Abstract
The lethal effect of an Escherichia coli pgsA null mutation, which causes a complete lack of the major acidic phospholipids, phosphatidylglycerol and cardiolipin, is alleviated by a lack of the major outer membrane lipoprotein encoded by the lpp gene, but an lpp pgsA strain shows a thermosensitive growth defect. Using transposon mutagenesis, we found that this thermosensitivity was suppressed by disruption of the rcsC, rcsF, and yojN genes, which code for a sensor kinase, accessory positive factor, and phosphotransmitter, respectively, of the Rcs phosphorelay signal transduction system initially identified as regulating the capsular polysaccharide synthesis (cps) genes. Disruption of the rcsB gene coding for the response regulator of the system also suppressed the thermosensitivity, whereas disruption of cpsE did not. By monitoring the expression of a cpsB′-lac fusion, we showed that the Rcs system is activated in the pgsA mutant and is reverted to a wild-type level by the rcs mutations. These results indicate that envelope stress due to an acidic phospholipid deficiency activates the Rcs phosphorelay system and thereby causes the thermosensitive growth defect independent of the activation of capsule synthesis.
The pgsA gene of Escherichia coli codes for phosphatidylglycerophosphate synthase, which catalyzes the committed step of biosynthesis of the major acidic phospholipids of the organism, phosphatidylglycerol and cardiolipin (14). A pgsA null mutation is lethal (25), and these phospholipids are considered essential to E. coli. Since a null mutation of the cls gene coding for cardiolipin synthase, which condenses two phosphatidylglycerol molecules to form cardiolipin, caused only a minor growth defect (40), the lethality was attributed to the lack of phosphatidylglycerol (14). However, it was then found that in a strain defective in the lpp gene coding for the major outer membrane lipoprotein, the pgsA null mutation did not result in a loss of viability, although it caused a complete lack of phosphatidylglycerol, indicating the dispensability of this phospholipid in E. coli (29, 35). The precursor of Lpp is modified with diacylglycerol derived from phosphatidylglycerol (47). This modification is a prerequisite for signal peptide processing and thus for translocation to the outer membrane (59). When Lpp was expressed in the pgsA null mutant, most of the molecules remained in the inner membrane because of inefficient modification, and the covalent linkage of their C-terminal Lys to peptidoglycan led to an anomalous association of the inner and outer membranes and eventually to cell lysis (54). The lpp pgsA strain accumulates small amounts of biosynthetic intermediates of the major phospholipids, specifically phosphatidic acid and CDP-diacylglycerol (29). These acidic phospholipids, quantitatively very minor in wild-type cells, may substitute for major acidic phospholipids in giving negative charges to the membrane surface in pgsA null cells (35, 54). They have been shown to serve as substrates for lipoprotein modification in vitro, but they do so inefficiently (47).
Even if it was defective in lpp, a pgsA null strain could not be cured of a replication-thermosensitive plasmid carrying the pgsA gene by elevating the growth temperature to 42°C (60). This result was misinterpreted as indicating that the lpp pgsA strain was inviable. However, the inability to cure the plasmid was explained by the thermosensitive growth of an lpp pgsA strain constructed by P1 transduction of the pgsA::kan allele into an lpp strain: when shifted to 42°C, the cells started to lyse in 2 to 3 h (29).
In this study, in order to identify the gene(s) involved in the thermosensitive defect of the lpp pgsA strain, we used transposon mutagenesis, screened for thermoresistant suppressor mutants, and found that disruption of the rcsC, rcsF, and yojN genes suppressed the defect. The rcsC gene codes for the sensor kinase of the Rcs phosphorelay signal transduction system, which was originally described as regulating the capsular polysaccharide synthesis genes (cps) (20, 52). RcsC activates RcsB, the response regulator of the system, by phosphoryl group transfer via the phosphotransmitter YojN (55). The rcsF gene product was reported to activate RcsB when overproduced (19), but its function in signal transduction was not clear until recently, when Hagiwara et al. (22) showed its involvement in Rcs signaling in response to glucose and zinc at 20°C. The Rcs system was shown to affect the expression of tolQRA (10), rcsA (15), ftsAZ (5), osmC (13), rprA (34), and flhDC (18). Recent transcriptome analyses using DNA arrays identified many other genes that are under direct or indirect control of the Rcs system (17, 22, 43).
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture media.
The E. coli K-12 derivative strains and the plasmids used for this study are listed in Table 1. According to the DDBJ/EMBL/GenBank DNA databases (accession no. D90851), the rcsC gene of the W3110 (Kohara) strain, from whose chromosomal DNA an ordered λ clone library was constructed (31), is disrupted by IS2 inserted at nucleotide (nt) 2374 of the 2,799-nt coding sequence with a duplication of nt 2370 to 2374. The C-terminal 142 residues, including Asp-859, which is essential for signal transduction (9), are missing from the gene product. However, the W3110 strain in our laboratory stock is rcsC+: PCR analysis of its rcsC region gave an amplified product with no apparent difference in size from that expected for the wild type, and its derivative S330 showed a thermosensitive growth phenotype in an rcsC-dependent manner, as described in Results.
TABLE 1.
Bacterial strains and plasmids used for this study
| Strain or plasmid | Relevant genotype or description | Construction, source, or reference |
|---|---|---|
| Strains | ||
| S301 | W3110 ksgB1 lpp-2a | 29 |
| S330 | S301 pgsA30::kan | 29 |
| SG20043 | MC4100 Δlon-100 cpsE3::Tn10 | 53, 56; NIGb collection |
| SG20781 | MC4100 cpsB10::lac-Mu-immλ | 3 |
| SG20797 | MC4100 cpsB10::lac-Mu-immλ Δlon-510 rcsB11::Tn10Δ16Δ17 | 3 |
| SG20803 | SG20781 rcsC137 ompC::Tn5 | 3 |
| UE29 | SG20781 lpp-2 | This study |
| UE44 | SG20781 lpp-2 Δara714c | This study |
| DHB6501 | λ− λssupF58 | 2 |
| UE38 | DHB6501 Δ(λattL-lom)::(bla araC PBAD-pgsA) | λ InCh procedure (2) using pHR702 |
| UE35 | MG1655 Δara714 | This study |
| UE39 | MG1655 Δara714 Δ(λattL-lom)::(bla araC PBAD-pgsA) | P1(UE38) × UE35 |
| UE42d | MG1655 Δara714 Δ(λattL-lom)::(bla araC PBAD-pgsA) pgsA30::kan | P1(S330) × UE39 |
| UE45 | SG20781 lpp-2 Δara714 Δ(λattL-lom)::(bla araC PBAD-pgsA) | P1(UE42) × UE44 |
| UE46 | SG20781 lpp-2 Δara714 Δ(λattL-lom)::(bla araC PBAD-pgsA) pgsA30::kan | P1(UE42) × UE45 |
| MDL12 | pgsA30::kan Φ(lacOP-pgsA+)1 lacZ lacY::Tn9 | 60 |
| CL330 | SG20781 lpp-2 pgsA30::kan | P1(MDL12) × UE29 |
| UE54 | MG1655 lpp-2 Δara714 rcsF::mini-Tn10 cam ΔpgsA::FRT-kan-FRT | λ Red recombinase procedure (12) |
| UE60d | MG1655 Δara714 Δ(λattL-lom)::(bla araC PBAD-pgsA) ΔpgsA::FRT-kan-FRT | P1(UE54) × UE39 |
| UE61d | MG1655 Δara714 Δ(λattL-lom)::(bla araC PBAD-pgsA) ΔpgsA::FRT | Yeast FLP recombinase procedure (12) |
| YK4516 | fliC::Tn10 | NIG collection |
| UE62d | MG1655 Δara714 Δ(λattL-lom)::(bla araC PBAD-pgsA) ΔpgsA::FRT fliC::Tn10 | P1(YK4516) × UE61 |
| CL332 | SG20781 lpp-2 ΔpgsA::FRT fliC::Tn10 | P1(UE62) × CL330 |
| ST261 | ΔyojN::kan | 55 |
| NFB216 | mdoH200::Tn10 | 33 |
| NFB732 | mdoB214::Tn10 | 32 |
| Plasmidse | ||
| pNK2884 | pBR322 derivative; mini-Tn10 cam Ptac-ATS transposasef | 30; American Type Culture Collection |
| pGB2 | pSC101 derivative; Spcr | 6 |
| pHR693 | pGB2 pgsA | This study |
| prcsC | pGB2 rcsC | This study |
| prcsF | pGB2 rcsF | This study |
| pHR718 | pGB2 lacIq Ptrc | This study |
| pHR719 | pGB2 lacIq P204 (down mutation in the −35 region of Ptrc) | This study |
| pHR722 | pGB2 lacIq Ptrc-rcsB | This study |
| pHR737 | pGB2 lacIq Ptrc-yojN | This study |
| pHR741 | pGB2 lacIq P204-djlA | This study |
| pBAD24 | pBR322 derivative; araC PBAD, Apr | 21 |
| pHR702 | pBAD24 pgsA | This study |
Formerly called lpo-5508.
NIG, National Institute of Genetics, Mishima, Japan.
SG20781, a derivative of MC4100, is sensitive to l-arabinose because of the araD139 mutation. Its Δara714 derivatives are not sensitive to l-arabinose.
These strains grow only in the presence of l-arabinose, which induces transcription from the PBAD promoter.
For details on the construction of plasmids, see Materials and Methods.
ATS transposase, altered target specificity transposase (30).
Recently, yojN was proposed to be renamed rcsD (18; also see the EcoGene web site [http://bmb.med.miami.edu/EcoGene/EcoWeb]), which may be confused with the previously reported rcsD mutation that is allelic to dsbB (20, 52). We use the gene designation yojN in this report.
For placement of the pgsA gene under the control of the PBAD promoter, a PCR-amplified pgsA fragment was inserted into the NheI-SmaI region of pBAD24 (21), and the resultant PBAD-pgsA construct in pHR702 was recombined, together with the araC and bla genes, into the chromosome at the λ attachment site by use of the λ InCh system (2). The chromosomal pgsA gene was deleted by Datsenko and Wanner's method utilizing λ Red and yeast FLP recombinases (12). First, the entire pgsA gene was replaced with the kanamycin resistance gene (kan) flanked by FLP recognition target sites (FRTs) in a strain with lpp and rcsF mutations, which remained thermoresistant even after the functional pgsA was lost. After ΔpgsA::FRT-kan-FRT was P1 transduced and confirmed for its lethality in an lpp+ strain carrying PBAD-pgsA at attλ, the kan cassette was excised to obtain the ΔpgsA::FRT allele.
For the construction of plasmids carrying the pgsA, rcsC, and rcsF genes, PCR-amplified fragments were cloned into the multiple cloning sites of pGB2 (6) in the same orientation as that of the spectinomycin resistance gene (aadA) of the vector. Low-copy-number vectors carrying Ptrc and its weakened derivative P204 (pHR718 and pHR719) were constructed by inserting a 1.8-kb NdeI (filled in)-HindIII fragment containing lacIq and the promoters of pTrc99A (1) and pDSW204 (58), respectively, into the EcoRI (filled in)-HindIII region of pGB2. PCR-amplified yojN and rcsB fragments were inserted into the NcoI (filled in)-BamHI region of pHR718 to construct pHR722 and pHR737, respectively. pHR741 was constructed by inserting a PCR-amplified djlA fragment into pHR719 in a similar way, but without filling the NcoI site, and the second residue of the product was changed from Gln to Glu.
Luria-Bertani (LB) medium (38), buffered LB medium in which the NaCl content was reduced to 3.5 g/liter and in which 10 ml of buffered salt solution (24) per liter was included, and M9 medium (38) were used as rich and minimal media. For culture plates, the media were solidified with 1.5% agar. When appropriate, antibiotics were included at the following concentrations (in micrograms per milliliter) for multicopy and single-copy resistance genes, respectively: ampicillin, 50 and 20; chloramphenicol, 50 and 10; kanamycin, 50 and 20; tetracycline, 3 (single copy); spectinomycin, 50 (multicopy). 5-Bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) was used at 40 μg/ml to examine the β-galactosidase levels of colonies on LB agar medium. Cell growth was monitored with a Klett-Summerson colorimeter equipped with a no. 54 filter.
Genetic and recombinant DNA procedures.
DNA procedures were based on standard methods (38, 46). Mutagenesis with mini-Tn10 cam (30) was performed essentially as described by Cao et al. (4). Briefly, strain S330(pNK2884) grown to early exponential phase was incubated for 30 min with 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) to induce the expression of transposase. The cells were then collected and resuspended in IPTG-free medium for the preparation of a phage P1 lysate. We estimated the number of transposed mini-Tn10 cam units per chromosome to be about 5 by comparing the transduction frequencies of kanamycin and chloramphenicol resistance markers. Chromosomally inserted minitransposons with their flanking regions were cloned by digesting the chromosomal DNAs of the insertion mutants with HindIII or PstI, or partially with Sau3AI, inserting the fragments into the HindIII, PstI, or BamHI site of pBR322, and screening for those that conferred chloramphenicol resistance. The insertion positions and orientations were determined by sequencing the cloned fragments in either direction from inside the transposon, using the primers 5′-CTACTGACGGGGTGGTGCGTAACGGC-3′ and 5′-CGATATGATCATTTATTCTGCCTCCC-3′, corresponding to nt 315 to 341 upstream of the initiation codon and nt 253 to 278 downstream of the termination codon, respectively, of the chloramphenicol resistance gene (cam). DNA sequencing was performed with a dye terminator cycle sequencing kit and a model 310 capillary DNA sequencer (Applied Biosystems).
The PCR primers used were as follows: 5′-GGGGTAAGCTTACTGACAACAG-3′ (48 to 27 nt upstream of the initiation codon with a substitution [underlined] to introduce a restriction site [italics]) and 5′-ggggatCCTGGCGCGATGAGTCAACG-3′ (68 to 48 nt downstream of the termination codon plus additional nucleotides [lowercase]) for cloning pgsA in pGB2; 5′-ACTGACAACAGCTAGCTACCCGTC-3′ (27 to 4 nt upstream of the initiation codon) and 5′-CCCGAATTCATCAAGCAATCAG-3′ (156 to 135 nt downstream of the termination codon) for placing pgsA under the control of the PBAD promoter; 5′-TAATCTTACTGACAACAGATAGTTACCCGTCATTATG-3′ (33 upstream nucleotides plus the initiation codon) joined to P4, a priming sequence for pKD13 (12), and 5′-TGATCGTTTGCTGAAAATTACGCCGAAACGATCACT-3′ (31 nt downstream to 2 nt upstream of the termination codon) joined to priming sequence P1 for deleting chromosomal pgsA; 5′-ggggaagcTTCGGTAATGGGGGCAAGTTCTGC-3′ (484 to 461 nt upstream of the initiation codon) and 5′-gcggaTCCGGCAGATAAAGACTAATCACCTGTAGG-3′ (215 to 186 nt downstream of the termination codon) for rcsC; 5′-gcctgcaGACTCCGGCGAAAGACGTATCTTT-3′ (297 to 274 nt upstream of the initiation codon) and 5′-ggaattCGAGCGAATAACGCCTATTTGCTC-3′ (38 to 15 nt downstream of the termination codon) for rcsF; 5′-CACTGCAGGATGATAAATATCACGGG-3′ (126 to 101 nt upstream of the initiation codon) and 5′-TTTGCGAATTCCGAACAAGAC-3′ (79 to 59 nt downstream of the termination codon) for PCR analysis of yojN; 5′-CGTCAGAAAGAGACAACGGCC-3′ (starting with the second codon) and 5′-gcggatCCTTGCTACAGCAAGCTCTTGAC-3′ (5 nt downstream to 15 nt upstream of the termination codon) for cloning yojN; 5′-ATTGAAGCTTAACTGGCGCAGGAAGAG-3′ (352 to 326 nt upstream of the initiation codon) and 5′-ggggatCCTGACGTTATATGCCGAGAG-3′ (249 to 229 nt downstream of the termination codon) for PCR analysis of rcsB; 5′-AACAATATGAACGTAATTATTGCCGATGACC-3′ (starting with the second codon) and 5′-gcggatCCTACAGGTGATTAGTCTTTATCTGCCGG-3′ (11 nt downstream to 15 nt upstream of the termination codon) for cloning rcsB; and 5′-catgccATGGAGTATTGGGGAAAAATCATTGGCG-3′ (28 nt, starting with the initiation codon) and 5′-ggcaagCTTTAATCGTGGGCAGTTACTCAGC-3′ (38 to 14 nt downstream of the termination codon) for djlA.
Biochemical procedures.
Cellular phospholipids were extracted and analyzed as described previously (41). A β-galactosidase assay using o-nitrophenyl-β-d-galactoside as a substrate and the definition of a unit were done as described by Wang and Doi (57).
RESULTS
Suppression of the thermosensitive growth defect of the pgsA null mutant by disruption of the rcsC, rcsF, yojN, and rcsB genes.
A pgsA null strain, S330, lacking detectable phosphatidylglycerol and cardiolipin grows well at 30 and 37°C but lyses when incubated at 42°C (29). The introduction of plasmid pHR693 carrying the pgsA gene corrected this thermosensitive defect. We sought suppressor mutations of this defect by transposon mutagenesis. Mini-Tn10 cam (30) was transposed randomly into the chromosome of S330 from plasmid pNK2884 by inducing the tac promoter-controlled transposase gene on the plasmid. Phage P1 lysates prepared from 16 independently transposition-induced cultures were used to infect S330, and chloramphenicol-resistant transductants were selected at 42°C on LB agar medium. Several transductants were picked from each independent experiment and examined for growth at 42°C in LB liquid medium. Forty-four transductants that were confirmed for the suppression of thermosensitivity were analyzed for their phospholipid composition. They all lacked detectable phosphatidylglycerol and cardiolipin, as did S330.
We found disruptions of the rcsC, rcsF, and yojN genes in one, four, and one independent suppressor mutant, respectively, by cloning and sequencing of the chromosomal fragments containing the inserted minitransposons. In the rcsC mutant, the insertion was at nt 2318 of the 2,799-nt coding sequence, and the 9 nt from positions 2310 to 2318 were duplicated. The cam gene was in the same orientation as rcsC. In the four rcsF mutants, the insertion positions and orientations were the same: the insertions were at nt 43 of the 399-nt coding sequence, with a duplication of nt 35 to 43, and the orientation was opposite to that of rcsF. In the yojN mutant, the transposon was inserted at nt 1358 of the 2,670-nt coding sequence in the same orientation. Although the transposition was catalyzed by altered target specificity transposase (30), the 9-nt target sequences for these insertions matched the consensus sequence 5′-NGCTNAGCN-3′ for the wild-type Tn10 or were mismatched at only a single AT base pair. Insertions of 1.5 kb in the rcsC, rcsF, and yojN genes in these suppressor mutants were also shown by PCR amplification of the respective genes.
Screening by PCR of the other 35 suppressor mutants identified 1.5-kb insertions in either the rcsC, rcsF, or yojN region in all but three mutants. For the transduction experiments described below, we used the mini-Tn10 cam insertion alleles with defined insertion positions that we first identified. Three mutants, two of which originated from one P1 lysate, did not have insertions in the genes that are known to be involved in the Rcs signal transduction system, and they are now under investigation.
Mini-Tn10 cam insertions in rcsC, rcsF, and yojN were transduced into S330 by the use of phage P1, and all of the chloramphenicol-resistant transductants were thermoresistant (Fig. 1A to D). Their phospholipid compositions were not altered from that of parent strain S330 (see Fig. 3). When the rcsF::mini-Tn10 cam and yojN::mini-Tn10 cam transductants were transformed with plasmids carrying rcsF and yojN, respectively, they became thermosensitive again, indicating that the disruption of rcsF and yojN was responsible for the suppression. The introduction of an rcsC-carrying plasmid to the rcsC::mini-Tn10 cam transductant resulted in much poorer growth at 42°C, but not in complete thermosensitivity. In this plasmid, the cloned fragment contained the putative promoter for the rcsC gene. Moreover, the rcsC gene was placed downstream of the aadA gene of the vector so that transcription from the aadA promoter would flow into rcsC. Although the plasmid has a low copy number, it is likely to moderately overexpress rcsC. When S330 was transformed with the same plasmid, it grew, albeit very poorly, at 42°C on LB agar medium. The sensor kinase RcsC was suggested to control the activity of the response regulator RcsB not only positively but also negatively, presumably via dephosphorylation (3, 20, 34, 52). We concluded that the rcsC disruption was responsible for the suppression of the thermosensitive growth while rcsC overexpression only partially suppressed the thermosensitivity.
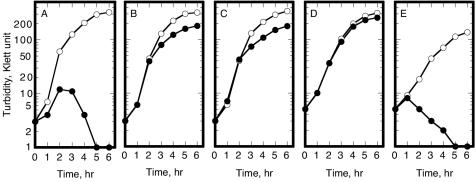
FIG. 1.
Suppression of the thermosensitive growth defect of the pgsA null mutant. The minitransposon insertions in rcsC, rcsF, and yojN identified by a screen for suppressor mutants were transduced by phage P1 into S330. Overnight cultures of S330 (A) and its rcsC::mini-Tn10 cam (B), rcsF::mini-Tn10 cam (C), yojN::mini-Tn10 cam (D), and cpsE::Tn10 (E) transductants were diluted to 3 to 5 Klett units in LB medium and grown at 37°C (open circles) and 42°C (closed circles), and the culture turbidities were monitored.
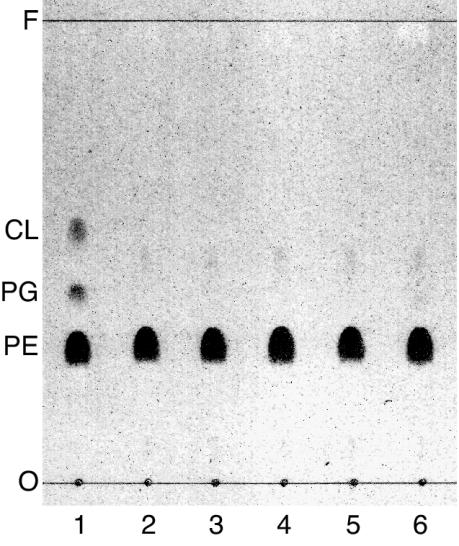
FIG. 3.
The mutations that suppress the thermosensitivity of the pgsA null mutant do not suppress the loss of phosphatidylglycerol and cardiolipin. UE29 (lane 1), CL330 (lane 2), and its rcsC::mini-Tn10 cam (lane 3), rcsF::mini-Tn10 cam (lane 4), yojN::mini-Tn10 cam (lane 5), and rcsB::Tn10Δ16Δ17 (lane 6) transductants were grown to mid-exponential phase (ca. 100 Klett units) in LB medium. The lipids were extracted and separated by thin-layer chromatography, and spots of phospholipids were visualized with Dittmer-Lester reagent. F, solvent front; CL, cardiolipin; PG, phosphatidylglycerol; PE, phosphatidylethanolamine; O, chromatographic origin. The faint spots at the positions between cardiolipin and phosphatidylglycerol in lanes 2 to 6 are phosphatidic acid, which accumulates in small amounts in pgsA null cells (29).
Among the suppressor mutants we isolated, none was found to have a minitransposon insertion in the rcsB region by PCR screening. However, when we transduced the rcsB::Tn10Δ16Δ17 mutation (3) into S330, the transductants were thermoresistant on LB agar medium while remaining devoid of the major acidic phospholipids (see Fig. 3). This suppression was reversed by the introduction of an rcsB-carrying plasmid. Thus, the disruption of rcsB also suppresses the thermosensitivity of S330.
For these complementation experiments, the yojN and rcsB genes were cloned under the control of the trc promoter and were expressed at the basal level in the absence of IPTG. The induction of either gene with 0.5 mM IPTG was inhibitory to the growth of S330, even at low temperatures.
Activation of Rcs phosphorelay signal transduction system in the pgsA null mutant.
Since a disruption of the genes for the components of the Rcs phosphorelay system suppressed the thermosensitive growth defect of S330, we supposed that this signal transduction system was activated in the pgsA null mutant and was responsible for the growth defect. The Rcs system positively regulates the cps genes for capsular polysaccharide synthesis (20, 52), and a cpsB′-lac transcriptional fusion (3, 56) provides a sensitive assay of the level of activation of the system. We first constructed a cpsB′-lac fusion strain in which the only functional pgsA gene was under the control of the arabinose-regulatable PBAD promoter. This strain, UE46, was grown in the presence of the inducer and plated on LB agar medium containing X-Gal. In the absence of arabinose, the colonies were blue, whereas in its presence the colonies were white, indicating that the Rcs system was activated when the expression of the pgsA gene was repressed.
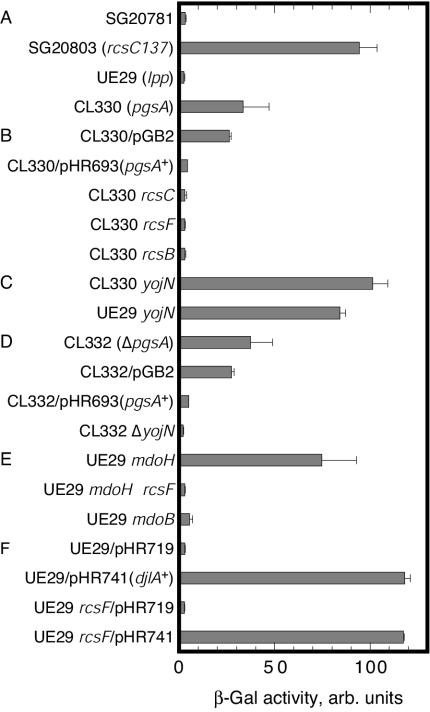
Although the PBAD promoter is tightly regulated (21), its repression may not produce a complete null phenotype, especially in cases of genes that are normally expressed at low levels (23). In the absence of arabinose, UE46 formed minute colonies at 42°C. Hence, we constructed the pgsA null, cpsB′-lac fusion strain CL330 and used it for quantitative measurements of β-galactosidase activity. This strain was thermosensitive, as was S330, and it formed no colonies at 42°C. Cells were grown at 30°C and assayed for enzyme activity at 28°C as described by Wang and Doi (57). As shown in Fig. 2A, cps transcription in CL330 was >10 times higher than that in the pgsA+ parent strain UE29. This transcription level was about one-third that found in strain SG20803, which has an rcsC137 mutation that causes constitutive activation of the Rcs system (3, 20, 52). On LB agar medium containing X-Gal, the colonies of CL330 were blue at 30 and 37°C.
FIG. 2.
Activation of Rcs phosphorelay signal transduction system in the pgsA null mutant. Cells were grown in buffered LB medium at 30°C to mid-exponential phase (ca. 100 Klett units), and the β-galactosidase activity was measured. The means and standard errors of at least three measurements are shown. The mutations transduced into strain UE29, CL330, or CL332 were rcsC::mini-Tn10 cam (rcsC), rcsF::mini-Tn10 cam (rcsF), rcsB::Tn10Δ16Δ17 (rcsB), yojN::mini-Tn10 cam (yojN), ΔyojN::kan (ΔyojN), mdoH::Tn10 (mdoH), and mdoB::Tn10 (mdoB). Three transductants were isolated and examined. They exhibited essentially the same results. For panel F, the cells were grown in the presence of 1 mM IPTG.
Transformation with a pgsA-carrying plasmid and transduction of the disrupted alleles of rcsC, rcsF, and rcsB corrected the thermosensitive growth defect of CL330 and lowered cps transcription to the wild-type level (Fig. 2B), demonstrating a correlation of activation of the Rcs phosphorelay system with thermosensitivity. CL330 contained no detectable phosphatidylglycerol or cardiolipin, nor did S330, and the mutations for the suppression of thermosensitivity did not alter the phospholipid composition (Fig. 3).
The yojN::mini-Tn10 cam allele unexpectedly activated Rcs signaling at 30°C.
The transduction of the yojN::mini-Tn10 cam allele into CL330 also suppressed the thermosensitive defect without suppressing the defect in major acidic phospholipid synthesis (Fig. 3). However, cpsB′-lac transcription in transductant cells grown at 30°C was as high as that in the rcsC137 mutant (Fig. 2C). When we examined the transductants on X-Gal-containing LB agar medium, we observed that they formed blue colonies at 30°C but that the colony color was very pale at 37°C and white at 42°C, indicating that yojN::mini-Tn10 cam suppressed activation of the Rcs system at the high temperature that was restrictive for the growth of the pgsA null mutant. We then transduced this yojN allele into UE29 and found that it caused a very high cps transcription level at 30°C, even in this pgsA+ strain (Fig. 2C). When this strain was examined on X-Gal-containing agar medium, the β-galactosidase activity was very low at 37°C and almost at the wild-type level at 42°C. A similar result was found for yojN::mini-Tn10 cam transductants of the lpp+ parent of UE29, SG20781. Thus, the high cps expression level in cells with this yojN allele was irrelevant to the pgsA or lpp defect and was observed only at low temperatures.
Transcription of the inserted cam gene might flow into the downstream rcsB gene and increase its expression, which could result in the increase in cps expression (3, 20). However, the introduction of the yojN-carrying plasmid into a yojN::mini-Tn10 cam derivative of SG20781 reduced the β-galactosidase activity at 30°C to the wild-type level, as examined on X-Gal-containing agar medium, indicating that the high cps expression level was due to the yojN mutation, not to an increase in rcsB expression. The transduction of rcsB::Tn10Δ16Δ17 abolished the cps induction, and the induction was likely due to the activation of RcsB. The truncated product encoded by this transposon-inserted yojN allele might have an unusual function at 30°C, although it was recessive to the wild type.
The unexpected activation of RcsB at 30°C was peculiar to this mini-Tn10 cam-inserted allele. The ΔyojN::kan allele constructed by Takeda et al. completely abolished Rcs signaling that was stimulated in response to DjlA overproduction at 30°C (55) and to glucose and zinc ions at 20°C (22). CL330 is resistant to kanamycin, and its ΔyojN::kan transductants could not be selected. Hence, we constructed a pgsA deletion allele with no antibiotic resistance markers and substituted it for the pgsA::kan allele in CL330. The resultant strain, CL332, showed the same phenotypes as CL330: it lacked the major acidic phospholipids, had the thermosensitive growth defect, and exhibited elevated cpsB′-lac transcription at 30°C, all of which were complemented by the plasmid-borne pgsA gene. When transduced into CL332, ΔyojN::kan corrected the growth defect and lowered cps transcription to the wild-type level (Fig. 2D). Thus, the thermosensitivity of the pgsA null mutant was correlated with the activation of the Rcs phosphorelay system, including YojN.
Capsular polysaccharide synthesis is not involved in the thermosensitivity of the pgsA null mutant.
The Rcs phosphorelay system was activated and caused the thermosensitive growth defect in CL330 and CL332. These strains were unable to produce capsules because the lac fusion inactivated the cpsB gene and probably exerted a polar effect on the downstream cps genes. The cpsB gene is the 14th in the 19-gene cps operon (51). Thus, the thermosensitive defect was not due to an increase in the amount of capsule. However, the first 13 genes of the operon were probably induced when the Rcs system was activated, and the imbalance in expression among the cps genes might have led to the accumulation of a capsule biosynthetic intermediate(s) with a deleterious effect on bacterial growth, as suggested by Brill et al. (3). We next examined the effect of a cpsE::Tn10 mutation (53, 56). The cpsE gene is positioned upstream of the other genetically identified cps genes, including cpsB (53), and according to the Profiling of E. coli Chromosome database (http://www.shigen.nig.ac.jp/ecoli/pec/), it is the first gene of the operon. When cpsE::Tn10 was transduced into S330, all of the transductants remained thermosensitive (Fig. 1E). We concluded that the activation of the capsule synthesis pathway was not involved in the thermosensitivity of the pgsA null mutant.
Defective modification of membrane-derived oligosaccharides is not responsible for activation of the Rcs phosphorelay system.
Phosphatidylglycerol is a substrate for phosphoglycerol modification of osmoregulated periplasmic glucans, which were hence named membrane-derived oligosaccharides (MDOs) (28). The pgsA3 point mutation leads to a low phosphatidylglycerol content and to reduced phosphoglycerol modification of MDOs (39). The pgsA null cells are probably devoid of phosphoglycerol-modified MDOs. Ebel et al. (16) reported that the disruption of mdoH activated the Rcs system, which we confirmed in mdoH::Tn10 transductants of UE29 (Fig. 2E), and we first suspected that defective MDOs could be responsible for Rcs activation in the pgsA null mutant. When the mdoH::Tn10 transductants were plated on LB agar medium containing X-Gal, the colonies were blue at 30°C but white at 37 and 42°C, which was in contrast to the blue colonies of CL330 at 37°C. The mdoH mutation leads to the loss of glucosyltransferase, and the mutant cells lack MDOs (33); an mdoB mutation leads to the loss of phosphoglycerol transferase, and the mutant cells produce MDOs in normal amounts but without phosphoglycerol substitutions (26). The pgsA null cells are presumably defective in the latter enzyme reaction because of the lack of the phosphoglycerol donor. We transduced mdoB::Tn10 into UE29 and found that the loss of phosphoglycerol modification did not activate the Rcs system (Fig. 2E). We concluded that defective modification of MDOs was not responsible for the activation of the Rcs system and thus for the thermosensitive growth defect of the pgsA null mutant.
Involvement of RcsF in activation of the Rcs phosphorelay system.
The RcsF protein was first reported to activate RcsB when it was overproduced (19) and was recently reported to be essential for Rcs signaling in response to glucose and zinc ions at 20°C (22). In this study, we showed that this protein was essential for Rcs activation in the pgsA null mutant. Rcs activation in the mdoH null strain was also dependent on RcsF: the transduction of rcsF::mini-Tn10 cam into an mdoH::Tn10 derivative of UE29 abolished the high level of cpsB′-lac expression (Fig. 2E). In contrast, Rcs activation in cells overproducing DjlA, or membrane-anchored DnaJ-like protein (8, 27), was observed in the absence of RcsF. IPTG-induced expression of djlA cloned under a weakened derivative of the Ptrc promoter led to elevated transcription of the cpsB′-lac fusion in UE29, and the introduction of rcsF::mini-Tn10 cam by P1 transduction did not lower the transcription level (Fig. 2F).
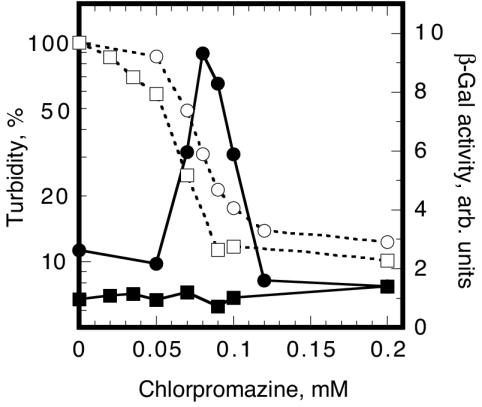
We were next interested in the activation of the Rcs system by the psychotropic drug chlorpromazine at sublethal concentrations (11). Chlorpromazine is a cationic amphipathic compound and potentially has an affinity for acidic phospholipids (48). When challenged with 70 to 90 μg of chlorpromazine/ml, SG20781 showed a severalfold induction of cps expression, whereas no induction was observed in the rcsF::mini-Tn10 cam transductant (Fig. 4), indicating the involvement of RcsF in the stimulation of Rcs signaling by the drug. The rcsF transductant was slightly more sensitive to the drug than the rcsF+ parent strain.
FIG. 4.
RcsF is essential for Rcs signal transduction in response to treatment with chlorpromazine. The experimental procedure was done essentially as described by Conter et al. (11). A cpsB′-lac fusion strain, SG20781 (circles), and its rcsF::mini-Tn10 cam transductant (squares) were grown in NaCl-free LB medium to ca. 25 Klett units at 37°C, and chlorpromazine was added to the indicated concentrations. After 2 h, the turbidity (open symbols) and β-galactosidase activity (closed symbols) were measured. The turbidity was normalized to the value of the culture that was not treated with the drug for each strain (ca. 200 Klett units) and is shown as a percentage.
DISCUSSION
In this study, we showed that the Rcs phosphorelay signal transduction system is activated in a pgsA null mutant lacking phosphatidylglycerol and cardiolipin and that the activation is responsible for the thermosensitive growth defect of the mutant. The growth defect could be interpreted as an indication of an essential role of these major acidic phospholipids at high temperatures. However, when Rcs signaling was blocked by disruption of the rcsC, rcsF, yojN, and rcsB genes, the pgsA null mutant became thermoresistant while remaining devoid of these phospholipids, indicating the dispensability of the major acidic phospholipids, even at high temperatures. Acidic phospholipids are considered to be required for essential cellular functions, including the recruitment of peripheral membrane proteins to the negatively charged membrane surface (14, 35). The minimum requirements for such functions can probably be fulfilled by the acidic intermediates phosphatidic acid and CDP-diacylglycerol, which accumulate, albeit in small amounts, in the pgsA null mutant (29, 35, 54).
Disrupted alleles of rcsF, rcsC, and yojN.
The mutations that suppressed thermosensitivity were isolated by minitransposon mutagenesis. In the rcsF::mini-Tn10 cam allele, five codons of unnatural sequence followed by two consecutive termination codons were introduced after the 13th codon, and thus this interrupted rcsF gene is a null allele. In the rcsC::mini-Tn10 cam allele, two consecutive termination codons were introduced just after codon 773. This mutation may leave the kinase domain intact, including the active center at His-463 (9, 20, 52, 55), although we do not know whether the truncated product is stable in cells. Even if it is stable, it is devoid of the receiver domain, including Asp-859 (9, 55), and the loss of Rcs signaling due to this mutation can be taken as an indication of the importance of the receiver domain for phosphorelay (9).
In the yojN::mini-Tn10 cam allele, two consecutive termination codons were introduced just after codon 453. The truncated product lacked the C-terminal phosphotransmitter domain, including His-842, that is essential for phosphorelay (55). Surprisingly, this mutation stimulated cps transcription in the wild-type background in the apparent absence of an external stimulus at 30°C. The truncated product may be stable in cells and manifest the unusual phenotype at low temperatures. This phenotype was dependent on the intact rcsB gene, indicating the involvement of activation of RcsB. The Rcs signal transduction system is postulated to relay a phosphoryl group from the autophosphorylated kinase domain of the sensor protein RcsC to its receiver domain, then to the YojN transmitter domain, and finally to the RcsB receiver domain to activate this response regulator (9, 20, 52, 55). In the presence of the YojN protein with a truncated transmitter domain encoded by the minitransposon-inserted allele identified in this study, however, RcsB may be activated by direct phosphotransfer from the RcsC kinase domain. Although the YojN truncation is artificial, a natural occurrence of a similar shortcut phosphotransfer in a multistep phosphorelay system has been reported for the Arc signal transduction system (36). The YojN protein has structural similarity to RcsC, except that the His residue for autophosphorylation is missing, and it has been suggested to form a heteromeric complex with RcsC in the cytoplasmic membrane (55). The truncation of YojN after residue 453 may not completely impair its ability to interact with RcsC, and RcsC in complex with truncated YojN may acquire an unusual activity of autophosphorylation without an external stimulus and of direct phosphotransfer to RcsB from its kinase domain. The truncated YojN protein is probably weaker in its interaction with RcsC than the wild-type protein and would be displaced from the complex in the presence of the wild type. Thus, plasmid-borne wild-type yojN corrected the unusual phenotype of yojN::mini-Tn10 cam at 30°C. At higher temperatures, the truncated YojN protein is likely unstable, and the phenotype disappeared at 42°C.
In our minitransposon mutagenesis experiments, suppressor mutations were isolated repeatedly in the rcsC, rcsF, and yojN genes, but none were isolated in the rcsB gene. However, transposon-inserted rcsB (3) suppressed the thermosensitivity of the pgsA null mutant. Thus, our screening for suppressors has not reached a saturating level, and further screening can be expected to lead to the identification of a gene(s) for an unknown factor(s) that is involved in the regulation of Rcs signaling or of a gene(s) whose Rcs-induced expression is responsible for the thermosensitive defect.
Rcs activation is responsible for the thermosensitive growth defect.
The suppression of activation of the Rcs signal transduction system and of the thermosensitive growth defect by disruption of the rcs genes was well correlated in the pgsA null mutant, indicating the responsibility of Rcs activation for the defect. In the case of yojN, the deletion allele constructed by Takeda et al. (55) showed a clear-cut correlation. Although the yojN::mini-Tn10 cam allele that we isolated showed an unusual Rcs-activating phenotype at 30°C, it blocked activation of the Rcs system at high temperatures and suppressed thermosensitivity.
Although the Rcs system positively regulates the expression of the cps genes, capsular polysaccharide synthesis is not involved in the growth defect, as revealed by the inability of a cpsB or cpsE disruption to suppress the defect. In addition to the cps genes and several other genes whose regulation by the Rcs system has been fairly thoroughly investigated (5, 10, 13, 15, 18, 34), many genes were recently identified as putative Rcs regulon members (17, 22, 43). Some are positively and others are negatively regulated. It has yet to be elucidated which of the members is directly responsible for the thermosensitivity of the pgsA null mutant. The responsible gene(s) may be found among suppressor mutations or multicopy suppressors.
Altered gene expression upon activated Rcs signaling causes a growth defect only in the absence of the major acidic phospholipids. In the pgsA+ background, Rcs activation does not cause the defect. SG20803, an rcsC137 cpsB′-lac fusion strain (3), grew well and formed blue colonies on X-Gal-containing agar medium at 42°C. Although overproduced DjlA is toxic in a pgsA+ strain even at low temperatures (7), the toxic effect cannot be ascribed solely to the activation of the Rcs system because in a preliminary experiment the growth inhibition due to the overexpression of djlA cloned under the strong inducible promoter Ptrc was not suppressed by the disruption of rcsC or yojN.
The growth defect was observed only at high temperatures. The pgsA null mutant may be vulnerable to an adverse effect of Rcs activation only at high temperatures. Alternatively, the stimulus to the Rcs system in the pgsA null mutant may be stronger at higher temperatures, causing more Rcs activation. At 30°C, the system was not fully activated compared with the activation in the rcsC137 mutant, and the overexpression of rcsF (Y. Shiba, K. Matsumoto, and H. Hara, unpublished data), yojN, or rcsB (this study), which would further stimulate Rcs signaling, caused a growth defect even at 30°C.
Envelope stresses that activate the Rcs signal transduction system.
The Rcs signal transduction system is activated in response to environmental stresses such as desiccation (42), osmotic shock (49), treatment with chlorpromazine (11), and growth in the presence of glucose and zinc ions at 20°C (22) and on a solid surface (17). Thus, the system is implicated in survival outside of a mammalian host (52) and in biofilm formation (17). Genetic modifications leading to a defect in lipopolysaccharides (45), the overproduction of DjlA (8, 27), and a lack of MDOs (16) also activate the Rcs system. A lack of phosphoglycerol substitutions in MDOs was not the cause of Rcs activation in the pgsA null mutant because a defect of the phosphoglycerol transferase MdoB had no stimulatory effect on the Rcs system. The environmental and genetic conditions that activate the Rcs system can be regarded as perturbing the cell envelope. The lack of the major acidic phospholipids in the pgsA null mutant is another example of such an envelope stress. It greatly decreases the negative charge on the membrane surfaces, which could affect the functions of peripheral and integral membrane proteins (14, 35). A decrease in the negative charge of MDOs because of the loss of phosphoglycerol residues could also affect periplasmic and membrane proteins (28).
Mileykovskaya and Dowhan (37) reported that the Cpx phosphorelay signal transduction system is activated in a pssA null mutant which completely lacks the major zwitterionic phospholipid phosphatidylethanolamine and contains the major acidic phospholipids in its place (14). We suspected that this dramatic alteration in phospholipid composition could also be an envelope stress to activate the Rcs phosphorelay system, but the pssA null mutation did not activate the Rcs system (A. Ito, K. Matsumoto, and H. Hara, unpublished data). Besides their zwitterionic versus anionic head groups, phosphatidylethanolamine and phosphatidylglycerol have distinct structural features: phosphatidylethanolamine has a propensity to form a nonbilayer phase owing to its small polar head group, whereas phosphatidylglycerol prefers to form a bilayer phase. Cardiolipin also has a non-bilayer-forming propensity, but only in the presence of high concentrations of divalent cations (14). The intrinsic curvature of the membrane with a higher content of non-bilayer-forming lipids would result in a lower packing density of head groups at the membrane surface. The pssA null mutant lacks non-bilayer-forming phosphatidylethanolamine, whereas the pgsA null mutant has a higher phosphatidylethanolamine content than the wild type (29). The physical properties of the membranes of the pgsA and pssA null mutants might produce distinct responses by the Rcs and Cpx phosphorelay systems. Activation of the Rcs system by treatment with chlorpromazine can also be ascribed to the altered physical properties of the membrane (11). This cationic amphipathic compound intercalates into the lipid bilayer and induces curvature stress (48).
The Cpx signal transduction system is implicated in biofilm formation, as is the Rcs system. The Cpx system is activated upon attachment to abiotic surfaces, and mutants defective in the system show a decreased level of attachment (44). Cpx activation occurs immediately upon attachment, probably in the initial stage of biofilm formation. In contrast, the Rcs system is induced during growth on a solid surface and affects the cell surface composition, and it is required for maturation of the biofilm (17). Along with the development of biofilms, cell-to-surface and cell-to-cell interactions may modulate the physical properties of the cell membrane, thereby eliciting responses by the phosphorelay systems that are appropriate for biofilm developmental stages.
Involvement of RcsF in Rcs signaling.
RcsF is an essential component of the Rcs signal transduction system when it responds to glucose and zinc ions at 20°C (22), a lack of the major acidic phospholipids, a lack of MDOs, and treatment with chlorpromazine. RcsF is predicted to be a lipoprotein located in the outer membrane because it has a lipobox sequence at the end of the putative signal peptide followed by a Ser residue (59). The stresses that stimulate Rcs signaling might affect the physical properties of the outer membrane and be sensed by RcsF. Among the Rcs-stimulating stresses that we examined, the overproduction of DjlA was the only example that was not dependent on RcsF. This is reasonable considering that DjlA is a cytoplasmic membrane protein whose major part is on the cytoplasmic side (7). The overproduced DjlA presumably interacts with RcsC to activate signal transduction.
RcsF was first identified as a protein whose overproduction stimulated Rcs signaling (19). In the Cpx signal transduction system, the overproduction of a minor outer membrane lipoprotein, NlpE, has a stimulatory effect (50). While this protein is not involved in Cpx activation in the pssA null mutant (37), it plays an essential role in the response to attachment to abiotic surfaces (44). It was suggested that in this response NlpE senses a stress in the outer membrane and mediates the signal to the sensor kinase CpxA in the cytoplasmic membrane (44). RcsF is likely to play a similar role in Rcs activation.
For the pgsA null mutant, there is another possible explanation for how RcsF is involved in Rcs activation. Phosphatidylglycerol, which is missing in the mutant, normally serves as a diacylglycerol donor for modification of the lipobox Cys residue, which is a prerequisite for the maturation of lipoproteins (59). The phosphatidic acid and CDP-diacylglycerol that the mutant accumulates in small amounts can also serve as donors, but only inefficiently (47). When the major outer membrane lipoprotein Lpp was expressed in the mutant, the product was poorly modified and the precursor form remained in the cytoplasmic membrane (54). RcsF may also be retarded in maturation and thus in localization to the outer membrane, which may lead to the stimulation of RcsC in the cytoplasmic membrane. However, we do not know how much this quantitatively minor lipoprotein is affected by the lack of phosphatidylglycerol.
A complete lack of a particular lipid species in the membrane is an artificial situation for wild-type bacteria. However, an alteration of the properties of the membrane due to an altered lipid composition may mimic the envelope perturbation brought about by environmental stresses, as we speculated for the pgsA and pssA null mutants of E. coli. We expect that mutants defective in lipid-synthesizing enzymes can be useful models for the elucidation of how phosphorelay signal transduction systems and other stress-responsive systems sense and respond to stresses exerted on the envelope.
Acknowledgments
We are grateful to Hirofumi Aiba, Mary Berlyn, Jean-Pierre Bohin, Dana Boyd, William Dowhan, Michael Ehrmann, Susan Gottesman, Akiko Nishimura, and Barry L. Wanner for bacterial strains and plasmids and to Hiroshi Matsuzaki, Hideki Nagahama, Yoshito Sadaie, Yutaka Sakamoto, and Isao Shibuya for stimulating discussions and continuous encouragement.
This work was supported in part by grants-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
REFERENCES
- 1.Amann, E., B. Ochs, and K.-J. Abel. 1988. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene 69:301-315. [DOI] [PubMed] [Google Scholar]
- 2.Boyd, D., D. S. Weiss, J. C. Chen, and J. Beckwith. 2000. Towards single-copy gene expression systems making gene cloning physiologically relevant: lambda InCh, a simple Escherichia coli plasmid-chromosome shuttle system. J. Bacteriol. 182:842-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brill, J. A., C. Quinlan-Wasche, and S. Gottesman. 1988. Fine-structure mapping and identification of two regulators of capsule synthesis in Escherichia coli. J. Bacteriol. 170:2599-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao, Y., R. R. R. Rowland, and T. Kogoma. 1993. DNA polymerase I and the bypassing of RecA dependence of constitutive stable DNA replication in Escherichia coli rnhA mutants. J. Bacteriol. 175:7247-7253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carballès, F., C. Bertrand, J.-P. Bouché, and K. Cam. 1999. Regulation of Escherichia coli cell division genes ftsA and ftsZ by the two-component system rcsC-rcsB. Mol. Microbiol. 34:442-450. [DOI] [PubMed] [Google Scholar]
- 6.Churchward, G., D. Belin, and Y. Nagamine. 1984. A pSC101-derived plasmid which shows no sequence homology to other commonly used cloning vectors. Gene 31:165-171. [DOI] [PubMed] [Google Scholar]
- 7.Clarke, D. J., A. Jacq, and I. B. Holland. 1996. A novel DnaJ-like protein in Escherichia coli inserts into the cytoplasmic membrane with a type III topology. Mol. Microbiol. 20:1273-1286. [DOI] [PubMed] [Google Scholar]
- 8.Clarke, D. J., I. B. Holland, and A. Jacq. 1997. Point mutations in the transmembrane domain of DjlA, a membrane-linked DnaJ-like protein, abolish its function in promoting colanic acid production via the Rcs signal transduction pathway. Mol. Microbiol. 25:933-944. [DOI] [PubMed] [Google Scholar]
- 9.Clarke, D. J., S. A. Joyce, C. M. Toutain, A. Jacq, and I. B. Holland. 2002. Genetic analysis of the RcsC sensor kinase from Escherichia coli K-12. J. Bacteriol. 184:1204-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clavel, T., J. C. Lazzaroni, A. Vianney, and R. Portalier. 1996. Expression of the tolQRA genes of Escherichia coli K-12 is controlled by the RcsC sensor protein involved in capsule synthesis. Mol. Microbiol. 19:19-25. [DOI] [PubMed] [Google Scholar]
- 11.Conter, A., R. Sturny, C. Gutierrez, and K. Cam. 2002. The RcsCB His-Asp phosphorelay system is essential to overcome chlorpromazine-induced stress in Escherichia coli. J. Bacteriol. 184:2850-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davalos-Garcia, M., A. Conter, I. Toesca, C. Gutierrez, and K. Cam. 2001. Regulation of osmC gene expression by the two-component system rcsB-rcsC in Escherichia coli. J. Bacteriol. 183:5870-5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dowhan, W. 1997. Molecular basis for membrane phospholipid diversity: why are there so many lipids? Annu. Rev. Biochem. 66:199-232. [DOI] [PubMed] [Google Scholar]
- 15.Ebel, W., and J. E. Trempy. 1999. Escherichia coli RcsA, a positive activator of colanic acid capsular polysaccharide synthesis, functions to activate its own expression. J. Bacteriol. 181:577-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebel, W., G. J. Vaughn, H. K. Peters III, and J. E. Trempy. 1997. Inactivation of mdoH leads to increased expression of colanic acid capsular polysaccharide in Escherichia coli. J. Bacteriol. 179:6858-6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrières, L., and D. J. Clarke. 2003. The RcsC sensor kinase is required for normal biofilm formation in Escherichia coli K-12 and controls the expression of a regulon in response to growth on a solid surface. Mol. Microbiol. 50:1655-1682. [DOI] [PubMed] [Google Scholar]
- 18.Francez-Charlot, A., B. Laugel, A. Van Gemert, N. Dubarry, F. Wiorowski, M.-P. Castanié-Cornet, C. Gutierrez, and K. Cam. 2003. RcsCDB His-Asp phosphorelay system negatively regulates the flhDC operon in Escherichia coli. Mol. Microbiol. 49:823-832. [DOI] [PubMed] [Google Scholar]
- 19.Gervais, F. G., and G. R. Drapeau. 1992. Identification, cloning, and characterization of rcsF, a new regulator gene for exopolysaccharide synthesis that suppresses the division mutation ftsZ84 in Escherichia coli K-12. J. Bacteriol. 174:8016-8022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gottesman, S. 1995. Regulation of capsule synthesis: modification of the two-component paradigm by an accessory unstable regulator, p. 253-262. In J. A. Hoch and T. J. Silhavy (ed.), Two-component signal transduction. ASM Press, Washington, D.C.
- 21.Guzman, L.-M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagiwara, D., M. Sugiura, T. Oshima, H. Mori, H. Aiba, T. Yamashino, and T. Mizuno. 2003. Genome-wide analysis revealing a signaling network of the RcsC-YojN-RcsB phosphorelay system in Escherichia coli. J. Bacteriol. 185:5735-5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haldimann, A., L. L. Daniels, and B. L. Wanner. 1998. Use of new methods for construction of tightly regulated arabinose and rhamnose promoter fusions in studies of the Escherichia coli phosphate regulon. J. Bacteriol. 180:1277-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hara, H., S. Yasuda, K. Horiuchi, and J. T. Park. 1997. A promoter for the first nine genes of the Escherichia coli mra cluster of cell division and cell envelope biosynthesis genes, including ftsI and ftsW. J. Bacteriol. 179:5802-5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heacock, P. N., and W. Dowhan. 1987. Construction of a lethal mutation in the synthesis of the major acidic phospholipids of Escherichia coli. J. Biol. Chem. 262:13044-13049. [PubMed] [Google Scholar]
- 26.Jackson, B. J., J.-P. Bohin, and E. P. Kennedy. 1984. Biosynthesis of membrane-derived oligosaccharides: characterization of mdoB mutants defective in phosphoglycerol transferase I activity. J. Bacteriol. 160:976-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelley, W. L., and C. Georgopoulos. 1997. Positive control of the two-component RcsC/B signal transduction network by DjlA: a member of the DnaJ family of molecular chaperones in Escherichia coli. Mol. Microbiol. 25:913-931. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy, E. P. 1996. Membrane-derived oligosaccharides (periplasmic beta-d-glucans) of Escherichia coli, p. 1064-1071. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 29.Kikuchi, S., I. Shibuya, and K. Matsumoto. 2000. Viability of an Escherichia coli pgsA null mutant lacking detectable phosphatidylglycerol and cardiolipin. J. Bacteriol. 182:371-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleckner, N., J. Bender, and S. Gottesman. 1991. Uses of transposons with emphasis on Tn10. Methods Enzymol. 204:139-180. [DOI] [PubMed] [Google Scholar]
- 31.Kohara, Y., K. Akiyama, and K. Isono. 1987. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell 50:495-505. [DOI] [PubMed] [Google Scholar]
- 32.Lacroix, J.-M., E. Lanfroy, V. Cogez, Y. Lequette, A. Bohin, and J.-P. Bohin. 1999. The mdoC gene of Escherichia coli encodes a membrane protein that is required for succinylation of osmoregulated periplasmic glucans. J. Bacteriol. 181:3626-3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lacroix, J.-M., I. Loubens, M. Tempête, B. Menichi, and J.-P. Bohin. 1991. The mdoA locus of Escherichia coli consists of an operon under osmotic control. Mol. Microbiol. 5:1745-1753. [DOI] [PubMed] [Google Scholar]
- 34.Majdalani, N., D. Hernandez, and S. Gottesman. 2002. Regulation and mode of action of the second small RNA activator of RpoS translation, RprA. Mol. Microbiol. 46:813-826. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto, K. 2001. Dispensable nature of phosphatidylglycerol in Escherichia coli: dual roles of anionic phospholipids. Mol. Microbiol. 39:1427-1433. [DOI] [PubMed] [Google Scholar]
- 36.Matsushika, A., and T. Mizuno. 1998. A dual-signaling mechanism mediated by the ArcB hybrid sensor kinase containing the histidine-containing phosphotransfer domain in Escherichia coli. J. Bacteriol. 180:3973-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mileykovskaya, E., and W. Dowhan. 1997. The Cpx two-component signal transduction pathway is activated in Escherichia coli mutant strains lacking phosphatidylethanolamine. J. Bacteriol. 179:1029-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller, J. H. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 39.Miyazaki, C., M. Kuroda, A. Ohta, and I. Shibuya. 1985. Genetic manipulation of membrane phospholipid composition in Escherichia coli: pgsA mutants defective in phosphatidylglycerol synthesis. Proc. Natl. Acad. Sci. USA 82:7530-7543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishijima, S., Y. Asami, U. Uetake, S. Yamagoe, A. Ohta, and I. Shibuya. 1988. Disruption of the Escherichia coli cls gene responsible for cardiolipin synthesis. J. Bacteriol. 170:775-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okada, M., H. Matsuzaki, I. Shibuya, and K. Matsumoto. 1994. Cloning, sequencing, and expression in Escherichia coli of the Bacillus subtilis gene for phosphatidylserine synthase. J. Bacteriol. 176:7456-7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ophir, T., and D. L. Gutnick. 1994. A role for exopolysaccharides in the protection of microorganisms from desiccation. Appl. Environ. Microbiol. 60:740-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oshima, T., H. Aiba, Y. Masuda, S. Kanaya, M. Sugiura, B. L. Wanner, H. Mori, and T. Mizuno. 2002. Transcriptome analysis of all two-component regulatory system mutants of Escherichia coli K-12. Mol. Microbiol. 46:281-291. [DOI] [PubMed] [Google Scholar]
- 44.Otto, K., and T. J. Silhavy. 2002. Surface sensing and adhesion of Escherichia coli controlled by the Cpx-signaling pathway. Proc. Natl. Acad. Sci. USA 99:2287-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parker, C. T., A. W. Kloser, C. A. Schnaitman, M. A. Stein, S. Gottesman, and B. W. Gibson. 1992. Role of the rfaG and rfaP genes in determining the lipopolysaccharide core structure and cell surface properties of Escherichia coli K-12. J. Bacteriol. 174:2525-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 47.Sankaran, K., and H. C. Wu. 1994. Lipid modification of bacterial prolipoprotein. Transfer of diacylglycerol moiety from phosphatidylglycerol. J. Biol. Chem. 269:19701-19706. [PubMed] [Google Scholar]
- 48.Sheetz, M. P., and S. J. Singer. 1974. Biological membranes as bilayer couplers. A molecular mechanism of drug-erythrocyte interaction. Proc. Natl. Acad. Sci. USA 71:4457-4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sledjeski, D. D., and S. Gottesman. 1996. Osmotic shock induction of capsule synthesis in Escherichia coli K-12. J. Bacteriol. 178:1204-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Snyder, W. B., L. J. Davis, P. N. Danese, C. L. Cosma, and T. J. Silhavy. 1995. Overproduction of NlpE, a new outer membrane lipoprotein, suppresses the toxicity of periplasmic LacZ by activation of the Cpx signal transduction pathway. J. Bacteriol. 177:4216-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stevenson, G., K. Andrianopoulos, M. Hobbs, and P. R. Reeves. 1996. Organization of the Escherichia coli K-12 gene cluster responsible for production of the extracellular polysaccharide colanic acid. J. Bacteriol. 178:4885-4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stout, V. 1994. Regulation of capsule synthesis includes interactions of the RcsC/RcsB regulatory pair. Res. Microbiol. 145:389-392. [DOI] [PubMed] [Google Scholar]
- 53.Stout, V. 1996. Identification of the promoter region for the colanic acid polysaccharide biosynthetic genes in Escherichia coli K-12. J. Bacteriol. 178:4273-4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suzuki, M., H. Hara, and K. Matsumoto. 2002. Envelope disorder of Escherichia coli cells lacking phosphatidylglycerol. J. Bacteriol. 184:5418-5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takeda, S., Y. Fujisawa, M. Matsubara, H. Aiba, and T. Mizuno. 2001. A novel feature of the multistep phosphorelay in Escherichia coli: a revised model of the RcsC→YojN→RcsB signalling pathway implicated in capsular synthesis and swarming behaviour. Mol. Microbiol. 40:440-450. [DOI] [PubMed] [Google Scholar]
- 56.Trisler, P., and S. Gottesman. 1984. lon transcriptional regulation of genes necessary for capsular polysaccharide synthesis in Escherichia coli K-12. J. Bacteriol. 160:184-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang, P.-Z., and R. H. Doi. 1984. Overlapping promoters transcribed by Bacillus subtilis σ55 and σ37 RNA polymerase holoenzymes during growth and stationary phases. J. Biol. Chem. 259:8619-8625. [PubMed] [Google Scholar]
- 58.Weiss, D. S., J. C. Chen, J.-M. Ghigo, D. Boyd, and J. Beckwith. 1999. Localization of FtsI (PBP3) to the septal ring requires its membrane anchor, the Z ring, FtsA, FtsQ, and FtsL. J. Bacteriol. 181:508-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu, H. C. 1996. Biosynthesis of lipoproteins, p. 1005-1024. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 60.Xia, W., and W. Dowhan. 1995. In vivo evidence for the involvement of anionic phospholipids in initiation of DNA replication in Escherichia coli. Proc. Natl. Acad. Sci. USA 92:783-787. [DOI] [PMC free article] [PubMed] [Google Scholar]