Abstract
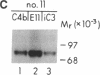
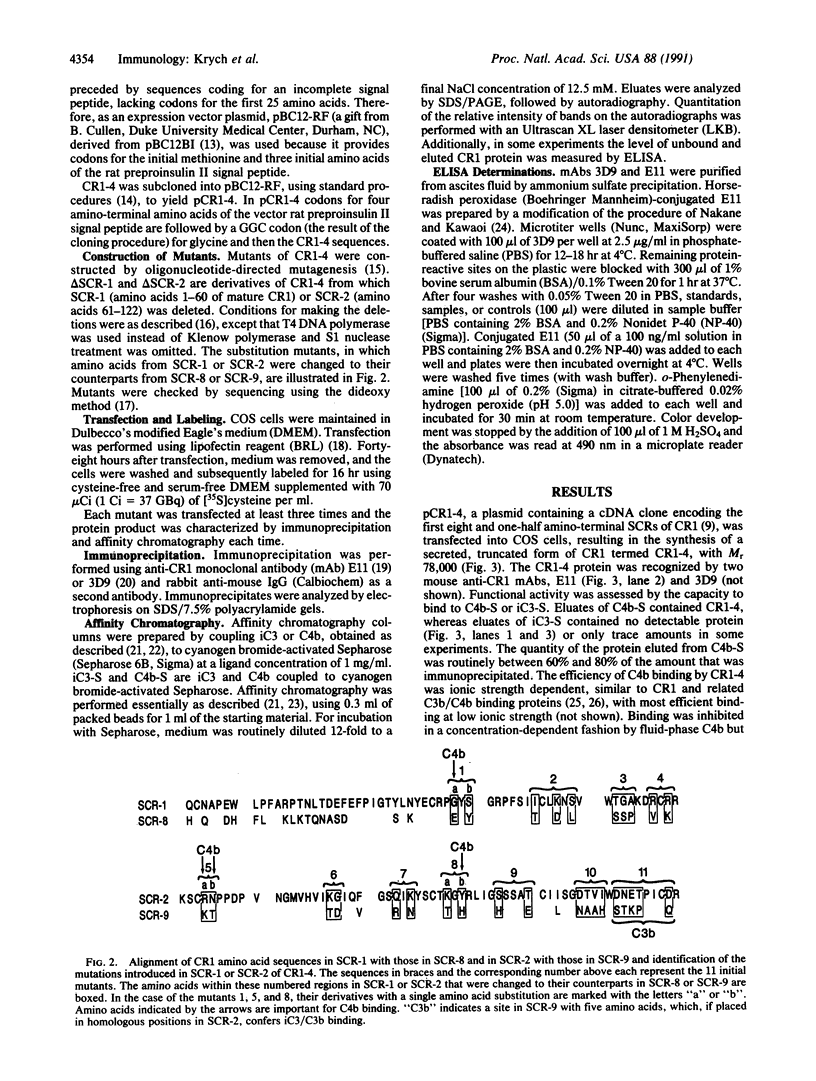
Cysteine-rich repeated units of 40-70 amino acids are building blocks of many mammalian proteins, including 12 proteins of the complement system. Human complement arranged motifs, designated short consensus repeats (SCRs), which constitute the entire extracellular portion of this protein. Klickstein et al. [Klickstein, L. B., Bartow, T. J., Miletic, V., Rabson, L. D., Smith, J. A. & Fearon, D. T. (1988) J. Exp. Med. 168, 1699-1717 (abstr.)] localized a C4b binding domain to SCR-1 and/or SCR-2 and a C3b binding domain to SCR-8 and/or SCR-9. These SCRs bind different ligands, although SCR-1 and SCR-8 are 55% homologous and SCR-2 and SCR-9 are 70% homologous. To examine if one or two SCRs are required for ligand binding and to define sites within the SCRs that determine specificity of binding, mutagenesis analysis of a truncated, secreted form of CR1, called CR1-4 by Hourcade et al. [Hourcade, D., Meisner, D. R., Atkinson, J. P. & Holers, V. M. (1988) J. Exp. Med. 168, 1255-1270], was undertaken. The latter, composed of the first eight and one-half amino-terminal SCRs of CR1, efficiently bound C4b but not iC3. SCR-1 and SCR-2 were necessary for this interaction. Analysis of the mutant CR1-4 proteins, in which amino acids in SCR-1 and SCR-2 were substituted a few at a time with the homologous amino acids of SCR-8 and SCR-9, led to the identification of one amino acid in SCR-1 and three amino acids in SCR-2 important for C4b binding. Furthermore, five amino acids at the end of SCR-9, if placed in the homologous positions of SCR-2, conferred iC3 binding and are likely essential for ligand binding activity of SCR-8 and SCR-9. This iC3 binding occurred only if SCR-1 was present, indicating that two contiguous SCRs are necessary for this interaction. These results provide identification of amino acids within SCRs that are important for ligand binding.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alsenz J., Becherer J. D., Nilsson B., Lambris J. D. Structural and functional analysis of C3 using monoclonal antibodies. Curr Top Microbiol Immunol. 1990;153:235–248. doi: 10.1007/978-3-642-74977-3_13. [DOI] [PubMed] [Google Scholar]
- Arnaout M. A., Dana N., Melamed J., Medicus R., Colten H. R. Low ionic strength or chemical cross-linking of monomeric C3b increases its binding affinity to the human complement C3b receptor. Immunology. 1983 Feb;48(2):229–237. [PMC free article] [PubMed] [Google Scholar]
- Becherer J. D., Alsenz J., Lambris J. D. Molecular aspects of C3 interactions and structural/functional analysis of C3 from different species. Curr Top Microbiol Immunol. 1990;153:45–72. doi: 10.1007/978-3-642-74977-3_3. [DOI] [PubMed] [Google Scholar]
- Brown E. J. The interaction of small oligomers of complement 3B (C3B) with phagocytes. High affinity binding and phorbol ester-induced internalization by polymorphonuclear leukocytes. J Biol Chem. 1989 Apr 15;264(11):6196–6201. [PubMed] [Google Scholar]
- Cole J. L., Housley G. A., Jr, Dykman T. R., MacDermott R. P., Atkinson J. P. Identification of an additional class of C3-binding membrane proteins of human peripheral blood leukocytes and cell lines. Proc Natl Acad Sci U S A. 1985 Feb;82(3):859–863. doi: 10.1073/pnas.82.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J. L., Housley G. A., Jr, Dykman T. R., MacDermott R. P., Atkinson J. P. Identification of an additional class of C3-binding membrane proteins of human peripheral blood leukocytes and cell lines. Proc Natl Acad Sci U S A. 1985 Feb;82(3):859–863. doi: 10.1073/pnas.82.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R. Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol. 1987;152:684–704. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- Dykman T. R., Cole J. L., Iida K., Atkinson J. P. Polymorphism of human erythrocyte C3b/C4b receptor. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1698–1702. doi: 10.1073/pnas.80.6.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykman T. R., Cole J. L., Iida K., Atkinson J. P. Structural heterogeneity of the C3b/C4b receptor (Cr 1) on human peripheral blood cells. J Exp Med. 1983 Jun 1;157(6):2160–2165. doi: 10.1084/jem.157.6.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghtedarzadeh M. K., Henikoff S. Use of oligonucleotides to generate large deletions. Nucleic Acids Res. 1986 Jun 25;14(12):5115–5115. doi: 10.1093/nar/14.12.5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farries T. C., Seya T., Harrison R. A., Atkinson J. P. Competition for binding sites on C3b by CR1, CR2, MCP, factor B and factor H. Complement Inflamm. 1990;7(1):30–41. doi: 10.1159/000463124. [DOI] [PubMed] [Google Scholar]
- Fearon D. T., Ahearn J. M. Complement receptor type 1 (C3b/C4b receptor; CD35) and complement receptor type 2 (C3d/Epstein-Barr virus receptor; CD21). Curr Top Microbiol Immunol. 1990;153:83–98. doi: 10.1007/978-3-642-74977-3_5. [DOI] [PubMed] [Google Scholar]
- Fearon D. T. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J Exp Med. 1980 Jul 1;152(1):20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Hebert L. A., Cosio G. The erythrocyte-immune complex-glomerulonephritis connection in man. Kidney Int. 1987 Apr;31(4):877–885. doi: 10.1038/ki.1987.81. [DOI] [PubMed] [Google Scholar]
- Hogg N., Ross G. D., Jones D. B., Slusarenko M., Walport M. J., Lachmann P. J. Identification of an anti-monocyte monoclonal antibody that is specific for membrane complement receptor type one (CR1). Eur J Immunol. 1984 Mar;14(3):236–243. doi: 10.1002/eji.1830140307. [DOI] [PubMed] [Google Scholar]
- Hourcade D., Miesner D. R., Atkinson J. P., Holers V. M. Identification of an alternative polyadenylation site in the human C3b/C4b receptor (complement receptor type 1) transcriptional unit and prediction of a secreted form of complement receptor type 1. J Exp Med. 1988 Oct 1;168(4):1255–1270. doi: 10.1084/jem.168.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klickstein L. B., Bartow T. J., Miletic V., Rabson L. D., Smith J. A., Fearon D. T. Identification of distinct C3b and C4b recognition sites in the human C3b/C4b receptor (CR1, CD35) by deletion mutagenesis. J Exp Med. 1988 Nov 1;168(5):1699–1717. doi: 10.1084/jem.168.5.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klickstein L. B., Wong W. W., Smith J. A., Weis J. H., Wilson J. G., Fearon D. T. Human C3b/C4b receptor (CR1). Demonstration of long homologous repeating domains that are composed of the short consensus repeats characteristics of C3/C4 binding proteins. J Exp Med. 1987 Apr 1;165(4):1095–1112. doi: 10.1084/jem.165.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lowell C. A., Klickstein L. B., Carter R. H., Mitchell J. A., Fearon D. T., Ahearn J. M. Mapping of the Epstein-Barr virus and C3dg binding sites to a common domain on complement receptor type 2. J Exp Med. 1989 Dec 1;170(6):1931–1946. doi: 10.1084/jem.170.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lublin D. M., Atkinson J. P. Decay-accelerating factor and membrane cofactor protein. Curr Top Microbiol Immunol. 1990;153:123–145. doi: 10.1007/978-3-642-74977-3_7. [DOI] [PubMed] [Google Scholar]
- McNearney T. A., Odell C., Holers V. M., Spear P. G., Atkinson J. P. Herpes simplex virus glycoproteins gC-1 and gC-2 bind to the third component of complement and provide protection against complement-mediated neutralization of viral infectivity. J Exp Med. 1987 Nov 1;166(5):1525–1535. doi: 10.1084/jem.166.5.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- O'Shea J. J., Brown E. J., Seligmann B. E., Metcalf J. A., Frank M. M., Gallin J. I. Evidence for distinct intracellular pools of receptors for C3b and C3bi in human neutrophils. J Immunol. 1985 Apr;134(4):2580–2587. [PubMed] [Google Scholar]
- Perkins S. J., Haris P. I., Sim R. B., Chapman D. A study of the structure of human complement component factor H by Fourier transform infrared spectroscopy and secondary structure averaging methods. Biochemistry. 1988 May 31;27(11):4004–4012. doi: 10.1021/bi00411a017. [DOI] [PubMed] [Google Scholar]
- Reid K. B., Day A. J. Structure-function relationships of the complement components. Immunol Today. 1989 Jun;10(6):177–180. doi: 10.1016/0167-5699(89)90317-4. [DOI] [PubMed] [Google Scholar]
- Ross G. D., Medof M. E. Membrane complement receptors specific for bound fragments of C3. Adv Immunol. 1985;37:217–267. doi: 10.1016/s0065-2776(08)60341-7. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]