Abstract
Menthol is used as a constituent of food and drink, tobacco and cosmetics nowadays. This cold receptor agonist has been used as a nasal inhalation solution in the daily life. The effect of menthol on nasal mucosa in vivo is well known; however, the effect of the drug on tracheal smooth muscle has been rarely explored. Therefore, during administration of the drug for nasal symptoms, it might also affect the trachea via oral intake or inhalation. We used our preparation to test the effectiveness of menthol on isolated rat tracheal smooth muscle. A 5 mm long portion of rat trachea was submersed in 30 ml Krebs solution in a muscle bath at 37ºC. Changes in tracheal contractility in response to the application of a parasympathetic mimetic agent were measured using a transducer connected to a Pentium III computer equipped with polygraph software. The following assessments of menthol were performed: (1) effect on tracheal smooth muscle resting tension; (2) effect on contraction caused by 10-6 M methacholine as a parasympathetic mimetic; (3) effect of the drug on electrically induced tracheal smooth muscle contractions. Results indicated that addition of a parasympathetic mimetic to the incubation medium caused the trachea to contract in a dose-dependent manner. Addition of menthol at doses of 10-5 M or above elicited a relaxation response to 10-6 M methacholine-induced contraction. Menthol could also inhibit electrical field stimulation (EFS) induced spike contraction. However, it alone had a minimal effect on the basal tension of trachea as the concentration increased. We concluded that the degree of drug-induced tracheal contraction or relaxation was dose-dependent. In addition, this study indicated that high concentrations of menthol might actually inhibit parasympathetic function of the trachea.
Keywords: menthol, trachea, smooth muscle, in vitro study.
Introduction
Menthol (2-isopropyl-5-methyl-cyclohexanol) is widely appreciated for its ability to produce a cooling sensation. It is used as a constituent of food, drink, tobacco and cosmetics in the daily life. It can reduce flatulence and colic pain in patients with irritable bowel syndrome 1. It was shown to reduce dyspnea in many respiratory conditions 2. It is recognized as a naturally-occurring cold receptor agonist that specifically activates the transient receptor potential melastatin 8 (TRPM8) channel in the skin and mucous membranes 3. It can induce a cooling sensation that appears to reduce the perception of respiratory effort without actually altering the temperature of the skin or mucous membranes 4-6. Because of the possible dual action of menthol on sensory nerves and smooth muscle, it may have a therapeutic role in upper respiratory tract infection, bronchitis and asthma. These properties of menthol point towards a compound of dual efficacy which may be an effective and well tolerated treatment for both respiratory tract infection and asthma by improving airways caliber and alleviating the associated cough.
To date, the effect of menthol on tracheal smooth muscle has been rarely explored. It has been used as a nasal inhalation solution in the daily life. Therefore, during administration of the menthol for nasal symptoms, it might also affect the trachea via oral intake or inhalation. Menthol, however, is a selective cold receptor agonist and offers an opportunity to examine the role of cold receptors in isolation. Since asthma is one of those allergic diseases, it is reasonable to explore the role of menthol in tracheal smooth muscle. During an asthmatic attack, the tracheal smooth muscle plays an important role in reducing pulmonary function as it becomes contracted. The aim of this study was to determine the effects of menthol on isolated tracheal smooth muscle in vitro.
Materials and methods
Chemicals used were of the highest purity available. All the chemical reagents were obtained from Sigma (St. Louis, MO, USA). We tested methacholine as a tracheal contraction drug. Eighteen rats were anesthetized by intraperitoneal administration of pentobarbital (45 mg/kg) and two pieces of trachea (about 5 mm in length) were removed from each rat. This study was approved by an animal experiment review board (LAC-2013-0098).
The tracheal specimen was mounted using two steel plates and submersed in a 30ml muscle bath at 37ºC as the previous report 7, 8. The bath (Fig. 1) was filled with 30 ml Krebs solution consisting of (mmol/L) NaCl, 118; KCl, 4.7; CaCl2, 2.5; MgSO4·7H2O, 1.2; KH2PO4, 1.2; NaHCO3, 25.0; and glucose, 10.0. Menthol was dissolved in dimethylsulphoxide (DMSO) and subsequently diluted in Krebs solution. Our preliminary studies showed that the vehicle (diluted DMSO) had no effect on the in vitro studies of rat tracheal smooth muscle. The upper side of the tracheal strip was attached to a Grass FT-03 force displacement transducer (AstroMed, West Warwick, RI, USA) using a steel plate and a 3-0 silk ligature. The other side of the strip was fixed to a steel plate attached to the bath. A passive tension of 0.3 g was applied to the strips and subsequent changes in tension were recorded continuously using Chart V4.2 software (PowerLab, ADInstruments, Colorado Springs, CO, USA). Preliminary tests showed that the tracheal strip immersed in the bath solution used for subsequent experiments did not contract when basal tension was applied. Before drug assays were conducted, isolated tracheas were equilibrated in the bath solution for 15-30 min, during which continuous aeration with a mixture of 95% O2 and 5% CO2 was applied. Stepwise increases in the amount of drugs used were employed to study contraction or relaxation responses of tracheal strips. All drugs were administered by adding a defined volume of stock solution to the tissue bath solution. In each experiment, one untreated strip served as a control.
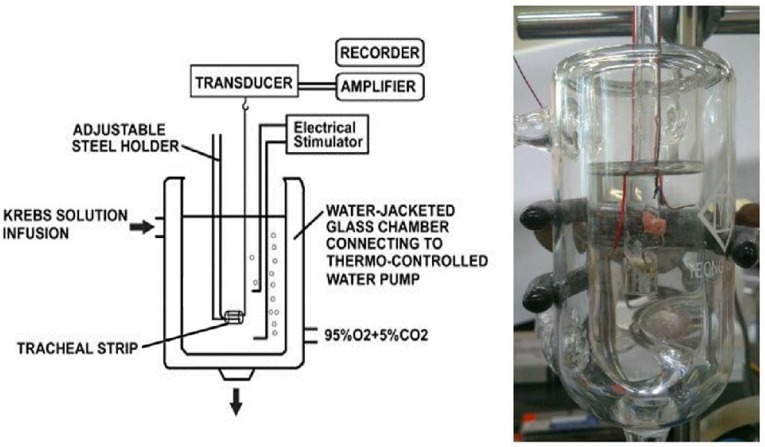
Figure 1.
Schematic diagram and actual photo describing the measurement of tension in isolated rat tracheal smooth muscle.
Electrical field stimulation (EFS) (5 Hz, 5 ms pulse duration, at a voltage of 50 V, trains of stimulation for 5 seconds) was applied to the trachea strip with two wire electrodes placed parallel to the trachea strip and connected to a direct-current stimulator (Grass S44, Quincy, MA, USA). An interval of 2 minutes was imposed between each stimulation period to allow recovery from the response. Stimulation was applied contiguously to the trachea at 37ºC.
The following assessments for menthol were performed: (1) effect on tracheal smooth muscle resting tension; (2) effect on contraction caused by 10-6 M methacholine; (3) effect of menthol on electrically induced tracheal smooth muscle contractions.
The concentrations of drugs were expressed as concentrations present in the 30ml bath solution. Data were presented as mean values and standard deviations (SDs). Differences between mean values were compared using Student's t-test. Differences were assumed to be significant at P < 0.05.
Results
The degree of contraction or relaxation of tracheal strips was estimated from the tension applied to the transducer. Tracheal contraction induced by a small dose of methacholine was easily detected (not shown), and the tissue remained in a contracted state until the drug was rinsed from the tissue.
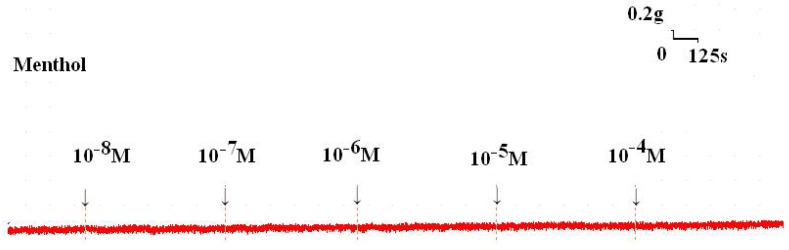
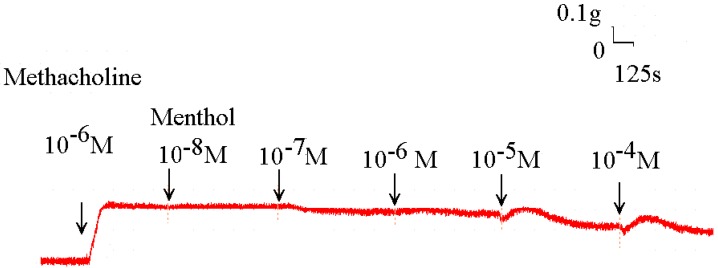
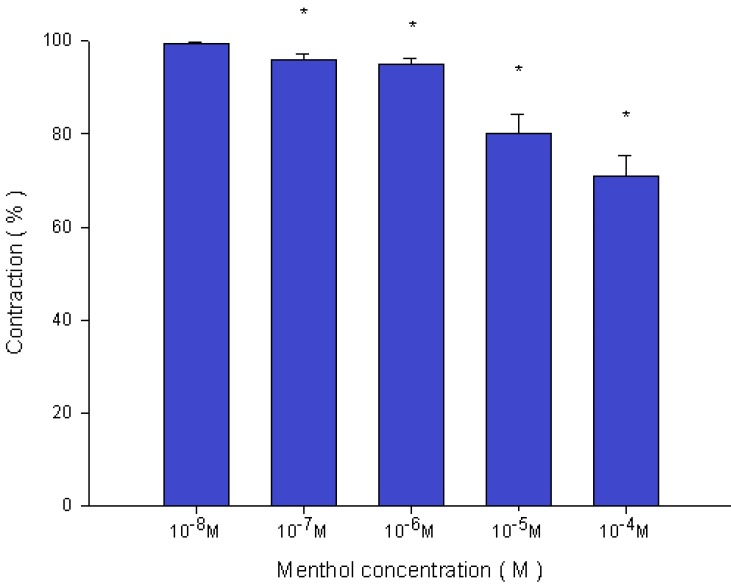
Addition of the cold receptor agonist, menthol, on the basal tension elicited a negligible response (Fig. 2), but resulted in a small relaxation of the trachea when introduced after the addition of a constricting agent such as 10-6 M methacholine (Fig. 3, 4). Low doses of menthol resulted in a mild effect on contraction and higher doses slightly relaxed the trachea smooth muscle (Fig. 4). At 10-8 M menthol, the tension was 99.5% of control values (Fig. 4). While at 10-5 M and 10-4 M menthol, the tensions were 80.1% and 71.0%, respectively (Fig. 4). The difference of tension among 10-8 M menthol and 10-5 M or 10-4 M menthol was statically significant (P<0.05).
Figure 2.
Tension changes in the rat trachea after the application of various menthol concentrations. Basal tension was 0.3 g.
Figure 3.
Original recording of the effects of menthol on 10-6 M methacholine-induced contraction of rat trachea.
Figure 4.
Effects of menthol on 10-6 M methacholine-induced contraction (contraction area was calculated at 100% with no addition of menthol) of rat trachea. The difference of tension between 10-8 M menthol and 10-5 M menthol or 10-4 M menthol was statically significant (P<0.05). Results were mean ± SD (n = 6).
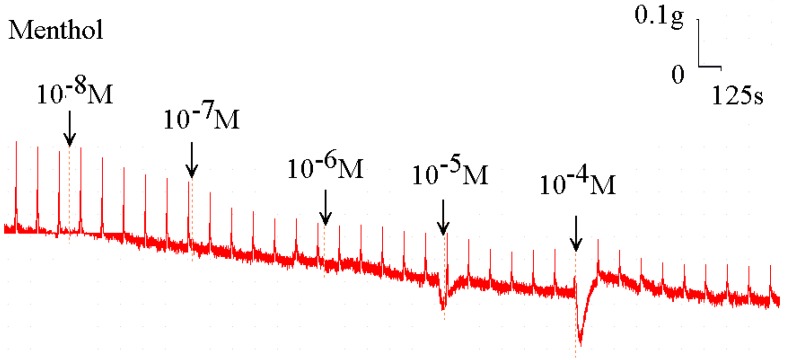
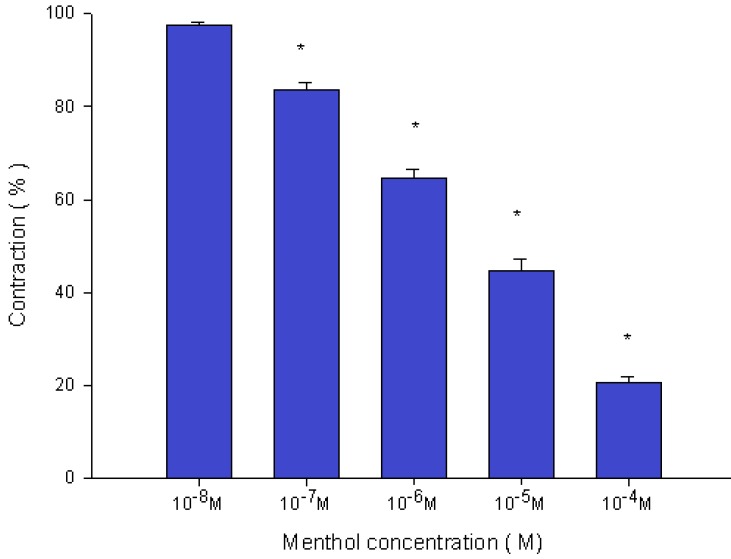
Menthol also inhibited the spike contraction induced by EFS (Fig. 5, 6). The peak tension of the tracheal strip evoked by EFS upon the addition of 10-8 M menthol was 97.4%, while at 10-5 M and 10-4 M menthol they were 44.6% and 20.7%, respectively (Fig. 6). The peak tension values of the tracheal strip evoked by EFS at 10-5 M and 10-4 M menthol addition were significantly lower than that at 10-8 M menthol (P<0.001).
Figure 5.
Original recording of the effects of menthol on electrically induced tracheal smooth muscle contractions was noted. Higher doses of menthol also decreased the spike contraction induced by EFS, and the basal tension was increased at the same time.
Figure 6.
Effects of menthol on electrically induced tracheal smooth muscle contractions (contraction area was calculated at 100% with no addition of menthol). The peak tension values of the tracheal strip evoked by EFS during the addition of 10-5 M and 10-4 M menthol were significantly lower than that at the addition of 10-8 M menthol (P<0.001). Results were mean ± SD (n = 6).
Discussion
Although tracheal responses to drugs by in vitro assays have been developed by other groups 9, 10, our method provides distinct advantages 7. Our test only requires a few millimeters of trachea, which is excised as an intact ring. An intact tracheal ring is an important component of our technique 7, and is in contrast to previous authors' use of tracheal smooth muscle strips to conduct drug tests 9-11. Our test is simpler and more robust than the tests in which tracheal rings are destroyed. Furthermore, an intact tracheal ring is much more representative of a physiological setting than smooth muscle strips.
Nevertheless, the results of our experiments should be interpreted within the context of the test materials used. Although it was difficult to determine which tissue component of the trachea was responsible for drug-induced contraction, the nature of specific tissues and their responses to specific drugs provided some indication. Firstly, the tracheal strips used in our study were crude preparations that contained cartilage and tracheal smooth muscle. The smooth muscle of the trachea appeared to be the main tissue component responsible for contraction, as the other components (epithelium, glands, connective tissue, nerves, and cartilage) did not contract to a significant extent. Because this method involved cross contraction, changes in tension were caused by radial contraction of the tracheal ring. Although responses to drugs and electrical stimulation have been verified for similar preparations 9-11, the contractile response observed in this study was probably an aggregate of the responses of various types of muscle tissue. Secondly, the isolated tracheal preparations used in our experiments were excised from rats without damaging the endothelium or smooth muscle. Therefore, it is reasonable to assume that tracheal responses to test agents in our study are comparable to those observed after application of a spray or drug inhalation to the trachea during an asthma attack.
The cholinergic contracting agent tested in this preparation is commonly used for research purposes. It is noteworthy that menthol-induced relaxation of tissue was dependent on prior partial contraction of smooth muscle using methacholine. Thus, it should thus be possible to assay the effects of common drugs and potential therapeutic agents supposedly responsible for relieving asthma. Menthol, a cold receptor agonist, could mildly reduce methacholine-induced contraction. It is known as a TMRP8 agonist 12, yet the mechanism by which this cold receptor agonist affected the trachea smooth muscle is unknown and further studies are needed to elucidate this question.
Electrical field stimulation is a common experimental tool and it activates the nerve terminals within the tissue to be tested and induces the release of endogenous neurotransmitters thereby triggering the smooth muscle to contract. EFS-induced spike contraction of canine nasal mucosa, which is believed to result from the contraction of vascular smooth muscles, disappeared after ipsilateral cervical sympathetic ganglionectomy 13. Thus, EFS-induced spike contraction of isolated canine nasal mucosa was proved to be meditated by sympathetic innervation 13. In this study, EFS-induced spike contraction of the tracheal smooth muscle was believed to be from the stimulation of parasympathetic innervation. Therefore, EFS-induced contraction of the trachea was decreased as the menthol concentration was increased. These findings suggest that a TMRP8 agonist could antagonize the parasympathetic innervation responsible for trachea smooth muscle contraction. Menthol non-selectively inhibits both Ca2+ influx pathways, voltage-dependent Ca2+ channels and receptor-operated Ca2+ entry in guinea pig tracheal smooth muscle 14, 15. Clearly, what was observed in this study is very interesting, but further study is needed to clarify these phenomena.
Menthol inhibited contractions induced by EFS. In general, Ca2+ influx plays an important role in determining the contractile force of smooth muscle by activating myosin light-chain kinase (MLCK) and subsequent phosphorylation of the myosin light chain 16. Methacholine are known to activate different Ca2+ influx pathways, i.e., receptor-operated Ca2+ entry and voltage-dependent Ca2+channels (mostly dihydropyridine-sensitive L-type Ca2+ channels), respectively 17. The inhibitory effects of menthol on Ca2+ influx and contraction were similar to those of non-selective inhibitors of Ca2+ channels such as SKF-96365, as reported previously 18, 19. Thus, the present results indicate that menthol attenuated both contractions induced by methacholine and EFS in rat tracheal smooth muscle. Menthol has been recognized as a compound that activates the cold receptors in sensory neurons 5. Involvement of the cold receptors sensitive to menthol has also been proposed in nonneuronal cells 5, 16. Taken together, these findings indicate that stimulation of an unknown cold receptor may be involved in the relaxation mediated by menthol in guinea pig tracheal smooth muscle.
The present study demonstrated that cold receptor did exist in the trachea. Some authors have demonstrated that nasal menthol inhalation is associated with a subjective sensation of improved airflow, but without any objective change in nasal resistance 20, 21. The current study extends those findings by demonstrating that menthol is not associated with any significant change in basal tension.
These properties of menthol point towards a compound of dual efficacy which may be an effective and well tolerated treatment for both respiratory tract infection and mild asthma by improving airways caliber and alleviating the associated cough.
Acknowledgments
This work was supported in part by Taipei Medical University (TMU-SHH -103-25) and Ministry of Science and Technology (MOST 104-2314-B038-58).
References
- 1.Hiki N, Kaminishi M, Yasuda K. et al. Multicenter phase II randomized study evaluating dose-response of antiperistaltic effect of L-menthol sprayed onto the gastric mucosa for upper gastrointestinal endoscopy. Dig Endosc. 2012;24:79–86. doi: 10.1111/j.1443-1661.2011.01163.x. [DOI] [PubMed] [Google Scholar]
- 2.Tamaoki J, Chiyotami A, Sakai A. et al. Effect of menthol vapour on airway hyperresponsiveness in patients with mild asthma. Respiratory Medicine. 1995;89:503–4. doi: 10.1016/0954-6111(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 3.Babes A, Ciobanu AC, Neacsu C. et al. TRPM8, a sensor for mild cooling in mammalian sensory nerve endings. Curr Pharm Biotechnol. 2011;12:78–88. doi: 10.2174/138920111793937835. [DOI] [PubMed] [Google Scholar]
- 4.Bharate SS, Bharate SB. Modulation of thermoreceptor TRPM8 by cooling compounds. ACS Chem Neurosci. 2012;3:248–67. doi: 10.1021/cn300006u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eccles R. Menthol and related cooling compounds. J Pharm Pharmacol. 1994;46:618–30. doi: 10.1111/j.2042-7158.1994.tb03871.x. [DOI] [PubMed] [Google Scholar]
- 6.Keh SM, Facer P, Yehia A. et al. The menthol and cold sensation receptor TRPM8 in normal human nasal mucosa and rhinitis. Rhinology. 2011;49:453–7. doi: 10.4193/Rhino11.089. [DOI] [PubMed] [Google Scholar]
- 7.Wang H-W, Wang Y-T, Wu C-C. A modified in vitro study of tracheal smooth muscle response to drugs. J Med Sci. 2007;27:203–6. [Google Scholar]
- 8.Lai W-S, Lin Y-Y, Chu Y-H. et al. Efficacy of fexofenadine in isolated rat trachea. Rhinology. 2013;51:376–80. doi: 10.4193/Rhino12.213. [DOI] [PubMed] [Google Scholar]
- 9.Yau KI, Hwang TL. The nonadrenergic noncholinergic system can modulate the effect of prokinetic agents on contractile response of isolated guinea pig trachea segments to electrical field stimulation. J Formos Med Assoc. 2002;101:695–9. [PubMed] [Google Scholar]
- 10.Bratton DL, Tanaka DT, Grunstein MM. Effects of temperature on cholinergic contractility of rabbit airway smooth muscle. Ame J Phy. 1987;63:1933–41. doi: 10.1152/jappl.1987.63.5.1933. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez O, Santacana GE. Effect of low temperature on tracheal smooth muscle contractile and relaxing responses evoked by electrical field stimulation. Physiol Res. 2001;20:237–43. [PubMed] [Google Scholar]
- 12.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–8. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 13.Wang H-W, Jackson RT. Do cholinergic neurons directly innervate nasal blood vessels? Rhinology. 1988;26:139–46. [PubMed] [Google Scholar]
- 14.Ito S, Kumea H, Shiraki A. et al. Inhibition by the cold receptor agonists menthol and icilin of airway smooth muscle contraction. Pulm Pharmacol Ther. 2008;21:812–7. doi: 10.1016/j.pupt.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Takeuchi S, Tamaoki J, Kondo M. et al. Effect of menthol on cytosolic Ca2+ levels in canine airway epithelium in culture. Biochem Biophys Res Commun. 1994;201:1333–8. doi: 10.1006/bbrc.1994.1850. [DOI] [PubMed] [Google Scholar]
- 16.Gerthoffer WT. Regulation of the contractile element of airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 1991;261:L15–28. doi: 10.1152/ajplung.1991.261.2.L15. [DOI] [PubMed] [Google Scholar]
- 17.Ito S, Kume H, Honjo H. et al. ML-9, a myosin light chain kinase inhibitor, reduces intracellular Ca2+ concentration in guinea pig trachealis. Eur J Pharmacol. 2004;486:325–33. doi: 10.1016/j.ejphar.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Hirota S, Helli P, Janssen LJ. Ionic mechanisms and Ca2+ handling in airway smooth muscle. Eur Respir J. 2007;30:114–33. doi: 10.1183/09031936.00147706. [DOI] [PubMed] [Google Scholar]
- 19.Oguma T, Kume H, Ito S. et al. Involvement of reduced sensitivity to Ca2+ in β-adrenergic action on airway smooth muscle. Clin Exp Allergy. 2006;36:183–91. doi: 10.1111/j.1365-2222.2006.02412.x. [DOI] [PubMed] [Google Scholar]
- 20.Eccles R, Griffiths DH, Newton CG. et al. The effects of menthol isomers on nasal sensation of airflow. Clin Otolaryngol Allied Sci. 1988;13:25–9. doi: 10.1111/j.1365-2273.1988.tb00277.x. [DOI] [PubMed] [Google Scholar]
- 21.Eccles R. Nasal airway resistance and nasal sensation of airflow. Rhinol. 1992;(Suppl 14):86–90. [PubMed] [Google Scholar]