D.H., Shaikh A.A., Desai A.R., Choksi H.H., Vaidya R.J., Ranch K.M., Koli A.R., Vyas B.A., and Shah D.O. In vitro and in vivo evaluation of novel implantation technology in hydrogel contact lenses for controlled drug delivery. J. Control. Release. 226:47–56, 2016 [DOI] [PubMed] [Google Scholar]
- 47.Ng X.W., Liu K.L., Veluchamy A.B., Lwin N.C., Wong T.T., and Venkatraman S.S. A biodegradable ocular implant for long-term suppression of intraocular pressure. Drug Deliv. Transl. Res. 5:469–479, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knight O.J., and Lawrence S.D. Sustained drug delivery in glaucoma. Curr. Opin. Ophthalmol. 25:112–117, 2014 [DOI] [PubMed] [Google Scholar]
- 49.Ciolino J.B., Stefanescu C.F., Ross A.E., Salvador-Culla B., Cortez P., Ford E.M., Wymbs K.A., Sprague S.L., Mascoop D.R., Rudina S.S., Trauger S.A., Cade F., and Kohane D.S. In vivo performance of a drug-eluting contact lens to treat glaucoma for a month. Biomaterials. 35:432–439, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giarmoukakis A., Labiris G., Sideroudi H., Tsimali Z., Koutsospyrou N., Avgoustakis K., and Kozobolis V. Biodegradable nanoparticles for controlled subconjunctival delivery of latanoprost acid: in vitro and in vivo evaluation. Preliminary results. Exp. Eye Res. 112:29–36, 2013 [DOI] [PubMed] [Google Scholar]
- 51.Kashiwagi K., Ito K., Haniuda H., Ohtsubo S., and Takeoka S. Development of latanoprost-loaded biodegradable nanosheet as a new drug delivery system for glaucoma. Invest. Ophthalmol. Vis. Sci. 54:5629–5637, 2013 [DOI] [PubMed] [Google Scholar]
- 52.Natarajan J.V., Darwitan A., Barathi V.A., Ang M., Htoon H.M., Boey F., Tam K.C., Wong T.T., and Venkatraman S.S. Sustained drug release in nanomedicine: a long-acting nanocarrier-based formulation for glaucoma. ACS Nano. 8:419–429, 2014 [DOI] [PubMed] [Google Scholar]
- 53.Wang T., Novack G., Ho C., Natarajan J., and Venkatraman S. A pilot study of the ocular hypotensive efficacy and safety of a subconjunctival injection of liposomal latanoprost. 24th Annual American Glaucoma Society Meeting Washington D.C., 2014, p. 80 [Google Scholar]
- 54.Akaishi T., Ishida N., Shimazaki A., Hara H., and Kuwayama Y. Continuous monitoring of circadian variations in intraocular pressure by telemetry system throughout a 12-week treatment with timolol maleate in rabbits. J. Ocul. Pharmacol. Ther. 21:436–444, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Chetoni P., Mariotti Bianchi L., Giannaccini B., Saettone M.F., Conte U., and Sangalli M.E. Ocular mini-tablets for controlled release of timolol: evaluation in rabbits. J. Ocul. Pharmacol. Ther. 12:245–252, 1996 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.