Abstract
The genome sequence of Lactococcus lactis revealed that the ycdB gene was recently exchanged between lactococci and enterobacteria. The present study of ycdB orthologs suggests that L. lactis was probably the gene donor and reveals three instances of gene transfer to enterobacteria. Analysis of ycdB gene transfer between two L. lactis subspecies, L. lactis subsp. lactis and L. lactis subsp. cremoris, indicates that the gene can be mobilized, possibly by conjugation.
Horizontal gene transfer between distant species is an important factor in prokaryotic evolution (for recent reviews, see references 8, 12, and 15). Genome analysis of Lactococcus lactis IL1403 suggested a relatively recent horizontal transfer of a gene between lactococci and gram-negative enterobacteria (4). The gene, ycdB, encodes a protein of unknown function, containing an PFAM DUF028 domain. Such proteins are ubiquitous in Eubacteria, present in Eucaryota, but absent in Archaea. Fully sequenced genomes of Escherichia coli and Shigella and Salmonella species have two DUF028 domain genes, yeeN and yebC, encoding proteins that have ∼96 and ∼40% of identity, respectively, to the YcdB protein of L. lactis IL1403. Divergence of the enterobacterial yeeN gene (which we designate ycdBEnt hereafter) and the lactococcal ycdB gene (ycdBLac) at synonymous nucleotide positions, where the mutations do not change the encoded amino acid, is about 10%, suggesting that the transfer could have taken place only about ten million years ago (4). Here we address the possibility that the transfer involved another lactococcal species and could have occurred more recently by examining the ycdBLac genes of a number of strains belonging to different lactococcal species. We also address the question of the mechanism of transfer by examining exchange of the gene between two subspecies of L. lactis, namely, L. lactis subsp. lactis and L. lactis subsp. cremoris (7, 13).
Enterobacterial ycdB orthologs were acquired from L. lactis.
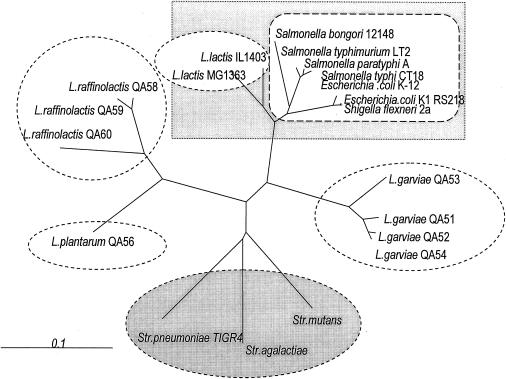
We sequenced the ycdBLac orthologs of 70 strains belonging to four different Lactococcus taxa (L. lactis, Lactococcus plantarum, Lactococcus garviae, and Lactococcus raffinolactis) and compared their sequences with ycdBEnt DNA sequences of gram-negative enterobacteria, which included Escherichia, Shigella, and Salmonella strains. The phylogenetic tree based on these results is shown in Fig. 1. Divergence of ycdBEnt and ycdBLac genes at synonymous nucleotide sites is about 10%, while that of ycdBLac orthologs from other lactococci is up to 30%. This indicates that L. lactis, rather than another species of Lactococcus, was involved in gene exchange with the enteric bacteria.
FIG. 1.
Phylogenetic tree inferred from nucleotide sequences of the ycdBLac orthologous genes. Closely related species are indicated by the dashed ovals. The proximity of L. lactis and enterobacterial ycdBEnt alleles is highlighted by the square shaded box. The data from the National Center for Biotechnology Information, Sanger Institute, and ERGO databases were used for streptococci, enteric bacteria, and L. lactis IL1403. Other sequences were determined in the course of this work. L. lactis subsp. hordniae data showing tight clustering to the L. lactis IL1403 group are not shown to avoid overcrowding of the figure. Str., Streptococcus. The scale bar indicates the expected number of nucleotide substitutions per site.
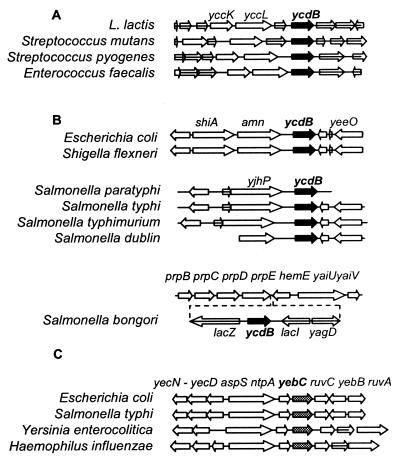
Three lines of evidence indicate that the direction of transfer was from lactococci to enteric bacteria. First, the average G+C content of the ycdBLac and ycdBEnt genes, ∼39%, is much closer to the average G+C content of the L. lactis genome than to that of enteric bacteria (35 and 51%, respectively). Second, species phylogenetically close to enteric bacteria, such as Yersinia and Klebsiella species, lack the ycbD orthologs. Third, there is conservation of the gene order upstream of the ycdB gene homologs among lactococci, streptococci, and even enterococci (yccK and yccL genes) (Fig. 2A). In contrast, conservation of the gene order in the vicinity of the ycdBEnt genes is found only among very closely related species of enteric bacteria, such as E. coli and Shigella flexneri or in different Salmonella serovars (Fig. 2B). This differs sharply for the region in the vicinity of the homolog, yebC, where the conservation extends even to the much more distant Yersinia and Haemophilus species (Fig. 2C). A conserved gene order in distant species indicates that the gene was present in a common ancestor, while the absence of conservation supports multiple instances of gene acquisition by horizontal transfer. We suggest that ycdBLac is an ancestral gene in lactococcus, as is yebC in enteric bacteria, whereas ycdBEnt gene was acquired by enteric bacteria more recently, most likely from lactococcus.
FIG. 2.
Genetic organization of the regions proximal to the ycdB (A and B) and yebC (C) genes in different bacteria. Alleles of conservative genome organization within the group of related species are indicated by black arrows. (A) Organization of the ycdB region in gram-positive lactobacilli. (B) Three different localizations of ycdBEnt in enterobacteria. (C) Conservative genome organization of the region proximal to yebC (paralog of ycdBEnt) in enterobacteria.
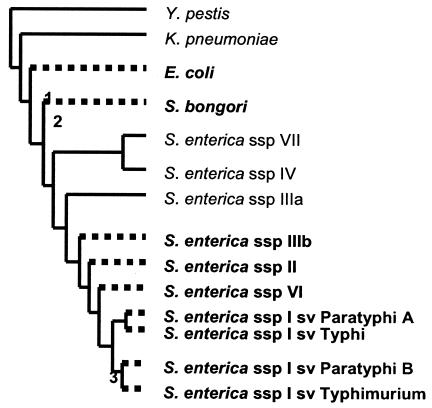
Recent microarray hybridization analysis of the enteric bacterial genomes (17) allowed us to assess the distribution of the ycdBEnt gene (Fig. 3). The gene is present in E. coli, Salmonella bongori, and biphasic Salmonella enterica but is absent in monophasic S. enterica. This distribution can be accounted for by three independent instances of horizontal transfer to enteric bacteria (Fig. 3), each to a different genome localization (Fig. 2B). However, we cannot exclude the possibility of an even higher number of transfer events, as the environment of the gene in S. enterica is known only for subspecies I. It should be noted also that although the initial ycdBLac transfer appears to have taken place between L. lactis and enteric bacteria, subsequent transfer events could have occurred between enteric bacteria. This is almost certainly the case for the S. bongori ycdBEnt gene. The gene is inserted in a region well conserved among Salmonella strains, together with three other genes, lacI, lacZ, and yagD, which share high levels of identity with E. coli genes (74, 78, and 79%, respectively, with the corresponding proteins) but lack orthologs in L. lactis and S. enterica. This particular gene association could have arisen in an enteric bacteria related to E. coli and transferred subsequently to S. bongori.
FIG. 3.
Presence of ycdBEnt gene among enteric bacteria. The data are from reference 17, and the phylogenetic tree is redrawn from a figure in the article. The species possessing the gene are shown in bold type. The numbers indicate different occurrences of the horizontal transfer. Y. pestis, Yersinia pestis; K. pneumoniae, Klebsiella pneumoniae; ssp, subspecies; sv, serovar.
The time of occurrence of the ycdBLac gene transfer to enteric bacteria can be estimated in several ways. First, assuming that the synonymous divergence rate in L. lactis is similar to that of E. coli, about 0.9% per million years (14), the transfer took place about 10 million years ago. Second, ycdBLac clustering indicates that the gene transfer to enterobacteria preceded divergence of L. lactis to two principal subspecies. Since the 16S rRNA of the two differ by 0.33%, the divergence and transfer could have taken place some 17 millions years ago, assuming that the rate of divergence of 16S RNA was 1% per 50 million years (14). Given the uncertainties involved, we assume that 10 million years is a reasonable estimate of the time of transfer of the ycdBLac gene to enteric bacteria.
The ycdBLac gene is horizontally transferred between L. lactis strains.
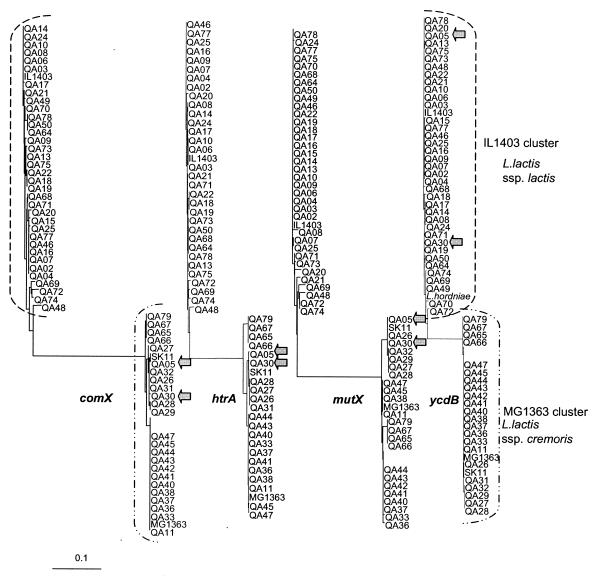
To examine a possible transfer of the ycdBLac gene among L. lactis strains, we first assigned ∼60 strains from our collection to two subspecies, L. lactis subsp. lactis and L. lactis subsp. cremoris, by analyzing sequences of three genes, htrA, comX, and mutX, encoding a housekeeping protease, a competence factor, and an antimutator protein, respectively. Phylogenetic trees were calculated for each gene (Fig. 4). This analysis clearly demonstrated the existence of two clusters of alleles for each gene, and for all three genes, nonambiguous strain assignment between these two clusters was obtained. The clusters obtained were confirmed by a discriminatory 16S rRNA PCR analysis (21), which gave identical patterns for all strains within a cluster and different patterns for strains in different clusters (data not shown). Similar analysis of the ycdBLac gene revealed that two strains of the L. lactis MG1363 cluster, strains QA5 and QA30, contain alleles of the IL1403 type (Fig. 4). This indicates that the ycdBLac gene was horizontally transferred among L. lactis strains.
FIG. 4.
Neighbor-joining unrooted phylogenetic trees inferred from L. lactis comX, htrA, mutX, and ycdBLac gene nucleotide sequences. The strains used are listed in Table and were previously characterized by randomly amplified polymorphic DNA (19). The primers used to amplify the genes follow: for ycdBLac, ATGGGACGTAAATGGGCCAATATT and GAGATTTGCAACGTTATGATAAACTT; for comX, ACTTGCTGAAATCGTTGAAGG and GTTCGTCCTGAGCCAGGATC; for htrA, AGGTATTATTAAGTGAGAGTAG and GCACGACCAATTCCTGAATG; for mutX (IL1403), GGGACTCCCCAATAAGTATCATG and TATGCTGGGATTGCTCGTAAAGC; and for mutX (MG1363), GTGCTCCCCAATAGGTATCATGA and TATGCTGGGATTGCTCGTAAAGC. Multiple nucleotide sequences were analyzed by CLUSTAL (11). Multilocus comparison was performed by using CLUSTER analysis (6) and equality-weighted PAUP distance matrices. The results of phylogenetic and correlation analyses were visually presented by using the TREEVIEW program (16). The two strains that carry the L. lactis subsp. lactis ycdBLac gene but have all the other genes of the L. lactis subsp. cremoris type are indicated by the shaded arrows. The YcdB tree contains the L. lactis subsp. hordniae (“L. hordniae”) gene, which is closely related to the L. lactis subsp. lactis gene. The scale bar indicates the expected number of nucleotide substitutions per site.
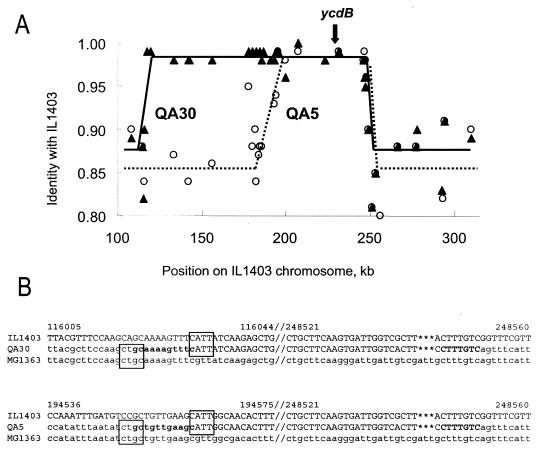
Organization of the regions flanking the ycdBLac gene in strains QA5 and QA30 was determined by long-range PCR mapping and sequencing. The mapping was initiated at the ycdBLac locus, which contains an allele of the L. lactis IL1403 cluster type, and extended in both directions until the alleles of the L. lactis MG1363 cluster type were detected. A large IL1403-like region is present in both strains, extending from the vicinity of the ycdBLac gene for 50 and 130 kb in strains QA05 and QA30, respectively (Fig. 5A).
FIG. 5.
(A) Long L. lactis subsp. lactis regions are present in the vicinity of the ycdB gene in strains QA5 and QA30, which belong to the L. lactis subsp. cremoris cluster. Mapping was performed by amplifying 5- to 10-kb regions, using primers deduced from the L. lactis IL1403 (4) or MG1363 (3) (GenBank accession no. BH770319 to BH771051) sequence and sequencing the resulting products. The genes were identified using CRITICA (1) and assigned to the IL1403 or MG1363 cluster. Levels of nucleotide identity of QA05 and QSA30 chromosome tags to L. lactis IL1403 are indicated by white circles and black triangles, respectively. (B) Nucleotide sequences in the transition zones between regions derived from L. lactis subsp. lactis and L. lactis subsp. cremoris in strains QA5 and QA30. The numbers refer to the coordinates in the IL1403 genome, the likely crossover sites are shown in bold type, and the conserved sequences at the left crossover sites are boxed.
Transfer of genetic material between bacteria can occur by three processes, namely, transformation, transduction, and conjugation. The maximal size of the transferred region depends on the process and is the highest for conjugation, which can mediate transfer of genome-size segments. In contrast, the size of the transferred region is limited by the phage capsid size in transduction and by the intact DNA size in transformation. Lactococcal phages have been extensively studied, mainly due to their importance to the dairy industry, and are known to fall into three different quasispecies, with the maximal genome size slightly above 40 kb (5). This value is too low to account for the transfer we detected, particularly for the 130-kb region. Similarly, it is very unlikely that intact DNA of such size can persist in the natural environment and mediate the transfer. We thus suggest that the transfer is likely to have occurred by conjugation, although we cannot fully rule out transduction by a putative lactococcal phage with a much larger genome, such as those known in some other bacterial species. A chromosomally located sex factor that mediates conjugational transfer in L. lactis has been described previously (18). If the ycdB gene is prone to exchange by conjugation among lactococci, it could have been transferred to enteric bacteria by the same process. It is known that conjugation can take place between gram-positive and enteric bacteria (20). In contrast, we are not aware of phages that can infect both lactococci and enteric bacteria. Similarly, there is no natural DNA uptake system in enteric bacteria, which should severely limit their ability to undergo transformation in situ. An ecological niche conducive to transfer between lactococci and enteric bacteria is the animal digestive tract. Lactococci, which are thought to be associated with plant materials in nature, could easily be brought in the proximity of enteric bacteria upon ingestion of plant by animals. Conjugational gene transfer from L. lactis that passed through the mouse digestive tract to the resident bacteria has been reported (9, 10).
Long-range PCR products corresponding to the transferred region borders were sequenced (Fig. 5B). The right crossover site, which is localized within the pepDA gene, is identical in the two strains, suggesting a similar type of transfer in the two cases. In contrast, the left crossover sites are different, and are localized within the acpD and ybjJ genes in strains QA30 and QA5, respectively. A common pattern CTGC-N8-CATT (Fig. 5B) was detected at the left crossover sites. It is often difficult to deduce the mechanism of recombination from analysis of the crossover sites, but it is conceivable that the common crossover site might be a recombinational hot spot. We have no explanation for the possible role, if any, of the common sequences at the other crossover sites.
Genes acquired by horizontal transfer from a distant species might be deleterious, neutral, or beneficial to the recipient. Deleterious genes should be eliminated by selection; neutral genes may be maintained, while beneficial genes should be selected for. It has been argued that only the strongly selected genes will become established in a bacterial species due to the very large sizes of bacterial populations (2). In keeping with this argument, we suggest that ycbD might be beneficial in enteric bacteria, notwithstanding the presence of an ancestral homologue, as that would most easily account for its fixation upon at least three different events of horizontal transfer, one each to E. coli, S. bongori, and S. enterica. Future work should allow the function of the ubiquitous ycdB gene to be determined.
Nucleotide sequence accession numbers.
The nucleotide sequences of the amplified products of the L. lactis comX, htrA, mutX, and ycdBLac genes were determined and deposited in GenBank under following accession numbers: AY708538 to AY708650.
TABLE 1.
Strains used in this study
| Strain | Lactococcus speciesc | Source | Geographical origina | Designationb |
|---|---|---|---|---|
| NCDO657 | L. garviae | Raw milk | QA51 | |
| NCDO2155 | L. garviae | Mastitis | QA52 | |
| NCDO2159 | L. garviae | Mastitis | QA53 | |
| NCDO2728 | L. garviae | QA54 | ||
| NCDO2181 | L. lactis subsp. hordniae | Leaf hopper | QA55 | |
| NCDO1869 | L. plantarum | Frozen peas | QA56 | |
| NCDO0617 | L. raffinolactis | Raw milk | QA58 | |
| NCDO2112 | L. raffinolactis | Garden carrots | QA59 | |
| NCDO2126 | L. raffinolactis | Termite gut | QA60 | |
| IL1403 | L. lactis subsp. lactis | France | QA00/IL1403 | |
| MG1363 | L. lactis subsp. cremoris | QA01/MG1363 | ||
| F36 | L. lactis subsp. lactis var. diacetylactis | France | QA02 | |
| IL584 | L. lactis subsp. lactis | Starter | France | QA03 |
| A15 | L. lactis subsp. cremoris | Raw milk | France | QA04 |
| A76 | L. lactis subsp. cremoris | Starter | France | QA05 |
| CNRZ124 | L. lactis subsp. lactis var. diacetylactis | Australia | QA06 | |
| IL1321 | L. lactis subsp. lactis | Milk | Mexico | QA07 |
| A13 | L. lactis subsp. lactis | Raw milk | France | QA08 |
| A108 | L. lactis subsp. lactis | Starter | France | QA09 |
| A152 | L. lactis subsp. lactis var. diacetylactis | Starter | France | QA10 |
| IL581 | L. lactis subsp. lactis | Starter | France | QA11 |
| NCDO2146 | L. lactis | Mastitis | QA13 | |
| A11 | L. lactis subsp. lactis | Raw milk | France | QA14 |
| A17 | L. lactis subsp. lactis | Reblochon cheese | France | QA15 |
| A310 | L. lactis subsp. lactis | Raw cream | France | QA16 |
| A26 | L. lactis subsp. lactis | Starter | France | QA17 |
| NCDO604 | L. lactis subsp. lactis | QA18 | ||
| A39 | L. lactis subsp. lactis | Starter | France | QA19 |
| A7 | L. lactis subsp. lactis | Tomme de Savoie cheese | France | QA20 |
| A8 | L. lactis subsp. lactis var. diacetylactis | Milk | France | QA21 |
| A27 | L. lactis subsp. lactis var. diacetylactis | Brie | France | QA22 |
| CNRZ379 | L. lactis subsp. cremoris | New Zealand | QA24 | |
| CNRZ380 | L. lactis subsp. cremoris | QA25 | ||
| CNRZ109 | L. lactis subsp. cremoris | UK | QA26 | |
| CNRZ357 | L. lactis subsp. cremoris | France | QA27 | |
| CNRZ359 | L. lactis subsp. cremoris | France | QA28 | |
| CNRZ353 | L. lactis subsp. cremoris | France | QA29 | |
| CNRZ112 | L. lactis subsp. cremoris | Starter | QA30 | |
| A170 | L. lactis subsp. cremoris | Starter | France | QA31 |
| A318 | L. lactis subsp. cremoris | Starter | France | QA32 |
| A16 | L. lactis subsp. lactis | Raw milk | France | QA33 |
| CNRZ269 | L. lactis subsp. lactis var. diacetylactis | France | QA36 | |
| NCDO276 | L. lactis subsp. lactis var. diacetylactis | Starter | QA37 | |
| CNRZ156 | L. lactis subsp. lactis | QA38 | ||
| NCDO763 | L. lactis subsp. lactis | New Zealand | QA40 | |
| CNRZ144 | L. lactis subsp. lactis | UK | QA41 | |
| LM0230 | L. lactis subsp. lactis | QA42 | ||
| NCDO2005 | L. lactis subsp. lactis | New Zealand | QA43 | |
| A140 | L. lactis subsp. lactis | QA44 | ||
| JIM578 | L. lactis subsp. lactis | Starter | France | QA45 |
| A171 | L. lactis subsp. cremoris | Starter | France | QA46 |
| JIM582 | L. lactis subsp. lactis | Starter | France | QA47 |
| NCDO2091 | L. lactis | Seeds | Japan | QA48 |
| NCDO2118 | L. lactis | Frozen beans | QA49 | |
| NCDO2633 | L. lactis | Cow rectum | QA50 | |
| NCDO2633 | L. lactis | Cow rectum | QA64 | |
| CO2 | L. lactis subsp. cremoris | Corn | USA | QA65 |
| CO4 | L. lactis subsp. cremoris | Corn | USA | QA66 |
| CO6 | L. lactis subsp. cremoris | Corn | USA | QA67 |
| NCDO1867 | L. lactis | Frozen beans | QA68 | |
| NCDO2108 | L. lactis | Frozen beans | QA69 | |
| NCDO2110 | L. lactis | Frozen beans | QA70 | |
| NCDO2111 | L. lactis | Frozen beans | QA71 | |
| NCDO2125 | L. lactis | Termite gut | QA72 | |
| NCDO2727 | L. lactis | Mung beans | QA73 | |
| NCDO2738 | L. lactis | “Anchu” mash | QA74 | |
| NCDO2146 | L. lactis | Mastitis | QA75 | |
| MS-46 | L. lactis subsp. lactis | Moroccan milk | QA77 | |
| F36 | L. lactis subsp. lactis | Colostrum | QA78 | |
| CM1-54 | L. lactis subsp. cremoris | Chinese milk | QA79 |
Abbreviations: UK, United Kingdom; USA, United States.
Strain designation used in this study. The cluster is given after a slash and strain designation for two strains.
All L. lactis strains are described in reference 19.
Acknowledgments
We thank Integrated Genomics, Inc., for the use of the ERGO suite and the Sanger Centre for the S. bongori sequence.
REFERENCES
- 1.Badger, J. H., and G. J. Olsen. 1999. CRITICA: coding region identification tool invoking comparative analysis. Mol. Biol. Evol. 16:512-524. [DOI] [PubMed] [Google Scholar]
- 2.Berg, O. G., and C. G. Kurland. 2002. Evolution of microbial genomes: sequence acquisition and loss. Mol. Biol. Evol. 19:2265-2276. [DOI] [PubMed] [Google Scholar]
- 3.Bolotin, A., S. D. Ehrlich, and A. Sorokin. 2002. Studies of genomes of dairy bacteria Lactococcus lactis. Sci. Aliment. 22:4-53. [Google Scholar]
- 4.Bolotin, A., P. Wincker, S. Mauger, O. Jaillon, K. Malarme, J. Weissenbach, S. D. Ehrlich, and A. Sorokin. 2001. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 11:731-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chopin, A., A. Bolotin, A. Sorokin, S. D. Ehrlich, and M. C. Chopin. 2001. Analysis of six prophages in Lactococcus lactis IL1403: different genetic structure of temperate and virulent phage populations. Nucleic Acids Res. 29:644-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisen, M. B., P. T. Spellman, P. O. Brown, and D. Botstein. 1998. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 95:14863-14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Godon, J.-J., C. Delorme, S. D. Ehrlich, and P. Renault. 1992. Divergence of genomic sequences between Lactococcus lactis subsp. lactis and Lactococcus lactis subsp. cremoris. Appl. Environ. Microbiol. 58:4045-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gogarten, J. P., W. F. Doolittle, and J. G. Lawrence. 2002. Prokaryotic evolution in light of gene transfer. Mol. Biol. Evol. 19:2226-2238. [DOI] [PubMed] [Google Scholar]
- 9.Gruzza, M., M. Fons, M. F. Ouriet, Y. Duval-Iflah, and R. Ducluzeau. 1994. Study of gene transfer in vitro and in the digestive tract of gnotobiotic mice from Lactococcus lactis strains to various strains belonging to human intestinal flora. Microb. Releases 2:183-189. [PubMed] [Google Scholar]
- 10.Gruzza, M., P. Langella, Y. Duval-Iflah, and R. Ducluzeau. 1993. Gene transfer from engineered Lactococcus lactis strains to Enterococcus faecalis in the digestive tract of gnotobiotic mice. Microb. Releases 2:121-125. [PubMed] [Google Scholar]
- 11.Higgins, D. G., J. D. Thompson, and T. J. Gibson. 1996. Using CLUSTAL or multiple sequence alignments. Methods Enzymol. 266:383-402. [DOI] [PubMed] [Google Scholar]
- 12.Jain, R., M. C. Rivera, J. E. Moore, and J. A. Lake. 2002. Horizontal gene transfer in microbial genome evolution. Theor. Popul. Biol. 61:489-495. [DOI] [PubMed] [Google Scholar]
- 13.Jarvis, A. W., and B. D. W. Jarwis. 1981. Deoxyribonucleic acid homology among lactis streptococci. Appl. Environ. Microbiol. 41:77-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ochman, H., S. Elwyn, and N. A. Moran. 1999. Calibrating bacterial evolution. Proc. Natl. Acad. Sci. USA 96:12638-12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ochman, H., J. G. Lawrence, and E. A. Groisman. 2000. Lateral gene transfer and the nature of bacterial innovation. Nature 405:299-304. [DOI] [PubMed] [Google Scholar]
- 16.Page, R. D. 1996. TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 17.Porwollik, S., R. M. Wong, and M. McClelland. 2002. Evolutionary genomics of Salmonella: gene acquisitions revealed by microarray analysis. Proc. Natl. Acad. Sci. USA 99:8956-8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shearman, C., J.-J. Godon, and M. Gasson. 1996. Splicing of a group II intron in a functional transfer gene of Lactococcus lactis. Mol. Microbiol. 21:45-53. [DOI] [PubMed] [Google Scholar]
- 19.Tailliez, P., J. Tremblay, S. D. Ehrlich, and A. Chopin. 1998. Molecular diversity and relationship within Lactococcus lactis, as revealed by randomly amplified polymorphic DNA (RAPD). Syst. Appl. Microbiol. 21:530-538. [DOI] [PubMed] [Google Scholar]
- 20.Trieu-Cuot, P., C. Carlier, and P. Courvalin. 1988. Conjugative plasmid transfer from Enterococcus faecalis to Escherichia coli. J Bacteriol. 170:4388-4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ward, L. J., J. C. Brown, and G. P. Bavey. 1998. Two methods for the genetic differentiation of Lactococcus lactis ssp. lactis and cremoris based on differences in the 16S rRNA gene sequence. FEMS Microbiol. Lett. 166:15-20. [DOI] [PubMed] [Google Scholar]