Abstract
Glyphosate, the active ingredient in the herbicide RoundUp, has increased dramatically in use over the past decade and constitutes a potent anthropogenic source of selection. In the southeastern United States, weedy morning glories have begun to develop tolerance to glyphosate, representing a unique opportunity to examine the evolutionary genetics of a novel trait. We found genetic variation for tolerance, indicating the potential for the population to respond to selection by glyphosate. However, the following significant evolutionary constraint exists: in the absence of glyphosate, tolerant genotypes produced fewer seeds than susceptible genotypes. The combination of strong positive directional selection in the presence of glyphosate and strong negative directional selection in its absence may indicate that the selective landscape of land use could drive the evolutionary trajectory of glyphosate tolerance. Understanding these evolutionary forces is imperative for devising comprehensive management strategies to help slow the rate of the evolution of tolerance.
Strong selection exerted by human technological innovations has wide-ranging evolutionary consequences and, as such, has caused accelerated cases of evolution in the natural world (1). For example, the introduction of herbicides and pesticides in the past century has intensified agricultural production significantly (2). However, the repeated use of herbicides exerting strong selection pressure on crop weeds has led to >250 documented cases of herbicide resistance (www.weedscience.org), and this process is likely to accelerate with increased reliance on herbicides.
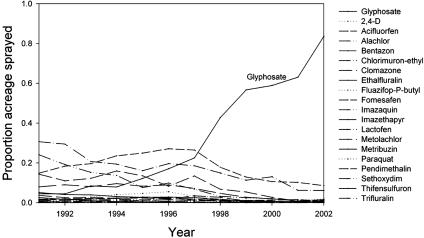
Since its introduction in 1974, glyphosate, the active ingredient in the herbicide RoundUp, has increased dramatically in use (3), particularly with the advent in the 1990s of crops genetically engineered to be tolerant of RoundUp (e.g., Roundup Ready canola, corn, cotton, soybeans, and sugar beets). Our analysis of U.S. Department of Agriculture (USDA) statistics shows that not only is the use of glyphosate increasing in U.S. soybean crops, but there is a concomitant decrease in the use of other herbicides (Fig. 1) (4). In addition, glyphosate is being used increasingly in a conservation context as a component of the management of invasive weeds (5). This widespread pattern of increased usage suggests that glyphosate is quickly becoming the predominant herbicide in managed systems.
Fig. 1.
The proportion of soybean acreage sprayed with glyphosate from 1991 to 2002 relative to other herbicides. Data are taken from ref. 4.
To date, of the 250 cases of herbicide resistance, only 6 cases of glyphosate resistance have been reported in plants (www.weedscience.org). If one considers the trajectory of evolution to every other major pesticide (1, 6), more cases of glyphosate resistance are likely to follow. However, tolerance to glyphosate, or the ability to sustain damage without a corresponding reduction in fitness (7) is also likely to be an important evolutionary strategy of weedy plants to circumvent the damaging effects of herbicide.
The distinction between tolerance and resistance was identified in the plant-herbivore literature (8). Resistance traits are defined as traits that reduce the amount of damage a plant experiences, whereas tolerance, or compensation, is defined as the ability of a plant to sustain a fixed amount of herbivore damage without a corresponding reduction in fitness (9). Unlike resistance, tolerance does not prevent herbivory but allows the plant to compensate for damage that herbivores have already inflicted. We have borrowed these definitions and applied them to the plant-herbicide system. Thus, resistance to an herbicide would involve a trait that prevented the plant from experiencing the damaging effects of the herbicide. For example, a plant enzyme that detoxified the herbicide would be considered a resistance trait. Tolerance to an herbicide is simply the ability of a plant to compensate for the damaging effects of the herbicide. Like that of tolerance to herbivores, there are likely to be a myriad of mechanisms that confer tolerance to herbicides (2). In Materials and Methods, we describe our operational definition of tolerance.
In the southeastern United States, Ipomoea purpurea (L.) Roth (the tall morning glory) is a noxious crop weed whose negative effects on agriculture have been largely mitigated by the use of glyphosate (10, 11). Our interviews with farmers in the southeastern United States suggest that morning glories can tolerate applications of glyphosate. In some cases, increasing concentrations of the herbicide have been required to control I. purpurea infestations. Such an increase in tolerance to glyphosate represents a unique opportunity to examine the evolutionary genetics of a novel trait, especially with regard to the constraints on the evolution of tolerance in natural plant populations. Understanding these constraints is also imperative for devising comprehensive management strategies to help slow the rate of the evolution of tolerance (12). In this article, we describe experimental evidence indicating the presence of genetic variation for tolerance to RoundUp in wild-collected I. purpurea maternal lines, as well as the presence of fitness costs associated with this tolerance. Also, we show significant positive selection for tolerance in the presence of RoundUp and significant negative directional selection against tolerance in the absence of RoundUp.
Materials and Methods
We collected seeds from 32 randomly selected, individual plants growing in an agricultural field in Oconee County, Georgia, that has been sprayed consistently with RoundUp for ≈8 years. Because I. purpurea possesses a mixed-mating system of out-crossing and selfing (13), all seeds collected from each plant share the maternal genetic contribution. We used the maternal line as the unit of our genetic analysis, which involved some design tradeoffs. Although using this maternal line design does not allow us to determine the additive genetic variance of tolerance, it does provide a broad-sense genetic measure. Of course, differences among these maternal lines represent genetic differences and the effects of the common parental environment. Because the main mode of action of glyphosate is to inhibit an enzyme (5-enolpyruvylshikimate-3-phosphate synthase) that occurs in the chloroplasts, including the maternal effect seemed to be appropriate. To minimize these environmentally derived differences, we planted five seeds from each grandmaternal line and selfed them for one generation. Maternal individuals were randomized in the greenhouse to account for potential environmentally induced differences within the greenhouse. Seeds were then collected from each plant, and seeds from each of the five maternal lines were bulked according to grandmaternal line.
We planted this experiment into an agricultural field at the University of Georgia Plant Sciences Farm (Oconee County, Georgia). This site supports a natural population of I. purpurea and was in the same area from which the maternal lines were collected. We randomized 10 replicates of each of the 32 maternal lines among five spatial blocks to account for habitat heterogeneity. To ensure germination, we scarified each seed before planting and marked planted seeds with plastic straws. Within each block, we planted seeds 1 m2 from the next experimental individual. We removed vegetation surrounding experimental individuals once to deter herbivory from cotton rats (Sigmodon hispidus) but otherwise let competitive weeds grow undeterred. Each plant was allowed to grow up a 1-m-tall bamboo stake, which mimics I. purpurea growth in agricultural fields and allows for easy identification of experimental plants. We applied glyphosate (RoundUp, Monsanto) at a rate of 1.121 kg·hectare-1 with a hand-held CO2 pressurized plot sprayer calibrated to a spray volume of 20 gallons per acre to half of the experimental individuals on July 17, 2002. This concentration of glyphosate has been found to reduce the biomass of I. purpurea by 90% (14). In I. purpurea, the physical symptoms of damage after glyphosate application are typically chlorosis and necrosis of the leaves and death of the apical meristem.
We collected mortality and damage data by assessing death of sprayed individuals and counting the total number of leaves per plant and the number of leaves exhibiting symptoms of glyphosate damage. We collected fruits during 10 rounds of collection and counted all viable seeds. Relative fitness was calculated by dividing all fitness values by overall mean fitness. Individuals that died before glyphosate application were not included in the analysis, and individuals that died as a result of glyphosate application were assigned a fitness of zero.
Our operational definition of tolerance is the ability of plants to reproduce after experiencing damage by herbicide. Tolerance was estimated for each maternal line because a single plant cannot be both damaged and undamaged. The mean relative fitness of each maternal line was regressed on environment (no herbicide and herbicide), and the level of tolerance was determined as the slope of relative fitness on environment after the effects of block were removed. A slope of zero would mean that the line was completely tolerant, whereas a significant negative slope would indicate low tolerance. A line exhibiting a positive slope would be overcompensating for damage (15). This method defines tolerance as a norm of reaction to glyphosate, which is analogous to studies that assess tolerance to herbivory (9, 16-18). If the maternal lines respond differently in the two environments (i.e., the slopes of the lines between fitness and environment differ across maternal lines), we would conclude that the lines exhibited genetic variation for tolerance. The statistical significance of such a response was tested with an analysis of covariance by examining the magnitude of the genotype by environment interaction, with fitness as the dependent variable. For this analysis, we used the general linear-model (GLM) procedure of the sas statistical software package (version 8.0, SAS Institute, Cary, NC) to conduct the analysis of covariance. In this analysis, the response variable was loge-transformed relative fitness, which we calculated by dividing each individual's fitness by overall mean fitness (16, 17). We used the residuals of relative fitness after the effects of block had been removed as the dependent variable in the model to reduce the effect of spatial heterogeneity.
To detect the costs of tolerance, we tested for the presence of a significant genetic covariance between relative fitness and mean level of tolerance for each maternal line. By using the same set of data to estimate both the slope (tolerance) and the fitness of undamaged plants produced an artifactual covariance (9, 16), which was subtracted from the calculated covariance for an unbiased estimate of the covariance. Standard errors of the covariances were made by jackknifing maternal-line estimates (19) with a two-tailed t statistic, which was then used to calculate a confidence interval.
To assess the pattern and magnitude of selection on tolerance to glyphosate, the partial regression analysis described by Rausher (20) and R.M. and Mojonnier (21) was used to determine coefficients of selection in both treatment environments. Again, we used maternal-line means as our unit of analysis. Before conducting the analyses, tolerance was standardized to a mean of zero and a variance of one. The response variable was the residual of relative fitness after the effects of block were removed to minimize the effects of spatial variation. Selection gradients in both treatments were estimated from the regression of fitness on tolerance by using maternal-line means according to standard methods (16, 20, 21). Only linear terms were included in the regressions for each maternal line because the initial analysis revealed no evidence of any nonlinear effects of treatment environment on fitness. Because the same artifactual covariance between fitness and tolerance in the fitness-cost analysis applies also in the selection analysis, standard errors of the covariances were made by jackknifing maternal-line estimates (19) with a two-tailed t statistic, which was then used to calculate a confidence interval to assess statistical significance of the selection gradients.
Results
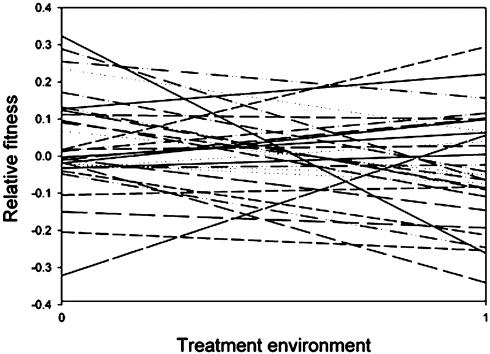
Maternal lines differed in tolerance to glyphosate damage, as revealed by the significant genotype by environment interaction in an analysis of covariance (Table 1), indicating that there is significant genetic variation for tolerance in this study population. In this analysis, loge-transformed fitness was the response variable, maternal line was the independent variable, and the treatment environment was the covariate. The maternal line by environment term was significant, indicating that the slopes of the relationship between fitness and environment (no herbicide and herbicide) differ among maternal lines. The fitness norm of reaction, which is the fitness of each maternal line regressed on treatment environment, illustrates the fitness tradeoff across environments (Fig. 2).
Table 1. Analysis of covariance for relative fitness (loge-transformed).
| Source of variation | df | Type III SS | F value | P value |
|---|---|---|---|---|
| Maternal line | 31 | 13.0788 | 2.08 | 0.0005 |
| Treatment | 1 | 1.1354 | 5.59 | 0.0182 |
| Maternal line × treatment | 31 | 9.9309 | 1.58 | 0.0235 |
| Error | 1,201 | 255.3189 | — | — |
By treatment interaction, the maternal line demonstrates the existence of genetic variation for tolerance.
Fig. 2.
Relationship between relative fitness and treatment environment for the 32 maternal lines. Residuals of fitness were used after the effect of block was removed. On the x axis, 0 = glyphosate absent, and 1 = glyphosate present. Slopes of the lines represent tolerance.
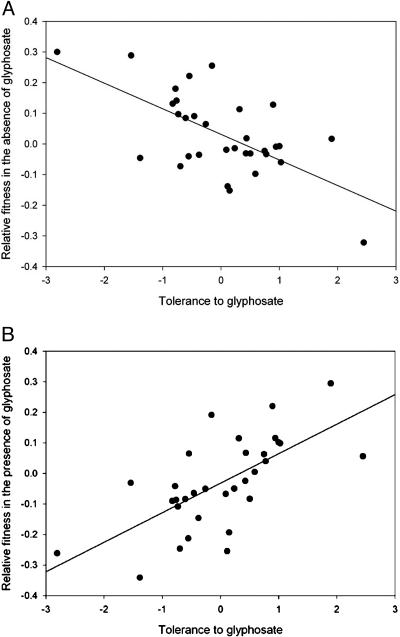
As indicated by a negative correlation between fitness and tolerance in the control treatment, substantial fitness costs are associated with tolerance to glyphosate (Fig. 3A). The corrected covariance between tolerance to glyphosate (slope) and the relative fitness of undamaged plants was -0.0200. By using the jackknife, we calculated the 95% confidence interval of this covariance to be ±0.0147. This corrected covariance was significantly different from zero at P = 0.002, indicating that there is a negative covariance between tolerance and fitness in the absence of glyphosate and, thus, evidence of a fitness cost of tolerance to glyphosate.
Fig. 3.
Costs and benefits of tolerance. (A) Costs of tolerance indicated by a significant negative genetic correlation between fitness and tolerance in the absence of glyphosate. (B) The benefits of tolerance in the presence of glyphosate as measured by a standardized selection gradient. In both graphs, the y axis depicts the residuals of relative fitness after the effects of block had been removed, and the x axis depicts the level of tolerance for each maternal line standardized to a mean of zero and a variance of one.
The magnitude of selection against tolerance associated with this cost, estimated as the coefficient of a standardized regression of fitness on tolerance by using maternal-line means (20, 21) is β2 = -0.0806 (P < 0.0001). The corrected covariance between fitness and tolerance determined by using maternal-line means is β2 = -0.0967. The jackknife procedure showed that this covariance was significantly different from zero at P = 0.03, with a confidence interval of ±0.0776.
In contrast, in the herbicide treatment, there is net selection for increased tolerance, as indicated by a positive correlation between fitness and tolerance. By using the same coefficient of a standardized regression of fitness on tolerance, we estimated the strength of positive selection on tolerance to be β1 = 0.0945 (P < 0.0001; Fig. 3B). After correcting for the artifactual covariance, β1 = 0.0882 and P = 0.015. There was no evidence of stabilizing or disruptive selection on tolerance in either environment.
Discussion
One prerequisite for the evolution of widespread tolerance to glyphosate is the presence of genetic variation for tolerance. Moreover, the rate of evolution of tolerance to selection imposed by glyphosate is expected to be proportional to the amount of genetic variation for tolerance that exists in natural populations (22). Consequently, a lack of appreciable genetic variation for tolerance could serve as a constraint on the evolution of tolerance. Unfortunately, this scenario does not appear to be the case; substantial genetic variation for tolerance appears to exist already in at least one natural population. Given the continued presence of glyphosate, the number of tolerant individuals should increase within the population over time, as might the overall level of tolerance of the population. This result is also significant in that glyphosate is an extremely effective selective agent that was put into widespread use recently, yet this plant species already exhibits a genetically based ability to tolerate the herbicide.
The presence of genetic variation alone does not guarantee that tolerance will evolve. A second prerequisite for the evolution of widespread tolerance to glyphosate is the presence of net selection favoring increased tolerance. The net selection acting on tolerance is determined by two components: fitness costs and benefits. Costs of resistance or tolerance are fitness reductions that are thought to arise from the diversion of limiting resources away from present and future growth and reproduction (23). Such costs are common, but not universal, for resistance and tolerance to herbivores (9, 16, 23, 24). Benefits of tolerance are increases in fitness that result from the ability to reduce the detrimental effects of damage on survival and reproductive success. Tolerance can evolve only if there is a net benefit (i.e., if the magnitude of fitness benefits exceeds the magnitude of the costs) (9, 16, 23-27).
We found that the most tolerant line produced 35% fewer seeds in the absence of RoundUp than the most susceptible line. Although this type of fitness cost has been well documented for genetically engineered resistance to the herbicide chlorsulfuron in Arabidopsis thaliana (28, 29) and resistance to the triazine herbicides (30), reports of fitness costs associated with tolerance to herbicide (more specifically, natural tolerance to glyphosate) are lacking. However, the magnitude of the fitness cost in our system was similar to the 34% reduction in fitness in transgenic A. thaliana (28, 29). These costs indicate that, in the absence of herbicide, natural selection would tend to minimize levels of tolerance.
Our data on the benefits of tolerance in the presence of glyphosate suggest that there is likely to be strong positive selection for tolerance in areas where glyphosate is sprayed. However, for areas where glyphosate is not sprayed, the costs of tolerance that we measured suggest that the trait will be strongly selected against. Clearly, glyphosate use is increasing dramatically in the United States. We obtained a preliminary estimate of the net selection for tolerance in I. purpurea by weighting the estimates of the magnitude of selection in each of our treatment environments by the proportions of U.S. crop acreage that were sprayed and not sprayed with glyphosate. We used the mean direction and magnitude of selection on glyphosate tolerance for I. purpurea, β, and a weighted average of the magnitude of selection with and without herbicide, β = p1β1 + p2β2, where p1 and p2 are the proportions of U.S. crop acreage sprayed and not sprayed and β1 and β2 are the coefficients of selection corresponding to the environment with and without glyphosate, respectively. To estimate p1 and p2, we used U.S. Department of Agriculture (USDA) data on acreage planted in soybeans, cotton, and corn, as well as acreage sprayed with glyphosate (4).
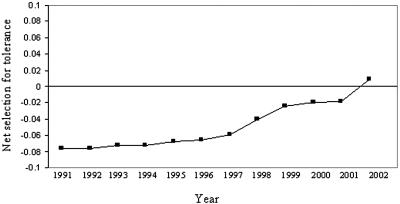
Our analysis revealed that, from 1991 to 2001, estimates of β are negative, indicating that net selection acted against an increase in tolerance (Fig. 4). During this period, the high cost of tolerance provided a successful evolutionary constraint. However, in 2002, β becomes positive, indicating net selection for increased tolerance (Fig. 4). Apparently, in this year, the continued increase in glyphosate use (Fig. 1) caused the proportion of acreage sprayed to cross a threshold such that herbicide use is common enough to tip the balance toward selection for the evolution of tolerance.
Fig. 4.
Relative proportion of U.S. agricultural land planted in soybean, cotton, and corn, subject to net selection (calculated by weighting acreage with a measure of selection) for tolerance to glyphosate over the past 12 years. A negative value indicates that the costs of glyphosate tolerance outweighed the benefits, and we predicted that tolerance would decrease in the weed populations growing in U.S. soybean, cotton, and corn agricultural lands. A positive value indicates that the acreage subject to benefits of glyphosate tolerance outweighed the costs, and we predicted that tolerance would increase in weed populations. Data are taken from ref. 4.
Admittedly, these calculations are crude. The analysis makes a number of simplistic assumptions, including the assumptions that (i) we can extrapolate the results from a single study population in a single year, (ii) there is little gene flow between sprayed and nonsprayed fields, and (iii) the species is localized to agricultural fields. However, this analysis does serve as a useful starting point for mathematical models of the spread of this important agricultural trait. Taking these calculations at face value, the analysis suggests that the amount of land experiencing a certain selective regime could influence the continued evolution of traits that then impact the efficacy of modern agriculture.
These calculations do suggest that serious and immediate consideration should be given to developing regional strategies for managing the evolution of tolerance in I. purpurea. Until now, little attention has been given to such efforts, particularly in contrast to the multitude of models that attempt to manage the evolution of Bt resistance (31). For glyphosate, such strategies could involve something as simple as periodically spraying with alternate herbicides, as long as there is little cross-tolerance with glyphosate. However, if there is cross-tolerance with other causes of plant damage, such as hail, herbivores, or pathogens, alternating spraying regimes may not be a viable mechanism for controlling the evolution of glyphosate tolerance.
An additional complication in modeling the evolution of tolerance to herbicide is the presence of a persistent seed bank. I. purpurea is known to sustain a viable seed bank of at least 7 years (32). Seed banks can preserve genetic variation (33) and can act as a buffer that could retard the evolution of traits in response to recent selection (34).
In conclusion, whether an evolutionary threshold has been crossed or not, the main findings of this work stand. There is evidence for genetic variation for tolerance in this species; in the presence of glyphosate, there is strong selection for tolerance, but in the absence of glyphosate, there is a significant cost to being tolerant. The estimates of these parameters will be critical for any serious attempt to model the evolutionary trajectory of this trait. Furthermore, our study illustrates the continuing relevance of basic evolutionary studies as a foundation for developing effective management strategies (6, 12).
Acknowledgments
We thank M. Rausher. C. Boake, P. Tiffin, A. Bouck, J. Estill, and J. Mank for invaluable assistance; anonymous reviewers for providing valuable comments on earlier drafts; and J. Estill, A. Bouck, J. Ferrell, W. Vencill, R. Smith, C. Spencer, A. McCollum, J. Williams, V. Koelling, D. Gotzek, C. Richards, and S. Held for assistance in the field. This work was supported by National Science Foundation Grants 0129191 and 9602223 and a Sigma-Xi Grant-in-Aid of Research grant (to R.S.B.).
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Palumbi, S. R. (2001) Science 293, 1786-1790. [DOI] [PubMed] [Google Scholar]
- 2.Cousens, R. & Mortimer, A. M. (1995) Dynamics of Weed Populations (Cambridge Univ. Press, Cambridge, U.K.).
- 3.Shaner, D. L. (2000) Pest Manag. Sci. 56, 320-326. [Google Scholar]
- 4.National Agricultural Statistics Service (2003) Agricultural Chemical Usage 1991-2002 Field Crops Summaries (U.S. Dept. Agric., Washington, DC).
- 5.Matarczyk, J. A., Willis, A. J., Vranjic, J. A. & Ash, J. E. (2002) Biol. Conserv. 108, 133-141. [Google Scholar]
- 6.Gould, F. (1995) Weed Technol. 9, 830-839. [Google Scholar]
- 7.Crawley, M. J. (1983) Herbivory: The Dynamics of Animal-Plant Interactions (Univ. of California Press, Berkeley).
- 8.Painter, R. H. (1958) Annu. Rev. Entomol. 3, 267-290. [Google Scholar]
- 9.Mauricio, R., Rausher, M. D. & Burdick, D. S. (1997) Ecology 78, 1301-1311. [Google Scholar]
- 10.Baylis, A. D. (2000) Pest Manag. Sci. 56, 299-308. [Google Scholar]
- 11.Hoss, N. E., Al-Khatib, K., Peterson, D. E. & Loughin, T. M. (2003) Weed Sci. 51, 110-117. [Google Scholar]
- 12.Rausher, M. D. (2001) Nature 411, 857-864. [DOI] [PubMed] [Google Scholar]
- 13.Ennos, R. A. (1981) Genetica (The Hague) 57, 93-98. [Google Scholar]
- 14.Culpepper, A. S., Gimenez, A. E., York, A. C., Batts, R. B. & Wilcut, J. W. (2001) Weed Technol. 15, 56-61. [Google Scholar]
- 15.Stowe, K. A., Marquis, R. J., Hochwender, C. G. & Simms, E. L. (2000) Annu. Rev. Ecol. Syst. 31, 565-595. [Google Scholar]
- 16.Tiffin, P. & Rausher, M. D. (1999) Am. Nat. 154, 700-716. [DOI] [PubMed] [Google Scholar]
- 17.Stinchcombe, J. R. & Rausher, M. D. (2002) Proc. R. Soc. London Ser. B 269, 1241-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abrahamson, W. G. & Weis, A. E. (1997) Evolutionary Ecology Across Three Trophic Levels: Goldenrods, Gall-Makers, and Natural Enemies (Princeton Univ. Press, Princeton).
- 19.Gray, H. L. & Schucany, W. R. (1972) The Generalized Jackknife Statistic (Dekker, New York).
- 20.Rausher, M. D. (1992) Evolution (Lawrence, Kans.) 46, 616-626. [DOI] [PubMed] [Google Scholar]
- 21.Mauricio, R. & Mojonnier, L. E. (1997) TREE 12, 433-436. [DOI] [PubMed] [Google Scholar]
- 22.Fisher, R. A. (1930) The Genetical Theory of Natural Selection (Oxford Univ. Press, Oxford).
- 23.Simms, E. L. & Triplett, J. (1994) Evolution (Lawrence, Kans.) 48, 1973-1985. [DOI] [PubMed] [Google Scholar]
- 24.Simms, E. L. & Rausher, M. D. (1987) Am. Nat. 130, 570-581. [Google Scholar]
- 25.Juenger, T., Lennartsson, T. & Tuomi, J. (2000) Evol. Ecol. 14, 393-419. [Google Scholar]
- 26.Lennartsson, T., Nilsson, P. & Tuomi, J. (1998) Ecology 79, 1061-1072. [Google Scholar]
- 27.Roy, B. A. & Kirchner, J. W. (2000) Evolution (Lawrence, Kans.) 54, 51-63. [DOI] [PubMed] [Google Scholar]
- 28.Bergelson, J., Purrington, C. B., Palm, C. J. & Lopez-Gutierrez, J.-C. (1996) Proc. R. Soc. London Ser. B 263, 1659-1663. [DOI] [PubMed] [Google Scholar]
- 29.Purrington, C. B. & Bergelson, J. (1997) Genetics 145, 807-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warwick, S. I. (1991) Annu. Rev. Ecol. Syst. 22, 95-114. [Google Scholar]
- 31.Adkisson, P., Abramson, S., Baenziger, S., Betz, F., Carrington, J. C., Goldburg, R. J., Gould, F., Hodgson, E., Jones, T., Levin, M., et al. (2000) Genetically Modified Pest-Protected Plants: Science and Regulation (Natl. Acad. Press, Washington, DC).
- 32.Baskin, C. C. & Baskin, J. M. (1998) Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination (Academic, San Diego).
- 33.Morris, A. B., Baucom, R. S. & Cruzan, M. B. (2002) Am. J. Bot. 89, 29-36. [DOI] [PubMed] [Google Scholar]
- 34.Templeton, A. R. & Levin, D. A. (1979) Am. Nat. 114, 232-249. [Google Scholar]