Abstract
The Escherichia coli nitric oxide sensor NorR was shown to bind to the promoter region of the norVW transcription unit, forming at least two distinct complexes detectable by gel retardation. Three binding sites for NorR and two integration host factor binding sites were identified in the norR-norV intergenic region. The derived consensus sequence for NorR binding sites was used to search for novel members of the E. coli NorR regulon and to show that NorR binding sites are partially conserved in other members of the proteobacteria.
NorR of Escherichia coli is a σ54-dependent transcriptional activator that regulates expression of the norVW genes encoding flavorubredoxin and its associated redox partner, respectively (4, 10, 15). The flavorubredoxin has a nitric oxide (NO) reductase activity and submicromolar affinity for NO and appears to have a role in NO tolerance under anoxic and microoxic growth conditions (9, 11, 13, 15). The norVW genes are induced ∼10-fold in Salmonella enterica serovar Typhimurium growing in macrophages (compared to in vitro growth), which is consistent with a role for the flavorubredoxin in protecting against macrophage-derived NO (7). In E. coli, NorR activates norVW transcription in anaerobic cultures in response to NO, nitrate, and nitrite (which may act as sources of endogenous NO) and nitroprusside and in aerobic cultures in response to S-nitrosoglutathione, acidified nitrite, and nitroprusside (4, 10, 15, 19). Transcription of the norVW genes can also be activated by NO in aerobic cultures of an hmp mutant, which lacks the flavohemoglobin that oxidizes NO to nitrate in the presence of oxygen (10). The fact that NorR can regulate gene expression under oxic conditions (which inactivate the NO reductase activity of the flavorubredoxin) raises the possibility that there are additional genes regulated by NorR, the products of which have roles in aerobically growing cultures. The homologous NorR protein of Ralstonia eutropha regulates expression of an NO reductase and can also be activated by nitroprusside and, presumably, NO (20).
A recent transcriptomic analysis using microarrays revealed norV and norW to be the most highly induced genes in aerobic cultures exposed to either S-nitrosoglutathione or acidified nitrite (19). Several E. coli regulatory proteins besides NorR have their activities modulated by sources of NO and nitrosative stress, including SoxR, OxyR, Fur, and FNR (3, 5, 6, 16). It has been argued that, of all of these proteins, NorR is perhaps the most important physiological NO sensor and is the only known regulatory protein in enteric bacteria that has evolved exclusively to serve this purpose (19). The array analysis also provided some evidence to suggest that there are other targets for NorR regulation in E. coli besides norVW (19).
Knowledge of the DNA binding specificity of a regulator is an important complement to array data in the identification of regulon members (23). As part of an effort to understand the mechanism of regulation of the norVW promoter and to help identify additional NorR targets, we have set out to determine the DNA sequence(s) recognized by NorR in the norR-norVW intergenic region. Taking advantage of the fact that the σ54-dependent transcriptional activators bind to DNA even when they are in their inactive configuration, we have defined three NorR binding sites by using purified protein in DNase I footprinting and methylation protection assays. The characterization of NorR binding sites will facilitate the identification of any novel members of the NorR regulon and leads to the conclusion that NorR DNA recognition sites are partially conserved in several proteobacteria.
Purified NorR binds to the norR-norVW intergenic region.
The start codon of the E. coli norR gene is probably incorrectly annotated in the GenBank database (accession no. NC 000913), because the predicted protein is ∼25 residues longer than its homologues at the N terminus. Accordingly, we and others (10) assume that the true start codon is located at coordinate 2830328 (accession no. NC 000913), as was predicted when the gene was first sequenced (22). To confirm that a protein initiating at this position is functional, an NdeI site incorporating the start codon was introduced into the norR gene, along with a VspI site adjacent to the stop codon. The NdeI-VspI fragment was cloned into pET21a (Novagen); the recombinant plasmid activated norV-lacZ expression in a norR mutant (14) when nitroprusside, nitrite, or nitrate was added to anaerobic cultures (data not shown), confirming that the expressed protein can fully complement the norR mutation. Complementation presumably requires the low level of expression that can occur from pET clones in a strain lacking the gene for T7 RNA polymerase (17).
NorR was overexpressed in strain BL21(DE3) and was purified by heparin agarose chromatography and gel filtration, using procedures similar to those described previously for other σ54-dependent activators, such as NtrC and NifA (1, 8). Gel retardation experiments in 10 mM Tris-HCl (pH 8.0)-5 mM MgCl2-30 mM KCl-0.4 mg of BSA/ml demonstrated that NorR binds to a 362-bp DNA fragment spanning the norR-norVW intergenic region (Fig. 1A). At relatively low concentrations of NorR, two distinct retarded species were evident (Fig. 1A, arrows A and B), suggesting the presence of multiple NorR binding sites. At NorR concentrations above 140 nM, only one retarded species (band A) was observed, presumably as a result of the complete occupation of NorR binding sites. No retardation of a labeled nifH promoter fragment was observed when incubated with 500 nM NorR, confirming specificity for the norVW promoter (Fig. 1A, lanes 15 and 16). Interestingly, retardation of the norR-norVW fragment was also observed with integration host factor (IHF) (Fig. 1A, arrow C), which could provide a mechanism for facilitating the interaction between NorR and the σ54 RNA polymerase (14, 24). When quantified (Fig. 1B), the NorR binding data give rise to a sigmoidal curve characteristic of cooperative binding, as observed with NtrC (21, 26). This suggests that NorR has a higher affinity for one site, the occupation of which facilitates further binding of NorR to the adjacent site(s).
FIG. 1.
Binding of NorR to the norR-norV intergenic region. (A) Gel mobility shift assays contained a 32P-labeled 362-bp EcoRI-BamHI fragment spanning the norR-norV intergenic region. NorR-retarded species are labeled A and B. Concentrations (nanomolar) of NorR were 0 (lane 2), 1 (lane 3), 20 (lane 4), 40 (lane 5), 60 (lane 6), 80 (lane 7), 100 (lane 8), 120 (lane 9), 140 (lane 10), 160 (lane 11), 180 (lane 12), 200 (lane 13), and 500 (lane 14). Lane 1 contained 1 μM IHF; the IHF-bound species is labeled C. A 360-bp fragment containing the nifH promoter (lane 15) was not bound by 500 nM NorR (lane 16). (B) Three independent gel mobility shift experiments (including the example shown in panel A) were quantified with a Fujix BAS 1000 phosphorimager. The total amount of retarded DNA is plotted as a percentage of the radioactivity present in each lane.
Footprinting NorR binding sites.
DNase I footprinting was performed under the same conditions as the gel retardation assays to identify the location of the NorR binding sites (Fig. 2). Three zones of protection were observed, denoted by bars labeled 1, 2, and 3 in Fig. 2. Similar protection was observed with the opposite strand of the DNA (data not shown). Protection by NorR in region 1 appears to be maintained at lower NorR concentrations (∼80 nM) than that observed for regions 2 and 3 (>∼140 nM), suggesting a mechanism for the cooperative binding of NorR to the norVW promoter that was indicated by the gel retardation experiments. Interestingly, the high-affinity site is ideally positioned to act as an upstream activator sequence for norVW, while sites 2 and 3 are located closer to the norR coding region. Although the significance of the multiple sites has not been established, it is possible that the high-affinity site is primarily required for NorR-dependent norVW regulation, while one or both of the lower affinity sites are involved in norR autoregulation (15).
FIG. 2.
DNase I footprinting of NorR with the template strand of the norVW promoter. The DNA fragment was the 362-bp EcoRI-BamHI fragment, 5′ end-labeled at the EcoRI site. G+A sequencing tracks prepared with the Maxam and Gilbert method are in lanes 1 and 15. Lane 14 contained no NorR. Binding reactions shown in lanes 3 to 14 contained increasing concentrations of NorR identical to those shown in Fig. 1. Regions of NorR protection were deduced from three independent experiments and are denoted by the solid lines to the right of the footprint, labeled 1 for the high-affinity site and 2 and 3 for the low-affinity sites.
To localize NorR binding sites more precisely and to establish which guanosine residues make close contacts with bound protein, methylation protection patterns were determined on both strands of the DNA fragment, using dimethyl sulfate as a footprinting reagent (Fig. 3A). Clear protection of guanosine residues by NorR was observed at all three of the sites identified by DNase I footprinting (compare Fig. 2 and 3A), thus confirming the locations of the NorR binding sites. There are very similar patterns of methylation protection and enhancement at each site, indicating that NorR interacts with each sequence in a similar manner. Confirmation of the predicted σ54 recognition site (25) was obtained from methylation protection experiments with the σ54-RNA polymerase holoenzyme (Fig. 3B). The pattern of protection was very similar to that demonstrated at other σ54-dependent promoters, with protection of G residues corresponding to the consensus GG positions at −25, −24, and −13 on the coding strand and residues corresponding to −23 and −12 on the noncoding strand (2, 18).
FIG. 3.
Methylation protection of the norR-norV intergenic region by NorR (A) and σ54 RNA polymerase (B). Binding reaction mixtures contained the 362-bp EcoRI-BamHI promoter fragment 5′ end-labeled at either the EcoRI end (lanes 1 and 2) or the BamHI end (lanes 3 and 4) and were treated with dimethyl sulfate. In each case, lanes 1 and 3 contained DNA only and lanes 2 and 4 contained either 500 nM NorR (A) or 1 μM σ54 plus 1.5 μM RNA polymerase (B). Residues are numbered with respect to the norV transcription start site, designated +1 (see Fig. 4 and 5). Protected G residues are marked with lollipops, and enhanced methylation at G residues is denoted by arrows.
To localize the norVW transcription start site, RNA was extracted from anaerobically grown cells and was analyzed by primer extension. As observed previously (4, 19), transcripts were not detectable in untreated cultures (Fig. 4, lane 1), but strong activation of the promoter was evident in cells treated with nitrite and nitroprusside (Fig. 4, lanes 2 and 4). Nitrate did not significantly induce transcription under these conditions, possibly because the treatment time (20 min) was too short for the generation of sufficient levels of nitrite and/or NO. As would be predicted, the major norV mRNA initiates at a cytidine residue 24 bp downstream of the GG motif of the σ54 promoter.
FIG. 4.
Localization of the norVW mRNA start site by primer extension. Anaerobic cultures of E. coli DH10B grown in LB medium supplemented with 0.1% glucose were grown to exponential phase (optical density at 650 nm, 0.45) and were treated with 4 mM NaNO2 (lane 2), 40 mM NaNO3 (lane 3), or 100 μM sodium nitroprusside (lane 4). After 20 min, total RNA was extracted and 20 μg was used for each reaction. Lane 1 contains the untreated control. Dideoxy sequencing reactions (lanes G, A, T, and C) were prepared with the same primer. The arrow marks the major norVW transcriptional start site.
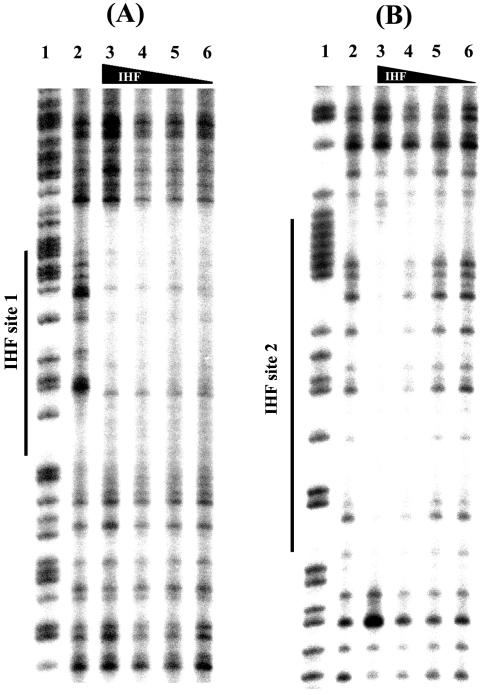
Footprinting IHF binding sites.
DNase I footprinting identified two distinct IHF binding sites in the norVW promoter region (Fig. 5). Site 1 is fully occupied at IHF concentrations lower than those for site 2, and it is located between the σ54 promoter and the promoter-proximal NorR binding site (Fig. 6). The location of this binding site is consistent with a role for IHF in bending DNA to bring NorR into contact with RNA polymerase. The lower affinity IHF site 2 is within the norV-transcribed region (Fig. 6). IHF binding sites have been observed in the transcribed regions of other promoters, such as in the csgD gene (12).
FIG. 5.
DNase I footprinting of IHF with the coding strand of the norVW promoter. The DNA fragment was the 362-bp EcoRI-BamHI fragment, 5′ end-labeled at the BamHI site. IHF site 1 (A) and IHF site 2 (B) are denoted by solid lines. G+A sequencing tracks prepared with the Maxam and Gilbert method are in lane 1 in each case. IHF concentrations in binding reaction mixtures (micromolar) were 0 (lanes 2), 1 (lanes 3), 0.1 (lanes 4), 0.03 (lanes 5), and 0.02 (lanes 6).
FIG. 6.
Protein binding sites in the norR-norV intergenic region. (A) The approximate extent of protection by NorR in DNase I footprinting experiments is indicated by underlining. Nucleotides contacted by NorR or σ54 RNA polymerase are shaded, those protected from methylation by NorR are marked by filled circles; enhancement of methylation is indicated by open circles. The predicted σ54 promoter is boxed. The high-affinity NorR binding site is labeled 1, the low-affinity sites are labeled 2 and 3. The major norV mRNA initiates at a cytidine residue indicated by an arrow. Start codons for norR and norV are shown in bold. IHF binding sites 1 and 2 are labeled and are indicated by wavy lines. (B) Conservation of NorR binding sites among selected bacterial species (Sty, S. enterica serovar Typhimurium; Sfl, S. flexneri; Pae, P. aeruginosa; Reu1, R. eutropha megaplasmid genes; Reu2, R. eutropha chromosomal genes). The three E. coli NorR binding sites are labeled 1, 2, and 3 as in panel A, and norR start codons are shaded. Conserved sequence elements in predicted NorR binding sites and σ54 promoters are highlighted. The promoter is located upstream of genes for flavorubredoxin (E. coli, S. enterica serovar Typhimurium, and S. flexneri), flavohemoglobin (P. aeruginosa), and NO reductase (R. eutropha).
NorR binding sites are present in several proteobacteria.
Alignment of the three E. coli NorR binding sites revealed that sites 1 and 3 both contain the inverted repeat GTCA-(N3)-TGAC, while site 2 is GTCA-(N3)-CGAC (Fig. 6). A weight matrix constructed from both strands of the three NorR binding sites was used with PROMSCAN (http://www.promscan.uklinux.net) to search for additional putative targets in the E. coli genome. No other high-scoring multiple NorR sites were found upstream of known or potential σ54-dependent promoters. We cannot exclude the possibility that NorR sites more divergent to those in the norV promoter (see below) are functional or that potential NorR sites mediate repression (and are therefore not associated with σ54-dependent promoters). The sequences of the three NorR binding sites that we have identified will facilitate future efforts to identify additional members of the NorR regulon by other techniques. Microarray analysis implicated additional targets for NorR regulation, either direct or indirect, such as ybiJ (19). The long noncoding region upstream of ybiJ contains neither a predicted σ54 promoter nor a strongly predicted site for NorR binding, suggesting that ybiJ is not directly activated by NorR.
Alignments of the norVW promoters from E. coli, S. enterica serovar Typhimurium, and Shigella flexneri and the chromosomal and megaplasmid copies of the norAB promoter from R. eutropha (20) indicate that each contains three conserved NorR binding sites with the minimal consensus GT-(N7)-AC (Fig. 5B). The analysis also suggests that NorR activates σ54-dependent promoters upstream of the hmp (fhp) gene (encoding a putative flavohemoglobin) in Pseudomonas aeruginosa (Fig. 5), Pseudomonas putida, and Vibrio cholerae. Conserved sites are also located upstream of a norV-like gene on chromosome II of Vibrio vulnificus (data not shown). Thus, there is, apparently, conservation of mechanisms among the proteobacteria for controlling genes encoding diverse enzymes that use NO as a substrate.
Acknowledgments
This work was funded by a BBSRC grant (83/P18536) to R.D. and S.S.
We thank Gary Sawers and Richard Little for useful discussions and advice.
REFERENCES
- 1.Austin, S., M. Buck, W. Cannon, T. Eydmann, and R. Dixon. 1994. Purification and in vitro activities of the native nitrogen fixation control proteins NIFA and NIFL. J. Bacteriol. 176:3460-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buck, M., and W. Cannon. 1992. Specific binding of the transcription factor sigma-54 to promoter DNA. Nature 358:422-424. [DOI] [PubMed] [Google Scholar]
- 3.Cruz-Ramos, H., J. Crack, G. Wu, M. N. Hughes, C. Scott, A. J. Thomson, J. Green, and R. K. Poole. 2002. NO sensing by FNR: regulation of the Escherichia coli NO-detoxifying flavohaemoglobin, Hmp. EMBO J. 21:3235-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Da Costa, P. N., M. Teixeira, and L. M. Saraiva. 2003. Regulation of the flavorubredoxin nitric oxide reductase gene in Escherichia coli: nitrate repression, nitrite induction, and possible post-transcription control. FEMS Microbiol. Lett. 218:385-393. [DOI] [PubMed] [Google Scholar]
- 5.D'Autréaux, B., D. Touati, B. Bersch, J.-M. Latour, and I. Michaud-Soret. 2002. Direct inhibition by nitric oxide of the transcriptional ferric uptake regulation protein via nitrosylation of the iron. Proc. Natl. Acad. Sci. USA 99:16619-16624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding, H., and B. Demple. 2000. Direct nitric oxide signal transduction via nitrosylation of iron-sulfur centers in the SoxR transcription activator. Proc. Natl. Acad. Sci. USA 97:5146-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eriksson, S., S. Lucchini, A. Thompson, M. Rhen, and J. C. Hinton. 2003. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol. Microbiol. 47:103-118. [DOI] [PubMed] [Google Scholar]
- 8.Farez-Vidal, M., T. Wilson, B. Davidson, G. Howlett, S. Austin, and R. Dixon. 1996. Effector-induced self-association and conformational changes in the enhancer-binding protein NTRC. Mol. Microbiol. 22:779-788. [DOI] [PubMed] [Google Scholar]
- 9.Gardner, A. M., and P. R. Gardner. 2002. Flavohemoglobin detoxifies nitric oxide in aerobic, but not anaerobic, Escherichia coli. Evidence for a novel inducible anaerobic nitric oxide-scavenging activity. J. Biol. Chem. 277:8166-8171. [DOI] [PubMed] [Google Scholar]
- 10.Gardner, A. M., C. R. Gessner, and P. R. Gardner. 2003. Regulation of the nitric oxide reduction operon (norRVW) in Escherichia coli. Role of NorR and σ54 in the nitric oxide stress response. J. Biol. Chem. 278:10081-10086. [DOI] [PubMed] [Google Scholar]
- 11.Gardner, A. M., R. A. Helmick, and P. R. Gardner. 2002. Flavorubredoxin, an inducible catalyst for nitric oxide reduction and detoxification in Escherichia coli. J. Biol. Chem. 277:8172-8177. [DOI] [PubMed] [Google Scholar]
- 12.Gerstel, U., C. Park, and U. Römling. 2003. Complex regulation of csgD promoter activity by global regulatory proteins. Mol. Microbiol. 49:639-654. [DOI] [PubMed] [Google Scholar]
- 13.Gomes, C. M., A. Giuffre, E. Forte, J. B. Vicente, L. M. Saraiva, M. Brunori, and M. Teixeira. 2002. A novel type of nitric-oxide reductase, Escherichia coli flavorubredoxin. J. Biol. Chem. 277:25273-25276. [DOI] [PubMed] [Google Scholar]
- 14.Hoover, T. R., E. Santero, S. Porter, and S. Kustu. 1990. The integration host factor stimulates interaction of RNA polymerase with NIFA, the transcriptional activator for nitrogen fixation operons. Cell 63:11-22. [DOI] [PubMed] [Google Scholar]
- 15.Hutchings, M. I., N. Mandhana, and S. Spiro. 2002. The NorR protein of Escherichia coli activates expression of the flavorubredoxin gene norV in response to reactive nitrogen species. J. Bacteriol. 184:4640-4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, S. O., K. Merchant, R. Nudelman, W. F. Beyer, Jr., T. Keng, J. DeAngelo, A. Hausladen, and J. S. Stamler. 2002. OxyR: a molecular code for redox-related signaling. Cell 109:383-896. [DOI] [PubMed] [Google Scholar]
- 17.Moore, L. J., and P. J. Kiley. 2001. Characterization of the dimerization domain in the FNR transcription factor. J. Biol. Chem. 276:45744-45750. [DOI] [PubMed] [Google Scholar]
- 18.Morett, E., and M. Buck. 1989. In vivo studies on the interaction of RNA polymerase-σ54 with the Klebsiella pneumoniae and Rhizobium meliloti nifH promoters: the role of NifA in the formation of an open promoter complex. J. Mol. Biol. 210:65-77. [DOI] [PubMed] [Google Scholar]
- 19.Mukhopadhyay, P., M. Zheng, L. A. Bedzyk, R. A. LaRossa, and G. Storz. 2004. Prominent roles of the NorR and Fur regulators in the Escherichia coli transcriptional response to reactive nitrogen species. Proc. Natl. Acad. Sci. USA 101:745-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pohlmann, A., R. Cramm, K. Schmelz, and B. Friedrich. 2000. A novel NO-responding regulator controls the reduction of nitric oxide in Ralstonia eutropha. Mol. Microbiol. 38:626-638. [DOI] [PubMed] [Google Scholar]
- 21.Porter, S., A. North, A. Wedel, and S. Kustu. 1993. Oligomerization of NTRC at the glnA enhancer is required for transcriptional activation. Gen. Dev. 7:2258-2273. [DOI] [PubMed] [Google Scholar]
- 22.Ramseier, T. M., R. M. Figge, and M. H. Saier, Jr. 1994. DNA sequence of a gene in Escherichia coli encoding a putative tripartite transcription factor with receiver, ATPase and DNA binding domains. DNA Seq. 5:17-24. [DOI] [PubMed] [Google Scholar]
- 23.Rhodius, V. A., and R. A. LaRossa. 2003. Uses and pitfalls of microarrays for studying transcriptional regulation. Curr. Opin. Microbiol. 6:114-119. [DOI] [PubMed] [Google Scholar]
- 24.Santero, E., T. R. Hoover, A. K. North, D. K. Berger, S. C. Porter, and S. Kustu. 1992. Role of integration host factor in stimulating transcription from the sigma 54-dependent nifH promoter. J. Mol. Biol. 227:602-620. [DOI] [PubMed] [Google Scholar]
- 25.Studholme, D. J., and R. Dixon. 2003. Domain architectures of sigma54-dependent transcriptional activators. J. Bacteriol. 185:1757-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Widdick, D., E. Farez Vidal, S. Austin, and R. Dixon. 1998. Properties of a mutant form of the prokaryotic enhancer binding protein, NTRC, which hydrolyses ATP in the absence of effectors. FEBS Lett. 437:70-74. [DOI] [PubMed] [Google Scholar]