Abstract
Growth of Lactococcus lactis in milk depends on the utilization of extracellular peptides. Up to now, oligopeptide uptake was thought to be due only to the ABC transporter Opp. Nevertheless, analysis of several Opp-deficient L. lactis strains revealed the implication of a second oligopeptide ABC transporter, the so-called Opt system. Both transporters are expressed in wild-type strains such as L. lactis SK11 and Wg2, whereas the plasmid-free strains MG1363 and IL-1403 synthesize only Opp and Opt, respectively. The Opt system displays significant differences from the lactococcal Opp system, which made Opt much more closely related to the oligopeptide transporters of streptococci than to the lactococcal Opp system: (i) genetic organization, (ii) peptide uptake specificity, and (iii) presence of two oligopeptide-binding proteins, OptS and OptA. The fact that only OptA is required for nutrition calls into question the function of the second oligopeptide binding protein (Opts). Sequence analysis of oligopeptide-binding proteins from different bacteria prompted us to propose a classification of these proteins in three distinct groups, differentiated by the presence (or not) of precisely located extensions.
The ABC transporter family is characterized by a high affinity for their substrates, and it uses ATP hydrolysis to drive a unidirectional accumulation of solutes into the bacterial cytoplasm. A structural similarity of five proteins is generally observed (10). The substrate-binding protein specifically captures the substrate and is therefore considered one of the determinants of transport specificity (5). The binding protein is located in the periplasm of gram-negative bacteria. In gram-positive bacteria, it is present as a lipoprotein anchored to the cell membrane via an N-terminal moiety. The structure of the protein consists of three distinct domains. Two distinct lobes are connected by a flexible hinge (29), which allows two different conformations: an open, unliganded form with a high affinity for the substrate, and a closed, liganded state, in which the substrate is entrapped into the binding protein (the so-called Venus flytrap model). The closed liganded state is characterized by a low affinity for the substrate. The closed liganded binding protein delivers the substrate to the translocon, which consists of two integral transmembrane proteins and two ATP-binding proteins located on the cytoplasmic side of the membrane. According to a recent model (38), a consequence of the substrate binding would be the transmission of a signal to the ATP subunits of the ABC system. This signal results in an increased affinity of the ATP-binding proteins for ATP, which in turn leads to the opening of the transmembrane channel and the simultaneous release of the solute from the binding protein to the opened pore (6).
In gram-positive bacteria, uptake of peptides by ABC transport systems is involved in nitrogen nutrition of the organism, as has been well established for lactic acid bacteria, for instance (11, 22), or in the regulation of several cellular processes, as has been shown for Bacillus subtilis, Enterococcus faecalis, and several pathogenic bacteria (23, 24). In Lactococcus lactis, by far the most studied lactic acid bacterium, oligopeptide transport is reported to be accomplished by only one ABC transporter, the OppLl system, despite the presence of several copies of such transporters on the chromosome (4, 22). OppLl presents several unique qualities, compared to other oligopeptide transport systems. For instance, it possesses only one binding protein (OppA), whereas the transport systems of Streptococcus thermophilus (Ami) and Lactobacillus bulgaricus are composed of three and two oligopeptide-binding proteins (OBPs), respectively (11, 27). OppA is able to bind peptides with up to 35 amino acid (aa) residues, which up to now was considered a unique feature of OBPs (8). Due to the length of liganded peptides, only the six N-terminal residues of the peptides are entrapped in the binding pocket of OppA, whereas the remainder of the peptide interacts with the surface of the binding protein in a nonopportunistic manner. Nevertheless, a recent study has demonstrated that the binding protein OppA is not the sole determinant of the specificity of peptide transport in L. lactis. Part of the specificity of peptide transport by OppLl is presumably imposed by the translocon itself (5). As a result, OppALl is capable to bind peptides that are not subsequently transported by the translocon (5, 18). Moreover, replacement of the binding protein OppA of L. lactis MG1363 by other lactococcal OppA proteins did not affect the pattern of peptide transport by the strain, suggesting quite a good conservation of OppLl specificity within the lactococcal genus, despite the variability of the amino acid sequences of OppALl binding protein (5).
In the present work, we demonstrate that in all L. lactis strains under study (except in MG1363), a second oligopeptide transport system is expressed and functional. This so-called Opt system differs from the well-known OppLl system in its genetic organization and in the number of OBPs synthesized. Analyses of peptide utilization and transport by Opp-deficient strains allowed us also to provide evidence for differences in peptide transport specificities between the Opp and Opt systems. Differences in specificities between both transporters and differences in expression of peptide transport systems between strains are very likely responsible for the previously observed variability in peptide uptake within the genus Lactococcus (5).
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The strains and plasmids used in this study are described in Table 1. Escherichia coli strains were grown at 37°C with aeration in Luria-Bertani medium. L. lactis strains were cultivated at 30°C without shaking in M17Lac medium (36), chemically defined medium (CDM) (28) supplemented with 0.5% (wt/vol) glucose and 0.25% (wt/vol) lactose, or reconstituted skim milk. In some cases, an essential amino acid (methionine, valine, histidine, glutamine, leucine, or isoleucine) was omitted from the CDM and replaced with a peptide containing the omitted amino acid at a final concentration of 0.08, 0.28, 0.03, 0.27, 0.36, or 0.16 mmol/liter, respectively. If necessary, erythromycin (2.5 mg/liter) or chloramphenicol (2.5 mg/liter) was added to the culture medium.
TABLE 1.
Strains and plasmids used in the present study
| Strain or plasmid | Characteristics and/or genotype | Source or reference |
|---|---|---|
| Strains | ||
| E. coli K-12 | ||
| NM522 | supE thi-1 Δ(lac-proAB) Δ(mcrB-hsdSM)5 (rK−mK−)/F′ proA proB lacIq Δ(lacZ)M15 | Amersham Biosciences |
| ML8166 | NM522/pQWTA | This work |
| L. lactis | ||
| SK11 | Wild type; Lac+ PrtP+ Opp+ Opt+ | 9 |
| SKM6 | SK11 derivative; Lac− PrtP− Opp− Opt+ | This work |
| Wg2 | Wild type; Lac+ PrtP+ Opp+ Opt+ | NIZOa |
| MG1363 | Plasmid-free derivative of NCDO 712; Lac− PrtP− Opp+ Opt− | 12 |
| IL1403 | Plasmid-free derivative of IL594; Lac− PrtP− Opp− Opt+ | 7 |
| MLA | IL-1403 derivative with integration of pRMA in the optA gene; Lac− PrtP− Opp− OptA−, Opt−, Eryr | This work |
| MLS | IL-1403 derivative with integration of pRMS in the optS gene; Lac− PrtP− Opp− OptS− Opt+ Eryr | This work |
| MLA521 | MLA/pNZ521; Lac− PrtP+ Opp− OptA− Opt− Eryr Cmr | This work |
| MLS521 | MLS/pNZ521; Lac− PrtP+ Opp− OptS− Opt+ Eryr Cmr | This work |
| ILM521 | IL-1403/pNZ521; Lac− PrtP+ Opp− Opt+ Cmr | This work |
| Plasmids | ||
| pQE30 | Expression vector of His6-tagged proteins; Ampr | QIAGEN S.A. |
| pQWTA | pQE30 derivative carrying the optA gene from L. lactis Wg2; OptA+ Ampr | This work |
| pNZ521 | pNZ122 derivative (pSH71 replicon) carrying the prtM and prtP genes from L. lactis SK11; PrtP+ Cmr | 9 |
| pRV300 | pBluescript SK(−) derivative; Ampr Eryr | 25 |
| pRMA | pRV300 derivative carrying a 500-bp PCR fragment of optA from L. lactis IL-1403; Eryr | This work |
| pRMS | pRV300 derivative carrying a 500-bp PCR fragment of optS from L. lactis IL-1403, Eryr | This work |
NIZO, Netherland Dairy Research Institute (Ede, The Netherlands).
Bacterial growth was estimated by measuring optical density at 600 nm (OD600) after treatment of 1-ml cultures with 3.5 ml EDTA (10 g/liter; pH 12.0) in the case of milk samples.
Peptide transport.
Peptide transport assays were performed at 22°C as previously described (5). Briefly, cells were grown in CDM (OD650 = 0.8), collected by centrifugation and washed twice in 50 mM KH2PO4-K2HPO4 (pH 7.5), and the intracellular pool of amino acids was exhausted by incubating cells in the presence of 1 mM 2-deoxyglucose (37). Prior to transport experiment, cells were energized with 25 mM glucose. Energized cells (OD650 = 1.0) were incubated at 22°C in the presence of 50 μmol of peptide/liter, unless otherwise stated. Peptide transport was determined by measuring the internal accumulation of the constitutive amino acids of the peptide under study. Amino acids were detected by reversed-phase high-performance liquid chromatography after precolumn derivatization with o-phthalaldehyde, as previously described (5).
Preparation of IL-1403 electrocompetent cells and electroporation conditions.
L. lactis IL-1403 was grown overnight in M17Lac supplemented with 0.5% (wt/vol) glucose, 0.17 mol of saccharose/liter, and 1% (wt/vol) glycine. Cells were collected (6,000 × g; 10 min at 4°C), washed twice, and finally resuspended in 0.5 M saccharose supplemented with 10% (vol/vol) glycerol. Cells were immediately used for electroporation as previously described (40), except that the electric conditions were 2.0 kV, 200 Ω, and 25 μF.
Cell surface proteinase activity and β-casein hydrolysis.
M17Lac-grown bacterial cells (OD600 = 1.0) were collected, washed, and resuspended in 100 mM HEPES (pH 6.5) and 10 mM CaCl2 to a final OD600 of approximately 7.0. A 5.5 μμ solution of dephosphorylated β-casein (Sigma) was hydrolyzed by cellular suspension as previously described (13). Hydrolysis products were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 15% polyacrylamide gels and stained with Coomassie brillant blue R250 (Prolabo, Fontenay-sous-Bois, France) as previously described (14).
RNA purification and RT-PCR.
RNA was purified from 1.25 ml cultures in CDM. Bacterial cells were harvested (OD600 = 0.8) and treated with the RNeasy Mini kit (QIAGEN) and DNase I (QIAGEN) as previously described (3). cDNAs were obtained with 0.1 μg of purified RNA with the QIAGEN OneStep RT-PCR kit according to the manufacturer's procedure. The cDNA amplification of the optA-optB region (2.5 kb) was obtained with the primers optAsens2 (5′-CGCGGATCCAGCGGTTCTAGCTCATCGTCA-3′) and optBrev1 (5′-GGCTGCAGCTAGAGACGAACTCGTGG-3′). The optS amplicon (1.6 kb) was generated with the primers optSS1 (5′-GTGGCATGCGGCACAAAAACAAGTGAGCAC-3′) and optSrev1 (5′-AACTGCAGTTATTTAACATAAGCCGATTTTAGG-3′). Before reverse transcriptase PCR (RT-PCR) assays, all primers were controlled by direct PCR with a ready-to-use PCR mixture (Fermentas) and the genomic DNAs from the different lactococcal strains. In RT-PCR assays, the DNA contaminations were assessed by adding the enzyme mixture at the second step of the protocol (95°C for 15 min), which corresponds to inactivation of the RT and activation of the hot-start polymerase.
Genomic DNA extraction and Southern blotting.
Genomic DNA was purified according to a rapid procedure as described previously (5). Then, the purified DNA was digested with HaeII, HindIII, AccI, or ClaI. The resulting DNA fragments were separated by electrophoresis on 0.8% (wt/vol) agarose gels and transferred onto a nylon membrane (Positive membrane; Q-BIOgene) as described by Southern (33). The 555-bp HaeII-BlgII fragment obtained from the plasmid pAML1 harboring the oppA gene from L. lactis Wg2 was used as a probe. The ECL direct nucleic acid labeling system (Amersham Biosciences Europe) was used to label the probe with horseradish peroxidase. Specific hybridizations were detected by chemiluminescence with the ECL detection system.
Construction of mutants disrupted in optA or optS.
The genes optA and optS were inactivated in strain IL-1403 by single-crossover homologous integration with plasmid pRV300 (25). Internal fragments of optS and optA genes were amplified by PCR with the DyNazyme polymerase (Finnzymes) and primer pairs optSmut1 (5′-GAATTCCAGTCACATTGGCACAAC-3′) and optSmut2 (5′-AAGCTTGTCGCAACGGTCGAAC-3′) and optAmut1 (5′-GAATTCCAGCTAACTCTGTTTATTACCCAC-3′) and optAmut2 (5′-AAGCTTGGTTGTGCTGCATACTTAGC-3′), respectively. Internal optS and optA PCR products were restricted with EcoRI and HindIII and cloned into the plasmid pRV300 treated with the same restriction enzymes. The resulting plasmids (pRMS and pRMA, respectively) were transferred into E. coli NM522, and the structure was confirmed by restriction digestion. Then, the two recombinant plasmids were independently transferred into L. lactis IL-1403 by electroporation. Transformants were selected on M17Lac agar medium supplemented with 0.5% (wt/vol) glucose and 1 mg of erythromycin/liter and controlled by Southern blotting for the single-crossover homologous integration of pRMS or pRMA in the optS or optA gene, respectively. In addition, the absence of OptA biosynthesis was checked by Western blotting.
Purification of L. lactis Wg2 OptA-His6 recombinant protein and preparation of rabbit anti-OptA polyclonal serum.
The optA open reading frame was amplified by PCR with the primers optAsens2 (5′-CGCGGATCCAGCGGTTCTAGCTCATCGTCA-3′) and optArev1 (5′-CCCAAGCTTGAGGTAAGCTGTTTTGAAGTCAT-3′). The PCR product was restricted with BamHI and HindIII and cloned into the Qiaexpress pQE30 vector (QIAGEN). The resulting plasmid, pQWTA, was introduced into E. coli NM522, leading to strain ML8166. Expression of the OptA-His6 recombinant protein in E. coli and the purification procedure were similar to the methods described previously for the OppA-His6 recombinant protein (5). This OptA-His6 purified protein was used to raise anti-OptA polyclonal antibodies in rabbit (Valbex-University Lyon I, Villeurbanne, France).
Western blot analysis of OppA and OptA.
Lactococcal cultures were carried out with CDM supplemented with 0.5% (wt/vol) glucose at an OD600 value of up to 0.8. Bacterial proteins were extracted by boiling and separated by electrophoresis with an sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis gel. Then, proteins were electrotransferred overnight (0.2 mA/cm2) onto reinforced cellulose nitrate membranes (Schleicher & Schuell). Immunodetection of the OptA protein was performed by the method of Harlow and Lane (17) with anti-OptA (or anti-OppA) polyclonal antibodies (1:4,000) and anti-rabbit immunoglobulin G-peroxidase conjugate (1:4,000; Sigma-Aldrich). Revelation was done with the BM chemiluminescence Western blotting substrate of Roche Molecular Biochemicals.
Nucleotide sequence accession number.
The nucleotide sequence reported in this paper has been submitted to GenBank with accession number AY445663.
RESULTS
Oligopeptide uptake. (i) In L. lactis SK11, oligopeptide uptake is not exclusively taken in charge by Opp.
Six spontaneous variants of L. lactis SK11 which were unable to develop in milk supplemented with glucose were obtained by successive cultures in CDM. One of the variants, designated SKM6, did not express OppA as indicated by immunoblot analyses. Southern hybridization with a total DNA preparation from SKM6 indicated that the plasmid oppA gene was not integrated in the chromosome (data not shown). As SKM6 was deprived of lactose-fermenting capacity, it did presumably lose the plasmid pSK11L harboring the lac and opp clusters (41). Besides, SKM6 was also devoid of cell surface proteinase activity (PrtP), as assessed by β-casein hydrolysis capability of the strain. This PrtP− phenotype probably reflected the loss of the plasmid pSK111 (41).
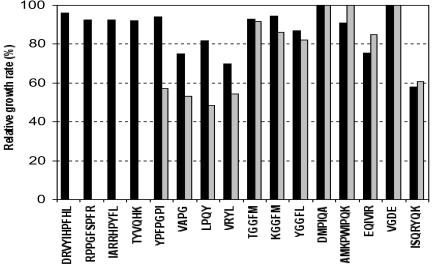
The sixteen peptides under study were able to support the growth of the strain SK11 in CDM (Fig. 1). Surprisingly, the Opp− variant SKM6 still used twelve peptides (YPFPGPI, VAPG, LPQY, VRYL, TGGFM, KGGFM, YGGFL, DMPIQA, AMKPWIQPK, EQIVIR, VGDE, and ISQRYQK) as a source of essential amino acid. Actually, only four peptides (DRVYIHPFHL, RPPGFSPFR, IARRHPYFL, and TYVQHK) were unable to sustain the growth of SKM6. It therefore suggests that 12 of these 16 peptides are (at least partly) taken in charge by an oligopeptide transport system other than the Opp system. This suggestion is reinforced by peptide transport experiments. The lack of growth of SKM6 in CDM containing DRVYIHPFHL, RPPGFSPFR, IARRHPYFL, or TYVQHK was due to the inability of the mutant to transport these peptides, as was exemplified with DRVYIHPFHL (Table 2). These four peptides are therefore specifically internalized via the Opp transporter. On the other hand, the 12 other peptides were effectively transported by the Opp− variant SKM6. It is worth mentioning that SKM6 exhibited a PrtP− phenotype, and no hydrolysis of the peptides prior to transport by SKM6 could be detected. This again supports the hypothesis of the involvement of a second functional oligopeptide transporter. Nevertheless, the transport rates of several peptides (VAPG, TGGFM, DMPIQA, and VGDE) in SKM6 were significantly reduced (Table 2), suggesting an overlapping specificity between this unknown, but functional, oligopeptide transport system and the well-known Opp system.
FIG. 1.
Growth of L. lactis SK11 and its Opp− derivative SKM6 in the presence of peptide as a source of essential amino acids. Growth rate was compared to that obtained in CDM containing all the amino acids in free form.
TABLE 2.
Initial rate of peptide transport by L. lactis SK11 and its Opp− derivative SKM6a
| Peptide | Rate of peptide uptake (nmol/min/mg of protein)
|
|
|---|---|---|
| SK11 | SKM6 | |
| DRVYIHPFHL | 7 | 0 |
| VAPG | 100 | 44 |
| TGGFM | 31 | 8 |
| DMPIQA | 40 | 27 |
| VGDE | 120 | 5 |
Peptide concentration used for transport assays was 50 μM, except for VGDE (300 μM). The amino acids used for monitoring the transport of DRVYIHPFHL, VAPG, TGGFM, DMPIQA, and VGDE were His, Val, Met, Met, and Val, respectively. Each value is the mean of two assays.
(ii) Opt, a second oligopeptide ABC transporter expressed in some Lactococcus strains.
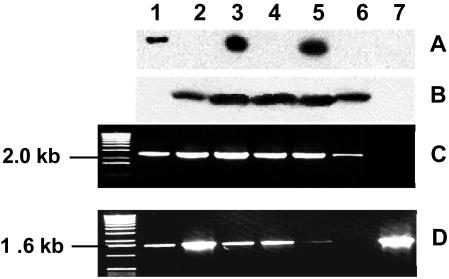
The hypothesis of a functional lactococcal oligopeptide transporter other than Opp is reinforced by analyzing in detail the peptide transport characteristics of strain IL-1403. Surprisingly, this strain did not synthesize OppA, whatever the culture medium used (M17 plus glucose or CDM plus glucose supplemented with amino acids, tryptone, or peptides) or the growth phase (exponential, early or late stationary) (Fig. 2). Under all these culture conditions, no cDNA amplification of the oppA, oppB, or oppC gene was obtained by RT-PCR (data not shown). Thus, the opp operon of IL-1403 was not transcribed. This could have been due to the opp promoter region, which is very different from those of L. lactis SSL135 or SK11, which both display −35 (TTGCAA) and −10 (TATACT) conserved boxes not found in strain IL-1403. Nevertheless, IL-1403 is able to transport peptides, and its peptide transport specificity is different from that of other L. lactis strains (5). Consequently, strain IL-1403 has to synthesize a functional oligopeptide transporter other than Opp.
FIG. 2.
Variability of oligopeptide transport system expression by L. lactis. Immunodetection of OBP proteins OppA (A) or OptA (B) or cDNA amplification of the region optA-optB (C) or optS (D) in L. lactis MG1363 (lane 1), IL-1403 (lane 2), SK11 (lane 3), SKM6 (lane 4), WG2 (lane 5), MLS (lane 6), and MLA (lane 7). The molecular mass marker used in cDNA fragment electrophoresis was the 1-kb DNA ladder (Invitrogen).
Genome sequence analysis of L. lactis IL-1403 demonstrated the presence of the optABCDF operon (4), coding for five proteins which display significant identities with the Opp proteins. A gene encoding a putative peptide binding protein, OptS, is located upstream of the opt operon. Transcription of the optS gene and the optABCDF operon in five lactococcal strains (IL-1403, MG1363, Wg2, SK11, and SKM6) was analyzed by RT-PCR. In all strains, the transcription was effective, as shown by cDNA amplification (Fig. 2). Moreover, Western blot analyses indicated that the binding protein OptA was synthesized by several lactococcal strains (IL-1403, Wg2, SK11, and SKM6). However, OptA was not synthesized by MG1363 (Fig. 2), due to the presence of a stop codon (TAG) at position 706 to 708 in the optA gene (sequence accession number, AY445663). Thus, the wild-type strains Wg2 and SK11 synthesize two oligopeptide ABC transporters (Opp and Opt), whereas the plasmid-free L. lactis strains under study synthesize only one oligopeptide transport system: Opp, in the case of MG1363, and Opt, in the case of IL-1403.
(iii) In silico analysis of the Opt transport system.
The opt gene order (optABCDF) is quite different from that of the opp operon (oppDFBCA). The genes optA, optB, optC, and optD are separated by short regions (78 nucleotides between optA and optB, 15 nucleotides between optB and optC, and 2 nucleotides between optC and optD). The genes optD and optF overlap by one nucleotide. Immediately upstream of the opt operon, the optS gene is transcribed in the same direction as the opt operon and encodes a second binding protein exhibiting 52.5% identity with OptA. The genes optS and optA are separated by 309 nucleotides. Both genes are preceded by potential promoter regions characterized by well-conserved −35 (TTGACA or TTGAAT, respectively) and −10 (TATAAT) boxes. These data suggest a possible independent transcription of optS and optABCDF.
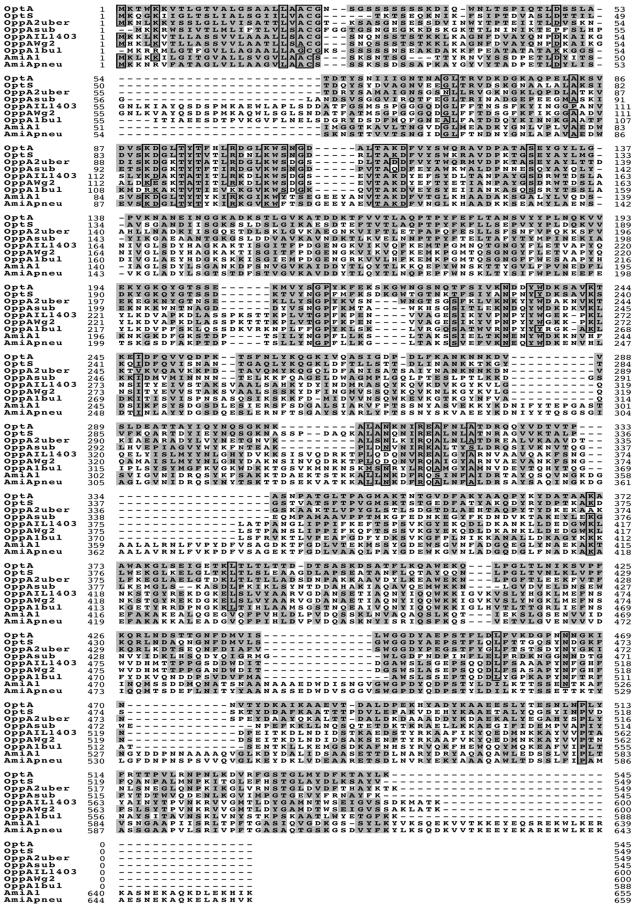
OptA and OptS are characterized by an N-terminus signal sequence typical of lipoproteins with a LXXC cleavage site. They also exhibit the extracellular peptide and nickel-binding protein family signature sequence (A X7 D X4 T X3 R X3 K) at position 83 of the OptA sequence (Fig. 3) (34). OptD and OptF display the Walker A motif, a glycine-rich loop involved in phosphoryl transfer, and the Walker B motif, exhibited by many nucleotide-binding proteins, especially ATP-binding proteins (39).
FIG.3.
Sequence comparison of OBPs from gram-positive bacteria. OptA, OptS, and OppA sequences of L. lactis IL-1403 were obtained from the corresponding sequenced genome (4). The sequences of OppA2 from S. uberis 0140J, OppA of B. subtilis 168, OppA of L. lactis Wg2, OppA1 of L. delbrueckii subsp. bulgaricus B14, AmiA1 of S. thermophilus, and AmiA of S. pneumoniae R800 were obtained from databases by their respective accession numbers (GenBank accession no. AY256913, P24141, AY189901, AAK72116, AAL68705.1, and AE007579, respectively). Identity of 75% and similarity of 85% of the amino acids are indicated by boxes and by the grey background, respectively.
Compared to other bacterial ABC peptide transporters, the most-conserved proteins of Opt are the ATP-binding proteins OptD and OptF (Table 3). The Opt proteins exhibit the highest identities with the corresponding Opp proteins from Streptococcus mutans UA159 (1). Similarly, OptA exhibits higher identities with streptococcal OBPs than with OppA proteins from Lactococcus or Lactobacillus species (4, 27): 48.5, 32.0, 30.0, 31.2, and 28.4% identities were observed with OppA2 from Streptococcus uberis 0140J, AmiA and AliB from Streptococcus pneumoniae R800, HppA from Streptococcus gordonii DL1, and AmiA1 from S. thermophilus St18, respectively (2, 11, 19, 35).
TABLE 3.
Homology between Opt of L. lactis IL-1403 and other ABC transport systems of oligopeptides from gram-positive bacteriaa
| Opt L. lactis IL-1403 (4) | % Identity of IL-1403 Opt with:
|
||||
|---|---|---|---|---|---|
| Opp L. lactis IL-1403 (4) | Opp S. mutans UA159 (1) | Opp B. subtilis 168 (30) | App B. subtilis 168 (21) | Opp L. delbrueckii supsp. bulgoricus B14 (27) | |
| OptA | 22.0 | 51.6 | 34.0 | 22.0 | 23.3 (OppA1); 20.7 (OppA2) |
| OptB | 31.7 | 65.7 | 46.4 | 26.2 | 27.3 |
| OptC | 30.8 | 60.1 | 44.6 | 33.9 | 31.8 |
| OptD | 45.4 | 73.9 | 62.5 | 49.0 | 48.6 |
| OptF | 49.4 | 74.8 | 61.0 | 51.5 | 43.5 |
| OptS | 24.5 | 44.2 | 33.4 | 20.5 | 25.5 (OppA1); 22.0 (OppA2) |
Percentages of identity between amino acid sequences were determined by the LFASTA program (26). Numbers in parentheses after Opt, Opp, and App systems are citations of references in this paper.
OptA (545 aa) and OptS (549 aa) are very short OBPs. However, compared to longer OBPs (up to 667 aa residues), both OptA and OptS are specifically characterized by a short extension of 4 residues (SKGM or DKGW) starting at position 218, following OptA numbering (Fig. 3). Compared to OptA, OptS exhibits an extra stretch of 7 aa positioned at residue 308 (following OppA sequence numbering). Besides, OppA proteins display several extensions compared to the sequences of OptS and OptA. The major extension ranges from 20 to 25 residues (at position 53, according to OptA numbering) and displays a variable sequence. S. thermophilus, S. pneumoniae, and S. gordonii display the longest OBPs, which are characterized by six conserved extensions of 5, 10, 21, 13, 12, or 41 residues, located at positions 110, 288, 334, 443, and 471 and at the C terminus, respectively (Fig. 3).
(iv) Characterization of the peptide uptake via the Opt transporter.
To unravel the respective roles of the two binding proteins of the Opt system, mutants of IL-1403 deficient for OptA or OptS protein expression were constructed. First, internal fragments of optA and optS genes were cloned in plasmid pRV300 with E. coli NM522 as a host. Second, the resulting plasmids (pRMA and pRMS, respectively) were transferred into L. lactis IL-1403. The integration of pRMA into the gene optA by homologous recombination resulted in the mutant MLA, and that of pRMS into optS yielded the mutant MLS. The integration loci were controlled by Southern hybridizations. The absence of expression of the optA gene in the mutant MLA and optS in the mutant MLS was checked by RT-PCR (data not shown).
Fifteen peptides were able to sustain the growth of the strain IL-1403 in CDM (5). The mutant MLA was unable to use any of these 15 peptides, which were not at all internalized, as shown by peptide transport analyses (Table 4). This results show that Opt is the unique transporter involved in the uptake of these oligopeptides by IL-1403. Moreover, Opt is unable to take up the peptide DRVYIHPFHL (Table 4), which confirms that this peptide is specifically internalized via the Opp system (Table 2).
TABLE 4.
Initial rate of peptide transport by L. lactis IL-1403 and its Opt mutants MLA and MLSa
| Strain or mutant | Rate of transport (nmol/min/mg of protein) with peptide
|
|||
|---|---|---|---|---|
| LPQY | RMPIQA | DGGFM | DRVYIHPFHL | |
| IL-1403 | 40 | 17 | 34 | 0 |
| MLA (OptA−) | 0 | 0 | 0 | 0 |
| MLS (OptS−) | ND | 18 | 39 | 0 |
Peptide concentration used for transport assays was 50 μM. The amino acids used to estimate the rate of uptake of LPQY, RMPIQA, DGGFM, and DRVYIHPFHL were Leu, Met, Met, and His, respectively. Each value represents the mean of two repetitions. ND, not determined.
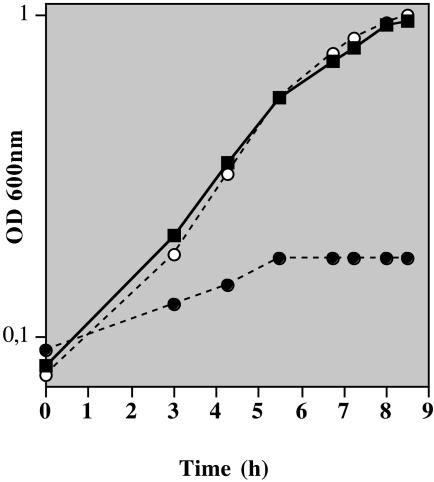
The transfer of the plasmid pNZ521 harboring the prtM and prtP genes of L. lactis SK11 (9) into IL-1403 (Lac− PrtP− Opp− Opt+) led to the biosynthesis of an active cell surface proteinase PrtP, which allowed the recombinant strain ILM521 (Lac− PrtP+ Opp− Opt+) to grow in milk supplemented with glucose (Fig. 4). In contrast, inactivation of the optABCDF operon (MLA521) prevented any bacterial growth in milk, despite the presence of PrtP. These results indicate that the Opt system is able to transport casein-derived peptides for the amino acid supply of the strain and therefore plays a nutritional role.
FIG. 4.
Growth of L. lactis ILM521 synthesizing the cell surface proteinase PrtP in milk (▪) and its opt derivatives MLA521 (OptA−; •) and MLS521 (OptS−; ○). Milk was supplemented with 0.5% (wt/vol) glucose. The assay was repeated twice.
Inactivation of the binding protein OptS had no detectable effect on (i) the pattern of peptide utilization, (ii) the rate of peptide uptake, or (iii) growth in milk (Fig. 4 and Table 4). As a consequence, OptS is not essential for the capture and the subsequent internalization of nutritional oligopeptides supporting bacterial growth in milk. Moreover, OptS does not appear to enhance the oligopeptide uptake capacity of the Opt system.
DISCUSSION
In L. lactis, extracellular oligopeptides were considered to be taken in charge via a unique ABC transporter named Opp (22). Our present work demonstrates the presence of a second functional oligopeptide ABC transporter in most L. lactis strains. This Opt transporter is characterized by a different genetic organization (optABCDF) than that of the opp gene (oppDFBCA) and by the presence of a second binding protein, OptS, encoded by a gene located immediately upstream of the operon optABCDF. The specificity of the Opt system is not the same as that of Opp, despite a large overlap between the two systems. This difference in peptide uptake specificity might result from differences in peptide-binding specificity between OppA and OptA/OptS. Nevertheless, it is also possible that the specificity of peptide uptake by the Opt system is not exclusively imposed by the binding proteins, as is the case for the Opp system (5). OppA and OptA might share similar binding specificities, whereas the specificities imposed by the two translocons OppBC and OptBC would differ. The construct of recombinant systems would probably answer this question.
The presence of multiple OBPs is a general feature of oligopeptide transporters from streptococci. For instance, S. uberis synthesizes two binding proteins, and three OBPs are recruited by the Ami transporters of S. pneumoniae or S. thermophilus and by the Hpp system of S. gordonii (2, 11, 19, 35). Moreover, a high level of identity was found between the Opt proteins from L. lactis and the corresponding oligopeptide ABC components of streptococci, suggesting a streptococcal origin of Opt. In the case of L. lactis, the benefit of the OBP-encoding gene duplication is not established. Inactivation of the OptS protein does not impair the peptide transport function of the Opt system. It therefore suggests that both binding proteins OptA and OptS act independently, instead of interacting to form a functional peptide-binding complex, as was previously suggested occurs with streptococci (2, 19). Since the OptS-defective mutant grows in milk, as does its parental strain, there may be a role other than a nutritional one (if any) for this binding protein. Moreover, the spectrum of oligopeptides that could be taken up by the Opt system is apparently not enlarged in the presence of a functional OptS protein, which calls the role of this second binding protein even more into question. Interestingly, OptS is highly identical to DppA (95% identity at the amino acid level) and is a lactococcal OBP which binds di-, tri- and tetrapeptides exclusively (31). The Dpp system transport is involved in transcriptional regulation of several components of the proteolytic system (16, 32). An exciting hypothesis would be that the Opt system is able to manage different functions via two peptide-binding proteins, i.e., a nutritional role played by OptA and a regulatory function exerted by OptS.
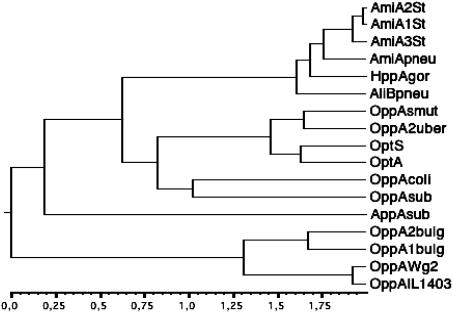
OptA and OptS exhibit the extracellular peptide and nickel-binding protein signature sequence (A X7 D X4 T X3 R X3 K), but are very different from OBPs of other lactic acid bacteria. Firstly, on the basis of their sequence length, OptA (545 aa) and OptS (549 aa) differ from OppA of L. lactis and AmiA1 of S. thermophilus St18 by 55 to 51 and 110 to 106 residues, respectively. Secondly, the phylogenetic tree revealed that OptA and OptS are more distant from OppA proteins of L. lactis and Lactobacillus delbrueckii subsp. bulgaricus than from OppA proteins of B. subtilis and E. coli (Fig. 5). Actually, OptA and OptS form a group closely related to OppA proteins from S. uberis and S. mutans.
FIG. 5.
Neighbor-joining phylogenetic tree of OBP sequences. The tree was inferred by using an expansion of CLUSTALW by Infobiogen (http://www.infobiogen.fr). Reading from top to bottom of the tree, the following sequences were obtained from databases by their respective GenBank accession numbers (in parentheses): AmiA1, AmiA2, and AmiA3 of S. thermophilus ST18 (AAL68705, AAL68706, and AAL68707), AmiA1 of S. pneumoniae R800 (AE007579), HppA of S. gordonii DL1 (AAB46614.1), AliB of S. pneumoniae R800 (Q51933), OppA of S. mutans UA159 (AAN58024.1), OppA2 from S. uberis 0140J (AY256913), OptS and OptA from the sequenced genome of L. lactis IL-1403 (4), OppA of E. coli K-12 (P23843), OppA of B. subtilis 168 (P24141), AppA of B. subtilis JH12795 (P42061), OppA2 and OppA1 of L. delbrueckii subsp. bulgaricus B14 (AAK72116), OppA of L. lactis Wg2 (AY189901), and OppA of L. lactis IL-1403 (4).
The biosynthesis of Opp and Opt varies between lactococcal strains. Actually, several combinations are possible, with oligopeptides being transported by Opp only (strain MG1363), by Opt only (IL-1403), or by both systems (SK11 and Wg2). As Opp and Opt do not share the same oligopeptide transport specificity, this variability contributes to the diversity of oligopeptide transport specificity between L. lactis strains.
The presence of two oligopeptide uptake systems is expected to confer a significant advantage to a lactococcal strain by broadening its substrate specificity and probably enhancing transport efficiency (38). Nevertheless, the presence of a unique transporter is sufficient to support the bacterial nutrition with extracellular oligopeptides. The transporter Opt can completely substitute Opp for the nutrition function and vice versa, as indicated by the growth capabilities of strains MG1363 and IL-1403. Once again, it calls the physiological meaning of the coexistence of the two oligopeptide transport systems in wild-type strains into question. In gram-positive bacteria, extracellular peptides are known to control many cellular processes such as the development of competence, sporulation, entry into stationary phase, conjugation, bacteriocin production, and virulence (15, 20, 23, 24). So, Opt and Opp transporters may differ in these nonnutritional functions.
Acknowledgments
We thank Willem de Vos for giving us plasmid pNZ521.
This work was supported by grants from C.N.R.S., I.N.R.A., and the Université Claude Bernard-Lyon I. M.L. and P.C. were the recipients of fellowships from the Ministère Français de la Recherche et de la Technologie.
REFERENCES
- 1.Ajdic, D., W. M. McShan, R. E. McLaughlin, G. Savic, J. Chang, M. B. Carson, C. Primeaux, R. Tian, S. Kenton, H. Jia, S. Lin, Y. Qian, S. Li, H. Zhu, F. Najar, H. Lai, J. White, B. A. Roe, and J. Ferretti. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. USA 99:14434-14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alloing, G., P. de Philip, and J.-P. Claverys. 1994. Three highly homologous membrane-bound lipoproteins participate in oligopeptide transport by the Ami system of the gram-positive Streptococcus pneumoniae. J. Mol. Biol. 241:44-58. [DOI] [PubMed] [Google Scholar]
- 3.Aubel, D., J. E. Germond, C. Gilbert, and D. Atlan. 2002. Isolation of the patC gene encoding the cystathionine beta-lyase of Lactobacillus delbrueckii subsp. bulgaricus and molecular analysis of inter-strain variability in enzyme biosynthesis. Microbiology 148:2029-2036. [DOI] [PubMed] [Google Scholar]
- 4.Bolotin, A., P. Wincker, S. Mauger, O. Jaillon, K. Malarme, J. Weissenbach, S. D. Ehrlich, and A. Sorokin. 2001. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 11:731-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charbonnel, P., M. Lamarque, J.-C. Piard, C. Gilbert, V. Juillard, and D. Atlan. 2003. Diversity of oligopeptide transport specificity in Lactococcus lactis species: a tool to unravel the role of OppA in uptake specificity. J. Biol. Chem. 278:14832-14840. [DOI] [PubMed] [Google Scholar]
- 6.Chen, J., S. Sharma, F. A. Quiocho, and A. L. Davidson. 2001. Trapping the transition state of an ATP-binding-cassette transporter: evidence for a concerted mechanism of maltose transport. Proc. Natl. Acad. Sci. USA 98:1525-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chopin, M.-C., A. Moillo-Batt, and A. Rouault. 1985. Plasmid-mediated UV-protection in Streptococcus lactis. FEMS Microbiol. Lett. 26:243-245. [Google Scholar]
- 8.Detmers, F. J. M., F. C. Lanfermeijer, R. Abele, R. W. Jack, R. Tampé, W. N. Konings, and B. Poolman. 2000. Combinatorial peptide libraries reveal the ligand binding mechanism of the oligopeptide receptor OppA of Lactococcus lactis. Proc. Natl. Acad. Sci. USA 97:12487-12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Vos, W. M., P. Vos, H. deHaard, and I. Boerrigter. 1989. Cloning and expression of the Lactococcus lactis subsp. cremoris SK11 gene encoding an extracellular serine proteinase. Gene 85:169-176. [DOI] [PubMed] [Google Scholar]
- 10.Driessen, A. J., B. P. Rosen, and W. N. Konings. 2000. Diversity of transport mechanisms: common structural principles. Trends Biochem. Sci. 25:397-401. [DOI] [PubMed] [Google Scholar]
- 11.Garault, P., D. Le Bars, C. Besset, and V. Monnet. 2002. Three oligopeptide-binding proteins are involved in the oligopeptide transport of Streptococcus thermophilus. J. Biol. Chem. 277:32-39. [DOI] [PubMed] [Google Scholar]
- 12.Gasson, M. J. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Germond, J.-E., M. Delley, C. Gilbert, and D. Atlan. 2003. Determination of the domain of the Lactobacillus delbrueckii subsp. bulgaricus cell surface proteinase PrtB involved in attachment to the cell wall after heterologous expression of the prtB gene in Lactococcus lactis. Appl. Environ. Microbiol. 69:3377-3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbert, C., B. Blanc, J. Frot-Coutaz, R. Portalier, and D. Atlan. 1997. Comparison of cell surface proteinase activities within the Lactobacillus genus. J. Dairy Res. 64:561-571. [Google Scholar]
- 15.Gominet, M., L. Slamti, N. Gilois, M. Rose, and D. Lereclus. 2001. Oligopeptide permease is required for expression of the Bacillus thuregensis plcR regulon and for virulence. Mol. Microbiol. 40:963-975. [DOI] [PubMed] [Google Scholar]
- 16.Guédon, E., P. Serror, S. D. Ehrlich., P. Renault, and C. Delorme. 2001. Pleiotropic transcriptional repressor CodY senses the intracellular pool of branched-chain amino acids in Lactococcus lactis. Mol. Microbiol. 40:1227-1239. [DOI] [PubMed] [Google Scholar]
- 17.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 18.Helinck, S., P. Charbonnel, J.-C. Piard, C. Foucaud, and V. Juillard. 2003. Charged casein-derived oligopeptides competitively inhibit the transport of a reporter oligopeptide by Lactococcus lactis. J. Appl. Microbiol. 94:900-907. [DOI] [PubMed] [Google Scholar]
- 19.Jenkinson, H. F., R. A. Baker, and G. W. Tannock. 1996. A binding-lipoprotein-dependent oligopeptide transport system in Streptococcus gordonii essential for uptake of hexa- and heptapeptides. J. Bacteriol. 178:68-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleerebezem, M., L. E. Quadri, O. P. Kuipers, and W. M. de Vos. 1997. Quorum sensing by peptide pheromones and two-component signal-transduction systems in gram-positive bacteria. Mol. Microbiol. 24:895-904. [DOI] [PubMed] [Google Scholar]
- 21.Koide, A., and J. A. Hoch. 1994. Identification of a second oligopeptide transport system in Bacillus subtilis and determination of its role in sporulation. Mol. Microbiol. 13:417-426. [DOI] [PubMed] [Google Scholar]
- 22.Kunji, E. R. S., A. Hagting, C. J. de Vries, V. Juillard, A. J. Haandrikman, B. Poolman, and W. N. Konings. 1995. Transport of β-casein-derived peptides by the oligopeptide transport system is a crucial step in the proteolytic pathway of Lactococcus lactis. J. Biol. Chem. 270:1569-1574. [DOI] [PubMed] [Google Scholar]
- 23.Lazazzera, B. 2001. The intracellular function of extracellular signaling peptides. Peptides 22:1519-1527. [DOI] [PubMed] [Google Scholar]
- 24.Lazazzera, B. 2000. Quorum sensing and starvation: signals for entry into stationary phase. Curr. Opin. Microbiol. 3:177-182. [DOI] [PubMed] [Google Scholar]
- 25.Leloup, L., S. D. Ehrlich, M. Zagorec, and F. Morel-Delville. 1997. Single-crossover integration in the Lactobacillus sake chromosome and insertional inactivation of the ptsI and lacL genes. Appl. Environ. Microbiol. 63:2117-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearson, W. R. 1990. Rapid and sensitive sequence comparison with FASTP and FASTA. Methods Enzymol. 183:63-98. [DOI] [PubMed] [Google Scholar]
- 27.Peltoniemi, K., E. Vesanto, and A. Palva. 2002. Genetic characterization of an oligopeptide transport system from Lactobacillus delbrueckii subsp. bulgaricus. Arch. Microbiol. 177:457-467. [DOI] [PubMed] [Google Scholar]
- 28.Poolman, B., and W. N. Konings. 1988. Relation of growth of Streptococcus lactis and Streptococcus cremoris to amino-acid transport. J. Bacteriol. 170:700-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quiocho, F. A., and P. S. Ledvina. 1996. Atomic structure and specificity of bacterial periplasmic receptors for active transport and chemotaxis: variation of common themes. Mol. Microbiol. 20:17-25. [DOI] [PubMed] [Google Scholar]
- 30.Rudner, D. Z., J. R. LeDeaux, K. Ireton, and A. D. Grossman. 1991. The spo0K locus of Bacillus subtilis is homologous to the oligopeptide permease locus and is required for sporulation and competence. J. Bacteriol. 173:1388-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanz, Y., F. Toldra, P. Renault, and B. Poolman. 2003. Specificity of the second binding protein of the peptide ABC-transporter (Dpp) of Lactococcus lactis IL1403. FEMS Microbiol. Lett. 227:33-38. [DOI] [PubMed] [Google Scholar]
- 32.Sanz, Y., F. C. Lanfermeijer, P. Renault, A. Bolotin, W. N. Konings, and B. Poolman. 2001. Genetic and functional characterization of dpp genes encoding a dipeptide transport system in Lactococcus lactis. Arch. Microbiol. 175:334-343. [DOI] [PubMed] [Google Scholar]
- 33.Southern, E. M. 1974. An improved method for transferring nucleotides from electrophoresis strips to thin layers of ion-exchange cellulose. Anal. Biochem. 62:317-318. [DOI] [PubMed] [Google Scholar]
- 34.Tam, R., and M. H. Saier, Jr. 1993. Structural, functional, and evolutionary relationships among extracellular solute-binding receptors of bacteria. Microbiol. Rev. 57:320-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor, D. L., P. N. Ward, C. D. Rapier, J. A. Leigh, and L. D. Bowler. 2003. Identification of a differentially expressed oligopeptide binding protein (OppA2) in Streptococcus uberis by representational difference analysis of cDNA. J. Bacteriol. 185:5210-5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terzaghi, B. E., and W. E. Sandine. 1975. Improved medium for lactic streptococci and their bacteriophages. Appl. Environ. Microbiol. 29:807-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson, J., and B. M. Chassy. 1983. Regulation of glycolysis and sugar phosphotransferase activities in Streptococcus lactis: growth in the presence of 2-deoxy-d-glucose. J. Bacteriol. 154:819-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van der Heide, T., and B. Poolman. 2002. ABC transporters: one, two or four extracytoplasmic substrate-binding sites? EMBO Rep. 3:938-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walker, J. E., M. Saraste, M. J. Runswick, and N. J. Gay. 1982. Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases and other ATP requiring enzymes and a common nucleotide binding fold. EMBO J. 1:945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wells, J. M., P. W. Wilson, and R. W. F. Le Page. 1993. A model system for the investigation of heterologous protein secretion pathways in Lactococcus lactis. J. Appl. Bacteriol. 74:629-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu, W., K. Gillies, J. K. Kondo, J. R. Broadbent, and L. L. McKay. 1996. Loss of plasmid-mediated oligopeptide transport system in lactococci: another reason for slow milk coagulation. Plasmid 35:145-155. [DOI] [PubMed] [Google Scholar]