Abstract
Streptococcus mutans and Streptococcus sobrinus are the bacteria most commonly associated with human dental caries. A major virulence attribute of these and other cariogenic bacteria is acid tolerance. The acid tolerance mechanisms of S. mutans have begun to be investigated in detail, including the adaptive acid tolerance response (ATR), but this is not the case for S. sobrinus. An analysis of the ATR of two S. sobrinus strains was conducted with cells grown to steady state in continuous chemostat cultures. Compared with cells grown at neutral pH, S. sobrinus cells grown at pH 5.0 showed an increased resistance to acid killing and were able to drive down the pH through glycolysis to lower values. Unlike what is found for S. mutans, the enhanced acid tolerance and glycolytic capacities of acid-adapted S. sobrinus were not due to increased F-ATPase activities. Interestingly though, S. sobrinus cells grown at pH 5.0 had twofold more glucose phosphoenolpyruvate:sugar phosphotransferase system (PTS) activity than cells grown at pH 7.0. In contrast, glucose PTS activity was actually higher in S. mutans grown at pH 7.0 than in cells grown at pH 5.0. Silver staining of two-dimensional gels of whole-cell lysates of S. sobrinus 6715 revealed that at least 9 proteins were up-regulated and 22 proteins were down-regulated in pH 5.0-grown cells compared with cells grown at pH 7.0. Our results demonstrate that S. sobrinus is capable of mounting an ATR but that there are critical differences between the mechanisms of acid adaptation used by S. sobrinus and S. mutans.
The oral cavity is a dynamic environment that undergoes rapid and often substantial changes in pH, nutrient availability, carbohydrate source, and oxygen tension. The capacity of oral bacteria to tolerate acidic environments is of major importance in the ecology of dental plaque and is directly related to the pathogenesis of dental caries. Two species of mutans streptococci, Streptococcus mutans and Streptococcus sobrinus, were implicated as etiologic agents of dental caries when elevated proportions of these organisms were detected in dental plaque from caries-active subjects (15, 32). S. mutans and S. sobrinus, which can grow and continue to carry out glycolysis at low pH, are thought to gain a competitive advantage over other plaque bacteria during the periods of sustained acidification that are conducive to caries development.
Acid tolerance by S. mutans has been studied in some detail, and it is established that this organism possesses an inherent acid resistance that distinguishes it from organisms not commonly associated with dental caries. S. mutans can grow and carry out glycolysis at pH values below 5.0 and can drive the pH to values well below 4.0 (3). The aciduricity of these organisms has been attributed in large part to the possession of a proton-extruding F-ATPase that is expressed at higher levels than in many oral bacteria and that functions well at pH values of 5.0 and below, allowing the organisms to maintain adequate ΔpH when the external pH falls to 4.0 and lower. In addition to having constitutive acid tolerance properties, S. mutans is also capable of mounting an adaptive acid tolerance response (ATR). Adaptation to growth in moderately acidic conditions renders the organisms less susceptible to lethal acidification and is associated with enhanced glycolytic capacities and increased activity of the proton-translocating F-ATPase (3, 16). Several other mechanisms of resistance to acid stress have been identified in S. mutans, including the induction of stress proteins (23, 30), DNA repair enzymes (14, 20), changes in the cell membrane composition (12, 36), an H+-glucose symporter that operates at pH 5.0 (8), and, recently, an ammonia- and polyamine-generating pathway (13). Moreover, response regulators and quorum-sensing genes have also been shown to play a role in acid adaptation by S. mutans (31, 48). Although S. sobrinus is considered to be the most acidogenic bacteria among the oral streptococci (9, 10, 21), very little is known of the responses of this organism to environmental acidification. While it is known that the organisms produce relatively high levels of F-ATPase, other acid resistance mechanisms have not been explored. In a previous study, mid-exponential-phase batch cultures of S. sobrinus CH125/43 were unable to induce an ATR after a 2-h incubation in medium buffered at an acidic, nonlethal pH value (43). In the same study, three different strains of S. mutans were shown to induce a strong ATR when grown under the same conditions. From this work emerged the current concept that S. mutans is capable of inducing a strong ATR and that S. sobrinus, although relatively acid tolerant, is unable to mount an ATR. Other than the ATR, the tacit assumption has been that the acid tolerance mechanisms of S. sobrinus are the same as those in S. mutans.
The ability of oral streptococci to efficiently transport and metabolize a wide variety of sugars, especially at low pH, is another characteristic that allows these bacteria to grow and persist in the mouth and is directly linked to their ability to elicit dental caries. The phosphoenolpyruvate:sugar phosphotransferase system (PTS) is the primary sugar transport system in oral streptococci, especially under carbohydrate-limiting conditions, and plays important roles in global control of gene expression (33, 35, 38, 41, 45). The PTS consists of two proteins that are common to all PTS substrates, enzyme I (EI) and the heat-stable phosphocarrier protein Hpr, as well as a variety of sugar-specific permeases, known as EII complexes, that catalyze the transport and concomitant phosphorylation of their cognate substrates. Previous research with S. mutans strain Ingbritt has demonstrated that glucose PTS activity is markedly decreased in cells grown at pH 5.5, compared to that for those grown at neutral pH values, and it was suggested that repression occurs at the level of EII synthesis (46, 47). It is believed that an H+-glucose symport system may be the primary transporter for glucose at low pH (8). Thus, while the PTS is believed to be critical for high-affinity and high-capacity transport of carbohydrate and a major contributor to acidogenesis, its role in acid tolerance is not established.
Continuous chemostat cultures are considered to be particularly advantageous for studies of acid tolerance and carbohydrate metabolism specifically because steady-state cultures that are grown at different pH values do not differ in growth rate, availability of nutrients, growth phase, and other important physiological parameters. By using chemostats to tightly control the physiology of the organisms, one can effectively rule out the possibility that variables other than pH influence the phenotypic properties. This has been demonstrated repeatedly in studies with S. mutans. The purpose of this report was to conduct a more detailed analysis of the responses of S. sobrinus to environmental acidification. The results indicate that S. sobrinus does indeed possess an ATR but that there are critical differences in the molecular mechanisms of acid adaptation by S. sobrinus and S. mutans.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. sobrinus 6715 and SL1 and S. mutans UA159 were routinely grown in brain heart infusion media in a 5% CO2 aerobic atmosphere at 37°C. To investigate the responses of S. sobrinus and S. mutans to acid shock and acid adaptation, cells were grown in a BioFloIII chemostat (New Brunswick Scientific, Edison, N.J.) with a working volume of 600 ml in TY base medium (3% tryptone, 0.5% yeast extract) containing 25 mM glucose, as previously described (23). The dilution rate of the culture was 0.3 h−1, corresponding to a generation time of 2.3 h, and the pH of the vessel was maintained by addition of 1 N KOH. Cells growing at steady state, pH 7.0, were acid shocked from pH 7.0 to 5.0 with 1 N HCl. The total time for titration of the vessel to pH 5 was about 2 min. Subsequently, cells were grown for a minimum of 10 generations to achieve steady state at pH 5.0. Samples were obtained from the chemostat by aspiration from the vessel into ice-cold tubes at selected time points. In all cases, cells from at least three independent chemostat runs were collected immediately by centrifugation at 4°C and subjected to acid killing and pH drop experiments or quick-frozen in a dry ice-ethanol bath and stored at −80°C for further analysis.
Acid killing and pH drop experiments.
For acid killing experiments, steady-state chemostat cells were washed once with 0.1 M glycine (pH 7.0) and resuspended in 0.1 M glycine (pH 2.8). Samples were stirred continuously at room temperature, and aliquots of cells were removed at 30, 60, and 90 min. Cells were serially diluted, plated on brain heart infusion-agar plates, and incubated at 37°C for 2 days before colonies were counted. Cell viability at each time point was expressed as the percentage of viable cells (CFU per milliliter) at time zero. The ability of S. sobrinus and S. mutans strains to lower the pH through glycolysis was monitored as described by Belli and Marquis (3). Briefly, cells from steady-state chemostat cultures were harvested, washed with 1 culture volume of cold distilled water, and resuspended in a solution of 50 mM KCl and 1 mM MgCl2 in 1/10 of the original culture volume. The suspension was titrated with 0.1 M KOH to a pH of 7.2, pH drops were initiated by addition of 55.6 mM glucose, and the pH was recorded over a 50-min period.
Biochemical assays.
For F-ATPase assays, cells collected from the chemostat were permeabilized with toluene and incubated with 5 mM ATP in ATPase buffer, as previously described (3). Samples were removed at various intervals and assayed for inorganic phosphate released from ATP with the Fiske-Subbarow reagent (Sigma, St. Louis, Mo.). To measure sugar transport by the PTS, cells obtained from the chemostat were washed twice with 0.1 M sodium-potassium phosphate buffer (pH 7.2) and suspended in 10% of the original volume with the same buffer. The cell suspension was then permeabilized with 50 μl of toluene-acetone (1:9) per ml of cells. Permeabilized cells (10 to 50 μl) were assayed by the method of LeBlanc et al. (28). Protein concentrations were determined by a bicinchoninic acid assay (Sigma) with bovine serum albumin as the standard.
DNA methods.
Chromosomal DNA was prepared from S. sobrinus 6715 as previously described (5). Sequences used to create oligonucleotide primers were obtained from the S. sobrinus genome sequencing project at The Institute for Genomic Research. The following primers were used: dnaK sense, 5′-GGTGGTGTCATCACTG-3′; dnaK antisense, 5′-GCAGCATCACGTTCAG-3′; atpB sense, 5′-CCTCATCAGAAATCTTGG-3′; atpB antisense, 5′-CGAAAGCTGTCGCTG-3′; ptsG sense, 5′-TGGGTGATGGCTTCGCTGTTC-3′; ptsG antisense, 5′-AGGAGCCCATAAGCGTGTTTCG-3′. PCRs were carried out with 100 ng of chromosomal DNA and Taq DNA polymerase, and PCR products were purified with the QIAquick kit (QIAGEN).
RNA methods.
Cell lysates were obtained by mechanical disruption with a Bead Beater (BioSpec, Bartlesville, Okla.) in the presence of chilled glass beads (0.1 mm diameter) for 3 cycles of 30 s, with cooling on ice for 1 min during the intervals. RNA was extracted from S. sobrinus and S. mutans chemostat-grown cells as described by Chen et al. (7). For quantitative slot blot analysis, equivalent amounts of denatured RNA were transferred to nylon membranes (GeneScreen Plus; NEN Life Science Products, Inc., Boston, Mass.) by a slot blot apparatus (Life Technologies Inc., Rockville, Md.) as described elsewhere (39). RNAs were UV cross-linked to the membranes, and filter membranes were probed with internal fragments of the genes of interest that had been labeled with psoralen-biotin with the Brightstar labeling kit (Ambion, Inc., Austin, Tex.). Hybridizations and washes were carried out under high-stringency conditions. Signals obtained on autoradiographs were quantified by densitometry with an IS1000 digital imaging system (Alpha Innotech Corp., San Leandro, Calif.).
Western blotting and 2-D gels.
Cells grown in the chemostat as described above were collected by centrifugation, washed twice with Tris-buffered saline (TBS; 10 mM Tris-HCl [pH 7.4], 0.9% NaCl), and suspended in sodium dodecyl sulfate (SDS) boiling buffer (60 mM Tris-HCl [pH 6.8], 5% SDS, 10% glycerol). Cell lysates were obtained by mechanical disruption with a Bead Beater. Protein lysates (10 μg) were subjected to SDS-polyacrylamide gel electrophoresis, blotted to Immunobilon-P membranes (Millipore, Bedford, Mass.), and analyzed by Western blotting (2). Membranes were incubated with antibodies raised against the Streptococcus pyogenes DnaK and GroEL proteins (29) or the Streptococcus salivarius EI or HPr protein at dilutions of 1:1,000, 1:500, 1:200, or 1:200, respectively, in TBS. Antibodies against EI and Hpr were a gift from C. Vadenbocoeur, Universite Laval, Quebec, Canada. Immune reactivity was detected by incubation with peroxidase-conjugated goat anti-rabbit immunoglobulin G, followed by detection with 4-chloro-1-naphthol. Two-dimensional (2-D) gel electrophoresis was performed at Kendrick Labs, Inc. (Madison, Wis.) by following the protocol described by O'Farrell (34), and the gels were stained with silver. Densitometric analysis was used to compare the intensities of the spots.
RESULTS
Acid-adaptive response of S. sobrinus.
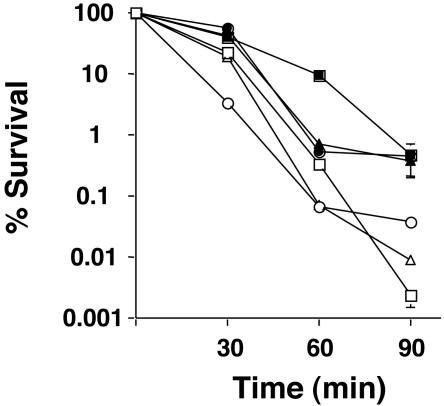
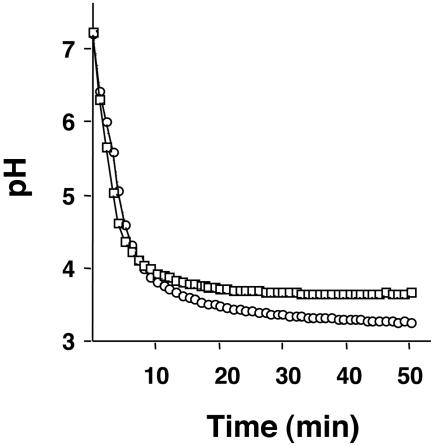
To determine if S. sobrinus had the ability to mount an ATR, acid killing and pH drop experiments were performed with cells obtained from steady-state chemostat cultures. To confirm that the growth conditions were adequate to induce an ATR, chemostat cultures of S. mutans UA159 grown under the same conditions were also analyzed. Steady-state pH 7.0 or 5.0 cultures of S. sobrinus 6715 and SL1 and S. mutans UA159 were subjected to acid killing at pH 2.8. After 90 min of incubation, all of the organisms showed an increase of at least 1 log unit in the number of survivors (Fig. 1 and Table 1). In pH drop experiments, cells grown at pH 5.0 were able to decrease the pH through glycolysis to values lower than that for cells grown at pH 7.0 (Fig. 2 and Table 1). In cells growing at pH 7.0, steady state, S. sobrinus 6715, S. sobrinus SL1, and S. mutans UA159 lowered the pH to 3.57 ± 0.09, 3.65 ± 0.02, and 3.52 ± 0.1, respectively, after 50 min. When cells cultured at pH 5.0 were used, the final pH values attained in the pH drops by the same strains were 3.21 ± 0.03, 3.15 ± 0.09, and 3.29 ± 0.18, respectively (Table 1).
FIG. 1.
Survival of S. sobrinus 6715 (squares), SL1 (circles), and S. mutans UA159 (triangles) during acid challenge. Cells were collected from a steady-state chemostat held at pH 7.0 (open symbols) or 5.0 (solid symbols) and subjected to acid killing at pH 2.85. Cell viability at each time point is expressed as the percentage of viable cells (CFU per milliliter of culture) at time zero.
TABLE 1.
Acid-adaptive response of S. sobrinus 6715 and SL1 and S. mutans UA159a
| Organism | Growth pH | 50-min glycolytic pH (final pH) | % Survival after 90 min |
|---|---|---|---|
| S. sobrinus 6715 | 7.0 | 3.57 (0.09) | 2.5 × 10−3 (1 × 10−3) |
| 5.0 | 3.21 (0.03) | 4.9 × 10−1 (2.7 × 10−1) | |
| S. sobrinus SL1 | 7.0 | 3.65 (0.02) | 3.8 × 10−2 (1 × 10−2) |
| 5.0 | 3.15 (0.09) | 4.7 × 10−1 (0.9 × 10−1) | |
| S. mutans UA159 | 7.0 | 3.52 (0.10) | 9.0 × 10−3 (1.0 × 10−3) |
| 5.0 | 3.29 (0.18) | 3.8 × 10−1 (1.8 × 10−1) |
The results are the means (± standard deviations) of at least three independent experiments.
FIG. 2.
Glycolytic pH profile of S. sobrinus 6715. Cells were collected from a steady-state chemostat held at pH 7.0 (squares) or 5.0 (circles), washed once with ice-cold water, and resuspended in a 50 mM KCl/1 mM MgCl2 solution. Glucose was added to the cell suspension to initiate glycolysis, and pH drops were continuously monitored for 50 min. Data points were collected every 10 s, and data for every 2 min 30 s are presented. The graph shown is a representative of five independent experiments.
Contribution of F-ATPases to acid tolerance by S. sobrinus and S. mutans.
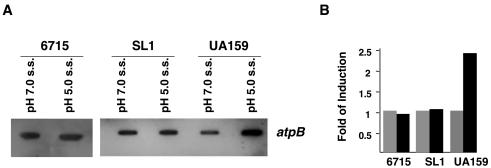
Previous data have shown that acid adaptation of S. mutans is correlated with an increase in proton-extruding F-ATPase activity (3, 16). Indeed, data from this study demonstrated about a twofold increase in F-ATPase activity in S. mutans UA159 growing at pH 5.0 compared to that in cells grown at pH 7.0. In contrast, there was no significant increase in the F-ATPase activity of the S. sobrinus strains when grown at pH 5.0 (Table 2). It has been suggested that increases in F-ATPase activity correlate with increased transcription of the F-ATPase operon (37). Quantitative slot blots of total RNA were used to test whether the levels of F-ATPase activity for S. mutans UA159 and S. sobrinus 6715 and SL1 correlated with mRNA for the F-ATPase genes. An internal fragment of the S. sobrinus atpB gene, which encodes the ATP synthase subunit of the F-ATPase enzyme, was used as a probe. In S. mutans, there was a 2.4-fold increase of atpB mRNA at pH 5.0, whereas in S. sobrinus 6715 and SL1 the transcriptional levels of atpB were not affected by growth pH (Fig. 3).
TABLE 2.
F-ATPase activity of S. sobrinus 6715 and SL1 and S. mutans UA159 grown in chemostat cultures
| Organism | Growth pH | ATPase activitya (μmol min−1 mg of protein−1) | Fold induction |
|---|---|---|---|
| S. sobrinus 6715 | 7.0 | 21 (±3) | 1.0 |
| 5.0 | 23 (±6.5) | 1.0 | |
| S. sobrinus SL1 | 7.0 | 19 (±1) | 1.0 |
| 5.0 | 23 (±12) | 1.2 | |
| S. mutans UA159 | 7.0 | 20 (±12) | 1.0 |
| 5.0 | 43 (±3) | 2.1 |
The values are the means (standard deviations) of at least three independent experiments.
FIG. 3.
Slot blot analysis of atpB mRNA in response to environmental acidification. (A) Total RNA was isolated from S. sobrinus 6715 and SL1 and S. mutans UA159 grown in the chemostat under the conditions indicated in the figure (s.s., steady state). Hybridization was performed with internal fragments of the S. sobrinus and S. mutans atpB genes against DNA from the parent organism. RNase-treated samples were used as controls (data not shown). (B) Bar graph of atpB mRNA levels as measured by densitometry.
PTS activity in S. sobrinus and S. mutans.
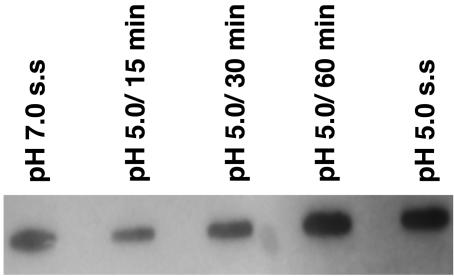
The glucose-specific PTS activities of chemostat-grown S. sobrinus and S. mutans cells were assessed. In agreement with previous results (17), the S. mutans glucose-specific PTS was repressed by 50% at pH 5.0, compared to that for cells grown at pH 7.0. Interestingly, glucose-specific PTS activity in both S. sobrinus strains was higher in cells grown at pH 5.0 than in cells grown at pH 7.0, particularly in strain SL1, which showed a 2.6-fold induction at pH 5 (Table 3). It has been demonstrated that the levels of the Hpr and EI proteins are not affected by changes in some environmental conditions, whereas synthesis of the sugar-specific EII enzymes can be down-regulated at low pH (47). Western blot analysis using antibodies raised against the Hpr and EI proteins from S. salivarius confirmed that the levels of these proteins are not affected by pH in S. sobrinus 6715 (data not shown). On the other hand, slot blot analysis using a ptsG probe, which encodes the glucose PTS permease EIIGlc, showed that the putative S. sobrinus ptsG was induced after 60 min of acid shock (titration of cells from pH 7 to 5 with dilute HCl over the course of 2 min) and remained elevated in pH 5 steady-state cells (Fig. 4).
TABLE 3.
Glucose-specific PTS activity of S. sobrinus 6715 and SL1 and S. mutans UA159 grown in the chemostat
| Organism | Growth pH | Glucose PTS activitya | Fold induction |
|---|---|---|---|
| S. sobrinus 6715 | 7.0 | 83 (±30) | 1.0 |
| 5.0 | 129 (±44) | 1.6 | |
| S. sobrinus SL1 | 7.0 | 73 (±38) | 1.0 |
| 5.0 | 197 (±88) | 2.7 | |
| S. mutans UA159 | 7.0 | 84 (±35) | 1.0 |
| 5.0 | 42 (±17) | 0.5 |
The values are the means (standard deviations) of at least three independent experiments. PTS activity is expressed as nanomoles of NADH oxidized in a PEP-dependent manner per minute per milligram of protein.
FIG. 4.
Slot blot analysis of the S. sobrinus 6715 putative ptsG gene in response to changes in environmental pH. Total RNA was isolated from S. sobrinus 6715 grown in the chemostat under the conditions indicated in the figure (s.s., steady state). Hybridization was performed with an internal fragment of the S. sobrinus ptsG gene. RNase-treated samples were used as controls (data not shown).
Studies with S. mutans have also demonstrated that sugar transport by other PTS permeases was repressed by low pH and that this repression was associated with reduced activity of the corresponding sugar-specific EII enzymes (47). To investigate whether the divergent regulation of the glucose PTS in S. sobrinus and S. mutans by pH was restricted to glucose transport, mannose and fructose PTS activity was measured. In S. mutans, mannose and fructose PTS activities were reduced at low pH, but there were no alterations in the levels of transport of these sugars in S. sobrinus 6715 or SL1 (data not shown).
Protein expression patterns of chemostat-grown cells.
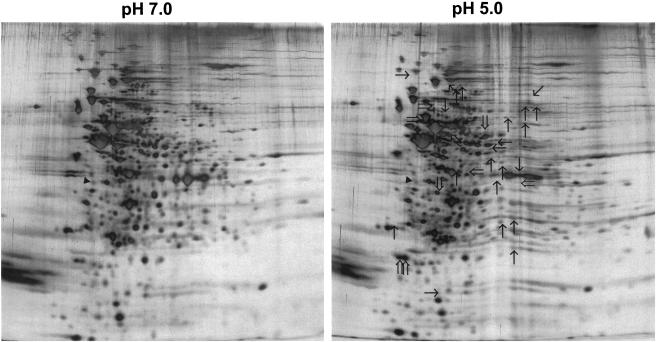
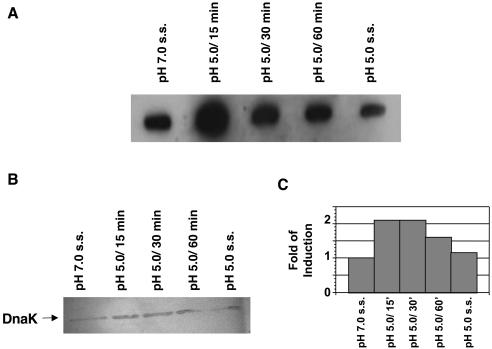
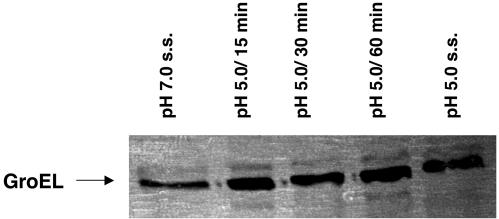
2-D electrophoresis was used to compare proteins from pH 7.0 and 5.0 steady-state cultures of S. sobrinus 6715 cells. By silver staining the gels, it was found that at least 9 proteins were up-regulated and 22 proteins were down-regulated in cells grown at pH 5.0 (Fig. 5), compared to results for cells grown at pH 7.0. In S. mutans UA159, the heat shock proteins GroEL and DnaK were up-regulated after acid shock (23, 30). Slot blot analysis revealed that the levels of S. sobrinus dnaK mRNA were increased about threefold when pH 7.0, steady-state cultures were subjected to acid shock but that there were no significant differences in the dnaK mRNA levels in steady-state cells grown at pH 7.0 or 5.0 (Fig. 6A). Western blotting confirmed that the levels of DnaK were elevated following acid shock but returned to basal levels in pH 5.0, steady-state cultures (Fig. 6B). Similar results were obtained in Western blot analyses using an anti-GroEL antibody (Fig. 7).
FIG. 5.
2D protein patterns of S. sobrinus 6715 grown in the chemostat. Double-line arrows, proteins with enhanced expression at pH 5.0, steady state; single-line arrows, proteins with reduced synthesis; solid triangle, tropomyosin, which was loaded on the gel as an internal control.
FIG. 6.
Induction of S. sobrinus 6715 dnaK mRNA and DnaK in response to environmental acidification. (A) Slot blot of total RNA isolated from cells grown in the chemostat under the conditions indicated (s.s., steady-state). Hybridization was performed with an internal fragment of the S. sobrinus dnaK gene. RNase-treated samples were used as controls (data not shown). (B) Western blot analysis of DnaK with a polyclonal antibody against S. pyogenes DnaK (1:1,000). (C) Bar graph of DnaK levels as measured by densitometry.
FIG. 7.
Western blot analysis of S. sobrinus 6715 GroEL in response to environmental acidification. Total cell lysates obtained from cells grown in the chemostat under the conditions indicated in the figure (s.s., steady-state) were probed with a polyclonal antibody against S. pyogenes GroEL (1:1,000).
DISCUSSION
Tolerance of acidic environments by cariogenic organisms is a critical factor in the ecology of oral biofilms and the pathogenesis of dental caries. S. mutans and S. sobrinus, along with lactobacilli, are the bacteria most commonly isolated from human dental caries. Some studies have indicated that S. sobrinus is more acidogenic and more cariogenic than S. mutans (9, 10). Several epidemiological studies found that S. mutans is more prevalent than S. sobrinus in dental plaque samples (6, 27, 32), although others have shown that high numbers of S. sobrinus cells are more closely associated with caries (10, 11, 22). These apparently contradictory results are likely attributable to heterogeneity among mutans streptococci in their acidogenic and aciduric properties, as well as in study populations, diet, and environmental factors. Regardless, S. mutans and S. sobrinus have consistently been implicated as the primary cariogenic organisms in human dental plaque directly as a result of their ability to tenaciously adhere to the tooth surface and to produce and tolerate copious quantities of organic acids.
The inherent acid tolerance of S. mutans and its ability to mount an ATR have been studied in some detail (3, 4, 16), but this is not the case for S. sobrinus. A single report classified S. sobrinus CH125/43 as a non-acid responder, i.e., unable to induce an ATR, while S. mutans Ingbritt and LT11 were considered strong responders (43). The results presented here demonstrate that S. sobrinus 6715 and SL1 do indeed have the ability to induce an ATR. The increased resistance to killing at low pH as a result of acid adaptation during growth at pH 5.0 was of a magnitude comparable to that observed for S. mutans UA159. Differences between the results obtained in this study and those of the previously published report (43) are most likely attributable to differences in growth conditions and strains. Most notably, a major difference is that we used steady-state cells grown in continuous chemostat culture instead of batch-cultivated cells and buffered media (43). In fact, when we tried to induce an ATR by using a batch culture approach, the enhanced survival of acid-adapted S. sobrinus cells was not appreciably different from that of cells cultivated at neutral pH (data not shown). We also noted that the ATR induced in S. mutans cells in batch culture is not as robust as that induced in chemostat-grown cells. The contrast in the phenotypic properties of steady-state chemostat cells and cells grown batchwise probably reflects other influences on the physiologic properties of the organisms arising from differences in growth rate, nutrient availability, and growth phase in the cells grown in batch culture with buffered media. Another important factor when comparing these studies is that it is generally recognized that there are significant variations in acidogenicity, aciduricity, and the ability to elicit caries in experimental animals among different strains of mutans streptococci. S. sobrinus 6715 is commonly used in experimental caries models specifically because of its ability to strongly induce both smooth-surface and sulcal caries. Likewise, S. mutans UA159 is strongly cariogenic in rats and capable of inducing a potent ATR. The cariogenic properties of S. sobrinus CH125/43, a nursing caries isolate, and of strain SL1 have not, to our knowledge, been assessed since the strains were isolated and subjected to laboratory passage. Thus, it is not possible to determine from existing information whether an ATR is a necessary trait for cariogenic microorganisms.
The ability of S. mutans to survive environmental acidification appears to be directly related to the membrane-bound, proton-translocating ATPase (F-ATPase) (37). In S. mutans, it was demonstrated that a decrease in the external pH causes an increase in F-ATPase activity and that this enhanced activity is correlated with the mounting of an ATR (3, 16, 37). Proton extrusion via the F-ATPase results in an internal pH that is more basic than that of the environment, conferring some protection to acid-sensitive glycolytic enzymes and maintaining a ΔpH for bioenergetic processes. Here, the enhanced acid tolerance and glycolytic capacities of pH 5.0-grown S. sobrinus could not be attributed to increased F-ATPase activities. Also of note, the enhanced acid resistance of S. sobrinus was not correlated with constitutive levels of ATPase activity higher than those for S. mutans or to differences in the optimum pH for enzyme activity. The S. sobrinus F-ATPase enzyme was expressed at a level of approximately 20 μmol min−1 mg of protein−1, essentially identical to the levels found for S. mutans UA159. Also, optimal activity at the S. sobrinus ATPase was near pH 6.0 (data not shown), similar to what was described for S. mutans (42). Collectively, these results reveal a striking and fundamental difference in the mechanisms underlying adaptive acid tolerance in two closely related oral bacteria.
Differences in acid tolerance mechanisms between these two organisms were not restricted to ATPase expression. In S. mutans UA159, acid-adapted cells were shown to have increased proportions of monounsaturated membrane fatty acids compared with unadapted cells grown at pH 7.0 (36). However, in the same study, S. sobrinus 6715 showed only minimal changes in its membrane fatty acid composition when grown at low pH. Similarly, a lack of sustained up-regulation of DnaK in S. sobrinus growing at pH 5.0, in contrast to what was been observed in S. mutans (23), is yet another major difference in the behavior of these organisms during acid adaptation. The lack of up-regulation of the DnaK chaperone also indicates that the cells are not perceiving a stress condition when grown at pH 5.0, indicating that the cells are maintaining an adequate ΔpH to avoid protein denaturation. Thus, S. sobrinus must possess other mechanisms to reduce proton permeability or to prevent acid damage to the cells.
Among the more intriguing findings in our results is the linkage of elevated glucose PTS activity with enhanced acid resistance. At low sugar concentrations, which are thought to exist for sustained periods in dental plaque due to fasting by the host, the high-affinity sugar PTS is the primary transport system and plays an important role in carbohydrate uptake (19, 25, 26, 45). Early chemostat studies with S. mutans Ingbritt suggested that the glucose PTS was repressed under a variety of conditions, including growth at low pH, faster growth rates, and growth under glucose excess conditions (17, 18). Subsequent continuous-culture studies with S. mutans Ingbritt demonstrated that repression of the glucose PTS was associated with reduced synthesis of the sugar-specific permease EIIGlc (47). Our results with the S. mutans strain UA159 followed the same patterns described above. On the other hand, S. sobrinus 6715 and SL1 grown at steady state at pH 5.0 had 1.6- and 2.7-fold more glucose PTS activity, respectively, than cells grown at pH 7.0, which correlated well with expression of the gene for EIIGlc. It is also noteworthy that, compared to that for S. mutans UA159 growing at pH 5.0, overall glucose PTS activity in S. sobrinus is some three- to fivefold higher. Increases in S. sobrinus PTS activity caused by acidification of the growth medium were restricted to the glucose PTS, since the fructose and mannose PTS activities were not affected by pH. Because the PTS also plays important roles in the regulation of gene expression (1, 24, 35, 38, 40, 41, 45), we speculate that changes in expression of the glucose PTS could modify S. sobrinus acid tolerance by modulating gene expression patterns in a way that enhances protection against environmental acidification. In Escherichia coli, EIIGlc was shown to negatively regulate expression of the σS (rpoS) subunit of RNA polymerase, which controls transcription of a variety of stationary-phase and stress survival genes (44). Although, to the best of our knowledge, oral streptococci lack rpoS, it is possible that other stress regulators are under EIIGlc control. Another possibility is that increases in the glucose PTS result in higher rates of ATP generation through glycolysis, thus enhancing the ability of the cells to maintain ΔpH. However, in our pH drop experiments with S. sobrinus 6715, the rates of glycolysis of pH 7.0- and pH 5.0-grown cells, as measured by the time it took the cells to lower the pH to 3.8, appear to be the same. Therefore, the role of the glucose PTS in acid tolerance is potentially complex, and additional studies are needed to better understand the linkage of the PTS enzymes with the ATR.
In summary, the data presented in this study indicate that S. sobrinus is constitutively acid tolerant and is capable of developing an adaptive ATR during cultivation at acidic pH values that can readily be achieved in dental plaque. Elucidation of the molecular genetics mechanisms governing adaptation of S. sobrinus to acid will lead to a better understanding of the pathogenic mechanisms of this bacterium. The opportunity to contrast the acid-adaptive strategies of S. sobrinus and S. mutans should provide much-needed insight into the genetic and physiologic control of acid tolerance in important human pathogens.
Acknowledgments
This work was supported by grants RO1 DE13239 and DE12236 from the NIDCR and by FAPESP (01/14271-9).
We thank The Institute for Genomic Research for early release of the preliminary data of the S. sobrinus 6715 genome sequence. We also thank Ann R. Griswold for critical evaluation of the manuscript and Robert E. Marquis for helpful discussions.
REFERENCES
- 1.Abranches, J., Y. Y. Chen, and R. A. Burne. 2003. Characterization of Streptococcus mutans strains deficient in EIIAB Man of the sugar phosphotransferase system. Appl. Environ. Microbiol. 69:4760-4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, E. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1987. Current protocols in molecular biology. John Wiley & Sons, New York, N.Y.
- 3.Belli, W. A., and R. E. Marquis. 1991. Adaptation of Streptococcus mutans and Enterococcus hirae to acid stress in continuous culture. Appl. Environ. Microbiol. 57:1134-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bender, G. R., S. V. Sutton, and R. E. Marquis. 1986. Acid tolerance, proton permeabilities, and membrane ATPases of oral streptococci. Infect. Immun. 53:331-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burne, R. A., K. Schilling, W. H. Bowen, and R. E. Yasbin. 1987. Expression, purification, and characterization of an exo-β-d-fructosidase of Streptococcus mutans. J. Bacteriol. 169:4507-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlsson, P., I. A. Gandour, B. Olsson, B. Rickardsson, and K. Abbas. 1987. High prevalence of mutans streptococci in a population with extremely low prevalence of dental caries. Oral Microbiol. Immunol. 2:121-124. [DOI] [PubMed] [Google Scholar]
- 7.Chen, Y.-Y. M., C. A. Weaver, D. R. Mendelsohn, and R. A. Burne. 1998. Transcriptional regulation of the Streptococcus salivarius 57.I urease operon. J. Bacteriol. 180:5769-5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cvitkovitch, D. G., D. A. Boyd, T. Thevenot, and I. R. Hamilton. 1995. Glucose transport by a mutant of Streptococcus mutans unable to accumulate sugars via the phosphoenolpyruvate phosphotransferase system. J. Bacteriol. 177:2251-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Soet, J. J., F. A. Toors, and J. de Graaff. 1989. Acidogenesis by oral streptococci at different pH values. Caries Res. 23:14-17. [DOI] [PubMed] [Google Scholar]
- 10.de Soet, J. J., C. van Loveren, A. J. Lammens, M. J. Pavicic, C. H. Homburg, J. M. ten Cate, and J. de Graaff. 1991. Differences in cariogenicity between fresh isolates of Streptococcus sobrinus and Streptococcus mutans. Caries Res. 25:116-122. [DOI] [PubMed] [Google Scholar]
- 11.Emilson, C. G., P. Carlsson, and D. Bratthall. 1987. Strains of mutans streptococci isolated in a population with extremely low caries prevalence are cariogenic in the hamster model. Oral Microbiol. Immunol. 2:183-186. [DOI] [PubMed] [Google Scholar]
- 12.Fozo, E. M., and R. G. Quivey, Jr. 2004. Shifts in the membrane fatty acid profile of Streptococcus mutans enhance survival in acidic environments. Appl. Environ. Microbiol. 70:929-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griswold, A. R., Y. Y. Chen, and R. A. Burne. 2004. Analysis of an agmatine deiminase gene cluster in Streptococcus mutans UA159. J. Bacteriol. 186:1902-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hahn, K., R. C. Faustoferri, and R. G. Quivey, Jr. 1999. Induction of an AP endonuclease activity in Streptococcus mutans during growth at low pH. Mol. Microbiol. 31:1489-1498. [DOI] [PubMed] [Google Scholar]
- 15.Hamada, S., and H. D. Slade. 1980. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol. Rev. 44:331-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamilton, I. R., and N. D. Buckley. 1991. Adaptation by Streptococcus mutans to acid tolerance. Oral Microbiol. Immunol. 6:65-71. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton, I. R., and D. C. Ellwood. 1978. Effects of fluoride on carbohydrate metabolism by washed cells of Streptococcus mutans grown at various pH values in a chemostat. Infect. Immun. 19:434-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamilton, I. R., P. J. Phipps, and D. C. Ellwood. 1979. Effect of growth rate and glucose concentration on the biochemical properties of Streptococcus mutans Ingbritt in continuous culture. Infect. Immun. 26:861-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamilton, I. R., and E. J. St Martin. 1982. Evidence for the involvement of proton motive force in the transport of glucose by a mutant of Streptococcus mutans strain DR0001 defective in glucose-phosphoenolpyruvate phosphotransferase activity. Infect. Immun. 36:567-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanna, M. N., R. J. Ferguson, Y. H. Li, and D. G. Cvitkovitch. 2001. uvrA is an acid-inducible gene involved in the adaptive response to low pH in Streptococcus mutans. J. Bacteriol. 183:5964-5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harper, D. S., and W. J. Loesche. 1984. Growth and acid tolerance of human dental plaque bacteria. Arch. Oral Biol. 29:843-848. [DOI] [PubMed] [Google Scholar]
- 22.Hirose, H., K. Hirose, E. Isogai, H. Miura, and I. Ueda. 1993. Close association between Streptococcus sobrinus in the saliva of young children and smooth-surface caries increment. Caries Res. 27:292-297. [DOI] [PubMed] [Google Scholar]
- 23.Jayaraman, G. C., J. E. Penders, and R. A. Burne. 1997. Transcriptional analysis of the Streptococcus mutans hrcA, grpE and dnaK genes and regulation of expression in response to heat shock and environmental acidification. Mol. Microbiol. 25:329-341. [DOI] [PubMed] [Google Scholar]
- 24.Kamionka, A., S. Parche, H. Nothaft, J. Siepelmeyer, K. Jahreis, and F. Titgemeyer. 2002. The phosphotransferase system of Streptomyces coelicolor. Eur. J. Biochem. 269:2143-2150. [DOI] [PubMed] [Google Scholar]
- 25.Keevil, C. W., P. D. Marsh, and D. C. Ellwood. 1984. Regulation of glucose metabolism in oral streptococci through independent pathways of glucose 6-phosphate and glucose 1-phosphate formation. J. Bacteriol. 157:560-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keevil, C. W., A. S. McDermid, P. D. Marsh, and D. C. Ellwood. 1986. Protonmotive force driven 6-deoxyglucose uptake by the oral pathogen, Streptococcus mutans Ingbritt. Arch. Microbiol. 146:118-124. [DOI] [PubMed] [Google Scholar]
- 27.Kohler, B., and B. Krasse. 1990. Human strains of mutans streptococci show different cariogenic potential in the hamster model. Oral Microbiol. Immunol. 5:177-180. [DOI] [PubMed] [Google Scholar]
- 28.LeBlanc, D. J., V. L. Crow, L. N. Lee, and C. F. Garon. 1979. Influence of the lactose plasmid on the metabolism of galactose by Streptococcus lactis. J. Bacteriol. 137:878-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemos, J. A., R. A. Burne, and A. C. Castro. 2000. Molecular cloning, purification and immunological responses of recombinants GroEL and DnaK from Streptococcus pyogenes. FEMS Immunol. Med. Microbiol. 28:121-128. [DOI] [PubMed] [Google Scholar]
- 30.Lemos, J. A., Y. Y. Chen, and R. A. Burne. 2001. Genetic and physiologic analysis of the groE operon and role of the HrcA repressor in stress gene regulation and acid tolerance in Streptococcus mutans. J. Bacteriol. 183:6074-6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li, Y. H., P. C. Lau, N. Tang, G. Svensater, R. P. Ellen, and D. G. Cvitkovitch. 2002. Novel two-component regulatory system involved in biofilm formation and acid resistance in Streptococcus mutans. J. Bacteriol. 184:6333-6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loesche, W. J. 1986. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 50:353-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreno, M. S., B. L. Schneider, R. R. Maile, W. Weyler, and M. H. Saier, Jr. 2001. Catabolite repression mediated by the CcpA protein in Bacillus subtilis: novel modes of regulation revealed by whole-genome analyses. Mol. Microbiol. 39:1366-1381. [DOI] [PubMed] [Google Scholar]
- 34.O'Farrell, P. H. 1975. High resolution two-dimensional electrophoresis of proteins. J. Biol. Chem. 250:4007-4021. [PMC free article] [PubMed] [Google Scholar]
- 35.Postma, P. W., J. W. Lengeler, and G. R. Jacobson. 1993. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol. Rev. 57:543-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quivey, R. G., Jr., R. Faustoferri, K. Monahan, and R. Marquis. 2000. Shifts in membrane fatty acid profiles associated with acid adaptation of Streptococcus mutans. FEMS Microbiol. Lett. 189:89-92. [DOI] [PubMed] [Google Scholar]
- 37.Quivey, R. G., Jr., W. L. Kuhnert, and K. Hahn. 2000. Adaptation of oral streptococci to low pH. Adv. Microb. Physiol. 42:239-274. [DOI] [PubMed] [Google Scholar]
- 38.Saier, M. H., Jr., S. Chauvaux, G. M. Cook, J. Deutscher, I. T. Paulsen, J. Reizer, and J. J. Ye. 1996. Catabolite repression and inducer control in gram-positive bacteria. Microbiology 142(Pt. 2):217-230. [DOI] [PubMed] [Google Scholar]
- 39.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cols Spring Harbor, N.Y.
- 40.Sondej, M., A. B. Weinglass, A. Peterkofsky, and H. R. Kaback. 2002. Binding of enzyme IIAGlc, a component of the phosphoenolpyruvate:sugar phosphotransferase system, to the Escherichia coli lactose permease. Biochemistry 41:5556-5565. [DOI] [PubMed] [Google Scholar]
- 41.Stulke, J., and W. Hillen. 1998. Coupling physiology and gene regulation in bacteria: the phosphotransferase sugar uptake system delivers the signals. Naturwissenschaften 85:583-592. [DOI] [PubMed] [Google Scholar]
- 42.Sturr, M. G., and R. E. Marquis. 1992. Comparative acid tolerances and inhibitor sensitivities of isolated F-ATPases of oral lactic acid bacteria. Appl. Environ. Microbiol. 58:2287-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Svensater, G., U. B. Larsson, E. C. Greif, D. G. Cvitkovitch, and I. R. Hamilton. 1997. Acid tolerance response and survival by oral bacteria. Oral Microbiol. Immunol. 12:266-273. [DOI] [PubMed] [Google Scholar]
- 44.Ueguchi, C., N. Misonou, and T. Mizuno. 2001. Negative control of rpoS expression by phosphoenolpyruvate:carbohydrate phosphotransferase system in Escherichia coli. J. Bacteriol. 183:520-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vadeboncoeur, C., and M. Pelletier. 1997. The phosphoenolpyruvate:sugar phosphotransferase system of oral streptococci and its role in the control of sugar metabolism. FEMS Microbiol. Rev. 19:187-207. [DOI] [PubMed] [Google Scholar]
- 46.Vadeboncoeur, C., S. St Martin, D. Brochu, and I. R. Hamilton. 1991. Effect of growth rate and pH on intracellular levels and activities of the components of the phosphoenolpyruvate:sugar phosphotransferase system in Streptococcus mutans Ingbritt. Infect. Immun. 59:900-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vadeboncoeur, C., L. Thibault, S. Neron, H. Halvorson, and I. R. Hamilton. 1987. Effect of growth conditions on levels of components of the phosphoenolpyruvate:sugar phosphotransferase system in Streptococcus mutans and Streptococcus sobrinus grown in continuous culture. J. Bacteriol. 169:5686-5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wen, Z. T., and R. A. Burne. 2004. LuxS-mediated signaling in Streptococcus mutans is involved in regulation of acid and oxidative stress tolerance and biofilm formation. J. Bacteriol. 186:2682-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]