Abstract
NifA is the general transcriptional activator of nitrogen fixation genes in diazotrophic bacteria. In Rhizobium leguminosarum bv. viciae UPM791, the nifA gene is part of a gene cluster (orf71 orf79 fixW orf5 fixABCX nifAB) separated by 896 bp from an upstream and divergent truncated duplication of nifH (ΔnifH). Symbiotic expression analysis of genomic nifA::lacZ fusions revealed that in strain UPM791 nifA is expressed mainly from a σ54-dependent promoter (PnifA1) located upstream of orf71. This promoter contains canonical NifA upstream activating sequences located 91 bp from the transcription initiation site. The transcript initiated in PnifA1 spans 5.1 kb and includes nifA and nifB genes. NifA from Klebsiella pneumoniae was able to activate transcription from PnifA1 in a heterologous Escherichia coli system. In R. leguminosarum, the PnifA1 promoter is essential for effective nitrogen fixation in symbiosis with peas. In its absence, partially efficient nitrogen-fixing nodules were produced, and the corresponding bacteroids exhibited only low levels of nifA gene expression. The basal level of nifA expression resulted from a promoter activity originating upstream of the fixX-nifA intergenic region and probably from an incomplete duplication of PnifA1 located immediately upstream of fixA.
NifA belongs to the bacterial enhancer-binding protein family of transcriptional regulators that activate gene expression in concert with RNA polymerase containing the specialized σ54 factor, which allows the polymerase core to recognize the −24/−12-type promoters (8, 15, 21). NifA normally binds to promoter sites designated upstream activating sequences (UAS) and interacts with the RNA polymerase holoenzyme via loop formation in the intervening DNA (25, 26). DNA loop formation is facilitated in many, but not all, cases by binding of the integration host factor to a site located between the UAS and the core promoter region (12, 25, 26). Transcription is promoted by interaction of NifA with the holoenzyme and subsequent catalysis of open complex formation through an ATP-dependent reaction.
In diazotrophic bacteria, NifA activates transcription of nitrogenase genes (nif genes) and, particularly in the legume endosymbiotic bacteria, also fix genes. Although nifA genes are conserved in diazotrophic bacteria, their organization within nif gene clusters differs (for a review see reference 8). In fast-growing rhizobia, such as Sinorhizobium meliloti, Rhizobium leguminosarum bv. trifolii, and R. leguminosarum bv. viciae, nifA is located in the symbiotic plasmid, between the fixABC operon and the nifB gene. In Bradyrhizobium japonicum, nifA is located in the chromosome as part of the fixR nifA operon.
Globally, NifA is regulated at both the transcriptional and activity levels mainly in response to oxygen or combined-nitrogen availability. In rhizobia, nifA gene expression is basically controlled by O2 availability, and it is maximal at low O2 concentrations, such as those available to the bacteroid inside the legume nodules. However, there are different mechanisms for regulation of nifA expression in root nodule bacteria. S. meliloti nifA promoter activity is regulated by the FixLJ cascade in free-living microaerobic cells and in symbiosis (7). In this bacterium, nifA expression is increased by feedback from the fixABCX promoter (5, 14). The nifA promoter is negatively controlled by FixK, a member of the Fnr/Crp family of transcriptional regulators, by an unknown mechanism (1). In B. japonicum, nifA is significantly expressed in aerobic conditions from the promoter of the fixR nifA operon, which is regulated by a two-component system, RegS/RegR (2). Expression of the fixR nifA operon is increased in microaerobic, anaerobic, and symbiotic conditions by the action of NifA in response to the redox state of the cell (33). Finally, the regulation of nifA expression in Azorhizobium caulinodans is complex and is controlled from overlapping promoter regions responding to both the nitrogen and oxygen status of the cell (18). The regulation of nifA expression in R. leguminosarum bv. viciae has not been studied in detail. Nitrogenase is not expressed in free-living cells, probably because NifA is not functional under these conditions. In symbiosis, NifA is essential for nitrogen fixation. In R. leguminosarum bv. viciae strain PRE, nifA mutants exhibit a Nif− Fix− phenotype (30). In this strain, a transcription start site was identified just upstream of the nifA gene (27), but no evidence for a nifA promoter was obtained from sequence analysis of this region in strain 3855 (9). In this work we found that R. leguminosarum bv. viciae nifA is expressed in symbiosis with peas from a σ54-dependent NifA-autoregulated promoter located upstream of the orf71 orf79 fixW orf5 fixABCX nifA nifB operon, although basal levels of symbiotic NifA expression were obtained from a second promoter located upstream of the fixX-nifA intergenic region.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Bacterial strains and plasmids used in this work are listed in Table 1. R. leguminosarum strains were routinely grown in tryptone-yeast extract (3) or yeast extract-mannitol (YMB) (34) media at 28°C. Escherichia coli strains were grown in Luria-Bertani medium. Antibiotics were added at the following concentrations: tetracycline, 12 μg ml−1 (6 μg ml−1 for Rhizobium); kanamycin, 50 μg ml−1; and ampicillin, 100 μg ml−1. Klebsiella pneumoniae nifA was aerobically expressed from plasmid pMJ220 in E. coli strain ET8000 at 28°C as previously described. Nonsuicide plasmids pSPM9 and pSPM10 were introduced into R. leguminosarum by conjugation by using E. coli strain S17.1, and transconjugants were selected in Rhizobium minimal medium (23) supplemented with tetracycline. Suicide plasmids derived from pVIK112 were introduced into R. leguminosarum by conjugation by using E. coli strain S17.1 λpir, and plasmid integration into the genome was selected in Rhizobium minimal medium supplemented with kanamycin.
TABLE 1.
Bacterial strains and plasmids used in this work
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| R. leguminosarum strains | ||
| UPM791 | 128C53 Strr | 16 |
| UPM791.5 | UPM791 cured of the symbiotic plasmid | 17 |
| GLF1 | UPM791::pVIKM1, nifA::lacZ fusion, Fix+ Kmr | This study |
| GLF2 | UPM791::pVIKM2, nifA::lacZ fusion, Fix+ Kmr | This study |
| GLF3 | UPM791::pVIKM3, nifA::lacZ fusion, Fix+ Kmr | This study |
| GLF4 | UPM791::pVIKM4, nifA::lacZ fusion, Fix− Kmr | This study |
| GLF8 | UPM791::pVIKM8, nifA::lacZ fusion, Fix− Kmr | This study |
| GLF12 | UPM791::pVIKM12, orf79::lacZ fusion, Fix+ Kmr | This study |
| GLF18 | UPM791::pVIKM18, nifA::lacZ fusion, Fix− Kmr | This study |
| E. coli strains | ||
| ET8000 | rbs lacZ::IS1 gyrA hutCka | 19 |
| S17.1 | thi pro hsdR hsdM+recA RP4 2-Tc::Mu-Km::Tn7 | 31 |
| S17.1 λpir | 294::[RP4 2-Tc::Mu-Km::Tn7]pro res ΔrecA mod+ λpir+ | 6 |
| Plasmids | ||
| pALM31 | UPM791 fixW upstream region in pLAFR1 cosmid; Tcr | This study |
| pALM35 | UPM791 nifA cluster in pLAFR1 cosmid; Tcr | This study |
| pCR 2.1-TOPO | PCR product cloning vector; Apr | Invitrogen |
| pCRnifA | UPM791 nifA gene in pCR2.1-TOPO; Apr | This study |
| pSKM2 | orf79-nifA fragment in pBluescript(SK); Apr | This study |
| pSKM3 | pSKM2 with a deletion of fixX-nifA intergenic fragment; Apr | This study |
| pSKM4 | ΔnifH-orf79 fragment in pBluescript(SK); Apr | This study |
| pVIK112 | R6K oriV lacZY; Kmr | 13 |
| pVIKM1 | ΔnifH orf71 orf79 fixW orf5 fixABCX nifA′::lacZ fusion in pVIK112; Kmr | This study |
| pVIKM2 | orf79 fixW orf5 fixABCX nifA′::lacZ fusion in pVIK112; Kmr | This study |
| pVIKM3 | orf79 fixW orf5 fixABCX nifA′::lacZ fusion in pVIK112; intergenic fixX-nifA fragment deleted; Kmr | This study |
| pVIKM4 | ′fixX nifA′::lacZ fusion in pVIK112; Kmr | This study |
| pVIKM8 | ′nifA::lacZ fusion in pVIK112; Kmr | This study |
| pVIKM12 | orf79 fixW orf5 fixABCX nifA′ fragment in opposite orientation with respect to lacZ in pVIK112; Kmr | This study |
| pVIKM18 | nifA fragment in opposite orientation with respect to lacZ in pVIK112; Kmr | This study |
| pSPV4 | pMP220 derivative; Tcr | 24 |
| pSPM9 | orf79::lacZ fusion in pSPV4; Tcr | This study |
| pSPM10 | ΔnifH::lacZ fusion in pSPV4; Tcr | This study |
| pMJ220 | K. pneumoniae NifA in pACYC184; Cmr | 22 |
DNA manipulation and analysis.
Plasmid DNA preparation, restriction enzyme digestion, agarose and polyacrylamide gel electrophoresis, DNA cloning, and transformation of DNA into E. coli cells were carried out by standard protocols (29). R. leguminosarum genomic DNA was extracted as previously described (16). For Southern hybridization, DNA probes were labeled with digoxigenin (Roche Molecular Biochemicals, Mannheim, Germany), and hybridization bands were visualized by luminography. DNA sequencing was carried out by using a rhodamine terminator cycle sequencing Ready Reaction kit and an ABI377 automatic sequencer (PE Biosystems, Foster City, Calif.) or by using a Sequenase kit (Sequenase, version 2.0; United States Biochemicals, Cleveland, Ohio).
Transcription start mapping.
The location of the 5′ end of the mRNA from the nifA gene was determined by primer extension analysis as described by Hidalgo et al. (10). For this assay, two synthetic oligonucleotides, RT-LP1 (5′-CCCGACGAAGACGATACCAG-3′) and RT-LP2 (5′-GCACGAGGCCATTCCAGAGC-3′), were used. These primers were complementary to the sequence corresponding to codons 16 to 22 and 52 to 58 of orf71, respectively. Total RNA for this analysis was isolated from microaerobic cells and bacteroids of R. leguminosarum UPM791 by using an RNeasy mini kit (QIAGEN, Hilden, Germany). The synthetic primers were labeled with [γ-32P]dATP, and the corresponding DNA extension products obtained by a reverse transcriptase reaction from RNA were visualized by autoradiography.
RT-PCR.
Reverse transcription (RT)-PCRs were performed with a GeneAmp PCR System 2400 DNA thermal cycler (PE Biosystems) by using the Titan One Tube RT-PCR system with avian myeloblastosis virus and Expand High Fidelity (Roche Molecular Biochemicals) as specified by the manufacturer. Total RNA from aerobic cells and bacteroids of R. leguminosarum UPM791 was isolated as described above and treated with RNase-free DNase (Roche Molecular Biochemicals) to remove contaminating chromosomal DNA. As a negative control, PCRs were carried out in parallel with each RT-PCR by using the Expand High Fidelity PCR system (Roche Molecular Biochemicals). The primers used are described in Table 2.
TABLE 2.
Primers used in RT-PCR experiments
| RT-PCR product length (bp) | Upper primer
|
Lower primer
|
||
|---|---|---|---|---|
| Designation (sequence) | 5′ Position | Designation (sequence) | 5′ Position | |
| 782 | RT-UP1 (5′-GGGCGGATTGCGACACGACA-3′) | 939a | RT-LP4 (5′-ACGCCCGTGTCGCTGAACTT-3′) | 1720a |
| 1,472 | RT-UP1 (5′-GGGCGGATTGCGACACGACA-3′) | 939a | RT-LP5 (5′-GCCCCCATAGCGGTTACGAA-3′) | 2410a |
| 1,270 | RT-UP2 (5′-GCTTCGGACGCCTGGTATCG-3′) | 1141a | RT-LP5 (5′-GCCCCCATAGCGGTTACGAA-3′) | 2410a |
| 2,108 | RT-UP6 (5′-CAAGGCGTACCGACCATCAT-3′) | 2330a | RT-LP8 (5′-GCGTCATCTCGGAAGTTTGG-3′) | 4437a |
| 1,874 | RT-UP8 (5′-GCAACCGCACAACTCTATCA-3′) | 4197a | PD-Inter2 (5′-TGGAGCCGCGCCTCTGGTTT-3′) | 995b |
| 954 | IDH549 (5′-GAAATGACGCGCATCATCTGG-3′) | 421c | IDHA982 (5′-CCAGGCGAAGATCGAGGCGAT-3′) | 1354c |
Generation of R. leguminosarum UPM791 nifA and fixW upstream region probes.
Two oligonucleotides (primer NIFA1 [5′-CGGAATTCGGCATGATTAAACCAGAGGC-3′] and primer NIFA2 [5′-CGGGATCCTGACTCCTTCTTCACATCGATA-3′]) derived from the R. leguminosarum nifA sequence (EMBL accession no. X05049 [9]) were used as primers for PCR amplification of total DNA from strain UPM791. Two other oligonucleotides (primer UPFixW [5′-GTCGGCAACCATACAAACCC-3′] and primer LPFixW [5′-CTGCGGGTGCGTGACATTGC-3′]), which were derived from another R. leguminosarum sequence (EMBL accession no. X16521 [11]), were used as primers for PCR amplification of total DNA from strain UPM791. The amplified 1.6- and 0.4-kb DNA fragments were sequenced to confirm that they corresponded to the nifA and fixW region sequences, respectively.
Plasmid construction.
The 1.6-kb DNA fragment containing nifA mentioned above was cloned in plasmid pCR 2.1-TOPO, generating plasmid pCRnifA. The nifA probe was used to isolate cosmid pALM35 from a previously constructed R. leguminosarum UPM791 genomic library (16).
Plasmid pSKM2 was generated by cloning a 1.1-kb SmaI/EcoRI fragment, which contained the fixX-nifA intergenic region, from the pALM35 cosmid in pBluescript. The fixX-nifA intergenic region in pSKM2 was deleted by sequentially introducing two AvrII cut sites, the first one immediately downstream of the fixX gene and the second one immediately upstream of the nifA gene. AvrII cut sites were introduced by site-directed mutagenesis by using a Quick Change site-directed mutagenesis kit (Stratagene) and following the manufacturer's protocol. Two synthetic, complementary oligonucleotides carrying the corresponding mutations were used to synthesize the entire plasmid. Oligonucleotide 5′-GGATGAGGAGTCCCTAGGTCCGGCGG-3′ and the complementary primer were used to create the AvrII cut site downstream of fixX. The AvrII cut site upstream of nifA was created by using primer 5′-CACCCTCCCCTGTTCCTAGGCATGATTAAA-3′ and the complementary primer. Introduction of the correct mutations into the plasmid was confirmed by sequencing of the fixX-nifA intergenic region. The resulting plasmid was cut with the AvrII enzyme and religated, giving plasmid pSKM3. Plasmid pSKM4 was generated by cloning in pBluescript a 1.3-kb SalI/EcoRI fragment from the pALM35 cosmid containing the PnifA1 promoter.
Generation of lacZ fusions.
A 4.8-kb EcoRI/XbaI fragment and the upstream 1.3-kb SalI/EcoRI fragment were sequentially cloned in pVIK112 to generate plasmid pVIKM1 containing a nifA::lacZ fusion. A 5.6-kb EcoRI fragment containing the 5′ end of nifA from cosmid pALM35 was cloned in the pVIK112 vector in both orientations, generating plasmids pVIKM2 and pVIKM12 containing nifA::lacZ and orf79::lacZ fusions, respectively. The 4.5-kb EcoRI/SmaI fragment from cosmid pALM35 and the 939-bp SmaI/EcoRI fragment from plasmid pSKM3 were sequentially cloned in pVIK112, giving plasmid pVIKM3. The 1.1-kb SmaI/EcoRI fragment from plasmid pSKM2 containing the 5′ end of nifA was cloned in the pVIK112 vector, generating plasmid pVIKM4. The 0.66-kb EcoRI fragment from plasmid pCRnifA was cloned in pVIK112 in both orientations, generating plasmids pVIKM8 and pVIKM18. The 1.3-kb SalI/EcoRI fragment from cosmid pALM35 was cloned in vector pSPV4, generating plasmids pSPM9 and pSPM10 containing orf79::lacZ and ΔnifH::lacZ fusions, respectively.
Plant tests and enzyme assays.
Pea (Pisum sativum L. cv. Frisson) plants were used as hosts for R. leguminosarum bv. viciae strains. The conditions used for plant inoculation and growth have been described previously (16). β-Galactosidase activities in E. coli and Rhizobium cell cultures and pea bacteroid suspensions were determined as described by Miller (20). Whole-root acetylene reduction was determined as described by Ruiz-Argüeso et al. (28) by using 120-ml flasks containing the whole pea plant root system. The protein contents of bacteroid suspensions and cell cultures were measured by the bicinchoninic acid method (32) after alkaline digestion in 1 N NaOH at 90°C for 10 min; bovine serum albumin was used as a standard.
RESULTS
Analysis of the nifA DNA region.
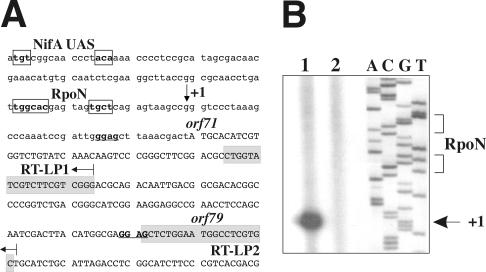
The nifA DNA region of R. leguminosarum bv. viciae strain UPM791 was located and sequenced as a first step towards identifying the regulatory DNA elements involved in NifA expression in this symbiotic bacterium. A gene library was screened with nifA and orf71 orf79 probes (see Materials and Methods) that were generated from available nifA (9) and fixW region (11) sequences from strains 3855 and PRE, respectively. Two overlapping cosmids, pALM31 and pALM35, spanning a 50-kb region that included the nifA region, nodI, and fixW3, were identified by using these probes (Fig. 1A). PCR and Southern hybridization analyses with strains UPM791 and UPM791.5 (cured of the symbiotic plasmid) indicated that only one copy of nifA was located in the symbiotic plasmid (data not shown). From this region, a 9.3-kb DNA fragment containing nifA was sequenced (EMBL accession no. AJ431175 and AJ520101), and the sequence analysis revealed a gene cluster (orf71 orf79 fixW orf5 fixA fixB fixC fixX nifA nifB) separated by 896 bp from a truncated duplication of nifH (ΔnifH). Genes and open reading frames in this cluster were designated on the basis of their similarity to genes and open reading frames previously described in R. leguminosarum bv. viciae 3855 (9) and PRE (11) and in other rhizobia. This is the first report of a complete fixABCX gene sequence from R. leguminosarum bv. viciae. The gene products FixA, FixB, FixC, and FixX were homologous (with overall levels of identity ranging from 53 to 97% [data not shown]) to the same proteins from other diazotrophic bacteria present in databanks. ΔnifH, orf71, orf79, and fixW were highly conserved with respect to corresponding genes and coding sequences from strain PRE (11). The ΔnifH-orf71 intergenic region contained two potential σ54-binding sequences preceded by two consensus NifA-binding sequences, suggesting the existence of two divergent NifA-dependent promoters that we designated PΔnifH and PnifA1 (Fig. 1A). An imperfect repetition of the PnifA1 region was found at the 5′ end of fixA. This region included the σ54-binding motif but not the NifA-binding site (Fig. 1B).
FIG. 1.
Structure of nifA DNA region from R. leguminosarum bv. viciae UPM791. (A) Gene organization. The grey arrows correspond to genes or open reading frames identified in a 6.9-kb SalI/EcoRI fragment containing the nifA gene from R. leguminosarum and adjacent DNA (EMBL accession no. AJ431175 and AJ520101). The pattern of restriction enzyme cutting sites is indicated below the genetic map. The positions of NifA upstream binding sequences (UAS) and σ54-binding sites are indicated by open vertical arrows and grey boxes, respectively. PΔnifH and PnifA1, indicated by horizontal lines below the restriction map, correspond to potential NifA and σ54-dependent promoters located upstream of ΔnifH and orf71, respectively. The DNA bordering the NifA region and the cosmids used in its identification are shown at the bottom. (B) Comparison of nucleotide sequences of the region upstream of orf71 plus the 5′ end of orf71 (top line) with the 3′ end of orf5, the intergenic fragment orf5-fixA, and the 5′ end of fixA (bottom line). The numbers on the left indicate the positions of the first nucleotide according to the EMBL accession no. AJ431175 sequence. Nucleotides conserved in both sequences are indicated by asterisks. NifA- and σ54-binding sites are enclosed in boxes. The transcription initiation site (+1) of the PnifA1 promoter is indicated by a vertical arrow.
Expression analysis of nifA.
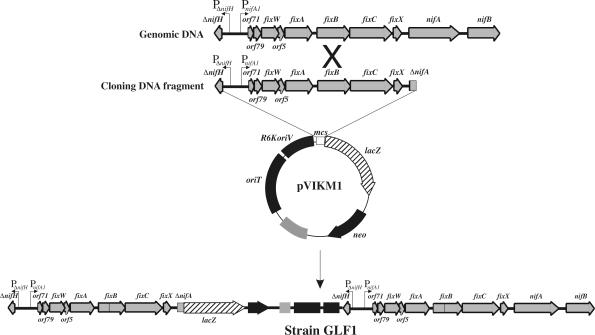
Expression analysis of R. leguminosarum UPM791 nifA was accomplished by generating genomic lacZ fusions to nifA and to other target genes within a 6.9-kb SalI/EcoRI fragment, including the potential nifA promoter region. The suicide vector pVIK112 (13) was used to clone fragments of the nifA region upstream of the lacZ reporter gene, and the genomic fusions were created in a single step by integrative homologous recombination, which resulted in UPM791 derivative strains belonging to the GLF series (Fig. 2 and 3A). This process is illustrated in Fig. 2 for strain GLF1. Except for GLF8 and GLF18, cloned fragments contained the intact 5′ end of the nifA gene with or without the PnifA1 promoter.
FIG. 2.
Construction of R. leguminosarum GLF1 genomic nifA::lacZ fusion. A 6.1-kb SalI/XbaI fragment spanning the region from ΔnifH to nifA was cloned in suicide vector pVIK112 upstream of a lacZ reporter gene, generating plasmid pVIKM1. Single homologous recombination between the cloned fragment and genomic DNA resulted in integration of the entire pVIKM1 plasmid in the genome. The resulting GLF1 strain harbored a duplication of the cloned DNA fragment and a genomic nifA::lacZ fusion.
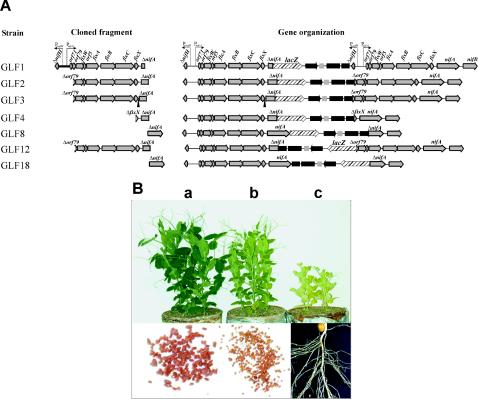
FIG. 3.
Expression analysis of nifA region. (A) Structure of lacZ fusion constructs: DNA fragments cloned in suicide vector pVIK112 and the genetic organization resulting after integration of the pVIKM plasmids in the genome. The designations of the derivative GLF reporter strains are indicated on the left. (B) Appearance of 21-day-old pea plants inoculated with the R. leguminosarum GLF1 (plant a), GLF2 (plant b), and GLF4 (plant c) strains containing nifA::lacZ genomic fusions. Root nodules are shown below the corresponding inoculated plants.
Only plants inoculated with R. leguminosarum strain GLF1 exhibited a wild-type phenotype (green plants and normal red nodules) (Fig. 3B), suggesting that normal amounts of NifA were present. Plants inoculated with the GLF2, GLF3, and GLF12 strains exhibited an intermediate yellow-green color and smaller red nodules (Fig. 3B), as well as a 65% decrease in the acetylene reduction rate and a 50% decrease in the dry weight (Table 3). Finally, plants inoculated with strains GLF4, GLF8, and GLF18 contained tiny, white nodules and could not be distinguished from noninoculated control plants on the basis of color (Fig. 3B), dry weight, and nitrogenase activity (Table 3).
TABLE 3.
Symbiotic characteristics of reporter GLF derivative strainsa
| Strain | Fix pheno- type | Plants
|
Bacteroids
|
||
|---|---|---|---|---|---|
| Color | Dry wt (g/pot)b | Nitro- genase activityc | β-Galacto- sidase activity (Miller units) | ||
| GLF1 | Fix+ | Green (wild type) | 4.85 ± 0.3 | 5.1 ± 1.6 | 1,021 ± 30 |
| GLF2 | Fix+ | Intermediate | 2.65 ± 0.25 | 1.9 ± 0.6 | 267 ± 65 |
| GLF3 | Fix+ | Intermediate | 2.25 ± 0.25 | 1.8 ± 0.45 | 172 ± 80 |
| GLF4 | Fix− | Yellow | 1.15 ± 0.25 | <10 | NDd |
| GLF8 | Fix− | Yellow | 1.20 ± 0.32 | <10 | ND |
| GLF12 | Fix+ | Intermediate | 2.67 ± 0.35 | 1.9 ± 0.7 | 40 ± 20 |
| GLF18 | Fix− | Yellow | 1.12 ± 0.2 | <10 | ND |
The strains were used as inoculants for peas, and the Fix phenotypes, plant colors, dry weights 32 days after inoculation, and nitrogenase and β-galactosidase activities of pea bacteroids were determined. All values are averages ± standard deviations for three assays.
Each pot contained five plants.
Nitrogenase activity is expressed in nanomoles of C2H2 reduced per hour per plant.
ND, not determined.
R. leguminosarum GLF strains were tested for β-galactosidase activity in free-living cells grown under aerobic or microaerobic conditions and in bacteroids from peas. No significant β-galactosidase activity was detected in free-living cultures of any of the GLF strains grown under either aerobic or microaerobic conditions (data not shown). In strain GLF1 bacteroids, in which high levels of nifA transcription were observed (Table 3), the nifA promoter must be included in the region upstream of nifA located in the fragment integrated in the GLF1 strain. This region contains PΔnifH and PnifA1 promoters and any other potential promoter located upstream of the nifA gene (Fig. 3A). The plant phenotypes suggested that the intact copy of the nifA gene was not transcribed at wild-type levels in bacteroids from the GLF2, GLF3, and GLF12 strains. These strains did not contain either PΔnifH or PnifA1 upstream of the intact copy of the nifA gene. Comparison of these results with those obtained with strain GLF1 indicated that nifA is mainly transcribed from PnifA1 and also that low levels of expression originated from the activity of a basal promoter located between orf79 and nifA. This potential promoter was designated PnifA2. The β-galactosidase activities observed in bacteroids from nifA::lacZ fusions (Table 3) demonstrated that expression of nifA in bacteroids from strain GLF2 was only 25% of the expression in bacteroids from strain GLF1. Given that PnifA1 has the structure of a NifA-dependent promoter (NifA UAS and properly spaced σ54 box), these results suggest that NifA is the activator of the PnifA1 promoter. The fact that GLF3, a strain lacking the intergenic region between fixX and nifA in the cloned fragment upstream of lacZ, exhibited β-galactosidase activities similar to those of strain GLF2 (Fig. 3) suggests that PnifA2 is located upstream of the intergenic fixX-nifA region.
β-Galactosidase activities could not be determined in strains GLF4, GLF8, and GLF18 since these strains initiated only incipient, inefficient nodules in pea plants. The plant phenotype obtained with strain GLF4 suggested that its intact copy of the nifA gene was not transcribed in bacteroids. Since the upstream region of this nifA gene contained only the 3′ end of fixX and the intergenic region between fixX and nifA, we concluded that there was no promoter activity in this intergenic region. This result implies that the basal promoter PnifA2 may be located between orf79 and the 3′ end of fixX. R. leguminosarum strains GLF8 and GLF18 were generated by integration of a pVIK112 derivative plasmid containing the 3′ end of nifA. This resulted in an intact copy of the nifA gene that was upstream of the plasmid DNA but was separated from nifB. Since these strains exhibited a Fix− phenotype when they were used as inocula for peas, although the intact copy of the nifA gene was fully transcribed from PnifA1, we suggest that nifB, which is normally cotranscribed with nifA, was not transcribed in these strains.
To confirm the functionality of the PnifA1 promoter, primer extension analysis of RNA from bacteroids was carried out by using primers RT-LP1 and RT-LP2 located upstream of orf79 (Fig. 4A). A clear DNA extension band identified the G located 13 bp downstream of the TGGCAC-N6-TGCT σ54-binding signature as the PnifA1 transcription initiation site (Fig. 4B). No transcription initiation site was detected by using RNA from free-living microaerobic cells. These results confirmed the functionality of the σ54-type promoter PnifA1 in bacteroids. Similarly, we attempted to show that transcription corresponding to the PnifA2 promoter was associated with the PnifA1 promoter homologous sequence located upstream of fixA (Fig. 1B). However, primer P3nifA (5′-ATTTGTCACCGGGTGGACGC-3′), corresponding to a fixA sequence, did not detect any transcription initiation site in the primer extension experiment with bacteroid RNA (data not shown). A similar, negative result was obtained with primer P2nifA (5′-AGAGCCGCTACTTAAACTAG-3′), which corresponded to a fixW sequence and was designed to identify promoter activity associated with the potential σ54-binding site within orf79 (data not shown).
FIG. 4.
Identification of the transcription initiation site of the PnifA1 promoter. (A) Nucleotide sequence (360 bp) that included the region upstream of orf71, orf71, and the 5′ end of orf79. Potential NifA- and σ54-binding sites are enclosed in boxes. The potential ribosome-binding sites for orf71 and orf79 are underlined and in boldface type. The transcription initiation site (+1) is indicated by a vertical arrow. The sequences in grey boxes designated RT-LP1 and RT-LP2 correspond to the two primers used in primer extension reactions. (B) Determination of the transcription start site. Total-RNA samples (10 μg) isolated from pea bacteroids (lane 1) and microaerobic free-living cells (lane 2) of R. leguminosarum bv. viciae UPM791 were used in primer extension reactions with primer RT-LP1. Lanes A, C, G, and T, dideoxynucleotide sequencing reactions carried out with plasmid pSKM4 and the same primer. The positions of the relevant sequence for RpoN and the transcription initiation site are indicated on the right. The same results were obtained with primer RT-LP2.
PnifA1 controls symbiotic expression of the nifA gene.
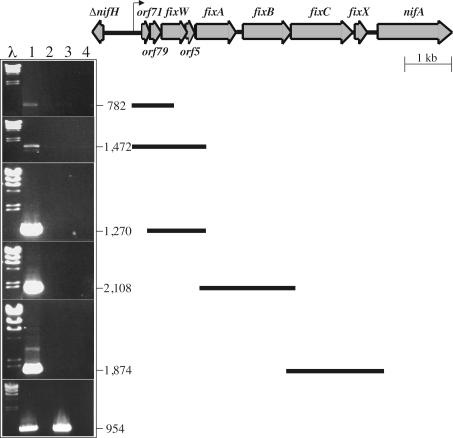
To confirm that nifA was transcribed from the PnifA1 promoter under symbiotic conditions, we investigated the existence of an mRNA extending from this promoter to nifA in pea bacteroids from wild-type strain UPM791 by performing RT-PCR experiments (Fig. 5). RNA isolated from bacteroids from 21-day-old pea plants and from free-living aerobic cells (as a control) served as templates for the RT-PCRs and PCRs with various primer sets (Table 2). These sets, covering the orf71 orf79 fixW orf5 fixA (1,270-bp fragment), fixA fixB fixC (2,108-bp fragment), and fixB fixC fixX nifA (1,874-bp fragment) DNA regions, yielded the expected products (Fig. 5, lane 1), providing evidence that the orf71 orf79 fixW orf5 fixA fixB fixC fixX nifA gene cluster forms a transcription unit. In contrast, a primer designed to anneal with the region upstream of the PnifA1 transcription start site gave weak RT-PCR products (782- and 1,472-bp fragments). No PCR products were obtained in the absence of reverse transcriptase (Fig. 5, lanes 2 and 4), which excludes the possibility that the PCR products observed resulted from contaminated chromosomal DNA. RT-PCR amplification of the isocitrate dehydrogenase gene was used as an internal standard. For this gene, PCR products (954 bp) with similar intensities were obtained from bacteroids and aerobically grown free-living cells (Fig. 5, lanes 1 and 3).
FIG. 5.
RT-PCR expression analysis of nifA region from R. leguminosarum bv. viciae UPM791. The gene organization of the nifA region is indicated at the top. The gel DNA band profiles are the profiles for RT-PCR (lanes 1 and 3) and PCR (lanes 2 and 4) products obtained with total RNA from bacteroids (lanes 1 and 2) or from aerobic free-living cells (lanes 3 and 4). The lengths of the resulting RT-PCR products (in base pairs) and their positions within the nifA region are indicated on the right. The primers used for RT-PCR and PCRs are described in Table 2. Amplification of an internal fragment of the isocitrate dehydrogenase gene (954 bp) was used as a control for RNA quantitation (bottom panel).
Promoter PnifA1 is regulated by NifA.
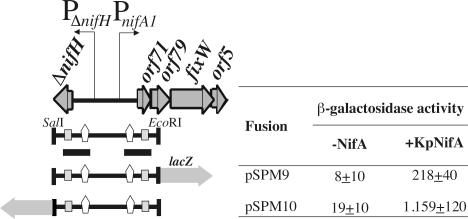
Since PnifA1 has the structure of a NifA-dependent promoter, we tested its activation in E. coli in the presence of K. pneumoniae NifA supplied by plasmid pMJ220. Reporter plasmids pSPM9 and pSPM10 containing the PΔnifH and PnifA1 promoters were transformed into E. coli strains ET8000 and ET8000(pMJ220), and the β-galactosidase activities of the resulting strains were assayed in cells from aerobic cultures (Fig. 6). For both plasmids, β-galactosidase activity was detected only in the presence of NifA. These results demonstrated that both the PnifA1 and PΔnifH promoters can be activated by NifA.
FIG. 6.
NifA-dependent expression of PΔnifH and PnifA1 promoters in E. coli. The physical and genetic map of a 1.3-kb SalI/EcoRI fragment containing the PΔnifH and PnifA1 promoters is shown on the left. The positions of NifA upstream binding sequences (UAS) and σ54-binding sites are indicated by open vertical arrows and grey boxes, respectively. The fragment fused to the lacZ gene in both orientations is shown at the bottom. β-Galactosidase activities associated with the lacZ fusions were measured in E. coli strain ET8000 expressing K. pneumoniae nifA (KpNifA) from plasmid pMJ220. The values (in Miller units) are averages ± standard deviations for three assays.
DISCUSSION
The nifA region of R. leguminosarum bv. viciae UPM791 contains a gene cluster (orf71 orf79 fixW orf5 fixA fixB fixC fixX nifA nifB) separated by 896 bp from an upstream truncated duplication of nifH (ΔnifH) in the opposite orientation. The gene organization of this region was similar to that described for strain PRE (11) and was identical to the gene organization of part of this region described for strain 3855 (9). Sequences corresponding to orf71, orf79, and fixW sequences have been detected only in certain strains of R. leguminosarum (11).
UPM791 reporter strains, constructed by insertion of suicide pVIKM plasmids into the genome (Fig. 3A), clearly demonstrated that nifA is exclusively transcribed in pea bacteroids and that this transcription originates from a σ54-dependent promoter (PnifA1) located upstream of orf71 at the expected distance (−25/−13) from the identified transcription initiation site (Fig. 4). mRNA analysis confirmed that orf71 orf79 fixW orf5 fixABC fixX and nifA form an operon in symbiotic conditions (Fig. 5). This operon might also include nifB since non-nitrogen-fixing plants were obtained when nifB was transcriptionally separated from nifA. Green, efficient nitrogen-fixing pea plants were obtained only in the presence of full transcription levels of nifA nifB that originated from PnifA1 (Fig. 3B).
PnifA1 contains canonical NifA-binding sequences (TGTN10ACA) located 91 bp upstream of the transcription start site. These NifA-binding sequences in a σ54-type promoter suggested that there was autoregulation of PnifA1 by NifA, and this was confirmed by showing that K. pneumoniae NifA promoted the expression of an orf79::lacZ fusion in E. coli (Fig. 6). No consensus sequences involved in bending the DNA (integration host factor) to facilitate contact of NifA with the RNA polymerase were identified between the UAS and the σ54-binding signature in the PnifA1 and PΔnifH promoters. The autoregulated PnifA1 promoter is required for effective nitrogen fixation by pea plants inoculated with the UPM791 strain. Derivative strains lacking PnifA1 induced pea nodules that were symbiotically inefficient, and the bacteroids exhibited only basal, although bacteroid-specific, levels of nifA expression. Autoregulation of nifA expression in symbiosis appears to be a frequent phenomenon in endosymbiotic bacteria. In S. meliloti, autoregulation of NifA expression occurs from the fixABCX promoter, but basal levels of symbiotic NifA expression are dependent on the FixLJ system acting on a nifA promoter (5, 7). Similarly, the fixR nifA operon of B. japonicum is regulated by RegR in aerobic cells and is autoregulated by NifA in symbiosis (33).
Deletion analysis of the DNA region upstream of nifA clearly showed that basal, bacteroid-specific nifA expression was prompted by a second, unidentified promoter (PnifA2) located downstream of orf79 and upstream of the 3′ end of fixX. No promoter activity associated with the nifA-fixX intergenic region was detected. This was confirmed by the absence of symbiotic nitrogen fixation with strains whose nifA expression was dependent on this intergenic DNA fragment. This conclusion is supported by the absence of recognizable promoter sequence motifs immediately upstream of nifA. Initially, it was hypothesized that the promoter responsible for basal levels of NifA expression (PnifA2) in bacteroids was located immediately upstream of fixA. This assumption was based on (i) the existence of a fixABCX operon in other bacteria (S. meliloti); (ii) the absence of promoter-like sequences upstream of fixX; (iii) the lack of intergenic space among the fixA, fixB, and fixC genes; and overall (iv) the observation that the DNA sequence preceding fixA is identical to the DNA sequence of PnifA1 except for the absence of the NifA-binding UAS. The existence of this sequence duplication suggests that PnifA1 was the original promoter of the fixABC operon but it was interrupted and partially duplicated, together with the N-terminal end of fixA, after insertion of fixW. However, primer extension assays, directed to identify a possible transcription initiation site downstream of the σ54-binding motif by using bacteroid RNA samples, were unsuccessful. It is possible that the PnifA2 promoter is indeed active and that the failure was due to its low activity. If this were the case, the existence of a symbiosis-specific, trans-acting regulatory factor should be postulated for PnifA2 activity, since σ54-dependent promoters possess no constitutive activity in the absence of an activator protein.
In conclusion, our analysis showed that in R. leguminosarum bv. viciae UPM791 nifA is expressed only under symbiotic conditions, and this explains the previously observed inability of this organism to express NifA-dependent systems, such as nitrogenase and hydrogenase (4), under free-living conditions. Symbiotic nifA expression is under positive autoregulation by NifA and originates from a promoter (PnifA1) located 4.7 kb upstream of nifA. This promoter determines transcription of the orf71 orf79 fixW orf5 fixABC fixX nifA operon. It is absolutely required for full nifA transcription and, consequently, for efficient nitrogen fixation. In its absence, basal nifA transcription originates from an uncharacterized promoter (PnifA2) located upstream of the intergenic fixX-nifA region and probably upstream of fixA. This mode of regulation of nifA is characteristic of R. leguminosarum bv. viciae and differs from models studied in other rhizobia (8).
Acknowledgments
This research was supported by a grant from Programa de Grupos Estratégicos (III PRICYT) of the Comunidad Autónoma de Madrid and by grant AGL2001-2295 from MCYT to T.R.-A.
REFERENCES
- 1.Batut, J., M. L. Daveran-Mingot, M. David, J. Jacobs, A. M. Garnerone, and D. Kahn. 1989. fixK, a gene homologous with fnr and crp from Escherichia coli, regulates nitrogen fixation genes both positively and negatively in Rhizobium meliloti. EMBO J. 8:1279-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer, E., T. Kaspar, H. M. Fischer, and H. Hennecke. 1998. Expression of the fixR-nifA operon in Bradyrhizobium japonicum depends on a new response regulator, RegR. J. Bacteriol. 180:3853-3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beringer, J. 1974. R factor transfer in Rhizobium leguminosarum. J. Gen. Microbiol. 84:188-198. [DOI] [PubMed] [Google Scholar]
- 4.Brito, B., M. Martinez, D. Fernandez, L. Rey, E. Cabrera, J. M. Palacios, J. Imperial, and T. Ruiz-Argüeso. 1997. Hydrogenase genes from Rhizobium leguminosarum bv. viciae are controlled by the nitrogen fixation regulatory protein NifA. Proc. Natl. Acad. Sci. USA 94:6019-6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buikema, W. J., W. W. Szeto, P. V. Lemley, W. H. Orme-Johnson, and F. M. Ausubel. 1985. Nitrogen fixation specific regulatory genes of Klebsiella pneumoniae and Rhizobium meliloti share homology with the general nitrogen regulatory gene ntrC of K. pneumoniae. Nucleic Acids Res. 13:4539-4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Lorenzo, V., M. Herrero, U. Jakubzik, and K. N. Timmis. 1990. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J. Bacteriol. 172:6568-6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ditta, G., E. Virts, A. Palomares, and C. H. Kim. 1987. The nifA gene of Rhizobium meliloti is oxygen regulated. J. Bacteriol. 169:3217-3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer, H. M. 1994. Genetic regulation of nitrogen fixation in rhizobia. Microbiol. Rev. 58:352-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grönger, P., S. S. Manian, H. Reilander, M. O'Connell, U. B. Priefer, and A. Pühler. 1987. Organization and partial sequence of a DNA region of the Rhizobium leguminosarum symbiotic plasmid pRL6JI containing the genes fixABC, nifA, nifB and a novel open reading frame. Nucleic Acids Res. 15:31-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hidalgo, E., J. M. Palacios, J. Murillo, and T. Ruiz-Argüeso. 1992. Nucleotide sequence and characterization of four additional genes of the hydrogenase structural operon from Rhizobium leguminosarum bv. viciae. J. Bacteriol. 174:4130-4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hontelez, J. G., R. K. Lankhorst, P. Katinakis, R. C. van den Bos, and A. van Kammen. 1989. Characterization and nucleotide sequence of a novel gene fixW upstream of the fixABC operon in Rhizobium leguminosarum. Mol. Gen. Genet. 218:536-544. [DOI] [PubMed] [Google Scholar]
- 12.Hoover, T. R., E. Santero, S. Porter, and S. Kustu. 1990. The integration host factor stimulates interaction of RNA-polymerase with NifA, the transcriptional activator for nitrogen fixation operons. Cell 63:11-22. [DOI] [PubMed] [Google Scholar]
- 13.Kalogeraki, V. S., and S. C. Winans. 1997. Suicide plasmids containing promoterless reporter genes can simultaneously disrupt and create fusions to target genes of diverse bacteria. Gene 188:69-75. [DOI] [PubMed] [Google Scholar]
- 14.Kim, C. H., D. R. Helinski, and G. Ditta. 1986. Overlapping transcription of the nifA regulatory gene in Rhizobium meliloti. Gene 50:141-148. [DOI] [PubMed] [Google Scholar]
- 15.Kustu, S., E. Santero, J. Keener, D. Popham, and D. Weiss. 1989. Expression of σ54- (NtrA)-dependent genes is probably united by a common mechanism. Microbiol. Rev. 53:367-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leyva, A., J. M. Palacios, T. Mozo, and T. Ruiz-Argüeso. 1987. Cloning and characterization of hydrogen uptake genes from Rhizobium leguminosarum. J. Bacteriol. 169:4929-4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leyva, A., J. M. Palacios, and T. Ruiz-Argüeso. 1987. Conserved plasmid hydrogen uptake (Hup)-specific sequences within Hup+ Rhizobium leguminosarum strains. Appl. Environ. Microbiol. 53:2539-2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loroch, A. I., B. G. Nguyen, and R. A. Ludwig. 1995. Interactive regulation of Azorhizobium nifA transcription via overlapping promoters. J. Bacteriol. 177:7210-7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacNeil, T., G. P. Roberts, D. MacNeil, and B. Tyler. 1982. The products of glnL and glnG are bifunctional regulatory proteins. Mol. Gen. Genet. 188:325-333. [DOI] [PubMed] [Google Scholar]
- 20.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 21.Morett, E., and M. Buck. 1989. In vivo studies on the interaction of RNA σ54-polymerase with the Klebsiella pneumoniae and Rhizobium meliloti nifH promoters: the role of NifA in the formation of an open promoter complex. J. Mol. Biol. 210:65-77. [DOI] [PubMed] [Google Scholar]
- 22.Morett, E., and M. Buck. 1988. NifA-dependent in vivo protection demonstrates that the upstream activator sequence of nif promoters is a protein binding site. Proc. Natl. Acad. Sci. USA 85:9401-9405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Gara, F., and K. T. Shanmugam. 1976. Regulation of nitrogen fixation by rhizobia. Export of fixed N2 as NH4+. Biochim. Biophys. Acta 437:313-321. [DOI] [PubMed] [Google Scholar]
- 24.Parry, S. K., S. B. Sharma, and E. A. Terzaghi. 1994. Construction of a bidirectional promoter probe vector and its use in analyzing nod gene expression in Rhizobium loti. Gene 150:105-109. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Martin, J., and V. de Lorenzo. 1997. Clues and consequences of DNA bending in transcription. Annu. Rev. Microbiol. 51:593-628. [DOI] [PubMed] [Google Scholar]
- 26.Perez-Martin, J., F. Rojo, and V. de Lorenzo. 1994. Promoters responsive to DNA bending: a common theme in prokaryotic gene expression. Microbiol. Rev. 58:268-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roelvink, P. W., J. G. Hontelez, A. van Kammen, and R. C. van den Bos. 1989. Nucleotide sequence of the regulatory nifA gene of Rhizobium leguminosarum PRE: transcriptional control sites and expression in Escherichia coli. Mol. Microbiol. 3:1441-1447. [DOI] [PubMed] [Google Scholar]
- 28.Ruiz-Argüeso, T., J. Hanus, and H. J. Evans. 1978. Hydrogen production and uptake by pea nodules as affected by strains of Rhizobium leguminosarum. Arch. Microbiol. 116:113-118. [Google Scholar]
- 29.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 30.Schetgens, R. M. P., J. G. J. Hontelez, R. C. Vandenbos, and A. Vankammen. 1985. Identification and phenotypical characterization of a cluster of fix genes, including a nif regulatory gene, from Rhizobium leguminosarum PRE. Mol. Gen. Genet. 200:368-374. [Google Scholar]
- 31.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 32.Smith, P. K., R. I. Krohn, G. T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olson, and D. C. Klenk. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76-85. [DOI] [PubMed] [Google Scholar]
- 33.Thöny, B., and H. Hennecke. 1989. The −24/−12 promoter comes of age. FEMS Microbiol. Rev. 5:341-357. [DOI] [PubMed] [Google Scholar]
- 34.Vincent, J. M. 1970. A manual for the practical study of root-nodule bacteria. Blackwell Scientific Publications, Oxford, United Kingdom.