Abstract
The Cool-2 (cloned-out of library-2) protein (identical to α-Pix for Pak-interactive exchange factor) has been implicated in various biological responses including chemoattractant signaling and in certain forms of mental retardation. We show that when Cool-2 exists as a dimer, it functions as a Rac-specific guanine nucleotide exchange factor (GEF). Dimerization of Cool-2 enables its Dbl (diffuse B-cell lymphoma) and pleckstrin homology domains to work together (in trans) to bind specifically to Rac-GDP. Dissociation of dimeric Cool-2 into its monomeric form allows it to act as a GEF for Cdc42 as well as for Rac. The binding of either PAK (p21-activated kinase) or Cbl (Casitas B-lymphoma) to the SH3 domain of monomeric Cool-2 is necessary for the functional interactions between GDP-bound Cdc42 or Rac and the Cool-2 monomer. The βγ subunit complex of large GTP-binding proteins, by interacting with PAK, stimulates the dissociation of the Cool-2 dimer and activates its GEF activity for Cdc42. Overall, these findings highlight novel mechanisms by which extracellular signals can direct the specific activation of Rac versus Cdc42 by Cool-2/α-Pix.
Keywords: Cdc42, Cool, Pix, Rac, signaling
Introduction
Members of the Rho family of GTP-binding proteins function as molecular switches in biological response pathways that result in changes in the actin cytoskeletal architecture, the stimulation of cell cycle progression and gene transcription, and the regulation of intracellular trafficking activities (Van Aelst and D'Souza-Schorey, 1997; Hall, 1998; Bar-Sagi and Hall, 2000; Erickson and Cerione, 2001). Three classes of proteins, GTPase-activating proteins (GAPs), guanine nucleotide dissociation inhibitors (GDIs), and guanine nucleotide exchange factors (GEFs), regulate the cycling of these GTP-binding proteins between their GDP-bound and GTP-bound states (Boguski and McCormick, 1993). Rho family proteins, such as Cdc42 and Rac, are activated by a number of upstream stimuli, as mediated by members of the Dbl (diffuse B-cell lymphoma) family of GEFs (Whitehead et al, 1997; Hoffman and Cerione, 2002). One subgroup of the Dbl family, the Cool/Pix proteins (Cool for cloned-out of library-2 and Pix for Pak-interactive exchange factor) (Oh et al, 1997; Bagrodia et al, 1998; Manser et al, 1998), is particularly interesting, as its members have been shown to exhibit a variety of functional activities and to elicit a diversity of cellular responses. We have characterized three members of the family: p50Cool-1, a longer splice variant form called p85Cool-1 (identical to β-Pix; Manser et al, 1998), and a distinct gene product called p90Cool-2 (identical to α-Pix but from here on called Cool-2).
In addition to containing Dbl homology (DH) and pleckstrin homology (PH) domains, Cool family members have an SH3 domain, which they use to bind to PAK (p21-activated kinase) (Bagrodia et al, 1998; Manser et al, 1998) and promote signaling activities in vivo (Ku et al, 2001; Lee et al, 2001). Recently, it was demonstrated that the Cbl (Casitas B-lymphoma) proteins were also able to bind to the Cool proteins through their SH3 domain and compete with PAK–Cool interactions (Flanders et al, 2003; Wu et al, 2003). Within the middle portions of the p85Cool-1 and Cool-2 proteins are a proline-rich region and a Cat (Cool-associated tyrosine phosphosubstrate)/Git (G protein-coupled receptor kinase interactor) binding domain (Premont et al, 1998; Bagrodia et al, 1999). The Cat/Git proteins, also known as Pkl (paxillin-kinase-linker) (Turner et al, 1999) and p95-APP1 (ArfGAP-putative, Pix-interacting, paxillin-interacting protein 1) (Di Cesare et al, 2000), are members of the ArfGAP family and regulate the dynamics of focal adhesion complexes (Turner et al, 2001). The Cool and Cat/Git proteins are often present in complexes containing paxillin and PAK that may mediate the integration of Arf and Rho family signaling activities at the cytoskeleton (Bagrodia and Cerione, 1999; Turner et al, 1999, 2001). Recently, it was also reported that a complex between p85Cool-1 and p95-APP1 was required for Rac1B-mediated neurite outgrowth (Albertinazzi et al, 2003).
The Cool-2 protein, unlike the Cool-1 proteins, contains a calponin homology (CH) domain that extends from its amino-terminus, and the disruption of this domain has been linked to certain (X-linked) forms of mental retardation (Kutsche et al, 2000; Ramakers, 2002). Both Cool-2 and p85Cool-1 also have a leucine zipper near their carboxyl-terminal ends that enables dimerization to occur. In the case of p85Cool-1, dimerization was suggested to promote the formation of microvillus-like structures and membrane ruffles (Kim et al, 2001; Koh et al, 2001).
The Cool proteins were initially assumed to function as Rho family GEFs, given that they contained conserved DH and PH domains. However, thus far, only one member of the family, Cool-2, has exhibited significant GEF activity in standard biochemical assays (Feng et al, 2002), whereas p85Cool-1 has been shown to bind preferentially to activated (GTP-bound) Cdc42 and to act as a target/scaffold protein in mediating the regulation of Cbl's E3 ubiquitin ligase activity (Wu et al, 2003). Cool-2 strongly stimulates PAK activity (Bagrodia et al, 1999; Daniels et al, 1999; Feng et al, 2002), and it has been assumed that this results from Cool-2 acting as a GEF for Cdc42 or Rac. It was also reported that Cool-2 played a key role in chemoattractant signaling through its ability to activate Cdc42 (Li et al, 2003).
In this study, we have focused on the Cool-2 protein, given its involvement in a variety of biological responses, with the aim of better understanding how it functions as a GEF. We show for the first time that two novel regulatory mechanisms control its GEF activity. Under conditions where Cool-2 exists as a dimer in vivo, it functions as a Rac-specific GEF. We found that the carboxyl-terminal portion of the PH domain of Cool-2 works together with the DH domain through dimerization (i.e. in trans) to mediate a highly specific interaction with GDP-bound Rac. However, when Cool-2 is a monomer, it exhibits GEF activity toward Cdc42, as well as Rac, but only upon the binding of PAK or Cbl to its SH3 domain. Thus, differential regulation of the monomer–dimer equilibrium of Cool-2 by specific extracellular signals can dictate whether Rac- and/or Cdc42-coupled signaling pathways are activated.
Results
Cool-2 functions as a GEF for Rac-
The Cool-2 protein contains tandem DH and PH domains, which is characteristic of Rho family GEFs (Figure 1A). We (Bagrodia et al, 1999) and others (Daniels et al, 1999) have previously shown that the SH3 domain of Cool-2 specifically interacts with a stretch of proline residues on PAK, resulting in the stimulation of PAK activity. Likewise, we found that a Cool-2 construct that contained just the SH3 and DH domains as well as most of the PH domain (SH3.PH(541).Cool-2 in Figure 1B) was able to activate PAK (Feng et al, 2002). We initially assumed that the Cool-2-mediated activation of PAK was the direct result of Cool-2 acting as a GEF for Cdc42 and/or Rac, as the GTP-bound forms of these proteins stimulate PAK activity (Manser et al, 1994; Bagrodia et al, 1995). However, as shown in Figure 2A, when Myc-tagged Cool-2 was expressed in COS-7 cells, then immunoprecipitated with anti-Myc and added to an assay incubation containing [3H]GDP-bound Cdc42, no detectable Cdc42-GEF activity was measured for Cool-2 as indicated by the fact that little dissociation of [3H]GDP from Cdc42 (i.e. the rate-limiting step for guanine nucleotide exchange) occurred over 20–25 min. This was similar to what was observed when Cdc42 was mixed with control lysates lacking Myc-Cool-2 (not shown) and in contrast to what occurred when EDTA was used to fully stimulate GDP dissociation by chelating Mg2+. On the other hand, when the same type of assay was performed with Rac1, Cool-2 stimulated the dissociation of [3H]GDP from Rac, indicating that full-length Cool-2 acts as a specific Rac-GEF. Likewise, in binding experiments, we found that Cool-2 formed a stable complex with Rac but not with Cdc42. Figure 2B (lanes 5–7) shows that the nucleotide-depleted Rac construct, GST-Rac(T17N), was able to precipitate Myc-tagged Cool-2 from cells, as well as Myc-p50Cool-1 and to a lesser extent Myc-p85Cool-1 (the relative expression of these different Myc-Cool proteins is shown in lanes 1–3), whereas the corresponding GST-Cdc42(T17N) protein did not show a detectable interaction with either the Cool-1 or Cool-2 proteins (Figure 2B, lanes 8–10).
Figure 1.

Domain structure of Cool-2 and different Cool-2 constructs. (A) Cool-2 contains CH, SH3, DH and PH domains, as well as a proline-rich region (PXXP), a Cat/Git binding domain (CBD), and a leucine zipper (LZ) region. The RSID (for Rac-specific interaction domain) is located at the C-terminal end of the PH domain. (B) Schematic representation of different truncated Cool-2 constructs and Cool-2 mutants.
Figure 2.
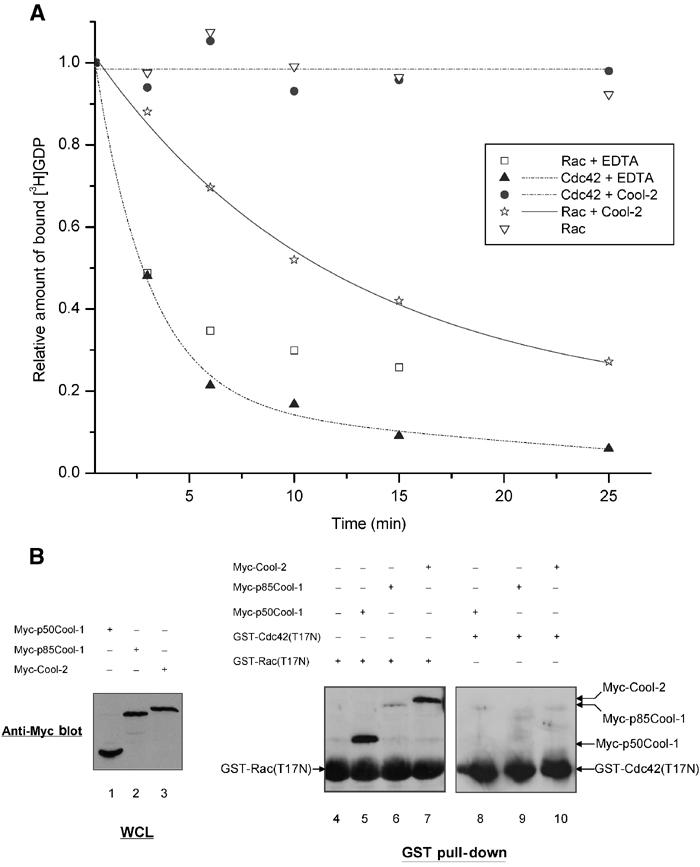
The abilities of wild-type Cool-2 to bind and exhibit GEF activity toward Cdc42 and Rac. (A) COS-7 cells were transiently transfected with a plasmid expressing Myc-Cool-2 and the Cool-2 proteins were immunoprecipitated using an anti-Myc antibody. Recombinant (E. coli) GST-Cdc42 or GST-Rac was preloaded with [3H]GDP. The exchange of bound [3H]GDP for GTPγS, as monitored by the dissociation of [3H]GDP from Cdc42 or Rac, was assayed in the presence of immunoprecipitated Myc-Cool-2 or in the presence of lysates from cells not expressing Myc-Cool-2. The data shown are representative of three experiments. (B) The GST-Rac(T17N) and GST-Cdc42(T17N) fusion proteins were prepared as described in Materials and methods. Lanes 1–3 show the relative expression of the different Myc-Cool constructs based on Western blots of whole-cell lysates (WCLs) with anti-Myc antibody. Lanes 4–10 show the results of GST pull-down experiments, in which ∼5 μg of GST fusion proteins, immobilized on glutathione-agarose beads, was incubated with COS-7 cell lysates expressing Myc-Cool-2, Myc-p85Cool-1, or Myc-p50Cool-1 as described in Materials and methods. The co-precipitated Myc-tagged Cool proteins were detected by Western blotting with anti-Myc antibody.
DH and PH domains of Cool-2 work together to exhibit GEF activity toward Rac-
Structural and mutagenesis studies have revealed a conserved mechanism by which Dbl family proteins catalyze nucleotide exchange on Rho-related GTP-binding proteins. For the case of Dbl, two conserved leucine residues within the DH domain are essential for activity (Hart et al, 1994). The corresponding leucine residues are present within the DH domain of Cool-2 as well. A good deal is also known regarding how certain members of the Dbl family are able to exhibit specificity for their GTP-binding protein targets (Worthylake et al, 2000; Gao et al, 2001; Hussain et al, 2001; Hoffman and Cerione, 2002; Rossman et al, 2002; Snyder et al, 2002). For example, the Intersectin proteins are highly specific GEFs for Cdc42 whereas Tiam1 is highly selective for Rac (Worthylake et al, 2000; Hussain et al, 2001; Hoffman and Cerione, 2002; Snyder et al, 2002). Because full-length Cool-2 acted as a Rac-specific GEF (Figure 2A), we compared its sequence with that of Tiam1. Isoleucine 1187 within the DH domain of Tiam1 has been suggested to play a critical role in determining specificity for Rac (Snyder et al, 2002). The corresponding residue in Cool-2 appeared to be Ile 544, because like Ile 1187 in Tiam1, Ile 544 was immediately downstream from a leucine residue and 10 residues downstream from a glutamine residue (these residues are in bold in the sequence shown in Figure 1A). However, Ile 544 is located within the carboxyl-terminal end of the PH domain rather than within the DH domain. To test the functional importance of this region for the Rac-GEF activity of Cool-2, we changed Leu 543 and Ile 544 to arginine and valine, respectively (designated LIm.Cool-2 in Figure 1B). The Myc-tagged LIm.Cool-2 construct was transiently expressed in COS-7 cells and then assayed for its ability to stimulate the guanine nucleotide exchange activity of Rac1.
Figure 3A, left panel, compares the relative expression of Myc-LIm.Cool-2 in COS-7 cells with wild-type Myc-Cool-2 and other Cool-2 mutants that will be described below, and the right panel compares the relative amounts of these same Cool-2 constructs that were immunoprecipitated with anti-Myc and used in the GEF assays. As shown in Figure 3B, LIm.Cool-2 was ineffective at stimulating the dissociation of [3H]GDP from Rac1. It was also incapable of stimulating PAK3 activity in cells (compare lane 1 (control) with lane 5 in the bottom panel of Figure 3C; the top panel shows the relative amounts of HA-tagged PAK3 expressed under the different experimental conditions).
Figure 3.
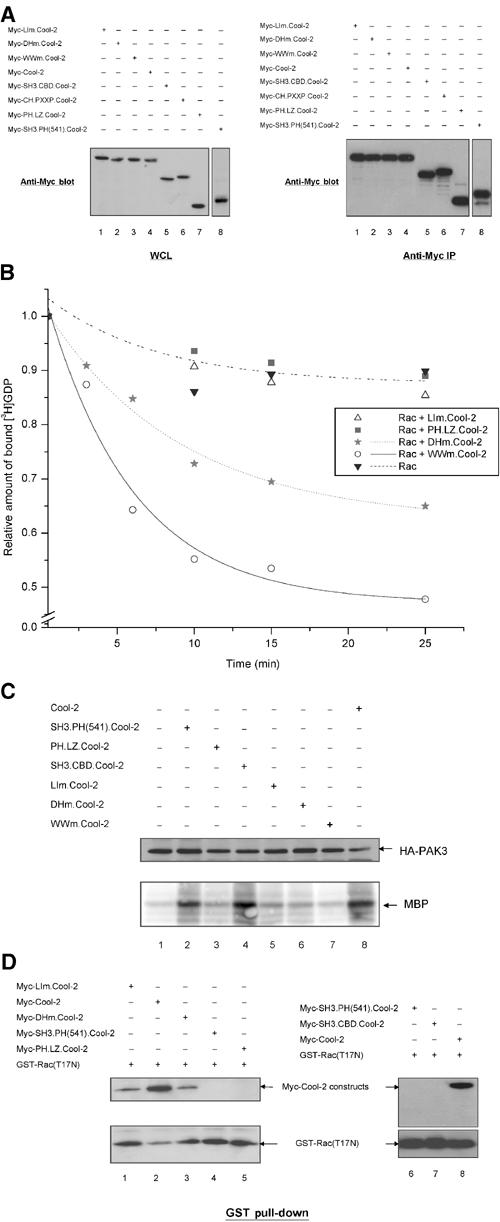
The DH and PH domains of Cool-2 work together to exhibit GEF activity toward Rac. (A) The left panel shows the relative expression in whole-cell lysates (WCLs) of different Myc-Cool-2 constructs used in the experiments shown in (B—D). The different Myc-Cool-2 constructs were expressed, alone (B, D) or together with HA-PAK3 (C) in COS-7 cells. The right panel shows the immunoprecipitation of the different Myc-Cool-2 proteins used in the GEF assays in (B) or in other experiments. (B) The dissociation of [3H]GDP from Rac1 catalyzed by different Cool-2 constructs was assayed as described in the legend to Figure 2A and the data shown are representative of three experiments. (C) Assays of PAK activity (shown in the bottom panel) were performed for the different conditions indicated, using myelin basic protein (MBP) as a phosphosubstrate (see Materials and methods). The top panel compares the relative amounts of PAK3 expressed in the different cell lysates. (D) The top panel shows the binding of the different Cool-2 constructs to GST-Rac(T17N), performed as described in the legend to Figure 2B, and the bottom panel shows the relative amounts of GST-Rac(T17N) precipitated in the different experiments.
We also generated a double mutant where the highly conserved leucine residues 383 and 384 within the DH domain of Cool-2 were changed to arginine and serine, respectively (designated DHm.Cool-2 in Figure 1B). We found that DHm.Cool-2 exhibited a relatively weak GEF activity (Figure 3B) and no detectable stimulation of PAK3 (lane 6 in the bottom panel of Figure 3C), consistent with the findings of Daniels et al (1999) when assaying PAK activation by a truncated version of Cool-2. Thus taken together, these results indicated that both the DH domain of Cool-2 and a newly identified region within the carboxyl-terminal end of the PH domain, which we designated as RSID (for Rac-specific interaction domain; see Figure 1A), were necessary for full Rac-GEF activity.
As a complimentary approach, we examined the ability of these and other Cool-2 mutants to bind to Rac. The LIm.Cool-2 and DHm.Cool-2 constructs were transiently expressed in COS-7 cells, as were full-length wild-type Cool-2, SH3.PH(541).Cool-2, and PH.LZ.Cool-2, which is comprised of the PH domain and everything downstream (Figure 1B). Lysates were prepared from the different transfectants and then incubated with GST-Rac(T17N). Compared to wild-type Cool-2, both LIm.Cool-2 and DHm.Cool-2 showed weaker binding to GST-Rac(T17N) (Figure 3D, compare lane 2 with lanes 1 and 3, respectively, in the top panel; the bottom panel shows the GST-Rac(T17N) used in the different pull-down experiments). Neither SH3.PH(541).Cool-2 (lanes 4 and 6, top panel), which ends at residue 541 and thereby lacks the complete RSID necessary for Rac-GEF activity, nor PH.LZ.Cool-2 (lane 5, top panel), which is missing the DH domain but includes the RSID, showed detectable binding to Rac. Thus, these results further indicate that the DH domain and residues within the carboxyl-terminal portion of the PH domain of Cool-2 are necessary for specific binding to Rac.
We have also examined whether a Cool-2 mutant that was unable to bind to PAK was able to stimulate PAK activity and/or exhibit GEF activity toward Rac1. A PAK-binding defective Cool-2 mutant was prepared by changing Trp 194 and Trp 195 to lysine residues. This Cool-2 mutant, designated as WWm.Cool-2 in Figure 1B, still exhibited GEF activity toward Rac1 (Figure 3B) but was incapable of stimulating PAK activity (Figure 3C, bottom panel, lane 7). Thus, while the binding of PAK to full-length Cool-2 was not essential for its Rac-GEF activity, this binding interaction was required for Cool-2-activated Rac molecules to stimulate PAK activity. This suggested that Cool-2 may not act catalytically to activate multiple Rac molecules and that once activated Rac is unable to dissociate from Cool-2 and engage PAK molecules that are not part of a Cool-2–PAK complex. Because Rac (or Cdc42) is typically in excess relative to the Cool-2 proteins in our assays, the inability of Cool-2 to act catalytically as a GEF may also explain as to why we often see less than complete dissociation of [3H]GDP from the total pool of GTP-binding proteins.
Dimerization of Cool-2 is necessary for its specific interaction with Rac1-
Based on the results described in the preceding section, we assumed that any Cool-2 construct that contained both the DH domain and the RSID would function as a Rac-specific GEF. We therefore examined the abilities of some additional Cool-2 constructs to act as Rac-GEFs, including one that contained both the DH domain and RSID as well as a portion of the proline-rich region (SH3.PX.Cool-2) (Figure 4A), and another that included the full proline-rich domain (SH3.PXXP.Cool-2). Surprisingly, neither of these truncated Cool-2 molecules alone exhibited GEF activity (data not shown).
Figure 4.

Dimerization of Cool-2 is necessary for its specific interaction with Rac1. (A) Schematic representation of different truncated Cool-2 constructs and Cool-2 mutants. (B) Top panel: COS-7 cells were transfected with a plasmid expressing HA-PAK3 together with plasmids expressing Myc-CH.PXXP.Cool-2 (lane 1), Myc-Cool-2 (lane 2), or control vector (lane 3). The middle panel compares the relative expression of HA-PAK3 for the different experimental conditions, and the bottom panel shows the results of PAK assays using MBP as a phosphosubstrate. (C) COS-7 cells were transfected with plasmids expressing Myc-CH.PXXP.Cool-2 or SH3.CBD.Cool-2, alone or together with a plasmid expressing HA-PAK3. The assays of [3H]GDP dissociation from Rac were performed as described in the legend to Figure 2A and the data shown are representative of three experiments.
This led us to consider other possibilities regarding how the DH domain and RSID might work together to activate Rac1 specifically. One interesting possibility stemmed from the findings that p85Cool-1 dimerizes through its carboxyl-terminal leucine zipper (Kim et al, 2001). Cool-2 also contains a leucine zipper and so we wondered whether the dimerization of Cool-2 might allow cooperation (in trans) between its DH domain and RSID. Such a mechanism would be consistent with our findings that truncated Cool-2 constructs lacking the leucine zipper did not exhibit Rac-GEF activity.
To test this idea, we examined two Cool-2 constructs. One, designated CH.PXXP.Cool-2 (Figure 4A), begins at the amino-terminal CH domain and terminates just downstream from the proline-rich region and thus lacks both the Cat/Git-binding domain (CBD) and the leucine zipper. A second construct, SH3.CBD.Cool-2 (Figure 4A), starts at the SH3 domain and terminates just after the Cat/Git-binding domain and also lacks the leucine zipper. Both SH3.CBD.Cool-2 and CH.PXXP.Cool-2 stimulated PAK activity (compare lanes 1 and 4 in the bottom panel of Figure 3C, and lanes 1 and 3 in the bottom panel of Figure 4B, respectively), but SH3.CBD.Cool-2 was unable to bind to GST-Rac(T17N) (Figure 3D, lane 7, top panel), and neither of these Cool-2 constructs alone stimulated [3H]GDP dissociation from Rac (Figure 4C). Thus, Cool-2 constructs that lack the leucine zipper region do not exhibit the same capability as full-length Cool-2 to function as Rac-GEFs. However, as will be discussed in more detail in the next section, when these truncated Cool-2 constructs were coexpressed with PAK3, they were then able to function as Rac-GEFs and stimulate [3H]GDP dissociation (Figure 4C).
To further demonstrate the importance of the leucine zipper for the Rac-specific GEF activity of full-length Cool-2, we generated a Cool-2 construct with specific mutations within this region. Leucine residues 711, 718, and 725 within the leucine zipper of Cool-2 conform to the ‘LxxxxxxL' motif. We therefore prepared a Cool-2 mutant in which leucine residues 711 and 718 were changed to arginine and serine, respectively (designated as LZm.Cool-2; Figure 4A). Myc-tagged LZm.Cool-2 or wild-type Cool-2 was then transiently expressed in COS-7 cells, either alone or together with HA-tagged p85Cool-1. As shown in Figure 5A (top panel, lanes 4 and 5), when these Cool-2 constructs were analyzed by SDS–polyacrylamide gel electrophoresis (SDS–PAGE), a single band with the expected mobility of the monomeric protein was always detected for Myc-LZm.Cool-2, whereas two bands were detected for full-length Cool-2 (lanes 1–3), with the slower mobility band reflecting dimer formation that was not fully disrupted even after treatment with SDS. We also found that HA-p85Cool-1 could be co-immunoprecipitated with Myc-Cool-2 (Figure 5B, bottom panel, lane 2), showing that heterodimerization between Cool-1 and Cool-2 can occur. However, HA-p85Cool-1 could not be co-immunoprecipitated with Myc-LZm.Cool-2 (Figure 5B, bottom panel, lane 1). Thus, the double mutation within the leucine zipper region of Cool-2 eliminated its ability to undergo dimerization. When we examined the ability of Myc-LZm.Cool-2, following its immunoprecipitation, to stimulate [3H]GDP dissociation from Rac, we found that it was ineffective (Figure 5C). This is consistent with the idea that dimerization is necessary for the proper juxtaposition of the DH domain and RSID for the Rac-specific GEF activity exhibited by the full-length Cool-2 protein.
Figure 5.
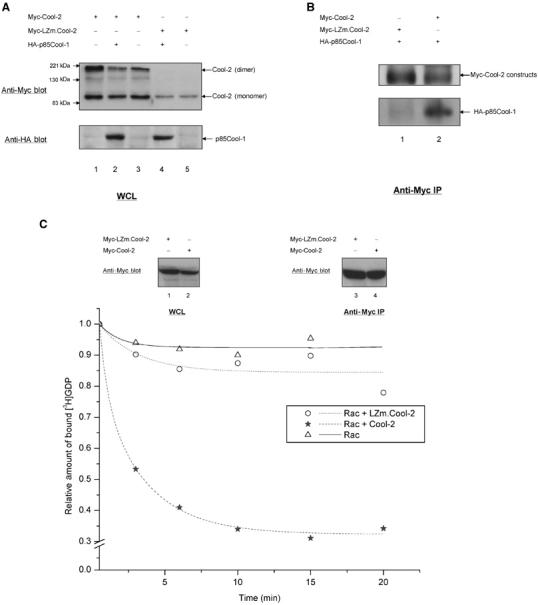
Dimerization of Cool-2 is necessary for its GEF activity toward Rac1. (A) SDS–PAGE profiles for Myc-Cool-2 or Myc-LZm.Cool-2, transiently expressed in COS-7 cells alone or together with HA-p85Cool-1. The higher bands in the top panel represent the Cool-2 dimer, whereas the lower bands represent the Cool-2 monomer. (B) Co-immunoprecipitation assays in which cell lysates coexpressing Myc-LZm.Cool-2 and HA-p85Cool-1, or Myc-Cool-2 and HA-p85Cool-1, were incubated with anti-Myc primary antibody. This experiment was carried out as described in Materials and methods. (C) The top panel compares the relative expression of Myc-Cool-2 and Myc-LZm.Cool-2 in whole-cell lysates (WCLs) (lanes 1 and 2) and the corresponding immunoprecipitation of the Cool-2 proteins (lanes 3 and 4). The bottom panel shows assays of [3H]GDP dissociation from Rac1, performed as described in the legend to Figure 2A.
SH3 domain interactions enable monomeric Cool-2 to exhibit GEF activity toward Rac and Cdc42-
Given the importance of the leucine zipper for the Rac-specific GEF activity of Cool-2, how was it that the SH3.PH(541). Cool-2 construct, lacking the leucine zipper, was still able to activate PAK when expressed in cells (Figure 3C, bottom panel, lane 2; see also, Feng et al, 2002)? We wondered whether the binding of PAK to dimerization-defective Cool-2 constructs might stimulate their GEF activity and thereby account for their ability to activate PAK. Indeed, the coexpression of PAK3 with truncated Cool-2 constructs lacking the leucine zipper (SH3.CBD.Cool-2 and CH.PXXP.Cool-2) enabled these Cool-2 proteins to stimulate the dissociation of [3H]GDP from Rac (Figure 4C). Likewise, coexpressing the shorter Myc-tagged SH3.PH(541).Cool-2 construct together with HA-tagged PAK3 (Figure 6A, panel a) gave rise to Rac-GEF activity (Figure 6B). Moreover, the coexpression of PAK3 together with SH3.PH(541).Cool-2 also enabled it to act as a GEF for Cdc42 (Figure 6B).
Figure 6.
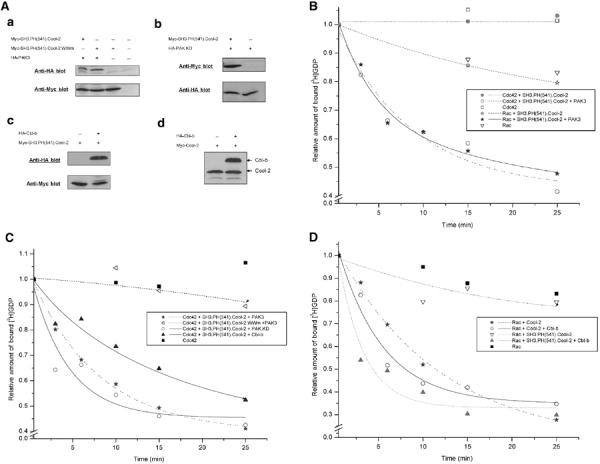
Assays of [3H]GDP dissociation from Rac or Cdc42 catalyzed by different Cool-2 constructs together with kinase-defective PAK3 or with Cbl-b. (A) Western blots for COS-7 cell lysates expressing Myc-SH3.PH(541).Cool-2 and HA-PAK3 (panel a), Myc-SH3.PH(541).Cool-2 and HA-PAK.KD (panel b), Myc-SH3.PH(541).Cool-2.WWm and HA-PAK3 (panel a), Myc-SH3.PH(541).Cool-2 and HA-Cbl-b (panel c), or Myc-Cool-2 and HA-Cbl-b (panel d). Assays of [3H]GDP dissociation from Rac (B, D) or Cdc42 (B, C) in the presence of the indicated Cool-2, PAK3, and Cbl-b constructs. The assays were performed as described in the legend to Figure 2A and the data shown are representative of three experiments.
To confirm that it was the direct interaction between Cool-2 and PAK that was responsible for the GEF activity exhibited by SH3.PH(541).Cool-2, we mutated the conserved tryptophan residues 194 and 195 in the SH3 domain of Cool-2 to lysines (designated SH3.PH(541).Cool-2.WWm), as these residues are essential for binding to PAK (Bagrodia et al, 1998). When SH3.PH(541).Cool-2.WWm was coexpressed with PAK3 (Figure 6A, panel a), it was unable to stimulate the guanine nucleotide exchange activity of Cdc42 (Figure 6C). The same was true for Rac (data not shown).
We next asked whether the kinase activity of PAK was essential for Cool-2 to functionally couple to Cdc42 or Rac. However, this was not the case, as the expression of a PAK3 construct that lacked the kinase domain (designated as PAK.KD in Figure 6A, panel b) was sufficient to enable SH3.PH(541).Cool-2 to stimulate [3H]GDP dissociation from Cdc42 (Figure 6C). Moreover, when the adaptor protein Cbl-b, a member of the Cbl family that is enriched in breast cancer cells and also binds to the SH3 domain of the Cool proteins (Flanders et al, 2003), was coexpressed with SH3.PH(541).Cool-2 (Figure 6A, panel c), this enabled SH3.PH(541).Cool-2 to act as a GEF for Cdc42 (Figure 6C), as well as for Rac (Figure 6D). The effects of Cbl-b (as well as PAK3) were specific for the SH3.PH(541).Cool-2 construct, as coexpression of Cbl-b with full-length Cool-2 (Figure 6A, panel d) did not alter its ability to act as a GEF for Rac (Figure 6D).
Apparently, the binding of PAK or Cbl to the SH3 domain of monomeric Cool-2 causes it to interact more effectively with Cdc42 or Rac. This is indicated from the binding experiments presented in Figure 7B. In these experiments, GST-Cdc42(T17N) was incubated with the lysates from cells expressing Myc-SH3.PH(541).Cool-2 together with either HA-Cbl-b or HA-PAK3 (Figure 7A is the Western blot showing the expression of these proteins), and then precipitated with glutathione-agarose beads. The precipitates were blotted with anti-Myc to detect Cool-2 that co-precipitated with GST-Cdc42(T17N). The data presented in Figure 7B show that GST-Cdc42(T17N) was not capable of binding to SH3.PH(541).Cool-2 alone (i.e. see lane 1 in the top panel; the bottom panel shows the relative amounts of GST-Cdc42 used in the different experiments). However, an interaction was detected when SH3.PH(541).Cool-2 was coexpressed with either Cbl-b or PAK3 (lanes 2 and 3, respectively, in the top panel). The apparent differences in the effectiveness of the Cdc42–Cool-2 interaction observed in the presence of Cbl-b versus PAK3 is likely attributed to differences in the relative expression of these proteins (see Figure 7A).
Figure 7.
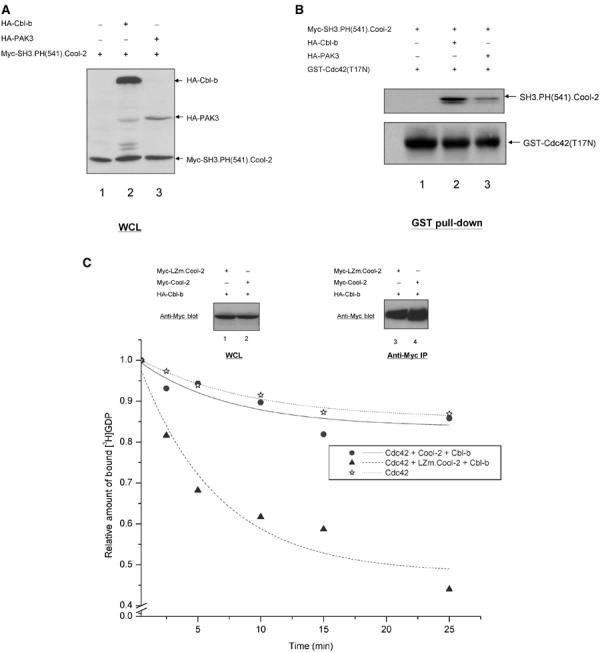
SH3 domain-mediated association enhances the interaction of Cdc42 with monomeric Cool-2 proteins. (A) Western blot of whole-cell lysates (WCLs) of COS-7 cells expressing Myc-SH3.PH(541).Cool-2 alone (lane 1) or together with HA-Cbl-b (lane 2) or HA-PAK3 (lane 3). (B) GST pull-down assays were performed by incubating GST-Cdc42(T17N) with lysates from COS-7 cells expressing Myc-SH3.PH(541).Cool-2, alone (lane 1) or together with HA-Cbl-b (lane 2) or HA-PAK3 (lane 3) as described in Materials and methods. The co-precipitated Myc-SH3.PH(541).Cool-2 was detected by Western blotting with anti-Myc antibody (top panel); the relative amounts of GST-Cdc42(T17N) precipitated in the different experiments are shown in the bottom panel. (C) Full-length Myc-Cool-2 or Myc-LZm.Cool-2 was coexpressed with HA-Cbl-b in COS-7 cells (top panel, lanes 1 and 2), and then the Myc-Cool-2 proteins were immunoprecipitated (top panel, lanes 3 and 4) and [3H]GDP dissociation from Cdc42 was assayed (bottom panel) as described in the legend to Figure 2A.
These findings, when taken together with those described in the preceding section, suggest that Cool-2 constructs that are incapable of forming dimers can exhibit GEF activity toward Cdc42 (or Rac), provided that their SH3 domain is engaged by PAK or Cbl. This is further supported by the data shown in Figure 7C. Full-length Myc-Cool-2, following its immunoprecipitation from cells, was incapable of stimulating the nucleotide exchange activity of Cdc42, even when coexpressed with Cbl-b (Figure 7C). However, the dimerization-defective LZm.Cool-2 protein, when expressed together with Cbl-b, was able to exhibit Cdc42-GEF activity. The same was true when LZm.Cool-2 was coexpressed with PAK3 (data not shown).
Recently, it was suggested that the Cool-2/α-Pix protein functioned in a chemoattractant signaling pathway to promote the activation of Cdc42 (Li et al, 2003). This activation event required both the binding of PAK to Cool-2 and the binding of the G protein βγ subunit complex to PAK. Given what we know about the necessity for Cool-2 to exist as a monomer to activate Cdc42, we suspected that the G protein βγ complex, upon binding to PAK, might promote the monomeric state of Cool-2. Indeed, the addition of the retinal G protein β1γ1 subunit complex, together with histidine (His)-tagged, purified Cool-2 protein, to cell lysates expressing Myc-Cool-2 and HA-PAK3 completely blocked the ability of His-Cool-2 to form a dimer with Myc-Cool-2, as read out by their co-immunoprecipitation with anti-Myc antibody (compare lanes 2 and 3 in the top panel of Figure 8A). As expected, Myc-LZm.Cool-2 was unable to dimerize with His-Cool-2, even in the absence of added β1γ1 complex (see lane 1 in the top panel of Figure 8A).
Figure 8.
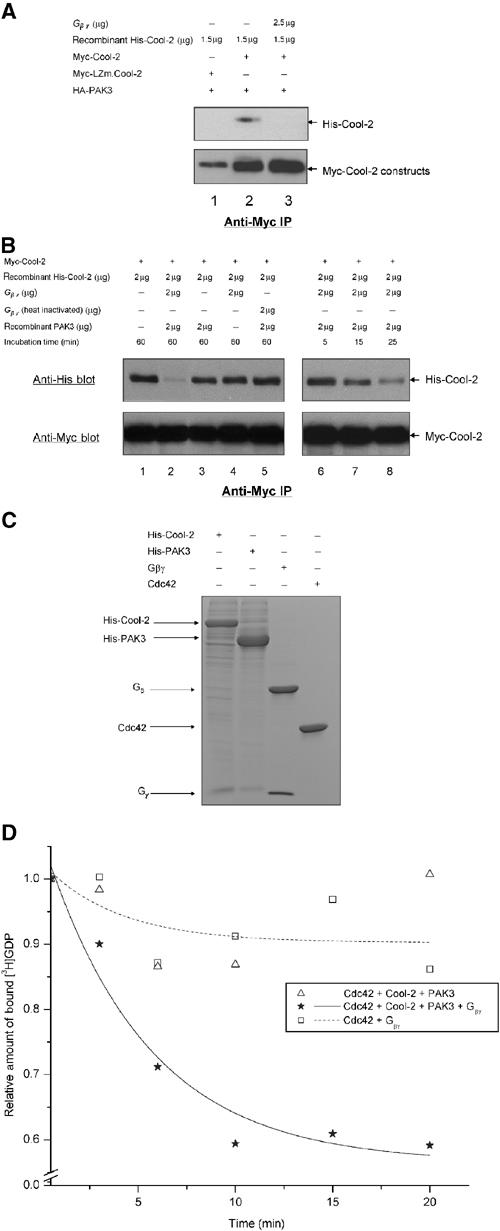
Gβγ helps to activate the Cdc42-GEF activity of Cool-2. (A) The ability of Myc-Cool-2 versus Myc-LZm.Cool-2 to dimerize was examined in the presence of PAK3, and in the presence and absence of bovine retinal β1γ1, by assaying the ability of insect cell-expressed His-Cool-2 to be co-immunoprecipitated with Myc-Cool-2 that was transiently expressed in COS-7 cells (see Materials and methods). (B) The time-dependent dissociation of the dimers formed between His-Cool-2 and Myc-Cool-2, upon the addition of retinal β1γ1 and insect cell-expressed His-PAK3, was assayed as described in Materials and methods. ‘Incubation time' refers to the time periods that β1γ1 and/or PAK3 were incubated with lysates expressing Myc-Cool-2 and containing His-Cool-2. (C) Coomassie blue staining of SDS–PAGE of purified protein preparations used in these experiments. Histidine-tagged Cool-2 and PAK3 were expressed and purified from insect cells (see Materials and methods), and GST-Cdc42 was expressed and purified from E. coli, with the GST tag being removed prior to the GEF assays. The retinal βγ subunit complex was isolated as previously described (Phillips et al, 1989). (D) E. coli-expressed Cdc42 (1 μg) was incubated with retinal βγ (5 μg) alone or with His-Cool-2 (4 μg) and His-PAK3 (2 μg) in the presence and absence of βγ, and then the dissociation of [3H]GDP from Cdc42 was assayed as described in the legend to Figure 2A.
Neither the addition of insect cell recombinant PAK3 nor retinal β1γ1 alone (Figure 8C shows the SDS–PAGE profiles for these purified proteins) was able to inhibit the ability of the purified His-Cool-2 protein to form a dimer with the transiently expressed Myc-Cool-2 (compare lanes 3 and 4, respectively, in the top panel of Figure 8B with lane 1). However, when β1γ1 was added together with PAK3, a time-dependent inhibition of Cool-2 dimerization was observed (see lanes 6–8 in the top panel of Figure 8B), culminating in a complete block of the co-immunoprecipitation of His-Cool-2 with Myc-Cool-2 (lane 2 in the top panel).
We then set out to reconstitute the Cool-2-promoted activation of Cdc42 using purified proteins (Figure 8C). When the insect cell recombinant Cool-2 was incubated with PAK3 alone, there was no detectable stimulation of [3H]GDP dissociation from Cdc42 (Figure 8D), consistent with the idea that PAK3 is not sufficient to promote the dissociation of dimeric Cool-2 into monomers. However, when Cool-2 was incubated with PAK3 together with the βγ complex (i.e. conditions that lead to the formation of Cool-2 monomers), we were able to reconstitute the Cdc42-GEF activity of Cool-2 successfully.
Discussion
The Cool/Pix proteins were originally identified through their abilities to bind to PAK (Bagrodia et al, 1998; Manser et al, 1998). Each of the members of the family share a common arrangement of SH3, DH, and PH domains, with the presence of tandem DH and PH domains leading to the expectation that these proteins would serve as upstream activators (GEFs) specifically complexed to a downstream target for Cdc42 or Rac. However, initially it was difficult to document that some of the members of the Cool/Pix family were indeed capable of GEF activity. For example, the expression of p50Cool-1 in cells resulted in an inhibition of Cdc42-stimulated PAK activity, whereas p85Cool-1 neither inhibited nor stimulated PAK activity (Bagrodia et al, 1998). On the other hand, Cool-2 strongly stimulated PAK activity. It was subsequently demonstrated that a truncated Cool-2 construct containing the SH3, DH, and PH domains, when assayed together with PAK3, was indeed capable of GEF activity as documented by its ability to stimulate the release of [3H]GDP from Cdc42 (Feng et al, 2002). In addition, it was determined that an 18-amino-acid sequence, designated as T1, acted to repress GEF activity in the Cool-1 proteins, such that its removal yielded Cool-1 constructs capable of stimulating GDP dissociation from Cdc42, whereas addition of the T1 region to Cool-2 constructs inhibited their GEF function.
Given these findings, the questions have shifted toward understanding the underlying mechanisms by which the Cool proteins might function as GEFs. There have been reports suggesting that p85Cool-1 gives rise to cellular responses that are consistent with the activation of Rac or Cdc42 (Kim et al, 2001; Koh et al, 2001). Presumably, some form of signaling regulation is responsible for reversing the negative regulatory activity of the T1 region, thus converting p85Cool-1 into a functioning GEF. We expected that the mechanisms underlying the GEF activity for Cool-2 would be more straightforward, given its readily detectable activation of PAK in cells. However, surprisingly, we have found that Cool-2 is susceptible to a novel and complex set of regulatory mechanisms. Full-length Cool-2 acts as a specific GEF for Rac, with the dimerization of Cool-2 being an essential requirement for this specificity. Previous studies had shown p85Cool-1 dimerizes and that dimerization might be important for its cellular functions (Koh et al, 2001). Here we demonstrate that full-length Cool-2 can form homodimers as well as heterodimers with p85Cool-1, and that the dimerization of Cool-2 is mediated via a leucine zipper located near the carboxyl-terminal end of the protein. Moreover, the dimerization of Cool-2 enables it to specifically recognize GDP-bound Rac through the combined actions of two regions, namely the DH domain from one of the partner monomers and a region near the carboxyl-terminal end of the PH domain (designated the Rac-specific interaction domain or RSID) from the other monomer partner (Figure 9A).
Figure 9.
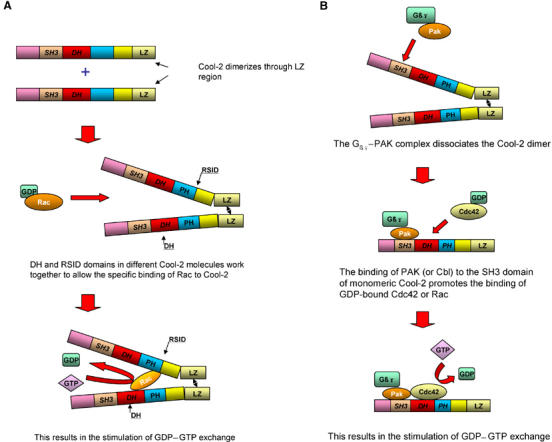
Dual regulatory mechanisms for the GEF activity of Cool-2. (A) Model depicting how the DH domain, RSID region, and dimerization work together to enhance the interaction between Rac and Cool-2. Cool-2 forms a dimer through its leucine zipper region. The DH domain from one Cool-2 molecule and the RSID region of the other Cool-2 molecule work together to bind Rac. (B) Monomeric Cool-2 shows GEF activity toward Cdc42 or Rac. The interaction of a G protein βγ subunit complex with PAK and the binding of the PAK–βγ complex to Cool-2 promote the dissociation of the Cool-2 dimer. The binding of PAK to the SH3 domain of monomeric Cool-2 induces a conformational change within the DH domain that enables GDP-bound Cdc42 or Rac to bind and undergo nucleotide exchange. The binding of Cbl-b to the SH3 domain of monomeric Cool-2 also activates its GEF activity (not shown), although Cbl-b alone is not sufficient to dissociate the Cool-2 dimer.
Perhaps even more interesting from a regulatory standpoint is the fact that a second type of GEF activity can be manifested, but only when Cool-2 is in its monomeric state and bound through its SH3 domain to either PAK or Cbl. Under these conditions, Cool-2 is able to stimulate the nucleotide exchange activity of either Cdc42 or Rac. Perhaps it is this functional interplay between PAK and Cool-2 that accounts for the findings that mutations in both PAK3 and Cool-2 give rise to certain forms of X-linked mental retardation (Ramakers, 2002). It is still unclear exactly how PAK or Cbl activates the GEF activity of monomeric Cool-2 proteins. One obvious possibility would be that the SH3 domain of monomeric Cool-2 folds over and blocks access to the DH domain. The binding of PAK or Cbl to the SH3 domain of Cool-2 might then prevent this intramolecular interaction from occurring and allow access to the DH domain. However, Cool-2 constructs that lack the SH3 domain and contain just the DH and PH domains would then be expected to show GEF activity, but this is not the case (data not shown). Rather, it seems more likely that the binding of PAK or Cbl to the SH3 domain of Cool-2 induces a conformational transition that changes the architecture of the DH domain, allowing it to couple to either Cdc42 or Rac functionally.
Thus, there may be receptor-coupled signaling systems that help to maintain Cool-2 in the dimeric state so that it acts specifically as a Rac-GEF and other types of signals that stimulate the dissociation of the Cool-2 dimer so that it can activate Cdc42. For example, it was recently reported that Cool-2 was acting downstream from G protein-coupled chemoattractant receptors in mouse neutrophils to specifically activate Cdc42, leading to F-actin formation and maintenance of cell polarity, two events crucial for the directional movement of these cells (Li et al, 2003). In this system, the activation of Cdc42 required the binding of PAK to Cool-2, as well as the interaction of PAK with the Gβγ complex that was released from the Gi protein when activated by the chemoattractant receptor. PAK alone was not sufficient to activate the Cdc42-GEF activity of Cool-2. These findings can be nicely explained based on what we now know about the mechanisms regulating Cool-2 function (Figure 9B). Specifically, we have shown that Gβγ complexes, by binding to PAK, are able to stimulate the dissociation of dimeric Cool-2 molecules to their monomeric state, resulting in the stimulated exchange of GDP for GTP on Cdc42. While PAK alone is not able to stimulate the dissociation of the dimer, the binding of PAK to the SH3 domain of monomeric Cool-2 is required for its GEF activity. Therefore, receptors that generate free Gβγ subunits can work together with PAK through Cool-2 to activate Cdc42, as apparently is the case in the chemoattractant-coupled signaling pathway. Still, some interesting questions remain. While chemoattractants activate Rac in human neutrophils (Benard et al, 1999), it is not clear that Cool-2 is essential for chemoattractant-dependent Rac activation (Li et al, 2003). Does this mean that in some cells Cdc42 (rather than Rac) preferentially couples to monomeric Cool-2? Exactly how is the binding of Gβγ to PAK translated into an effect on the monomer–dimer equilibrium of Cool-2? Are there other mechanisms by which this equilibrium can be perturbed, perhaps by the binding of proteins to Cbl, or through direct interactions with Cool-2 (e.g. via the Cat/Git/Pkl proteins)? How does PAK or Cbl alter the conformation of monomeric Cool-2 such that it becomes a functional GEF? Future studies will be directed toward addressing these issues.
Materials and methods
Plasmid construction
Plasmids pJ3HmPAK3 and pcDNA3-Myc-Cool-2 were prepared as described previously (Bagrodia et al, 1995, 1998; Flanders et al, 2003). The HA-tagged Cbl-b construct was a kind gift of Dr Stan Lipkowitz (National Cancer Institute, MD). The different Cool-2 and PAK3 constructs were prepared by amplifying the appropriate bases of the open reading frame of pJ3HmPAK3 and pcDNA3-Myc-Cool-2 and subcloning the PCR products into pcDNA3. Site-directed mutagenesis was performed using the QuikChange Site-Directed Mutagenesis Kit (Stratagene).
Cell culture, transfections, and co-immunoprecipitation assays
COS-7 cells were maintained in Dulbecco's modified Eagle's medium plus 10% fetal bovine serum (Invitrogen). Plasmids pJ3HmPAK3 (0.3–0.5 μg/100 mm dish) and pcDNA3-Myc-Cool-2, as well as pcDNA3 encoding different Cool mutants (1.5–5.0 μg/100 mm dish), were cotransfected into COS-7 cells using LipofectAMINE (Invitrogen). Cells were lysed after 24–48 h in lysis buffer (20 mM Hepes, pH 7.4, 1% Nonidet P-40, 150 mM NaCl, 1 mM EDTA, 1 mM sodium orthovanadate, 10 μg/ml leupeptin, and 10 μg/ml aprotinin) at 4°C for 20 min. Lysates were prepared by centrifugation at 12 000 g for 10 min at 4°C. Cell lysates coexpressing Myc- and HA-tagged proteins were incubated with anti-Myc primary antibody for 1.5 h on ice followed by mixing with protein A-Sepharose beads (Invitrogen) for 1 h. The beads were washed three times with lysis buffer and then resuspended in 2 × SDS–PAGE sample buffer. Proteins were eluted by boiling for 5 min, resolved on a 10 or 12% SDS–polyacrylamide gel and then transferred to an Immobilon-P membrane (Millipore). Proteins were probed with mouse monoclonal anti-Myc and anti-HA antibodies. Primary antibodies were detected with horseradish peroxidase-coupled sheep anti-mouse antibody by ECL (Amersham Biosciences).
Binding assays
The GST-Rac(T17N) and GST-Cdc42(T17N) fusion proteins were each expressed in Escherichia coli and purified by glutathione-agarose affinity chromatography. Cell lysates (∼500 μl) from COS-7 cells transiently expressing Myc- or HA-tagged proteins were combined with 50 μl of a suspension of GST fusion proteins (∼5 μg) bound to glutathione-Sepharose beads and incubated at 4°C for 2–3 h. The beads were precipitated by microcentrifugation, washed three times with lysis buffer, and resuspended in 2 × SDS–PAGE sample buffer and Western blotting was performed as described in the preceding section.
Effects of Gβγ on Cool-2 dimerization
In one set of experiments, 1 ml of cell lysates coexpressing Myc-Cool-2 and HA-PAK3 was divided into two aliquots of 400 and 600 μl. The first aliquot was incubated with 1.5 μg of insect cell His-Cool-2 for 1 h on ice, while the other was mixed with 1.5 μg of His-Cool-2 and 2.5 μg of retinal Gβ1γ1 (prepared as described by Phillips et al, 1989) for 1 h on ice. Both incubations were then incubated with anti-Myc primary antibody for 1 h by mixing with protein A-Sepharose beads (Invitrogen) prior to immunoprecipitation and Western blotting.
In a second set of experiments, 5.4 ml of cell lysates expressing Myc-Cool-2 was incubated with 18 μg of His-Cool-2 and 9 μl of anti-Myc antibody for 1.5 h on ice. This mixture was then divided into aliquots (600 μl) to which was added either 2 μg of insect cell His-PAK3 alone or 2 μg of either retinal Gβ1γ1 or heat-denatured Gβ1γ1 alone or in combination with 2 μg of His-PAK3, for 60 min. The Myc-Cool-2 was immunoprecipitated from these incubations using protein G-Sepharose beads (Invitrogen), prior to Western blotting. When examining the time-dependent dissociation of the dimers formed between His-Cool-2 and Myc-Cool-2, the initial aliquots were incubated with protein G-Sepharose beads for 45 min, and then β1γ1 and PAK3 (2 μg each) were added and the incubations were terminated at different times by the addition of 5 × SDS loading buffer.
Purification of PAK3 and Cool-2 from insect cells
Viruses expressing His-PAK3 and His-Cool-2 were produced using the Bac-to-Bac Baculovirus Expression Kit (Invitrogen). Insect (Sf21) cells were infected with virus at an MOI of 5 for 72 h. Cells were then lysed in buffer A (20 mM Tris–HCl, pH 8.0, 20 mM imidazole, 0.2% CHAPS, 10 μg/ml leupeptin, and 10 μg/ml aprotinin). The lysate was centrifuged at 14 000 rcf for 10 min and the supernatant was incubated for 30 min at 4°C with NTA agarose beads conjugated to nickel. The beads were washed three times with buffer A and then eluted in 10 ml of buffer B (20 mM Tris–HCl, pH 8.0, 200 mM imidazole, and 0.2% CHAPS).
PAK assays
COS-7 cells transiently expressing Myc-Cool-2 proteins and HA-PAK3 were maintained in Dulbecco's modified Eagle's medium plus 10% fetal bovine serum (Invitrogen) for 24–36 h, and then starved for 18–24 h. Cells were lysed in buffer A (40 mM Tris–HCl, pH 7.4, 1% Triton, 100 mM NaCl, 1 mM EDTA, 1 mM sodium orthovanadate, 10 μg/ml leupeptin, and 10 μg/ml aprotinin) and washed by centrifugation at 12 000 g for 10 min at 4°C. The lysates were incubated with anti-PAK3 primary antibody (Bagrodia et al, 1998) or anti-HA antibody for 2 h followed by mixing with protein A-Sepharose beads (Invitrogen) for 45 min. The beads were washed three times with buffer A, twice with 2 × phosphorylation buffer (10 mM MgCl2 and 40 mM Tris–HCl, pH 7.4), and mixed with 1.0 μg/sample of the substrate myelin basic protein (Sigma). PAK assays were initiated by adding 25 μM ATP and 10 μCi of [γ-32P]ATP (3000 Ci/mmol) to the immunoprecipitation mixture and were maintained at 25°C for 15 min. The reactions were stopped by adding 2 × SDS–PAGE sample buffer containing 20 mM EDTA. The phosphoproteins were resolved by SDS–PAGE (10 or 12% gel) and visualized by PhosphorImager analysis (Amersham Biosciences) prior to immunoblotting.
[3H]GDP dissociation assays
Lysates prepared from COS-7 cells expressing different Myc-tagged Cool-2 constructs were incubated with anti-Myc antibody for 1.5 h followed by mixing with protein A-Sepharose beads for 45 min. The beads were washed twice with lysis buffer and then with HMN buffer (10 mM Hepes, 5 mM MgCl2, and 100 mM NaCl, pH 7.5). The immunoprecipitated Cool-2 proteins were incubated with 1 μg of E. coli-expressed GST-Cdc42 or GST-Rac1, preloaded with [3H]GDP, in 140 μl of reaction buffer that contained 500 μM GTPγS (to allow for [3H]GDP–GTPγS exchange) at room temperature, and then 23-μl aliquots were diluted into 1.5 ml of ice-cold termination buffer (20 mM Tris–HCl, 10 mM MgCl2, and 100 mM NaCl, pH 7.4) at various time points. The percent [3H]GDP remaining on the filters was detected by scintillation counting.
References
- Albertinazzi C, Za L, Paris S, de Curtis I (2003) ADP-ribosylation factor 6 and a functional PIX/p95-APP1 complex are required for Rac1B-mediated neurite outgrowth. Mol Biol Cell 14: 1295–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagrodia S, Bailey D, Lenard Z, Hart M, Guan JL, Premont RT, Taylor SJ, Cerione RA (1999) A tyrosine-phosphorylated protein that binds to an important regulatory region on the cool family of p21-activated kinase-binding proteins. J Biol Chem 274: 22393–22400 [DOI] [PubMed] [Google Scholar]
- Bagrodia S, Cerione RA (1999) Pak to the future. Trends Cell Biol 9: 350–355 [DOI] [PubMed] [Google Scholar]
- Bagrodia S, Taylor SJ, Creasy CL, Chernoff J, Cerione RA (1995) Identification of a mouse p21Cdc42/Rac activated kinase. J Biol Chem 270: 22731–22737 [DOI] [PubMed] [Google Scholar]
- Bagrodia S, Taylor SJ, Jordan KA, Van Aelst L, Cerione RA (1998) A novel regulator of p21-activated kinases. J Biol Chem 273: 23633–23636 [DOI] [PubMed] [Google Scholar]
- Bar-Sagi D, Hall A (2000) Ras and Rho GTPases: a family reunion. Cell 103: 227–238 [DOI] [PubMed] [Google Scholar]
- Benard V, Bohl BP, Bokoch GM (1999) Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem 274: 13196–13204 [DOI] [PubMed] [Google Scholar]
- Boguski MS, McCormick F (1993) Proteins regulating Ras and its relatives. Nature 366: 643–654 [DOI] [PubMed] [Google Scholar]
- Daniels RH, Zenke FT, Bokoch GM (1999) alphaPix stimulates p21-activated kinase activity through exchange factor-dependent and -independent mechanisms. J Biol Chem 274: 6047–6050 [DOI] [PubMed] [Google Scholar]
- Di Cesare A, Paris S, Albertinazzi C, Dariozzi S, Andersen J, Mann M, Longhi R, de Curtis I (2000) p95-APP1 links membrane transport to Rac-mediated reorganization of actin. Nat Cell Biol 2: 521–530 [DOI] [PubMed] [Google Scholar]
- Erickson JW, Cerione RA (2001) Multiple roles for Cdc42 in cell regulation. Curr Opin Cell Biol 13: 153–157 [DOI] [PubMed] [Google Scholar]
- Feng Q, Albeck JG, Cerione RA, Yang W (2002) Regulation of the Cool/Pix proteins: key binding partners of the Cdc42/Rac targets, the p21-activated kinases. J Biol Chem 277: 5644–5650 [DOI] [PubMed] [Google Scholar]
- Flanders JA, Feng Q, Bagrodia S, Laux MT, Singavarapu A, Cerione RA (2003) The Cbl proteins are binding partners for the Cool/Pix family of p21-activated kinase-binding proteins. FEBS Lett 550: 119–123 [DOI] [PubMed] [Google Scholar]
- Gao Y, Xing J, Streuli M, Leto TL, Zheng Y (2001) Trp(56) of rac1 specifies interaction with a subset of guanine nucleotide exchange factors. J Biol Chem 276: 47530–47541 [DOI] [PubMed] [Google Scholar]
- Hall A (1998) Rho GTPases and the actin cytoskeleton. Science 279: 509–514 [DOI] [PubMed] [Google Scholar]
- Hart MJ, Eva A, Zangrilli D, Aaronson SA, Evan T, Cerione RA, Zheng Y (1994) Cellular transformation and guanine nucleotide exchange activity are catalyzed by a common domain on the dbl oncogene product. J Biol Chem 269: 62–65 [PubMed] [Google Scholar]
- Hoffman GR, Cerione RA (2002) Signaling to the Rho GTPases: networking with the DH domain. FEBS Lett 513: 85–91 [DOI] [PubMed] [Google Scholar]
- Hussain NK, Jenna S, Glogauer M, Quinn CC, Wasiak S, Guipponi M, Antonarakis SE, Kay BK, Stossel TP, Lamarche-Vane N, McPherson PS (2001) Endocytic protein intersectin-l regulates actin assembly via Cdc42 and N-WASP. Nat Cell Biol 3: 927–932 [DOI] [PubMed] [Google Scholar]
- Kim S, Lee SH, Park D (2001) Leucine zipper-mediated homodimerization of the p21-activated kinase-interacting factor, beta Pix. Implication for a role in cytoskeletal reorganization. J Biol Chem 276: 10581–10584 [DOI] [PubMed] [Google Scholar]
- Koh CG, Manser E, Zhao ZS, Ng CP, Lim L (2001) Beta1PIX, the PAK-interacting exchange factor, requires localization via a coiled-coil region to promote microvillus-like structures and membrane ruffles. J Cell Sci 114: 4239–4251 [DOI] [PubMed] [Google Scholar]
- Ku GM, Yablonski D, Manser E, Lim L, Weiss A (2001) A PAK1–PIX–PKL complex is activated by the T-cell receptor independent of Nck, Slp-76 and LAT. EMBO J 20: 457–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsche K, Yntema H, Brandt A, Jantke I, Nothwang HG, Orth H, Bovida MG, David D, Chelly J, Fryns JP, Moraine C, Ropers HH, Hamel BCJ, vanBokhoven H, Gal A (2000) Mutations in ARHGEF6, encoding a guanine nucleotide exchange factor for Rho GTPases, in patients with X-linked mental retardation. Nat Genet 26: 247–250 [DOI] [PubMed] [Google Scholar]
- Lee SH, Eom M, Lee SJ, Kim S, Park HJ, Park D (2001) BetaPix-enhanced p38 activation by Cdc42/Rac/PAK/MKK3/6-mediated pathway. Implication in the regulation of membrane ruffling. J Biol Chem 276: 25066–25072 [DOI] [PubMed] [Google Scholar]
- Li Z, Hannigan M, Mo Z, Liu B, Lu W, Wu Y, Smrcka AV, Wu G, Li L, Liu M, Huang CK, Wu D (2003) Directional sensing requires G beta gamma-mediated PAK1 and PIX alpha-dependent activation of Cdc42. Cell 114: 215–227 [DOI] [PubMed] [Google Scholar]
- Manser E, Leung T, Salhuddin H, Zhao ZS, Lim L (1994) A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature 367: 40–46 [DOI] [PubMed] [Google Scholar]
- Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, Tan L, Tan I, Leung T, Lim L (1998) PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell 1: 183–192 [DOI] [PubMed] [Google Scholar]
- Oh WK, Yoo JC, Jo D, Song YH, Kim MG, Park D (1997) Cloning of a SH3 domain-containing proline-rich protein, p85SPR, and its localization in focal adhesion. Biochem Biophys Res Commun 235: 794–798 [DOI] [PubMed] [Google Scholar]
- Phillips WJ, Trukawinski S, Cerione RA (1989) An antibody-induced enhancement of the transducin-stimulated cyclic GMP phosphodiesterase activity. J Biol Chem 264: 16679–16688 [PubMed] [Google Scholar]
- Premont RT, Claing A, Vitale N, Freeman JLR, Pitcher JA, Patton WA, Moss J, Vaughan M, Lefkowitz RJ (1998) beta2-Adrenergic receptor regulation by GIT1, a G protein-coupled receptor kinase-associated ADP ribosylation factor GTPase-activating protein. Proc Natl Acad Sci USA 95: 14082–14087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakers GJA (2002) Rho proteins, mental retardation and the cellular basis of cognition. Trends Neurosci 25: 191–199 [DOI] [PubMed] [Google Scholar]
- Rossman KL, Worthylake DK, Snyder JT, Siderovski DP, Campbell SL, Sondek J (2002) A crystallographic view of interactions between Dbs and Cdc42: PH domain-assisted guanine nucleotide exchange. EMBO J 21: 1315–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JT, Worthylake DK, Rossman KL, Betts L, Pruitt WM, Siderovski DP, Der CJ, Sondek J (2002) Structural basis for the selective activation of Rho GTPases by Dbl exchange factors. Nat Struct Biol 9: 468–475 [DOI] [PubMed] [Google Scholar]
- Turner CE, Brown MC, Perrotta JA, Riedy MC, Nikolopoulus SN, McDonald AR, Bagrodia S, Thomas S, Leventhal PS (1999) Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: a role in cytoskeletal remodeling. J Cell Biol 145: 851–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CE, West KA, Brown MC (2001) Paxillin-ARF GAP signaling and the cytoskeleton. Curr Opin Cell Biol 13: 593–599 [DOI] [PubMed] [Google Scholar]
- Van Aelst L, D'Souza-Schorey C (1997) Rho GTPases and signaling networks. Genes Dev 11: 2295–2322 [DOI] [PubMed] [Google Scholar]
- Whitehead IP, Campbell S, Rossman KL, Der CJ (1997) Dbl family proteins. Biochim Biophys Acta 1332: F1–23 [DOI] [PubMed] [Google Scholar]
- Worthylake DK, Rossman KL, Sondek J (2000) Crystal structure of Rac1 in complex with the guanine nucleotide exchange region of Tiam1. Nature 408: 682–688 [DOI] [PubMed] [Google Scholar]
- Wu WJ, Tu S, Cerione R (2003) Activated Cdc42 sequesters c-Cbl and prevents EGF receptor degradation. Cell 114: 715–725 [DOI] [PubMed] [Google Scholar]


