Abstract
Motility responses triggered by changes in the electron transport system are collectively known as energy taxis. In Azospirillum brasilense, energy taxis was shown to be the principal form of locomotor control. In the present study, we have identified a novel chemoreceptor-like protein, named Tlp1, which serves as an energy taxis transducer. The Tlp1 protein is predicted to have an N-terminal periplasmic region and a cytoplasmic C-terminal signaling module homologous to those of other chemoreceptors. The predicted periplasmic region of Tlp1 comprises a conserved domain that is found in two types of microbial sensory receptors: chemotaxis transducers and histidine kinases. However, the function of this domain is currently unknown. We characterized the behavior of a tlp1 mutant by a series of spatial and temporal gradient assays. The tlp1 mutant is deficient in (i) chemotaxis to several rapidly oxidizable substrates, (ii) taxis to terminal electron acceptors (oxygen and nitrate), and (iii) redox taxis. Taken together, the data strongly suggest that Tlp1 mediates energy taxis in A. brasilense. Using qualitative and quantitative assays, we have also demonstrated that the tlp1 mutant is impaired in colonization of plant roots. This finding supports the hypothesis that energy taxis and therefore bacterial metabolism might be key factors in determining host specificity in Azospirillum-grass associations.
Azospirillum brasilense, a free-living diazotroph that belongs to the alpha-subdivision of proteobacteria, associates with the roots of many agriculturally important crops, including wheat, corn, and rice. Azospirilla colonize the root surface and may significantly promote plant growth and crop yield, properties that make them attractive candidates for the development of biological fertilizers for these crops (26-28). The ability of Azospirillum to attain significant populations on the root surfaces of the host is essential for its beneficial effect on plant growth and requires that the bacteria come in close contact with the roots (9, 27, 28, 35). The abilities of sensing chemicals released by the host plant and navigating toward the root system are likely to be important for the establishment of bacteria, including Azospirillum (9, 10, 42), in the rhizosphere. Experimental evidence supporting this hypothesis was obtained by demonstrating that nonchemotactic and nonmotile mutants of A. brasilense are severely impaired in surface colonization of wheat roots (41). Although there is no strict host specificity in Azospirillum-plant associations, a strain-specific chemotaxis was reported: strains isolated from the rhizosphere of a particular grass demonstrated preferential chemotaxis toward chemicals found in root exudates of that grass (31). These results suggested that chemotaxis may contribute to host-plant specificity and could largely be determined by metabolism (31).
Most motility responses in A. brasilense are a particular form of metabolism-dependent taxis called energy taxis (1). In energy taxis, a flow of reducing equivalents through an electron transport system is required for a behavioral response. Thus, energy taxis typically encompasses various (but not all) types of aerotaxis, taxis to alternative electron acceptors, phototaxis, redox taxis, and chemotaxis to oxidizable substrates (donors of reducing equivalents) (4, 38, 39).
Aerotaxis is the strongest behavioral response in A. brasilense. It guides the bacteria to an oxygen concentration optimal for energy generation and nitrogen fixation (8, 52). It has also recently been shown that a functional electron transport system is required not only for aerotaxis and taxis to alternative electron acceptors, as expected, but also for chemotaxis, suggesting that A. brasilense is attracted to most chemicals via energy taxis (1). In addition, redox molecules that interact directly with the electron transport system and inhibit the flow of reducing equivalents through the respiratory chain are repellents for A. brasilense. Therefore, the signal for major behavioral responses in A. brasilense originates within the electron transport system (1). In energy taxis, any changes in electron transport trigger a behavioral response: changes leading to a step-up in intracellular energy level produce an attractant signal, whereas a step-down in energy level causes a repellent signal (4, 38, 39). Because energy taxis couples energy metabolism and motility, this behavior may be significant in the ecology of Azospirillum spp. and other species that are not dependent on specific plant signals to form associations with host plants (2).
Motility responses in bacteria depend on the cellular signal transduction machinery that transmits information from the environment to the motility apparatus. The molecular mechanism governing bacterial chemotaxis is best understood in Escherichia coli (15, 36). Changes in concentration of various effectors are detected by specialized transmembrane chemoreceptors, also termed methyl-accepting chemotaxis proteins (MCPs) or transducers. Upon binding of a chemoeffector, chemotaxis transducers undergo conformational changes that are transmitted to the flagellar motors by a phosphoryl transfer cascade between cytoplasmic signal transduction proteins. The CheA histidine kinase, which is docked to chemoreceptors via CheW, serves as a phosphodonor for CheY. In its phosphorylated form, CheY is able to bind to the switch of the flagellar motor, an event that changes the direction of rotation of the flagellum and causes the cell to tumble (36). Two additional proteins, CheB methylesterase and CheR methyltransferase, comprise the adaptation pathway (15, 36). E. coli has five chemotaxis transducers: four transmembrane MCPs (Tsr, Tar, Trg, and Tap) (15) and one membrane-associated cytoplasmic receptor (Aer) (13, 30).
The chemotaxis operon in A. brasilense has recently been identified, and it has been shown that the operon comprises genes coding for the central excitation and adaptation chemotaxis pathway, which is homologous to that of E. coli. Mutations in the A. brasilense chemotaxis operon abolish taxis to all known stimuli and affect the pattern of motility (20), suggesting that this operon is the major regulator of motile behavior. It has also been shown that, despite their divergence in sequence, the chemotaxis proteins of A. brasilense are functional homologs of their E. coli counterparts (5).
In this study, we describe the first chemoreceptor identified in A. brasilense, Tlp1 (for transducer-like protein 1), and demonstrate that it serves as an energy taxis transducer. We also show that Tlp1 promotes colonization of plant roots by A. brasilense. This finding is significant because it provides experimental support for the hypothesis that energy taxis, and therefore bacterial metabolism, might be a key factor in determining the host specificity in Azospirillum-grass associations. To our knowledge, Tlp1 is the only chemotaxis transducer with a known function that is directly implicated in host-microbe interactions.
MATERIALS AND METHODS
Media, bacterial strains, and growth conditions.
A. brasilense Sp7 (ATCC 29145), a wild type for chemotaxis, was used throughout this study. Bacterial strains and plasmids are listed in Table 1. The A. brasilense cells were grown at 28°C in a minimal medium (MMAB) (43) supplemented with the carbon source of choice at a final concentration of 10 mM. For aerobic growth, the cells were incubated at 200 rpm on a rotary shaker. For anaerobic growth, cells were incubated in a GasPak anaerobic system (Becton Dickinson Microbiology Systems, Cockeysville, Md.). The growth medium was supplemented with the antibiotics kanamycin (30 μg/ml) and tetracycline (10 μg/ml) for A. brasilense. Ampicillin (100 μg/ml) was used with E. coli.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Properties | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | General cloning strain | Gibco-BRL |
| S17-1 | thi endA recA hsdR with RP4-2Tc::Mu-Km::Tn7 Integrated in chromosome | 34 |
| A. brasilense | ||
| Sp7 | Wild-type strain; ATCC 29145 | 37 |
| SG323 | tlp1 mutant; Kmr | This work |
| Plasmids | ||
| pSG312 | pUC18 with a 1.6-kb EcoRI/SalI fragment from pHCD12 | This work |
| pSG316 | pUC18 with a 2.9-kb EcoRI/BstBI fragment from pHCD12 | This work |
| pSG318 | pSG312 with the Kmr cassette from pHP45Ω-Km blunt inserted in the StuI site (plus direction); Kmr Apr | This work |
| pSG319 | pSUP202 with EcoRI linearized pSG318 inserted at the EcoRI site; Tcr Kmr Apr | This work |
| pHCD12 | pLAFR1 clone from genome bank of A. brasilense Sp7, containing tlp1, Tcr | This work |
| pHP45Ω-Km | Apr Kmr | 16 |
| pRK2013 | Helper plasmid, carries tra genes; Kmr | 17 |
| pSUP202 | Mobilizable plasmid, suicide vector for A. brasilense; Cmr Tcr Apr | 34 |
| pUC18 | Cloning vector; Apr | 48 |
Growth of the wild-type strain Sp7 and the tlp1 mutant (see below) was compared by following the optical density at 600 nm (OD600) over time of cultures in MMAB minimal medium containing either malate, fumarate, succinate, or fructose as the sole carbon source at two different concentrations, 5 and 10 mM (final concentration).
Identification of a new chemotaxis transducer.
Twenty-four sequences of transducer-like proteins and MCPs from the α-proteobacteria Caulobacter crescentus, Rhodobacter capsulatus, Rhodobacter sphaeroides, Rhizobium leguminosarum, Rhizobium meliloti, Agrobacterium tumefaciens, and the γ-proteobacterium E. coli were aligned with CLUSTAL_X (40). A 16-residue highly conserved domain (HCD) sequence (NLLALNAGVEAARAG) was identified in the C-terminal region, and a degenerate oligonucleotide (HCD probe) (5′-AACCTGCTGGCCCTGAACGCCGGCGTCGAGGCCGCCCGCGCCGGC) was synthesized (Sigma Genosys, The Woodlands, Tex.) and labeled with digoxigenin-11-ddUTP with the DIG Oligonucleotide 3′End labeling kit (Roche Applied Science, Indianapolis, Ind.). The A. brasilense Sp7 genomic library in the pLAFR1 cosmid (44) was screened with the HCD probe by colony hybridization according to the manufacturer's protocols (Roche Applied Science). One of the cosmids yielding a positive hybridization with the probe was named pHCD12 (Table 1). The pHCD12 cosmid was digested with several enzymes and rehybridized with the probe to identify smaller restriction fragments suitable for sequencing. A 1.6-kb EcoRI/SalI fragment and a 2.9-kb BstBI/EcoRI fragment that still hybridized with the HCD probe were subcloned into pUC18 digested with the same enzymes, resulting in plasmids pSG312 and pSG316, respectively (Table 1). Direct sequencing and primer walking from pHCD12, pSG312, and pSG316 were used in automated DNA sequencing (ABI Prism) to obtain the DNA sequence of the region encompassing the tlp1 gene and flanking regions.
Recombinant DNA techniques.
Preparation of cosmid, plasmid, and genomic DNA, transformations, restriction endonuclease digestions, DNA extraction from agarose gels, ligation reactions; PCR and Southern hybridization, and DNA transformation into E. coli were carried out by standard protocols (33) and the manufacturers' instructions. The enzymes for DNA manipulation and PCR amplification were purchased from Roche Applied Science, New England Biolabs (Beverly, Mass.) and Epicentre (Madison, Wis.). To construct a tlp1 insertion mutant, an aphII cassette (encoding Kmr and two transcription stop signals) was obtained from pHP45Ω-Km digested with BamHI (16) and polished with the Klenow fragment of DNA polymerase I (New England Biolabs). The cassette was blunt-end ligated at an StuI site within the tlp1 gene cloned into pSG312, resulting in plasmid pSG318. The pSG318 construct contains the kanamycin cassette in the same orientation of transcription as the tlp1 gene (Table 1). pSG318 was linearized by digestion with EcoRI and ligated into the pSUP202 suicide vector, also digested with EcoRI, yielding pSG319 (Table 1). The pSG319 plasmid was introduced into A. brasilense by triparental mating with the pRK2013 vector as a helper, as described previously (20). Recombinants were screened for the loss of the recombinant plasmid and for double homologous recombination by replica plating on the appropriate antibiotics (Kmr and Tets). The correct allelic replacement in putative mutants was verified by PCR and Southern hybridization, with DNA fragments from the tlp1 gene, the suicide vector, and the Kmr cassette as probes. One of the tlp1 mutants (SG323) was chosen for further characterization.
Computational DNA and protein sequence analysis.
Computational gene finding was carried out using the FramePlot program, which is designed to predict protein-coding regions in bacterial DNA with a high G+C content (21). The start codon of the tlp1 gene was verified with the GeneMarkS program (11). Similarity searches against the nonredundant database at the National Center for Biotechnology Information (Bethesda, Md.) were carried out with the BLASTP and PSI-BLAST programs (6). Domain architecture of the predicted Tlp1 protein was obtained by searching against the SMART domain database (24). Multiple-sequence alignment of the conserved N-terminal periplasmic region of Tlp1 (residues 78 to 176) was constructed with CLUSTAL_X (40). A bootstrapped neighbor-joining tree was generated from the multiple alignment with the MEGA phylogenetic package (22).
Behavioral assays.
The swarm plate and temporal gradient assays for chemotaxis and the miniplug assay for redox taxis and chemotaxis in A. brasilense were performed essentially as previously described (1). For swarm plate assays, the same number of cells of the wild type and the mutant were inoculated by using a 5-μl aliquot of cells in exponential phase adjusted to the same OD600 value. The final concentration of the chemical to be tested as a chemoeffector was 10 mM. Taxis on swarm plates under anaerobic conditions was assessed with nitrate as a terminal electron acceptor, succinate or fructose as an electron donor, and the sole carbon source in the MMAB medium (43) lacking ammonium ions. The plates were incubated under anaerobic conditions in a GasPak anaerobic system (Becton Dickinson) and were inoculated with cells previously grown anaerobically with nitrate as a terminal electron acceptor in minimal liquid medium. The final concentration of nitrate was 10 mM. The optimum concentrations of the carbon sources added as electron donors to observe a sharp chemotaxis ring in this assay were determined in preliminary experiments and corresponded to 5 mM for succinate and 10 mM for fructose.
The spatial gradient assay for aerotaxis was used as previously described, with modifications (1). Cells were washed three times and resuspended in chemotaxis buffer (10 mM phosphate buffer [pH 7.0], 1 mM EDTA). Nine microliters of cells was mixed with 1 μl of the appropriate carbon source to give a final concentration of 2.5 mM and introduced into an optically flat capillary tube. The positions of the aerotactic bands formed by the wild type and the tlp1 mutant in the capillary tube were compared by measuring the relative distance of each aerotactic band from the meniscus. The temporal gradient assay for aerotaxis was used to measure the time until adaptation to oxygen removal and was performed as described by Zhulin et al. (52). Response times were measured in triplicate in three independent experiments.
Measurement of respiration.
Respiration (oxygen consumption) in bacterial suspensions of the wild type (Sp7) and the tlp1 mutant was measured as previously described (1).
Preparation of wheat seeds and plant root colonization assays.
Seeds of wheat (Triticum aestivum cv. Jagger) were provided by R. L. Bowden (U.S. Department of Agriculture-Agricultural Research Service, Manhattan, Kans.). Seeds were surface-sterilized as described by Ramos et al. (29) and germinated by incubation in the dark for 3 days on nutrient agar plates (8 g of Bacto nutrient broth/liter and 15 g of agar/liter) at 23°C. A. brasilense cells (Sp7 and the tlp1 mutant) were harvested at mid-logarithmic-growth phase (OD600, 0.4), washed three times in sterile chemotaxis buffer, and concentrated to 108 cells/ml. The density of the cell suspensions was verified by serial dilution and plating onto MMAB plates. For each strain, 107 cells were added to 26- by 150-mm glass tubes containing 15 ml of molten Farhaeus semisoft agar (4% [wt/vol] agar) (50). After the agar had solidified, one sterile germinated seedling was aseptically transferred into each tube. The seedlings were grown at 23°C, with a photoperiod of 16 h of light and 8 h of dark. Ten days after inoculation, the seedlings were washed briefly in sterile chemotaxis buffer to remove excess agar still adhering to the roots, blotted briefly on sterile Whatman 3MM filter paper, and weighed. Equal-sized roots from five plants were individually crushed in 50 ml of sterile chemotaxis buffer with a Waring blender. Serial dilutions were plated on MMAB medium supplemented with kanamycin (25 μg/ml) and incubated for 4 days at 28°C to count colonies of the tlp1 mutant.
In situ detection of bacteria on the root surface.
To monitor the pattern of colonization of the surface of sterile wheat roots, we introduced a stable plasmid, pJBA21TC, that constitutively expresses gusA from the Paph promoter (45) from E. coli into the wild-type strain Sp7 and the tlp1 mutant of A. brasilense by triparental matings, as described previously (44). There was no difference in the growth of A. brasilense Sp7 and tlp1 cells carrying the pJBA21TC plasmid. This plasmid contains the parDE genes and was reported to be very stable under nonselective conditions (45). Similarly, we found that this plasmid was stable in A. brasilense for at least 100 generations under nonselective conditions. Sterile wheat seedlings were prepared and inoculated as described above. The β-glucuronidase activity was measured 10 days after inoculation on seedlings with equal-sized roots by the procedure described by Ramos et al. (29), except that the final concentration of 5-bromo-4-chloro-3-indoxyl-β-d-glucuronide used was 100 μg/ml. The root systems of at least five plants were visually inspected and photographed.
Statistical analysis of data.
A two-tailed t test, assuming unequal variances and with a 0.01 confidence level, was used to determine if the differences between the mutant and the wild type in the spatial gradient assay for aerotaxis and in the spatial gradient assay for chemotaxis and anaerobic nitrate taxis were statistically significant. A t test, with similar parameters, was also performed to compare the respiration rates of the wild type and the tlp1 mutant. Data obtained for these two strains in the quantitative colonization of wheat were compared by analysis of variance.
Nucleotide sequence accession number.
The nucleotide sequences determined in this study have been deposited in the GenBank database under the accession number AY584240.
RESULTS
Identification of the tlp1 gene, sequence analysis, and mutant construction.
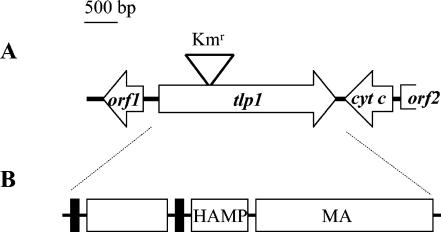
The genome of A. brasilense has not yet been sequenced. Therefore, the genomic library of A. brasilense Sp7 (20) was screened by colony hybridization with a degenerate probe to the HCD of the chemoreceptors (23, 51). This screen resulted in the identification of several candidate cosmids, including pHCD12. Sequencing and analysis of the pHCD12 insert revealed a 1,995-bp open reading frame encoding a putative chemoreceptor-like protein that we named Tlp1. Eleven base pairs upstream of the predicted start codon of the tlp1 gene was a predicted ribosomal binding site, as identified by the GeneMark program (11). A gene coding for a cytochrome c was identified immediately downstream and in the opposite orientation of tlp1. An open reading frame coding for a hypothetical protein was detected upstream and in the opposite orientation of the tlp1 gene (Fig. 1A).
FIG. 1.
Physical map of the 4,113-bp DNA region encompassing the tlp1 gene (A) and predicted domain architecture of Tlp1 (B). (A) The arrows indicate the direction of transcription. The triangle above the tlp1 gene indicates the insertion of the Kmr cassette. (B) Domains in the predicted Tlp1 protein, as defined by the SMART database (24): HAMP domain (7); MA, methyl-accepting chemotaxis-like domain (23). Black rectangles depict transmembrane regions.
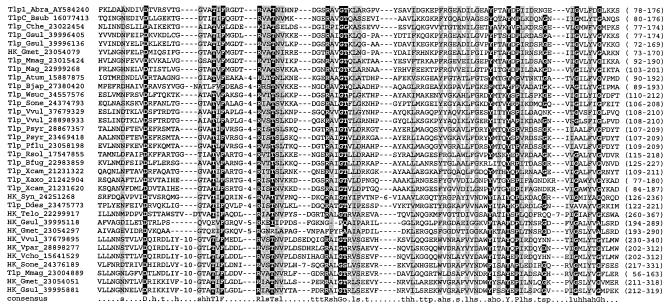
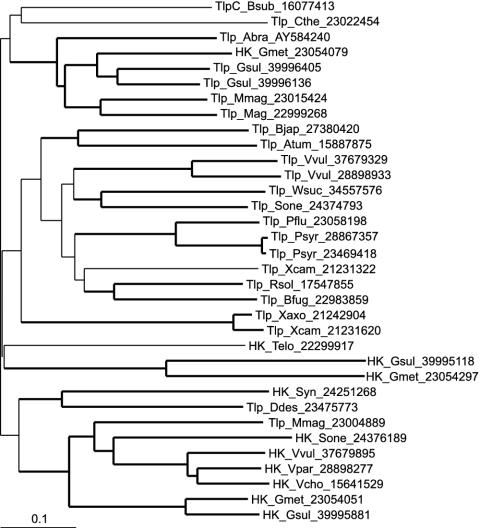
The inferred Tlp1 protein has a predicted molecular mass of 70 kDa. It has a membrane topology typical of classical transmembrane chemoreceptors (Fig. 1B). Two transmembrane regions demarcate the N-terminal periplasmic domain of Tlp1, and the C-terminal region consists of a HAMP (histidine kinases, adenylyl cyclases, methyl binding proteins, and phosphatases) domain (7) and a signaling module containing the HCD and two methylation regions typical of chemoreceptors (23). Similarity searches using the BLASTP program (6) revealed that the Tlp1 C-terminal signaling module is homologous to those of chemoreceptors from closely related α-proteobacteria, namely Magnetospirillum magnetotacticum, Rhodospirillum rubrum, Rhodopseudomonas palustris, and Bradyrhizobium japonicum (data not shown). PSI-BLAST searches with the N-terminal periplasmic region of Tlp1 followed by multiple alignment of related sequences showed that it comprises a novel domain of unknown function (Fig. 2). This domain was found exclusively in the extracellular regions of two classes of receptor proteins from various distantly related bacterial species: chemotaxis transducers and sensor histidine kinases (Fig. 3).
FIG. 2.
A protein domain family exemplified by the N-terminal periplasmic region of Tlp1 from A. brasilense. Multiple alignment of homologous amino acid sequences was constructed with the CLUSTAL_X program (40). The start and end positions (domain boundaries) are shown to the right of each sequence. A consensus for multiple alignment (85% threshold) determined by using the CONSENSUS script (www.bork.embl-heidelberg.de/Alignment/consensus.html) is shown at the bottom. Identical residues are highlighted in black, and chemically similar residues are highlighted in gray. Each sequence in the alignment is identified by its GenBank protein identification number (except for the Tlp1 protein, for which the GenBank accession number of the corresponding DNA region is given) and by the abbreviated name of the organism. Abbreviations: HK, histidine kinase; Abra, A. brasilense; Atum, A. tumefaciens; Bjap, B. japonicum; Bsub, B. subtilis; Cthe, Clostridium thermocellum; Ddes, Desulfovibrio desulfuricans; Gmet, G. metallireducens; Gsul, Geobacter sulfurreducens; Mmag, M. magnetotacticum, Mag, Magnetococcus sp.; Pflu, P. fluorescens; Psyr, Pseudomonas syringae; Rsol, Ralstonia solanacearum; Bfug, Burkholderia fungorum; Sone, Shewanella oneidensis; Syn, Synechococcus; Telo, Thermosynechococcus elongatus; Vcho, Vibrio cholerae; Vpar, Vibrio parahaemolyticus; Vvul, Vibrio vulnificus; Wsuc, Wolinella succinogenes; Xaxo, Xanthomonas axonopodis; Xcam, Xanthomonas campestris.
FIG. 3.
Neighbor-joining tree showing the phylogenetic relationships within the protein domain family exemplified by the N-terminal periplasmic region of Tlp1 from A. brasilense. The tree was constructed from the multiple alignment shown in Fig. 2 with the MEGA phylogenetic package (22). Thick lines mark branches with significant (≥60%) bootstrap support (1,000 replicates). GenBank accession numbers and species abbreviations are the same as defined in the legend to Fig. 2.
Computational analysis did not suggest any specific sensory function for Tlp1. There are no known conserved motifs for binding prosthetic groups. Interestingly, there is a conserved set of aromatic amino acids (Phe102, Tyr152, and Tyr156) (Fig. 2) that is usually present in the contact sites where two proteins interact (25).
We constructed a mutant defective in the tlp1 gene (SG323) and characterized its motile behavior by a variety of qualitative and quantitative assays. The average swimming speed and reversal frequency of the mutant were essentially the same as that of the wild type. Growth of the mutant in rich and minimal media containing various chemicals as a sole carbon source (see Material and Methods) was indistinguishable from that of the wild type.
The tlp1 mutant is impaired in chemotaxis to rapidly oxidizable substrates.
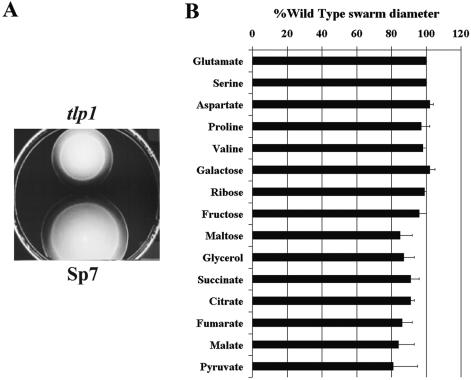
Chemotaxis to various carbon sources known to be attractants for A. brasilense was tested on swarm plates (1). Selected results are shown in Fig. 4. We found that chemotaxis to amino acids and certain sugars (galactose and ribose) was indistinguishable in the mutant and the wild type. However, the mutant was significantly impaired in chemotaxis to organic acids, glycerol, and maltose. The results of the swarm plate assay also indicated that the tlp1 mutant might be impaired in chemotaxis to fructose, but the difference was not statistically significant.
FIG. 4.
The tlp1 mutant is deficient in chemotaxis. Chemotaxis of the A. brasilense wild-type strain (Sp7) and the tlp1 mutant was compared by the swarm plate assay. Swarm diameters were measured after incubation at 28°C for 48 h. (A) Representative swarm plate with fumarate as the sole carbon source. (B) The average swarm diameters are expressed as the percentage relative to that of wild-type strain (defined as 100%). Error bars represent standard deviations from the mean calculated from at least six repetitions. Differences in the swarming diameters of the wild type and the tlp1 mutant were found to be statistically significant with the following chemoeffectors: maltose, glycerol, succinate, citrate, fumarate, malate, and pyruvate.
In swarm plates, bacteria metabolize the carbon source and move along its gradient. Therefore, in this assay, the size of the chemotactic ring is affected by both chemotaxis and metabolism. We have shown that there was no difference in growth between the mutant and wild-type cells on organic acids, sugars, and glycerol. Therefore, we infer that the diminished diameters of the chemotactic rings in these experiments are due to a defect in chemotaxis.
It is important to stress that chemotaxis was not abolished in the tlp1 mutant and that the mutant only displayed a reduced ability to move along gradients of oxidizable substrates (Fig. 4). The difference in the behavior of the wild type and the tlp1 mutant was most significant in the presence of substrates that were previously identified as the strongest chemoattractants for A. brasilense, such as sugars and organic acids (1). We have confirmed the chemotaxis phenotype by two additional behavioral assays: a miniplug method and a quantitative temporal gradient assay (Table 2). Cell metabolism is not required for the formation of the gradient of the chemoeffector tested in either assay. We found that the tlp1 mutant was significantly impaired in taxis to chemicals that are strong attractants for A. brasilense, whereas no significant difference could be detected in the response of the wild type and the tlp1 mutant to weaker chemoeffectors, such as amino acids. Data from both the temporal gradient assay and the miniplug method also suggested that the tlp1 mutant was deficient in chemotaxis to fructose, a strong attractant for A. brasilense. Altogether, these results suggest that the tlp1 mutant is deficient in chemotaxis to rapidly oxidizable substrates that have been previously shown to elicit energy taxis in A. brasilense (1).
TABLE 2.
Chemotaxis of A. brasilense wild type and tlp1 mutant in spatial (miniplug) and temporal gradient assays
| Attractanta | Threshold in miniplug assay (μM)
|
Mean response time ± SD temporal assayb
|
||
|---|---|---|---|---|
| Sp7 | tlp1 | Sp7 | tlp1 | |
| Fructose | 0.1 | 1 | 72 ± 5 | 66 ± 5 |
| Fumarate | 1 | 10 | 78 ± 4 | 43 ± 3 |
| Malate | 1 | 100 | 71 ± 10 | 46 ± 7 |
| Succinate | 1 | 100 | 67 ± 8 | 47 ± 5 |
| Glycerol | 10 | 100 | 37 ± 7 | 32 ± 5 |
| Aspartate | 100 | 100 | 28 ± 7 | 21 ± 6 |
Cells were grown with the chemical to be tested as an attractant and prepared as described in Materials and Methods.
The chemicals were tested at a final concentration of 10 mM. Values are in seconds.
The tlp1 mutant is impaired in taxis to electron acceptors.
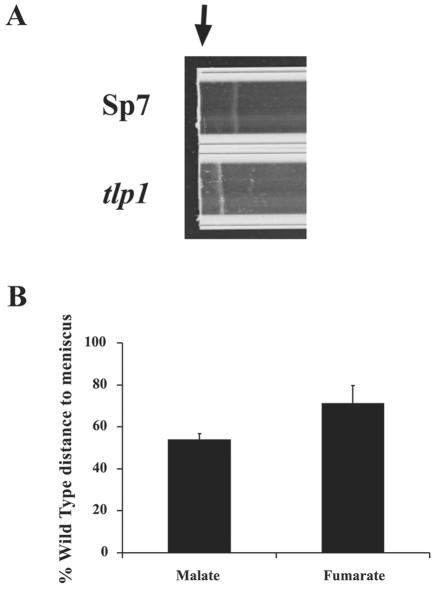
Aerotaxis is the strongest motility response in A. brasilense (8, 52) and is part of the overall energy taxis in this organism (1). We measured aerotaxis in the tlp1 mutant and the wild type by the capillary assay described previously (1, 52). Cells in a minimal medium placed in an optically flat capillary tube form an aerotactic band at a preferred oxygen concentration within 0.5 to 5 min. In aerotaxis, cells respond to changes in the rate of electron transport between the electron donor (substrate) and electron acceptor (oxygen) (1, 39, 52). Because the substrate in the capillary is distributed uniformly in the cell suspension, the behavior observed in this assay is in response to the oxygen gradient formed by oxygen diffusion into the capillary tube and oxygen consumption by respiring cells. The tlp1 mutant cells were capable of aerotaxis, i.e., they formed a sharp aerotactic band similar to that of the wild type; however, the band formed by the mutant was always located closer to the meniscus relative to the wild type, i.e., it formed at a higher oxygen concentration (Fig. 5A). This behavior was consistent regardless of the electron donor used (Fig. 5B). Furthermore, we measured oxygen consumption (respiration) by the mutant and wild type. We found no difference in the respiration rates of the wild type and the tlp1 mutant with malate, fumarate, or fructose as a substrate (data not shown), whereas the differences in the position of the aerotactic band were statistically significant.
FIG. 5.
The tlp1 mutant is altered for aerotaxis. The ability of the A. brasilense wild-type strain (Sp7) and the tlp1 mutant to form a sharp aerotactic band in a gradient of oxygen formed in a capillary tube was compared. (A) Representative capillary assay for aerotaxis showing the position of the aerotactic band in the gradient for the wild-type strain (Sp7) and the tlp1 mutant. The arrow indicates the position of the meniscus at the air-liquid interface. Fumarate (2.5 mM) was added as the sole carbon and energy source to the suspension of motile cells. Magnification, ×35. (B) The average distances of the aerotactic band to the meniscus expressed as a percentage of the distance for the wild-type strain (defined as 100%). Malate or fumarate was added at 2.5 mM as the sole substrate to the suspension of motile cells, and the distance of the aerotactic band to the meniscus was measured. Error bars represent standard deviations from the mean calculated from three independent experiments. Similar results were obtained with succinate as the sole carbon source.
We confirmed that the tlp1 mutant was impaired in aerotaxis by measuring the response time of cells to the removal of oxygen in a temporal gradient assay. The wild-type cells adapted to the repellent effect of oxygen removal in 35 ± 2 s, whereas cells of the tlp1 mutant responded in 24 ± 2 s (five independent experiments).
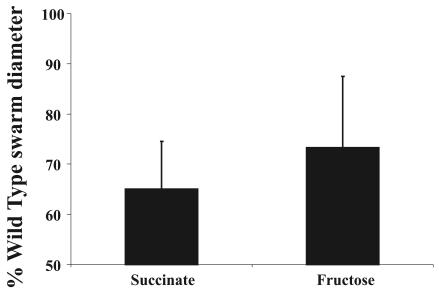
To determine whether the difference in the behavior of the wild type and the tlp1 mutant is limited to oxygen sensing or is representative of overall energy taxis, we compared tactic responses under anaerobic conditions in the presence of NO3− as an alternative electron acceptor. We found that the tlp1 mutant cells had a reduced response in a spatial gradient assay, regardless of the electron donor used in this experiment (Fig. 6).
FIG. 6.
The tlp1 mutant shows altered energy taxis under anaerobic conditions. Tactic responses of the A. brasilense wild-type strain (Sp7) and the tlp1 mutant were measured in the presence of nitrate as electron acceptor and succinate or fructose as electron donor. Plates were incubated anaerobically. Control plates containing no electron acceptor or no electron donor did not support growth. The average swarm diameters are expressed as a percentage of the wild-type swarm diameter (defined as 100%). Error bars represent standard deviation from the mean calculated from three independent experiments. The differences in the swarm diameters of the wild type and the tlp1 mutant were statistically significant.
The tlp1 mutant is less sensitive to the repellent effect of a substituted quinone.
Substituted quinones, such as 1,4-benzoquinone, are redox-active chemicals that trigger a repellent response for A. brasilense by interfering with electron transport through the respiratory chain (1, 19). We compared the wild type (Sp7) and tlp1 mutant responses to 10 μM 1,4-benzoquinone by two methods: a miniplug assay and a temporal gradient assay. In both assays, the tlp1 mutant showed a significantly reduced response to the redox repellent. The threshold in the miniplug assay was 100 μM for Sp7 and 1,000 μM for the tlp1 mutant. The response time in the temporal gradient assay was 93 ± 15 s for Sp7 and 55 ± 6 s for the tlp1 mutant (means ± standard deviations). A higher concentration of repellent was required to elicit the response from the mutant in a spatial gradient assay, and the mutant was also less sensitive (i.e., had a shorter response time) than the wild type in a temporal gradient assay. Because the tlp1 mutant was impaired in all behavioral responses that are known to constitute energy taxis in A. brasilense, we conclude that Tlp1 is an energy taxis transducer.
The tlp1 mutant is impaired in colonization of plant roots.
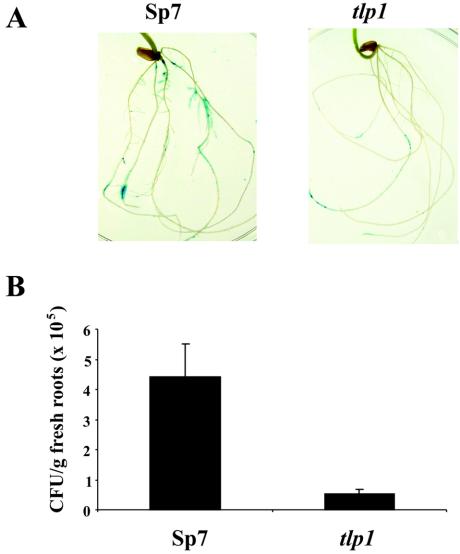
The construction and characterization of a mutant of A. brasilense lacking an energy taxis transducer and significantly impaired in energy taxis provided us with the unique opportunity to investigate the role of this behavior in colonization of plant roots. We employed a qualitative (46) and a quantitative (3) assay to compare the ability of the tlp1 mutant and the wild type to colonize the surface of sterile wheat roots. In the first approach, the spatial pattern of root colonization was visualized by detecting β-glucuronidase activity constitutively expressed from a stable plasmid, pJBA21TC (45), introduced in both strains. The wild type and the mutant cells were inoculated individually under sterile conditions at a final concentration of approximately 107 cells/ml to 10 different sterile wheat plants, and the plants were incubated for 10 days. Figure 7A shows the dramatic difference in the efficiency of root surface colonization between the mutant and the wild type.
FIG. 7.
The tlp1 mutant is impaired in colonization of wheat root surfaces. (A) The pattern of root surface colonization was visualized on wheat roots 10 days after inoculation with similar levels of the A. brasilense Sp 7 wild type and the tlp1 mutant. Each strain harbored plasmid pJBA21TC, which constitutively expresses β-glucuronidase, with activity indicated by the blue color. (B) Colonization levels of wheat roots by A. brasilense wild-type strain Sp7 and the tlp1 mutant 10 days after inoculation with similar numbers of cells. The error bars represent the standard deviations about the mean calculated for five plants. Similar results were obtained in three independent experiments.
We confirmed this difference in a quantitative assay by determining the number of wild-type and tlp1 cells colonizing the root surface of wheat plants 10 days after inoculation under conditions similar to those used for the qualitative assay (Fig. 7B). In agreement with the results from the qualitative assay, we found that the number of tlp1 mutant cells recovered from the wheat root surface was significantly lower than the number of wild-type cells. In summary, the data indicate that the tlp1 mutant is impaired in colonization of wheat root surfaces and suggest that energy taxis contributes to root colonization in A. brasilense.
DISCUSSION
During energy taxis, cells seek a position in gradients of metabolized compounds at which the intracellular energy level is optimum. This behavior in E. coli has been studied extensively (12, 13, 18, 30, 32) and has been described for other bacterial species, including A. brasilense (1). However, the Aer and Tsr chemotaxis transducers of E. coli are the only two proteins that have been conclusively shown to transduce signals from energy taxis (13, 30).
We have identified and characterized a novel energy taxis transducer in A. brasilense. The Tlp1 protein has a predicted membrane topology typical of classical transmembrane receptors in E. coli (15); however, its extracellular domain has no sequence similarity to the N-terminal domains of E. coli chemoreceptors and a rather limited similarity in the C-terminal domain. Tlp1 contains the cytoplasmic C-terminal signaling module, which consists of an HCD (23) flanked by methylation helices. The C-terminal domain of Tlp1 is highly similar to those of most closely related α-proteobacterial species.
The predicted periplasmic N-terminal domain is the first member of a newly identified domain family, which is found in two types of microbial sensor molecules: chemotaxis transducers and sensor histidine kinases. Such domain sharing between different classes of microbial sensors has recently been described for several unrelated extracellular sensory domains in prokaryotes (53). The presence of a conserved sensory domain in different classes of signal transduction proteins clearly indicates its importance to the biology of the organisms.
The N-terminal domain of Tlp1 is more closely related to a homologous domain of a histidine kinase from a distantly related delta-proteobacterial species, Geobacter metallireducens, than to homologous domains of chemotaxis transducers from closely related α-proteobacterial species. This relationship is indicative of an independent evolutionary history of the extracellular and intracellular domains of Tlp1, which is consistent with the recently reported independent domain evolution of cyanobacterial chemotaxis transducers (47). The biological function of the newly identified domain remains unknown.
The following experimental results strongly indicate that Tlp1 mediates energy taxis in A. brasilense, likely by monitoring a parameter linked to the electron transport system. In the absence of a functional Tlp1 transducer, mutant cells did not respond as effectively as cells of the wild type to gradients of electron donors (rapidly oxidizable substrates) or to terminal electron acceptors (oxygen or nitrate under anaerobic conditions) and were less sensitive to the repellent effect of a competitive electron transport inhibitor (a substituted quinone). All our results were consistent with the hypothesis that Tlp1 is an energy taxis transducer.
Although Tlp1 is an energy taxis transducer, the tlp1 mutant does not have a null phenotype. Therefore, we conclude that there are other energy taxis transducers in A. brasilense. Similarly, mutation of either Aer or Tsr, the two energy taxis transducers in E. coli, does not result in a null phenotype for energy taxis because the intact transducer can compensate for the mutated one (30). In addition, the aer and tsr mutants form an aerotactic band in a capillary at a lower or higher oxygen concentration, respectively, than wild-type cells (30), which is similar to the behavior of the tlp1 mutant.
The molecular mechanism of energy sensing is understood only for the Aer transducer of E. coli (12, 32). The Aer transducer has a PAS domain and a flavin-adenine dinucleotide cofactor that is proposed to sense redox potential via interaction with a component of the electron transport system (12, 32). The molecular mechanism by which Tlp1 senses energy remains unknown, as it is for Tsr, and Tlp1 has a membrane topology similar to that of Tsr. The N-terminal periplasmic domain of the Tsr chemoreceptor lacks any oxygen- or redox-responsive prosthetic group. Similarly, sequence analysis of Tlp1 did not reveal any conserved motifs for putative binding sites for oxygen-and redox-responsive cofactors. It is important to mention that, although Tsr and Tlp1 are both energy taxis transducers, there is no sequence similarity between their N-terminal periplasmic domains. Whether Tsr and Tlp1 sense energy via similar mechanisms remains to be determined.
The involvement of microbial chemotaxis in colonization of plant roots has been previously addressed (for a review, see reference 42). However, most of these studies used genetically uncharacterized mutants with chemotactic behavior that was not analyzed. Only recently, the role of chemotaxis in root colonization by Pseudomonas fluorescens has been demonstrated directly by using a cheA mutant (14). Similarly, a R. leguminosarum mutant deficient in a chemotaxis transducer was shown to be less competitive in nodulation of the host plant (49). However, the function of this transducer in R. leguminosarum and the behavior of the corresponding mutant were not established.
Root exudates serve as growth substrates for soil microorganisms. This factor ultimately contributes to the selection of the soil bacteria that colonize the rhizosphere by affecting the growth of bacteria in the vicinity of the roots. It was previously suggested that root colonization by azospirilla is dependent on the bacterial metabolism, and metabolism-dependent chemotaxis was proposed to contribute to host selection by the bacteria (31). It has been demonstrated that metabolism-dependent chemotaxis in A. brasilense is largely energy taxis (1). The findings of the present study suggested that energy taxis might be involved in root colonization and the establishment of Azospirillum-grass associations.
The identification of Tlp1 as an energy taxis transducer in A. brasilense allowed us to test this hypothesis. We have determined that a tlp1 mutant is significantly less efficient than the wild-type strain in root colonization when sterile wheat seedlings were inoculated to in vitro. Since the tlp1 mutant had a reduced ability to navigate in gradients of chemoeffectors that affect intracellular energy levels, it is likely that the smaller populations of the tlp1 mutant resulted from reduced sensitivity of the mutant to root-generated gradients of chemoeffectors that affect energy levels.
It was previously proposed that active motility and chemotaxis to chemicals present in cereal root exudates are responsible for the first steps of root colonization in various host microbe associations, including in Azospirillum spp. (41, 42). Our results suggest that energy taxis is important for the establishment of A. brasilense in the rhizosphere of wheat. We hypothesize that previously observed strain-specific chemotaxis of Azospirillum to chemicals typical of plant root exudates (31) can be attributed to energy taxis. Through energy taxis, azospirilla are attracted to a broad range of chemicals that support metabolism. Such metabolites are found in the root exudates of various host plants. This broad response is in contrast to the strict host specificity in which specific chemical signals establish the association. It is therefore tempting to speculate that energy taxis might be a significant factor that contributes to the broad host specificity seen in Azospirillum-plant associations.
A recent analysis of the number of chemotaxis transducers in different microbial genomes indicates that many soil bacteria possess significantly more chemoreceptors than the model organisms studied for chemotaxis (E. coli and Bacillus subtilis). The sensory specificity for the large majority of these transducers is unknown (2). However, given that chemotaxis is probably a major factor in bacterial adaptation to changing conditions, future studies focusing on characterizing the sensory specificity of chemotaxis transducers should unravel the diversity of environmental cues that trigger adaptive responses in microorganisms. They will also aid in defining how chemotaxis contributes to the biology of these organisms.
Acknowledgments
This work was supported in part by a National Science Foundation CAREER award (MCB-0347218), the Georgia Research Foundation, and the Office of Institutional Research, Georgia State University (to G.A.).
We thank Jos Vanderleyden (Katholieke Universiteit Leuven, Leuven, Belgium) for providing the A. brasilense Sp7 genomic library, Robert L Bowden (USDA-ARS, Manhattan, Kans.) for providing the wheat seeds, and Anna Skorupska (M. Curie-Sklodowska University, Lublin, Poland) for the pJBA21TC plasmid. We are grateful to Barry Taylor and Igor Zhulin for access to their laboratories' facilities at the early stage of this project. We thank Mark Johnson, Barry Taylor, Igor Zhulin, and Sandy Parkinson for expert advice, stimulating discussions, and critical reading of the manuscript.
REFERENCES
- 1.Alexandre, G., S. E. Greer, and I. B. Zhulin. 2000. Energy taxis is the dominant behavior in Azospirillum brasilense. J. Bacteriol. 182:6042-6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexandre, G., S. E. Greer-Phillips, and I. B. Zhulin. 2004. Ecological role of energy taxis in microorganisms. FEMS Microbiol. Rev. 28:113-126. [DOI] [PubMed] [Google Scholar]
- 3.Alexandre, G., C. Jacoud, D. Faure, and R. Bally. 1996. Population dynamics of a motile and non-motile Azospirillum lipoferum strain during rice colonization and motility variation in the rhizosphere. FEMS Microbiol. Ecol. 19:271-278. [Google Scholar]
- 4.Alexandre, G., and I. B. Zhulin. 2001. More than one way to sense chemicals. J. Bacteriol. 183:4681-4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexandre, G., and I. B. Zhulin. 2003. Different evolutionary constraints on chemotaxis proteins CheW and CheY revealed by heterologous expression studies and protein sequence analysis. J. Bacteriol. 185:544-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aravind, L., and C. P. Ponting. 1999. The cytoplasmic helical linker domain of receptor histidine kinase and methyl-accepting proteins is common to many prokaryotic signalling proteins. FEMS Microbiol. Lett. 176:111-116. [DOI] [PubMed] [Google Scholar]
- 8.Barak, R., I. Nur, Y. Okon, and Y. Henis. 1982. Aerotactic response of Azospirillum brasilense. J. Bacteriol. 152:643-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bashan, Y. 1986. Migration of the rhizosphere bacteria Azospirillum brasilense and Pseudomonas fluorescens towards wheat roots in the soil. J. Gen. Microbiol. 132:3407-3414. [Google Scholar]
- 10.Bashan, Y., and G. Holguin. 1994. Root-to-root travel of the beneficial soil bacterium Azospirillum brasilense. Appl. Environ. Microbiol. 60:2120-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Besemer, J., A. Lomsadze, and M. Borodovsky. 2001. GeneMarkS: a self-training method for prediction of gene starts in microbial genomes. Implications for finding sequence motifs in regulatory regions. Nucleic Acids Res. 29:2607-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bibikov S. I., L. A. Barnes, Y. Gitin, and J. S. Parkinson. 2000. Domain organization and flavin adenine dinucleotide-binding determinants in the aerotaxis signal transducer Aer of Escherichia coli. Proc. Natl. Acad. Sci. USA 97:5830-5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bibikov, S. I. R. Biran, K. E. Rudd, K. E., and J. S. Parkinson. 1997. A signal transducer for aerotaxis in Escherichia coli. J. Bacteriol. 179:4075-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Weert S., H. Vermeiren, I. H. Mulders, I. Kuiper, N. Hendrickx, G. V. Bloemberg, J. Vanderleyden, R. De Mot, B. J. Lugtenberg. 2002. Flagella-driven chemotaxis towards exudate components is an important trait for tomato root colonization by Pseudomonas fluorescens. Mol. Plant-Microbe Interact. 15:1173-1180. [DOI] [PubMed] [Google Scholar]
- 15.Falke, J. J., and G. L. Hazelbauer. 2001. Transmembrane signaling in bacterial chemoreceptors. Trends Biochem. Sci. 26:257-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fellay, R., J. Frey, and H. Krisch. 1987. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of gram-negative bacteria. Gene 52:147-154. [DOI] [PubMed] [Google Scholar]
- 17.Figurski, D. H., and D. R. Helinski. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 76:1648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greer-Phillips, S. E., G. Alexandre, B. L. Taylor, and I. B. Zhulin. 2003. Aer and Tsr guide Escherichia coli in spatial gradients of oxidizable substrates. Microbiology 149:2661-2667. [DOI] [PubMed] [Google Scholar]
- 19.Grishanin, R. N., I. I. Chalmina, and I. B. Zhulin. 1991. Behaviour of Azospirillum brasilense in a spatial gradient of oxygen and in a “redox” gradient of artificial electron acceptor. J. Gen. Microbiol. 137:2781-2785. [Google Scholar]
- 20.Hauwaerts, D., G. Alexandre, S. K. Das, J. Vanderleyden, and I. B. Zhulin. 2002. A major chemotaxis gene cluster in Azospirillum brasilense and relationships between chemotaxis operons in alpha-proteobacteria. FEMS Microbiol. Lett. 208:61-67. [DOI] [PubMed] [Google Scholar]
- 21.Ishikawa, J., and K. Hotta. 1999. FramePlot: a new implementation of the frame analysis for predicting protein-coding regions in bacterial DNA with a high G + C content. FEMS Microbiol. Lett. 174:251-253. [DOI] [PubMed] [Google Scholar]
- 22.Kumar, S. K. Tamura, and M. Nei. 1994. MEGA: molecular evolutionary genetics analysis software for microcomputers. Comput. Appl. Biosci. 10:189-191. [DOI] [PubMed] [Google Scholar]
- 23.Le Moual, H., and D. E. Koshland, Jr. 1996. Molecular evolution of the C-terminal cytoplasmic domain of a superfamily of bacterial receptors involved in taxis. J. Mol. Biol. 261:568-585. [DOI] [PubMed] [Google Scholar]
- 24.Letunic, I., R. R. Copley, S. Schmidt, F. D. Ciccarelli, T. Doerks, J. Schultz, C. P. Ponting, and P. Bork. 2004. SMART 4.0: towards genomic data integration. Nucleic Acids Res. 32:D142-D144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo Conte, L., C. Chothia, and J. Janin. 1999. The atomic structure of protein-protein recognition sites. J. Mol. Biol. 285:2177-2198. [DOI] [PubMed] [Google Scholar]
- 26.Okon, Y., and R. Itzigsohn. 1995. The development of Azospirillum as a commercial inoculant for improving crop yields. Biotechnol. Adv. 13:415-424. [DOI] [PubMed] [Google Scholar]
- 27.Okon, Y., and C. A. Labandera-Gonzalez. 1994. Agronomic applications of Azospirillum: an evaluation of 20 years worldwide field inoculation. Soil Biol. Biochem. 26:1591-1601. [Google Scholar]
- 28.Okon, Y., and J. Vanderleyden. 1997. Root-associated Azospirillum species can stimulate plants. ASM News 63:366-370. [Google Scholar]
- 29.Ramos, H. J. O., L. D. B. Roncato-Maccari, E. M. Souza, J. R. L. Soares-Ramos, M. Hungria, and F. O. Pedrosa. 2002. Monitoring Azospirillum-wheat interaction using the gfp and gusA genes constitutively expressed from a new broad-host range vector. J. Biotechnol. 97:243-252. [DOI] [PubMed] [Google Scholar]
- 30.Rebbapragada, A., M. S. Johnson, G. P. Harding, A. J. Zuccarelli, H. M. Fletcher, I. B. Zhulin, and B. L. Taylor. 1997. The Aer protein and the serine chemoreceptor Tsr independently sense intracellular energy levels and transduce oxygen, redox, and energy signals for Escherichia coli behavior. Proc. Natl. Acad. Sci. USA 94:10541-10546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reinhold, B., T. Hurek, and I. Fendrik. 1986. Strain-specific chemotaxis of Azospirillum spp. J. Bacteriol. 162:190-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Repik, A., A. Rebbapragada, M. S. Johnson, J. O. Haznedar, J. O., I. B. Zhulin, and B. L. Taylor. 2000. PAS domain residues involved in signal transduction by the Aer redox sensor of Escherichia coli. Mol. Microbiol. 36:806-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook, J., E. F. Fritsch, and T. A. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 34.Simon, R., U. Priefer, and A. Pülher. 1983. A broad host range mobilization system for in vivo genetic engineering transposon mutagenesis in gram-negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 35.Steenhoudt, O., and J. Vanderleyden. 2000. Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: genetic, biochemical and ecological aspects. FEMS Microbiol Rev. 24:487-506. [DOI] [PubMed] [Google Scholar]
- 36.Stock, J. B., and M. G. Surette. 1996. Chemotaxis. pp. 1103-1129. In C. N. Neidhart, R. Curtiss III, J. I. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella, vol. 1. ASM Press, Washington, D. C.
- 37.Tarrand, J. J., N. R. Krieg, and J. Döbereiner. 1978. A taxonomic study of the Spirillum lipoferum group, with description of a new genus, Azospirillum gen. nov. and two species, Azospirillum lipoferum (Beijerinck) comb. nov. and Azospirillum brasilense sp. nov. Can. J. Microbiol. 24:967-980. [DOI] [PubMed] [Google Scholar]
- 38.Taylor, B. L., and I. B. Zhulin. 1998. In search of higher energy: metabolism-dependent behaviour in bacteria. Mol. Microbiol. 28:683-690. [DOI] [PubMed] [Google Scholar]
- 39.Taylor, B. L., I. B. Zhulin, and M. S. Johnson. 1999. Aerotaxis and other energy-sensing behavior in bacteria. Annu. Rev. Microbiol. 53:103-128. [DOI] [PubMed] [Google Scholar]
- 40.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vande Broek, A., M. Lambrecht, and J. Vanderleyden. 1998. Bacterial chemotactic motility is important for the initiation of wheat root colonization by Azospirillum brasilense. Microbiology 144:2599-2606. [DOI] [PubMed] [Google Scholar]
- 42.Vande Broek, A., and J. Vanderleyden. 1995. The role of bacterial motility, chemotaxis, and attachment in bacteria-plant interactions. Mol. Plant-Microbe Interact. 8:800-810. [Google Scholar]
- 43.Vanstockem, M., K. Michiels, J. Vanderleyden, and A. P. Van Gool. 1987. Transposon mutagenesis of Azospirillum brasilense and Azospirillum lipoferum, physical analysis of Tn5 and Tn5-Mob insertion mutants. Appl. Environ. Microbiol. 53:410-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verreth, C., B. Cammue, P. Swinnen, D. Crombez, A. Michielsen, K. Michiels, A. Van Gool, and J. Vanderleyden. 1989. Cloning and expression in Escherichia coli of the Azospirillum brasilense Sp7 gene encoding ampicillin resistance. Appl. Environ. Microbiol. 55:2056-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wielbo, J., and A. Skorupska. 2001. Construction of improved vectors and cassettes containing gusA and antibiotic resistance genes for studies of transcriptional activity and bacterial localization. J. Microbiol. Methods 45:197-205. [DOI] [PubMed] [Google Scholar]
- 46.Wilson, K. J., A. Sessitsch, J. C. Corbo, K. E. Giller, A. D. Akkermans, and R. A. Jefferson. 1995. β-Glucuronidase (GUS) transposons for ecological and genetic studies of rhizobia and other gram-negative bacteria. Microbiology 141:1691-1705. [DOI] [PubMed] [Google Scholar]
- 47.Wuichet, K., and I. B. Zhulin. 2003. Molecular evolution of sensory domains in cyanobacterial chemoreceptors. Trends Microbiol. 11:200-203. [DOI] [PubMed] [Google Scholar]
- 48.Yanish-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 49.Yost, C. K., P. Rochepeau, and M. F. Hynes. 1998. Rhizobium leguminosarum contains a group of genes that appear to code for methyl-accepting chemotaxis proteins. Microbiology 144:1945-1956. [DOI] [PubMed] [Google Scholar]
- 50.Zamudio, M., and F. Bastarrachea. 1994. Adhesiveness and root hair deformation capacity of Azospirillum strains for wheat seedlings. Soil Biol. Biochem. 26:791-797. [Google Scholar]
- 51.Zhulin, I. B. 2001. The superfamily of chemotaxis transducers: from physiology to genomics and back. Adv. Microbiol. Physiol. 45:157-198. [DOI] [PubMed] [Google Scholar]
- 52.Zhulin, I. B., V. A. Bespalov, M. S. Johnson, and B. L. Taylor. 1996. Oxygen taxis and proton motive force in Azospirillum brasilense. J. Bacteriol. 178:5199-5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhulin, I. B., A. N. Nikolskaya, and M. Y. Galperin. 2003. Common extracellular sensory domains in transmembrane receptors for diverse signal transduction pathways in bacteria and archaea. J. Bacteriol. 185:285-294. [DOI] [PMC free article] [PubMed] [Google Scholar]