Abstract
Flagella act as semirigid helical propellers that are powered by reversible rotary motors. Two membrane proteins, MotA and MotB, function as a complex that acts as the stator and generates the torque that drives rotation. The genome sequence of Pseudomonas aeruginosa PAO1 contains dual sets of motA and motB genes, PA1460-PA1461 (motAB) and PA4954-PA4953 (motCD), as well as another gene, motY (PA3526), which is known to be required for motor function in some bacteria. Here, we show that these five genes contribute to motility. Loss of function of either motAB-like locus was dispensable for translocation in aqueous environments. However, swimming could be entirely eliminated by introduction of combinations of mutations in the two motAB-encoding regions. Mutation of both genes encoding the MotA homologs or MotB homologs was sufficient to abolish motility. Mutants carrying double mutations in nonequivalent genes (i.e., motA motD or motB motC) retained motility, indicating that noncognate components can function together. motY appears to be required for motAB function. The combination of motY and motCD mutations rendered the cells nonmotile. Loss of function of motAB, motY, or motAB motY produced similar phenotypes; although the swimming speed was only reduced to ∼85% of the wild-type speed, translocation in semisolid motility agar and swarming on the surface of solidified agar were severely impeded. Thus, the flagellar motor of P. aeruginosa represents a more complex configuration than the configuration that has been studied in other bacteria, and it enables efficient movement under different circumstances.
Many bacteria are rapidly propelled through aqueous environments by flagella. Swimming is achieved by rotating a long helical filament that acts as a propeller and is connected to the cell via a flexible hook (reviewed in reference 31). The hook serves as a universal hinge linking the filament to the basal body, which consists of a rod, bushings, and the MS and C rings. The hook-basal body-rod structure acts as a drive shaft in transmitting torque from the rotary motor to the filament. Two cytoplasmic membrane proteins, MotA and MotB, generate the force necessary to drive the propeller. These proteins form a complex that acts as the stator and use membrane potential to conduct ions and supply the torque for rotation (reviewed in references 3 and 28). Flagellar motors can be fueled by protons, as is the case in Escherichia coli and Salmonella enterica serovar Typhimurium, or sodium ions, as in the alkaliphilic Bacillus and marine Vibrio species (19, 30, 34).
Freeze-fracture electron micrograph images show a circular array of particles embedded in the membrane surrounding the flagellar basal body (24). These stud-like particles are believed to be ∼10 MotA/MotB complexes. Gel filtration and cross-linking studies suggest that the stoichiometry of each complex is MotA4MotB2 (29, 50). Controlled expression of motAB and successive inactivation with covalently bound inhibitors produce stepwise changes in the rotation rate, suggesting that each complex can function independently to generate torque (5, 6, 43). The four transmembrane domains of MotA and the one transmembrane domain of MotB form the channel through which ions are conducted (reviewed in reference 4). MotB is thought to additionally serve as a structural support that anchors the MotA/MotB complex to the cell wall via a peptidoglycan-binding domain in the C terminus (9, 11). Another protein with a peptidoglycan-binding domain is required for motor function in some bacteria. MotY was originally discovered as an essential component of the sodium-driven motor in Vibrio species (16, 36, 44). However, MotY does not seem to be a peculiarity of the sodium-type motor. More recently, a MotY homolog (LafY) has been shown to be required for proton-driven motility of the lateral flagellar system of Vibrio parahaemolyticus (54).
Although the molecular mechanism by which the flagellar motor generates torque is not completely understood, it is known that there is tight coupling of the passage of specific ions through the torque-generating complex formed by the MotA and MotB proteins to the rotation of the flagellum (38). Current evidence suggests a model for torque generation resulting from conformational changes in the stator that occur as protons associate and dissociate from the Asp-32 residue in MotB (reviewed in reference 4).
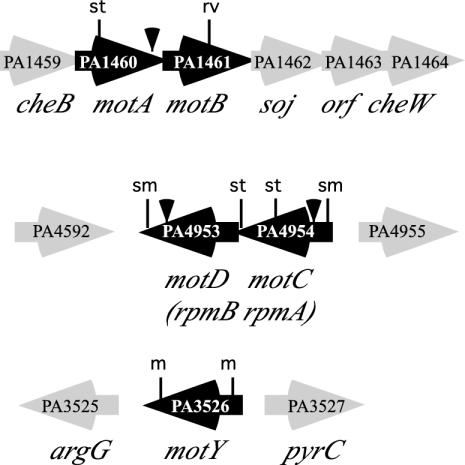
Pseudomonas aeruginosa is a gram negative, rod-shaped, motile bacterium with a polar flagellum. The flagellar genes and hierarchy of transcriptional control have recently been elegantly elucidated by using a combination of mutant analyses and RNA profiling (10); however, little is known about the flagellar motor in this organism. Surprisingly, the genome sequence contains two gene sets encoding MotA and MotB homologs: PA1460/PA1461 and PA4954/PA4953 (Fig. 1). PA1460 and PA1461 are located within a potential operon encoding chemotaxis proteins, whereas PA4954 and PA4953 are uniquely located in an operon elsewhere on the chromosome. BLAST analyses have shown that these predicted motor proteins are more similar to the motor proteins of other organisms than to each other. For example, the gene products of one locus are quite similar to Bacillus subtilis motor proteins; the PA1460 product shows 38% identity and 59% similarity (E = 5e-37) to B. subtilis MotA, and the PA1461 product shows 33% identity and 51% similarity (E = 2e-26) to B. subtilis MotB. The products of PA4954 and PA4953 are quite similar to the S. enterica serovar Typhimurium homologs (42% identity and 66% similarity for MotA [E = 7e-62] and 38% identity and 58% similarity for MotB [E = 2e-54]). In comparison, PA1460 and PA4954 have only 24% identity and 42% similarity (E = 7e-08) and PA1461 and PA4953 have 26% identity and 39% similarity (E = 1e-09).
FIG. 1.
P. aeruginosa flagellar motor genes. Open reading frames are indicated by arrows, and the directions of transcription are indicated by the arrowheads. The solid triangles indicate the positions of the Tn5 (Tetr) insertions. Restriction sites that were used to create drug resistance cassette mutations are indicated as follows: st, StuI; rv, EcoRV; sm, SmaI; and m, MscI. The motAB locus, the rpmAB locus (which we renamed motCD due to its role in motor function), and the motY gene were cloned from strain PAK by using high-fidelity PCR. A gentamicin resistance cassette was inserted into the StuI site to make motA2::Genr, into the EcoRV site to make motB2::Genr, into the StuI and EcoRV sites to make ΔmotAB3::Genr, and into the SmaI sites to make ΔmotCD6. A tetracycline cassette was introduced into StuI sites to create ΔmotC7::Tetr, into SmaI sites to create ΔmotCD8, and into MscI sites to create ΔmotY2::Tetr. In-frame deletions that removed the coding regions for motAB, motCD, and motY in strain PAO1 were also created.
Previously published data do not provide clear evidence about the nature of the flagellar motor in P. aeruginosa. Kato et al. examined the role of PA1460 and PA1461 by constructing mutations with a kanamycin resistance cassette (23). Their studies suggested that these genes are not required for swimming motility, as the insertion mutants were fully motile as determined by microscopy. Insertion mutants with mutations in the other potential motor-encoding locus were obtained while P. aeruginosa-macrophage interactions were being investigated (52). In that study, the workers isolated a mutant with a defect in a gene, designated rpmA, that was required for phagocytosis by macrophages. Sequencing revealed that RpmA is the MotA homolog encoded by PA4954. The phagocytosis phenotype of this mutant could be complemented only when both rpmA and rpmB were supplied in trans, suggesting that the two genes comprised an operon. However, no effect on cell motility was detected in a motility plate or by microscopy. It was therefore concluded that the function of rpmA in P. aeruginosa was different from that of motA in enteric bacteria; i.e., rpmA was not required for motor function.
Thus, the nature of the flagellar motor and the roles of the Mot homologs in P. aeruginosa were not clear. In this work we describe an analysis of mutants with defects in all of the homologs and the contribution of each gene. To test the hypothesis that the dual sets of motor genes have overlapping functions, a series of strains with single and multiple mot gene lesions were constructed. Analyses of mot mutants were performed with PAK and PAO1 strains, and similar results were obtained.
MATERIALS AND METHODS
Bacterial strains, plasmids and media.
The strains and plasmids used in this study are listed in Table 1. Strains were derived from the P. aeruginosa PAK (obtained from Pathogenesis Corporation, Seattle, Wash.), PAO1 (obtained from Barbara Iglewski via E. P Greenberg), or MPAO1 (obtained from C. Manoil). Strains were routinely propagated at 37°C in Luria-Bertani (LB) broth, which contained (per liter) 10 g of tryptone, 5 g of yeast extract, and 5 g of NaCl. Escherichia coli strains S17-1 and SM10 DAP− were used for conjugational transfer to P. aeruginosa (55). Tryptone motility medium contained 10 g tryptone per liter and 5 g NaCl per liter and was supplemented with 0.325% (wt/vol) Bacto Agar (Difco) unless indicated otherwise. Swarming plates were prepared by using M9 minimal medium with 0.2% (wt/vol) glucose as the carbon source and 0.05% (wt/vol) glutamate as the nitrogen source (27). The plates were wrapped in Parafilm and incubated at 37°C for ∼2 days. The inocula used for swarming were liquid cultures grown overnight in the same minimal medium. PAK strains swarmed best on plates with 0.5% agar, whereas PAO1 strains swarmed best on plates with 0.6% agar. Motility plates were sometimes incubated at lower temperatures (room temperature or 30°C) for photodocumentation. Antibiotics were used at the following concentrations: for E. coli ampicillin was used at a concentration of 75 μg/ml, gentamicin was used at a concentration of 40 μg/ml, and tetracycline was used at a concentration of 10 μg/ml; for P. aeruginosa carbenicillin was used at a concentration of 300 μg/ml, gentamicin was used at a concentration of 40 μg/ml in rich medium and at a concentration of 100 μg/ml in minimal medium, and tetracycline was used at a concentration of 50 μg/ml. dl-α,ɛ-Diaminopimelic acid was used at a final concentration of 0.32 mM.
TABLE 1.
P. aeruginosa strains and plasmids used in this study
| Strain or plasmid | Genotype or description | Parent, source, and/or reference |
|---|---|---|
| PAK strains | ||
| PAK | Wild-type P. aeruginosa | Pathogenesis Corp. |
| LMP1 | PAK containing Tn5 (Tetr); wild-type swimming and swarming | PAK; Pathogenesis Corp. |
| LMP2 | Genr PAK containing Tn5 lux(Genr); wild-type swimming and swarming | PAK; Pathogenesis Corp. |
| LMP4 | motA1::Tn5 (Tetr); Tn5 disrupts gene at position coding for amino acid 229 of MotA | PAK; Pathogenesis Corp. (= 074G05) |
| LMP5 | motA2::Genr; Gen cassette disrupts gene at position coding for amino acid 93 of MotA | PAK; this study |
| LMP6 | motB3::Genr; Gen cassette disrupts gene at position coding for amino acid 148 of MotB | PAK; this study |
| LMP7 | motC1::Tn5 (Tetr); Tn5 disrupts gene at position coding for amino acid 49 of MotC | PAK; Pathogenesis Corp. (= 028C02) |
| LMP8 | motD2::Tn5 (Tetr); Tn5 disrupts gene at position coding for amino acid 310 of MotD | PAK; Pathogenesis Corp. (= 024E04) |
| LMP9 | ΔmotAB4::Genr; deletion of 0.9-kb region coding from amino acid 93 of MotA to amino acid 148 of MotB with insertion of a Genr cassette | PAK; this study |
| LMP13 | ΔmotCD6::Genr; deletion of 1.6-kb region coding from amino acid 49 of MotC to amino acid 310 of MotD with insertion of a Genr cassette | PAK; this study |
| LMP15 | ΔmotAB4::GenrmotC1::Tn5 (Tetr) | LMP7; this study |
| LMP16 | ΔmotAB4::GenrmotD2::Tn5 (Tetr) | LMP8; this study |
| LMP17 | motA2::GenrmotC1::Tn5 (Tetr) | LMP7; this study |
| LMP18 | motA2::GenrmotD2::Tn5 (Tetr) | LMP8; this study |
| LMP19 | motB3::GenrmotC1::Tn5 (Tetr) | LMP7; this study |
| LMP20 | motB3::GenrmotD2::Tn5 (Tetr) | LMP8; this study |
| LMP44 | ΔmotC7::Tetr | PAK; this study |
| LMP47 | motA2::Genr ΔmotC7::Tetr | LMP5; this study |
| LMP48 | ΔmotCD8::TetrmotA2::Genr | LMP5; this study |
| LMP49 | motB3::Genr ΔmotC7::Tetr | LMP6; this study |
| LMP50 | motB3::Genr ΔmotCD8::Tetr | LMP6; this study |
| LMP54 | ΔmotY2::Tetr | PAK; this study |
| LMP55 | ΔmotAB4::Genr ΔmotY2::Tetr | LMP9; this study |
| LMP57 | flgC1::Tn5 (Tetr) | PAK; Pathogenesis Corp. (= 056E02) |
| LMP60 | ΔmotCD6::Genr ΔmotY2::Tetr | LMP13 |
| LMP61 | cheB1::Tn5 (Tetr) | PAK; Pathogenesis Corp. (= 024A09) |
| LMP65 | Genr Tetr PAK; wild-type swimming and swarming motility | PAK; this study |
| PAO1 strains | ||
| IgPAO1 | Wild type | Iglewski via Greenberg |
| MPAO1 | Wild type | 20 |
| PAO1000 | ΔcheW | 13 |
| PAO1001 | ΔmotAB | MPAO1; this study |
| PAO1002 | ΔmotCD | MPAO1; this study |
| PAO1003 | ΔmotY | MPAO1; this study |
| PAO2000 | motY::Tn5 (Genr) | IgPAO1; this study |
| Plasmids | ||
| pEX18Gm | GenroriT+sacB+ allelic exchange vector | 18 |
| pKV69 | Tetr Camr allelic exchange vector | K. L. Visick (Loyola University) |
| pGP704 | Ampr allelic exchange with R6K origin | 39 |
DNA manipulations.
Plasmid DNA was prepared by using a QIAprep Spin Miniprep kit (QIAGEN, Chatsworth, Calif.). Agarose gel electrophoresis, DNA conjugation, DNA restriction digestion, DNA ligation, Southern blotting, and transformation were performed essentially as previously described (48). Chromosomal DNA was isolated as described by Woo et al. (58).
Mutant construction.
Mutant strains were constructed by PCR amplification of the genes of interest by using a high-fidelity polymerase (Pfu or Herculase from Stratagene, La Jolla, Calif.). PCR products were ligated into the vectors pKV69 (Tetr Camr), pGP704 (Apr), and pEX18Gm (Genr). The gentamicin cassette was amplified from pGMΩ1 (51), and it was inserted into the StuI site to make motA2::Genr, into the EcoRV site to make motB2::Genr, and into the StuI and EcoRV sites to make ΔmotAB3::Genr. The tetracycline cassette was amplified from pBR322 (New England Biolabs, Beverly, Mass.) and was inserted into the StuI sites to create ΔmotC7::Tetr, into the SmaI sites to create ΔmotCD8, and into the MscI sites to create ΔmotY2::Tetr. Mutated genes were introduced into the chromosome by homologous recombination. In-frame deletion mutant strains were constructed by overlap extension PCR as described previously (13). Regions of DNA upstream (963 bp) and downstream (999 bp) of motAB were amplified and used as templates for a third PCR amplification. The resulting 1,968-bp product had a 1,638-bp deletion of motAB, which left only the ATG of motA, an engineered BamHI site, and the TGA codon of motB. The in-frame deletion of motCD (PA4954-PA4953) was constructed in a similar manner. The upstream (944-bp) and downstream (1,023-bp) products were used as templates for a third PCR amplification. The resulting product had a 1,898-bp deletion of motCD, which left only the first three codons of motC, a BamHI site, and the last three codons of motD. To construct the in-frame deletion of motY (PA3526), the upstream (933-bp) and downstream (1,021-bp) PCR products were used produce a product containing a 939-bp deletion of motY, which left only the first eight codons, a BamHI site, and the TGA codon of motY. The products with deletions were cloned into the suicide vector pEX18Gm. Suicide plasmids bearing the mot mutations were mobilized from E. coli into P. aeruginosa by conjugation. Recombinants were selected by plating in the presence of antibiotics. Single recombinants derived from the SacB vector were selected for by plating the mating mixture on M63 minimal medium (48) containing 10 mM succinate and 100 μg of gentamicin per ml. Double recombinants were then selected for by plating single recombinants on medium containing 5% sucrose. All strain constructions were confirmed by Southern blot hybridization and/or PCR analysis.
Motility assays.
To document swimming motility, plates were incubated for the times indicated below and photographed with a Kodak Company Digital Science imaging system. The average rate of expansion was determined in triplicate by measuring the diameter as a function of time (7). All expansion rates were normalized to the rate of a control wild-type strain inoculated on the same plate.
Swimming speeds were obtained by using a Hobson Tracker, Bacteria Edition (Hobson Tracking Systems Ltd., Sheffield, England). Cells were prepared by subculturing an overnight culture 1:50 and incubating the subculture in LB medium for ∼3 h at 30°C. Five microliters of cells was then placed on a microscope slide and viewed. Swimming speed was defined as the length of a run (in micrometers) divided by the duration of the run (in seconds). A value was calculated by adding the incremental distances moved in each frame along the path sampled and dividing by the total time for the track. Sample data were recorded for 30 s, and each sample consisted of at least 10 tracked events. Fifty samples were recorded for each strain analyzed. All events for each strain were averaged, and the standard deviation was calculated.
Chemotaxis assays.
Agarose plug assays were carried out as described previously (47). Briefly, the plugs contained 1% low-melting-point agarose (GibcoBRL, Grand Island, N.Y.) in chemotaxis buffer (40 mM potassium phosphate [pH 7.0]), a few crystals of methylene blue to provide contrast for photography, and either no added attractant or 10 mM l-arginine HCl (Sigma Chemical Co., St. Louis, Mo.) as an attractant. A 10-μl drop of the melted agarose mixture was placed on a microscope slide, and it was surrounded by two strips cut from a plastic coverslip and covered with a no. 1 1/2 glass coverslip. The chamber formed in this way was filled with approximately 150 μl of a suspension of motile bacteria (optical density at 660 nm, ∼0.8) in chemotaxis buffer. Chambers were then photographed under phase-contrast lighting conditions at the time intervals specified below. Assays were performed in duplicate on at least two different days, and representative photographs are shown below.
RESULTS
Mutations in either the motAB or motCD locus affect, but do not abolish, swimming motility.
The starting point for these analyses was an observation made with a set of Tn5 (Tetr) mutants with defects in flagellar genes that were provided by Pathogenesis Corporation. Specifically, insertions in either of the two potential mot loci affected swimming in semisolid motility agar compared to the swimming of the parental P. aeruginosa PAK strain. Transposon insertions within motA, motC, or motD resulted in mutants with decreased rates of radial expansion in a semisolid motility plate compared to the wild-type PAK strain rates (Fig. 2). These results suggested that both sets of motor genes contribute to motility. To explore this observation and this hypothesis, additional strain PAK mutants were constructed by introducing either a gentamicin resistance cassette to create ΔmotCD6::Genr, motA2::Genr, motB3::Genr, and ΔmotAB4::Genr or a tetracycline resistance cassette to create ΔmotC7::Tetr and ΔmotCD8::Tetr.
FIG. 2.
Transposon insertions in either the motAB or motCD locus affect movement in semisolid motility agar. The strains and normalized rates of radial expansion were as follows: LMP4 (motA1::Tn5Tetr), 0.66 ± 0.10; LMP7 (motC1::Tn5Tetr), 0.51 ± 0.05; and LMP8 (motD2::Tn5Tetr), 0.53 ± 0.06. The rates of expansion were normalized to the rate for reference strain LMP1, which contained Tn5 (Tetr) and showed wild-type swimming motility. Motility plates contained 50 μg of tetracycline per ml and were incubated at 30°C for 20 h. Similar results were observed in plates without an antibiotic.
Strains with the motC1::Tn5 (Tetr), motD2::Tn5 (Tetr), and ΔmotCD6::Genr alleles showed decreased radial expansion in soft agar plates compared to the wild-type parent strain PAK (Fig. 3). Swimming speeds are shown in Fig. 4. Wild-type strain PAK was found to swim at a speed of 41 ± 3 μm/s. The observed speeds of the three mutants were lower than the speed of the wild type: 28 ± 2, 27 ± 3, and 28 ± 2 μm/s, respectively. Thus, the motCD locus contributes to, but is not exclusively responsible for, motor function.
FIG. 3.
Deletion of either the motAB or motCD locus affects motility in semisolid agar. (A) Motility of strains LMP7, LMP8, LMP13, and wild-type strain PAK. The normalized rates of radial expansion were as follows: LMP7 (motC1::Tn5Tetr), 0.51 ± 0.05; LMP8 [motD2::Tn5 (Tetr)], 0.53 ± 0.06; and LMP13 (ΔmotCD6::Genr), 0.55 ± 0.03. (B) Motility of strains LMP5 (motA2::Genr), LMP6 (motB3::Genr), LMP9 (ΔmotAB4::Genr), and LMP2. The reference strain LMP2 contained Tn5 (Genr) and showed wild-type swimming motility. Radial expansions rates were not calculated because the strains failed to significantly migrate in the motility plate. (C) Motility of strains LMP13, LMP9, and LMP2. The plate in panel A contained no antibiotic and was incubated for 15 h at 30°C. The plates in panels B and C contained 50 μg of gentamicin per ml and were incubated for 20 h at 30°C.
FIG. 4.
Swimming speeds of the wild type and motor mutants. The average swimming rates were determined by using the Hobson Tracker for cells growing exponentially in liquid LB medium. Strains PAK, LMP44 (ΔmotC7), LMP7 (motC1), LMP8 (motD2), LMP13 (ΔmotCD6), LMP5 (motA2), LMP6 (motB3), LMP9 (ΔmotAB4), LMP54 (ΔmotY2), LMP55 (ΔmotAB4 ΔmotY2), LMP60 (ΔmotCD6 ΔmotY2), LMP18 (motA2 motD2), LMP49 (motB3 motC7), LMP17 (motA2 motC1), and LMP20 (motB3 motD2) were examined.
The Tn5 insertion in motA in strain LMP4 occurred near the end of the coding region (disrupting the gene at the position coding for amino acid 229 of 246 amino acids). Three additional mot alleles were created in the PA1460-PA1461 locus and transferred to the chromosome. The mutations, motA2::Genr, motB3::Genr, and ΔmotAB4::Genr, caused severe swimming motility defects in soft agar plates (Fig. 3B). However, the strains were highly motile as determined by light microscopy, and the calculated swimming speeds for motA2::Genr (40 ± 3 μm/s) and motB3::Genr (40 ± 3 μm/s) were very similar to the calculated swimming speed for wild-type strain PAK (41 ± 3 μm/s). The swimming speed for ΔmotAB4::Genr (35 ± 5 μm/s) was slightly less than that of the wild type.
These results are similar to those observed by Kato et al. (23) and seem to be consistent with the potential for lesions in motAB to affect expression of downstream chemotaxis genes. Radial expansion in semisolid agar plates measures chemotaxis as well as motility. The motAB mutants were motile as determined by microscopic observations, and yet the motAB mutant strains showed severe defects in semisolid motility plates compared to the ΔmotCD strain or the wild type (Fig. 3C). Even though the gentamicin resistance cassette that was introduced into motAB was designed to create a nonpolar mutation (21), chemotaxis genes occur upstream and downstream of motAB. It is possible that an imbalance in the specific ratio of chemotaxis signaling components could perturb the chemotaxis phosphotransfer cascade and affect radial expansion in plates. Even though they are competent for swimming (with little or no defect in the observed swimming speed), mutants with defects in the motAB locus could be lost in motility plates. However, unlike known chemotaxis mutants (e.g., cheW mutants), no profound motor bias that altered swimming patterns was observed by microscopy, and, as demonstrated below, chemotaxis in the motAB mutants was not affected.
Disruptions in both motAB and motCD loci abolish motility.
Since deletion of either set of operons encoding the Mot homologs was not sufficient to abolish swimming, mutants with combinations of mot lesions were constructed. Introduction of motC and motD alleles into the ΔmotAB strain to make LMP15 and LMP16, respectively, or introduction of motA and motB into the ΔmotCD strain to make LMP48 and LMP50 completely abolished motility in semisolid motility plates (Fig. 5, plate A) and motility determined by microscopy (Fig. 4). We next asked whether double mutations that resulted in loss of the homologous genes (i.e., motA and motC or motB and motD) were sufficient to disrupt swimming. Like the triple-mutant strains, LMP47 (motA2::Genr ΔmotC7::Tetr) and LMP20 (motB3::Genr motD2::Tn5) were nonmotile in plates (Fig. 5, plate B) and in liquid (data not shown). Thus, the two loci appear to encode proteins with overlapping functions; motility was abolished when both sets of motA-like or motB-like genes were deleted.
FIG. 5.
Disruption of genes in both motAB-like loci abolishes swimming motility. (Plate A) Triple gene knockouts. Strains LMP15 (ΔmotAB4 motC1), LMP16 (ΔmotAB4 motD2), LMP57 (flgC1; nonmotile Fla− control), LMP48 (motA2 ΔmotCD8), LMP50 (motB3 ΔmotCD8), and PAK were included. (Plate B) Double gene knockouts. Strains LMP57 (flgC1; nonmotile control), LMP47 (motA2 ΔmotC7), LMP20 (motB3 motD2), LMP18 (motA2 motD2), LMP49 (motB3 ΔmotC7), and PAK were included. The motility plates were incubated overnight at room temperature.
Noncognate pairs can work together to generate torque.
The additional combinations of alleles that were constructed included motA2 and motD2 to make LM18 and motB3 and motC7 to make LMP49. In motility agar (Fig. 5, plate B), LMP18 was motile (although less motile than the wild type), whereas LMP49 was severely defective. As determined by microscopy, both strain LMP18 and strain LMP49 were motile; the swimming speeds were determined to be 28 ± 2 and 38 ± 2 μm/s, respectively, compared to 41 ± 3 μm/s for the wild type (Fig. 4). Thus, motility was retained when one copy of a motA-like gene and one copy of a motB-like gene remained. These data suggest that the pairs of gene products do not require their cognate partners to work and can function with a partner from the alternate locus.
Discovery of an additional gene required for motor function in P. aeruginosa: motY.
While a transposon mutant library in P. aeruginosa strain PAO1 was being analyzed, a mutant that had a severe defect in a motility plate was isolated. The insertion of this mutant was sequenced and was found to disrupt PA3526. The gene product is most similar to the Vibrio cholerae motY product (30% identity and 51% similarity; E = 3e-29). MotY resembles MotB in that it contains a single transmembrane domain and a peptidoglycan interaction domain. A motY mutant was constructed in the PAK background and examined. As observed for PAO1, deletion of motY in PAK severely affected swimming motility in semisolid motility plates (Fig. 6). The radial expansion rate for LMP54 (ΔmotY::Tetr) normalized to the wild-type rate was 0.22 ± 0.03. However, in liquid the observed swimming rate for LMP54 was only slightly lower than that of wild-type strain PAK (35 ± 3 μm/s versus 41 ± 3 μm/s) and was equivalent to the speed observed for the ΔmotAB strain. Thus, for some reason, the motility defect in motY mutants (in PAK and PAO1 backgrounds) appeared to be more profound in a semisolid motility plate than in liquid. The motY strain is a prototroph, even though the motY coding region is located upstream of and transcribed in the same direction as argG, and there are no linked genes that are expected to play additional roles in motility or chemotaxis.
FIG. 6.

The fifth motor gene, motY. Strains LMP9 (ΔmotAB), LMP54 (ΔmotY), and LMP55 (ΔmotAB ΔmotY) were included in plate A, and strains LMP13 (ΔmotCD), LMP54 (ΔmotY), and LMP60 (ΔmotCD ΔmotY) were included in plate B. The motility plates were incubated overnight at room temperature.
The movement of the motY mutant resembled that of the motAB mutants in semisolid motility agar and in liquid. To probe the role of motY, the ΔmotY2 allele was introduced into the ΔmotAB strain to make LMP55 and into the ΔmotCD strain to make LMP60. Strains LMP55 (ΔmotAB ΔmotY), LMP9 (ΔmotAB), and LMP54 (ΔmotY) showed little radial expansion in semisolid motility plates. In liquid, the swimming speeds of LMP55, LMP9, and LMP54 were similar and only slightly less than the wild-type speed (i.e., ∼85% of the wild-type speed). In contrast, the motility phenotype of LMP60 (ΔmotCD ΔmotY) was unlike that of its parental ΔmotCD strain. LMP60 was nonmotile in plates (Fig. 6, plate B) and in liquid (Fig. 4). Thus, mutation of motY and motCD resulted in a complete loss of motility. Disruption of motY caused a phenotype similar to loss of motAB or loss of motAB and motY. Taken together, these results suggest that motY is required for MotA/B function, but not for MotC/D function.
Chemotaxis is not affected in the motAB mutants.
The motY gene was unlinked from other potential flagellar or chemotaxis genes. Since motY and motAB mutants displayed similar phenotypes, which included poor motility on surfaces or in semisolid agar but good motility in dilute aqueous environments, we decided to investigate the motAB mutants further. Additionally, we extended our findings by creating in-frame deletions (ΔmotAB and ΔmotCD) in another P. aeruginosa strain, strain PAO1. The resulting new strains had motility phenotypes comparable to those observed in PAK strains (Fig. 7 and data not shown). Thus, the in-frame deletion of motAB in PAO1 caused the same phenotype as the insertion of the (putative) nonpolar gentamicin cassette in PAK. An agarose plug assay was used to examine the chemotaxis phenotypes of these mutants. In this assay, a chemotactic response consisted of the formation of a ring of accumulated bacteria that surrounded an agarose plug containing the chemoattractant arginine. For PAK (Fig. 8A) and PAO1 (Fig. 8B) strains similar rings of cell accumulation were visualized for the wild-type and ΔmotAB strains, and there was no accumulation for strains with known chemotactic defects (LMP61 and PAO1000). Therefore, the radial expansion defect observed for motAB strains in semisolid motility agar cannot be attributed to polar effects that disturb chemotaxis.
FIG. 7.
Swimming and swarming phenotypes of mot mutants in PAK and PAO1 backgrounds. Swimming motility plates contained 0.325% semisolid agar, and solidified swarming plates contained 0.5% agar for PAK strains and 0.6% agar for PAO1 strains. The PAK strains used (top row of plates) were PAK, LMP9 (ΔmotAB), LMP13 (ΔmotCD), LMP54 (ΔmotY), LMP47 (motA2 motC7), LMP20 (motB3 motD2), LMP57 (flgC1), LMP18 (motA2 motD2), and LMP49 (motB3 motC7). The PAO1 strains used (bottom row of plates) were MPAO1, PAO1001 (ΔmotAB in MPAO1), PAO1002 (ΔmotCD in MPAO1), PAO2000 (ΔmotY in IgPAO1), and IgPAO1. Swimming plates were incubated overnight at room temperature, and swarming plates were incubated for 2 days at 37°C.
FIG. 8.
Chemotactic responses of PAK, PAO1, and isogenic mutants in the presence of an agarose plug containing arginine (ARG) or chemotaxis buffer (CB) containing no chemoattractant. A positive response resulted in a light ring around the dark plug.
Effects of motor mutations on swarming: MotA, MotB, and MotY are necessary to power movement on surfaces.
Flagellum-assisted motility can be used for locomotion in liquid (called swimming) or over surfaces (called swarming) (reviewed in reference 17). Figure 7 shows the movement of the mot mutant strains in the PAK and PAO1 backgrounds over the surface of solidified agar (0.5 and 0.6%, respectively); for comparison, movement within semisolid agar (0.3%) is also shown. Although some differences in the swarming pattern for the three wild-type strains were observed, all motor mutants that showed defects in translocation in semisolid swimming plates showed similar defects on solidified swarming plates. Not all movement was abolished on the surface. This was likely because the strains remained competent for twitching motility, which can also promote movement on surfaces and is powered by a distinctly different mechanism (27, 35).
The mutants with lesions in motAB or motY were profoundly defective for translocation on surfaces and in semisolid agar, and yet they displayed good motility (i.e., almost wild-type swimming speeds) in liquid (Table 2). These observations suggested that torque generators composed of MotC and MotD worked poorly under high-viscosity conditions. To test this idea, radial expansion in semisolid agar was examined by using a range of agar concentrations. Significant radial expansion was observed for motAB or motY mutants in motility plates with reduced agar concentrations (Fig. 9). Decreasing the agar concentration also allowed better discrimination of phenotypes. Radial expansion of LMP9 (ΔmotAB), radial expansion of LMP54 (motY), and radial expansion of LMP55 (ΔmotAB motY) were not identical; LMP54 moved better than LMP9, which moved better than LMP55. Figure 9 also shows that a hybrid MotA/D-type motor does not work well in high-viscosity conditions, whereas a MotB/C-type motor does function well on plates; in liquid the efficiency is reversed and the MotA/D-type motor produces higher speeds than the MotB/C-type motor (38 and 28 μm/s, respectively) (Fig. 4 and Table 2).
TABLE 2.
Comparison of movement in liquid and plates
| PAK strain | Relevant genotypea | Normalized motility in liquidb | Normalized motility in semisolid agarc | Motility on solidified agare |
|---|---|---|---|---|
| PAK | Wild type | 1.0 | 1.0 | + |
| LMP9 | ΔmotAB | 0.85 | No expansiond | − |
| LMP13 | ΔmotCD | 0.68 | 0.55 | + |
| LMP54 | motY | 0.85 | 0.22 | − |
| LMP18 | motA motD | 0.68 | 0.29 | + |
| LMP49 | motB motC | 0.93 | No expansiond | − |
| LMP47 | motA motC | Nonmotile | Nonmotile | − |
| LMP20 | motB motD | Nonmotile | Nonmotile | − |
| LMP55 | ΔmotAB motY | 0.85 | No expansiond | − |
| LMP60 | ΔmotCD motY | Nonmotile | Nonmotile | − |
Gene designations correspond to the following PA numbers in the annotated genome: motA, PA1460; motB, PA1461; motC, PA4954; motD, PA4953; and motY, PA3526.
The swimming speeds were normalized to the wild-type value.
The Rates of radial expansion in 0.325% semisolid agar motility plates were normalized to the wild-type value.
No significant radial migration from the point of inoculation.
Motility on the surface of minimal swarming agar (0.5% agar).
FIG. 9.
Movement in motility agar solidified with different concentrations of agar. Tryptone motility agar was solidified with the concentrations of agar indicated. Strains LMP57 (flgC1), LMP47 (motA2 motC7), LMP18 (motA2 motD2), LMP49 (motB3 motC7), LMP9 (ΔmotAB), LMP54 (ΔmotY), LMP55 (ΔmotAB ΔmotY), LMP60 (ΔmotCD ΔmotY), and PAK were used. The plates were incubated overnight at room temperature.
DISCUSSION
Motility is often a key factor in adaptation and survival, and flagella are one of the most widespread and effective means of locomotion for bacteria (2). Acting as propellers, flagella promote rapid swimming motility in dilute aqueous environments. Flagella allow bacteria to move towards more favorable environments and away from less favorable environments. Flagella can also enable movement through viscous environments and over surfaces (called swarming), as well as biofilm formation and architecture (17, 25, 45). As a result of this multiplicity of roles, it is not surprising that many studies have indicated that flagellar systems are important for pathogenesis of many organisms (reviewed in references 40 and 46), including P. aeruginosa (12, 14, 41, 42, 49).
In this paper we describe characterization of the P. aeruginosa flagellar motor, which is more complex than previously studied motors of other bacteria. The genome contains two sets of homologous motAB-like genes. We show here that both loci contribute to motility. These sets of genes are not simply redundant gene sets because the motility phenotypes of mutants are distinct. The products of one locus, MotA-MotB (PA1460-PA1461), require another protein MotY (PA3526) for optimal function. Loss of function of any of the three genes reduces the ability of P. aeruginosa to move in semisolid agar or over surfaces, but it does not dramatically reduce swimming motility. Although by themselves MotC and MotD do not seem to generate sufficient torque to move bacteria when there is a high external load, they can function well in liquid in the absence of MotA/B. In fact, MotC/D-driven motors seem to outperform MotA/B-driven motors in liquid; motAB mutants achieved swimming speeds approaching the wild-type speed. Elimination of MotA or MotB caused no significant change in swimming speed, and elimination of MotY, of MotA and MotB, or of MotA and MotB and MotY caused a reduction to 85% of the wild-type speed. Elimination of MotC and/or MotD reduced the swimming speed to 65% of the wild-type speed and caused a similar reduction in radial expansion in semisolid agar.
Why is there a complex flagellar motor, and how does it work? The products of five genes contribute to flagellar motor function in P. aeruginosa, whereas only two proteins are necessary for torque generation in E. coli. The analysis of mutants showed that generation of torque by MotA/B/Y or MotC/D is sufficient to promote motility in liquid; however, MotA/B/Y units are necessary for movement in viscous environments and over surfaces. E. coli and other peritrichous bacteria solve the problem of motility in the presence of increasing external load by upregulating the number of propellers (17). However, P. aeruginosa is polarly flagellated. V. parahaemolyticus is also polarly flagellated, and this organism induces a second, peritrichous system under high-viscosity conditions that enables translocation (37). P. aeruginosa appears to have solved the problem of motor energization under conditions under which there is a varying load by having a more complex torque-generating system, which may allow the bacterium to effectively function as an all-terrrain organism with a single mode of locomotion.
Two scenarios can be imagined for the P. aeruginosa motor: dual generators composed of discrete MotA/B/Y and MotC/D torque generators surrounding the rotor and functioning independently and MotA/B/C/D/Y complexes functioning together. We considered the possibility that distinct torque generators are powered differently (i.e., by the proton and sodium motive forces), but the motility phenotypes of the wild type and selected mutants do not change in the presence of the sodium-channel-blocking drug phenamil (80 μM) (data not shown). Data presented here demonstrate that MotA/MotD or MotB/MotC can interact productively to generate torque necessary to drive the flagellar motor; however, such data simply show the potential for an interaction in mutant backgrounds and do not address the findings for wild-type cells. The slight difference in plate motility for motY, motAB, and motAB motY mutants, as well as the observed difference in swimming speed between motA or motB and ΔmotAB mutants, also suggests that there is potential interaction of the remaining motor components. However, additional experiments are needed to reveal the roles and relationships of these proteins in promoting the generation of torque.
It is intriguing that motCD lesions were originally identified as lesions that cause an altered virulence phenotype (52). Specifically, the motCD locus was demonstrated to be required for ingestion by macrophages. Although in the original report the workers concluded that there was no effect on motility, we surmise that this phenotype may have been overlooked because it is subtle and different from the predicted and well-characterized E. coli motAB phenotype. The discovery that a motC mutant was resistant to phagocytosis is consistent with other findings showing that motility and flagella are necessary for phagocytosis (33). Thus, perhaps the dual motors are not coordinately regulated or are differentially functional in the host. Analysis of the microarray data provided by Dasgupta et al. provided evidence that PA1460 (motA), PA1461 (motB), and PA3526 (motY) are coordinately regulated as part of the flagellar biogenesis hierarchy, while PA4594 (motC) and PA493 (motD) show no change in gene expression compared with the wild type in strains with rpoN, fleQ, fleR, or fliA lesions (10).
It seems clear from a number of studies with P. aeruginosa and other bacteria that at times it is advantageous for a bacterium to be highly motile, while at other times it is advantageous to be more adhesive and sessile (8, 22, 25, 26). One common means to control motility is stringent transcriptional regulation. Numerous environmental factors and global regulators control the flagellar gene hierarchy (reviewed in references 1 and 53). Moreover, flagellar genes can be down-regulated once infection is established in a host or in biofilms (56, 57). Another known solution controlling motility is mutational loss of flagellation once infection is established (15, 32). However, this work raises an intriguing new possibility. Perhaps a bacterium can choose to be nonmotile by having motors that function or are expressed differentially under certain conditions. Such an option might allow a bacterium to be sessile under one condition and yet poised for motility in response to changing environments. This may have important implications for the development and maintenance of multicellular communities in biofilms.
Acknowledgments
This work was supported by a pilot grant from the Iowa Cystic Fibrosis Foundation to L.L.M.
We thank Pathogenesis Corporation and especially Wendy Hufnagle for provision of the transposon insertion mutants in the strain PAK background. We thank Carrie Harwood for support and fruitful discussions. Undergraduate students in the microbial genetics laboratory class at the University of Iowa discovered motY.
REFERENCES
- 1.Aldridge, P., and K. T. Hughes. 2002. Regulation of flagellar assembly. Curr. Opin. Microbiol. 5:160-165. [DOI] [PubMed] [Google Scholar]
- 2.Armitage, J. P. 1999. Bacterial tactic responses. Adv. Microb. Physiol. 41:229-289. [DOI] [PubMed] [Google Scholar]
- 3.Berg, H. C. 2003. The rotary motor of bacterial flagella. Annu. Rev. Biochem. 72:19-54. [DOI] [PubMed] [Google Scholar]
- 4.Blair, D. F. 2003. Flagellar movement driven by proton translocation. FEBS Lett. 545:86-95. [DOI] [PubMed] [Google Scholar]
- 5.Blair, D. F., and H. C. Berg. 1988. Restoration of torque in defective flagellar motors. Science 242:1678-1681. [DOI] [PubMed] [Google Scholar]
- 6.Block, S. M., and H. C. Berg. 1984. Successive incorporation of force-generating units in the bacterial rotary motor. Nature 309:470-472. [DOI] [PubMed] [Google Scholar]
- 7.Boles, B. R., and L. L. McCarter. 2000. Insertional inactivation of genes encoding components of the sodium-type flagellar motor and switch of Vibrio parahaemolyticus. J. Bacteriol. 182:1035-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boles, B. R., and L. L. McCarter. 2002. Vibrio parahaemolyticus scrABC, a novel operon affecting swarming and capsular polysaccharide regulation. J. Bacteriol. 184:5946-5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chun, S. Y., and J. S. Parkinson. 1988. Bacterial motility: membrane topology of the Escherichia coli MotB protein. Science 239:276-278. [DOI] [PubMed] [Google Scholar]
- 10.Dasgupta, N., M. C. Wolfgang, A. L. Goodman, S. K. Arora, J. Jyot, S. Lory, and R. Ramphal. 2003. A four-tiered transcriptional regulatory circuit controls flagellar biogenesis in Pseudomonas aeruginosa. Mol. Microbiol. 50:809-824. [DOI] [PubMed] [Google Scholar]
- 11.De Mot, R., and J. Vanderleyden. 1994. The C-terminal sequence conservation between OmpA-related outer membrane proteins and MotB suggests a common function in both gram-positive and gram-negative bacteria, possibly in the interaction of these domains with peptidoglycan. Mol. Microbiol. 12:333-334. [DOI] [PubMed] [Google Scholar]
- 12.Feldman, M., R. Bryan, S. Rajan, L. Scheffler, S. Brunnert, H. Tang, and A. Prince. 1998. Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect. Immun. 66:43-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrandez, A., A. C. Hawkins, D. T. Summerfield, and C. S. Harwood. 2002. Cluster II che genes from Pseudomonas aeruginosa are required for an optimal chemotactic response. J. Bacteriol. 184:4374-4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleiszig, S. M., S. K. Arora, R. Van, and R. Ramphal. 2001. FlhA, a component of the flagellum assembly apparatus of Pseudomonas aeruginosa, plays a role in internalization by corneal epithelial cells. Infect. Immun. 69:4931-4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garrett, E. S., D. Perlegas, and D. J. Wozniak. 1999. Negative control of flagellum synthesis in Pseudomonas aeruginosa is modulated by the alternative sigma factor AlgT (AlgU). J. Bacteriol. 181:7401-7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gosink, K. K., and C. C. Hase. 2000. Requirements for conversion of the Na+-driven flagellar motor of Vibrio cholerae to the H+-driven motor of Escherichia coli. J. Bacteriol. 182:4234-4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harshey, R. M. 2003. Bacterial motility on a surface: many ways to a common goal. Annu. Rev. Microbiol. 57:249-273. [DOI] [PubMed] [Google Scholar]
- 18.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77-86. [DOI] [PubMed] [Google Scholar]
- 19.Imae, Y., and T. Atsumi. 1989. Na+-driven bacterial flagellar motors. J. Bioenerg. Biomembr. 21:705-716. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs, M. A., A. Alwood, I. Thaipisuttikul, D. Spencer, E. Haugen, S. Ernst, O. Will, R. Kaul, C. Raymond, R. Levy, L. Chun-Rong, D. Guenthner, D. Bovee, M. V. Olson, and C. Manoil. 2003. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 100:14339-14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jain, S., and D. E. Ohman. 1998. Deletion of algK in mucoid Pseudomonas aeruginosa blocks alginate polymer formation and results in uronic acid secretion. J. Bacteriol. 180:634-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Josenhans, C., and S. Suerbaum. 2002. The role of motility as a virulence factor in bacteria. Int. J. Med. Microbiol. 291:605-614. [DOI] [PubMed] [Google Scholar]
- 23.Kato, J., T. Nakamura, A. Kuroda, and H. Ohtake. 1999. Cloning and characterization of chemotaxis genes in Pseudomonas aeruginosa. Biosci. Biotechnol. Biochem. 63:155-161. [DOI] [PubMed] [Google Scholar]
- 24.Khan, S., R. Zhao, and T. S. Reese. 1988. Architectural features of the Salmonella typhimurium flagellar motor switch revealed by disrupted C-rings. J. Struct. Biol. 122:311-319. [DOI] [PubMed] [Google Scholar]
- 25.Klausen, M., A. Aaes-Jorgensen, S. Molin, and T. Tolker-Nielsen. 2003. Involvement of bacterial migration in the development of complex multicellular structures in Pseudomonas aeruginosa biofilms. Mol. Microbiol. 50:61-68. [DOI] [PubMed] [Google Scholar]
- 26.Klausen, M., A. Heydorn, P. Ragas, L. Lambertsen, A. Aaes-Jorgensen, S. Molin, and T. Tolker-Nielsen. 2003. Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol. Microbiol. 48:1511-1524. [DOI] [PubMed] [Google Scholar]
- 27.Kohler, T., L. K. Curty, F. Barja, C. van Delden, and J. C. Pechere. 2000. Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J. Bacteriol. 182:5990-5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kojima, S., and D. F. Blair. 2004. The bacterial flagellar motor: structure and function of a complex molecular machine. Int. Rev. Cytol. 233:93-134. [DOI] [PubMed] [Google Scholar]
- 29.Kojima, S., and D. F. Blair. 2004. Solubilization and purification of the MotA/MotB complex of Escherichia coli. Biochemistry 43:26-34. [DOI] [PubMed] [Google Scholar]
- 30.Larsen, S. H., J. Adler, J. J. Gargus, and R. W. Hogg. 1974. Chemomechanical coupling without ATP: the source of energy for motility and chemotaxis in bacteria. Proc. Natl. Acad. Sci. USA 71:1239-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macnab, R. M. 2003. How bacteria assemble flagella. Annu. Rev. Microbiol. 57:77-100. [DOI] [PubMed] [Google Scholar]
- 32.Mahenthiralingam, E., M. E. Campbell, and D. P. Speert. 1994. Nonmotility and phagocytic resistance of Pseudomonas aeruginosa isolates from chronically colonized patients with cystic fibrosis. Infect. Immun. 62:596-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahenthiralingam, E., and D. P. Speert. 1995. Nonopsonic phagocytosis of Pseudomonas aeruginosa by macrophages and polymorphonuclear leukocytes requires the presence of the bacterial flagellum. Infect. Immun. 63:4519-4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manson, M. D., P. Tedesco, H. C. Berg, F. M. Harold, and C. Van der Drift. 1977. A protonmotive force drives bacterial flagella. Proc. Natl. Acad. Sci. USA 74:3060-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mattick, J. S. 2002. Type IV pili and twitching motility. Annu. Rev. Microbiol. 56:289-314. [DOI] [PubMed] [Google Scholar]
- 36.McCarter, L. L. 1994. MotY, a component of the sodium-type flagellar motor. J. Bacteriol. 176:4219-4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCarter, L. L. 2001. Polar flagellar motility of the Vibrionaceae. Microbiol. Mol. Biol. Rev. 65:445-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meister, M., G. Lowe, and H. C. Berg. 1987. The proton flux through the bacterial flagellar motor. Cell 49:643-650. [DOI] [PubMed] [Google Scholar]
- 39.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moens, S., and J. Vanderleyden. 1996. Functions of bacterial flagella. Crit. Rev. Microbiol. 22:67-100. [DOI] [PubMed] [Google Scholar]
- 41.Montie, T. C., D. Doyle-Huntzinger, R. C. Craven, and I. A. Holder. 1982. Loss of virulence associated with absence of flagellum in an isogenic mutant of Pseudomonas aeruginosa in the burned-mouse model. Infect. Immun. 38:1296-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montie, T. C., D. Drake, H. Sellin, O. Slater, and S. Edmonds. 1987. Motility, virulence, and protection with a flagella vaccine against Pseudomonas aeruginosa infection. Antibiot. Chemother. 39:233-248. [DOI] [PubMed] [Google Scholar]
- 43.Muramoto, K., S. Sugiyama, E. J. Cragoe, Jr., and Y. Imae. 1994. Successive inactivation of the force-generating units of sodium-driven bacterial flagellar motors by a photoreactive amiloride analog. J. Biol. Chem. 269:3374-3380. [PubMed] [Google Scholar]
- 44.Okabe, M., T. Yakushi, M. Kojima, and M. Homma. 2002. MotX and MotY, specific components of the sodium-driven flagellar motor, colocalize to the outer membrane in Vibrio alginolyticus. Mol. Microbiol. 46:125-134. [DOI] [PubMed] [Google Scholar]
- 45.O'Toole, G. A., and R. Kolter. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30:295-304. [DOI] [PubMed] [Google Scholar]
- 46.Ottemann, K. M., and J. F. Miller. 1997. Roles for motility in bacterial-host interactions. Mol. Microbiol. 24:1109-1117. [DOI] [PubMed] [Google Scholar]
- 47.Parales, R. E., J. L. Ditty, and C. S. Harwood. 2000. Toluene-degrading bacteria are chemotactic towards the environmental pollutants benzene, toluene, and trichloroethylene. Appl. Environ. Microbiol. 66:4098-4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 49.Sato, H., K. Okinaga, and H. Saito. 1988. Role of pili in the pathogenesis of Pseudomonas aeruginosa burn infection. Microbiol. Immunol. 32:131-139. [DOI] [PubMed] [Google Scholar]
- 50.Sato, K., and M. Homma. 2000. Functional reconstitution of the Na+-driven polar flagellar motor component of Vibrio alginolyticus. J. Biol. Chem. 275:5718-5722. [DOI] [PubMed] [Google Scholar]
- 51.Schweizer, H. D. 1993. Small broad-host-range gentamycin resistance gene cassettes for site-specific insertion and deletion mutagenesis. BioTechniques 15:831-834. [PubMed] [Google Scholar]
- 52.Simpson, D. A., and D. P. Speert. 2000. RpmA is required for nonopsonic phagocytosis of Pseudomonas aeruginosa. Infect. Immun. 68:2493-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soutourina, O. A., and P. N. Bertin. 2003. Regulation cascade of flagellar expression in Gram-negative bacteria. FEMS Microbiol. Rev. 27:505-523. [DOI] [PubMed] [Google Scholar]
- 54.Stewart, B. J., and L. L. McCarter. 2003. Lateral flagellar gene system of Vibrio parahaemolyticus. J. Bacteriol. 185:4508-4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watnick, P. I., and R. Kolter. 1999. Steps in the development of a Vibrio cholerae El Tor biofilm. Mol. Microbiol. 34:586-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whiteley, M., M. G. Bangera, R. E. Bumgarner, M. R. Parsek, G. M. Teitzel, S. Lory, and E. P. Greenberg. 2001. Gene expression in Pseudomonas aeruginosa biofilms. Nature 413:860-864. [DOI] [PubMed] [Google Scholar]
- 57.Wolfgang, M. C., J. Jyot, A. L. Goodman, R. Ramphal, and S. Lory. 2004. Pseudomonas aeruginosa regulates flagellin expression as part of a global response to airway fluid from cystic fibrosis patients. Proc. Natl. Acad. Sci. USA 101:6664-6668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Woo, T. H., A. F. Cheng, and J. M. Ling. 1992. An application of a simple method for the preparation of bacterial DNA. BioTechniques 13:696-698. [PubMed] [Google Scholar]