Abstract
Amphiphysin is a major dynamin-binding partner at the synapse; however, its function in fission is unclear. Incubation of large unilamellar liposomes with mice brain cytosol led to massive formation of small vesicles, whereas cytosol of amphiphysin 1 knockout mice was much less efficient in this reaction. Vesicle formation from large liposomes by purified dynamin was also strongly enhanced by amphiphysin. In the presence of liposomes, amphiphysin strongly affected dynamin GTPase activity and the recruitment of dynamin to the liposomes, but this activity was highly dependent on liposome size. Deletion from amphiphysin of its central proline-rich stretch dramatically potentiated its effect on dynamin, possibly by relieving an inhibitory intramolecular interaction. These results suggest a model in which maturation of endocytic pits correlates with the oligomerization of dynamin with either amphiphysin or other proteins with similar domain structure. Formation of these complexes is coupled to the activation of dynamin GTPase activity, thus explaining how deep invagination of the pit leads to fission.
Keywords: amphiphysin, dynamin, endocytosis, GTPase, liposome
Introduction
Clathrin-mediated endocytosis initiates with the recruitment of clathrin coat components to the plasma membrane, which is followed by the invagination of the membrane to form a clathrin-coated pit. It is completed by the fission reaction in which dynamin GTPase plays a key role (Slepnev and De Camilli, 2000; Takei and Haucke, 2001). Dynamin polymerizes into rings at the neck of clathrin-coated pits, but its precise mechanism of action in fission remains unclear. In vitro studies have shown that dynamin binds to lipids, and it can deform lipid bilayers into narrow tubules and fragment them in a GTP-hydrolysis-dependent way. Thus, one model proposes that dynamin acts as a mechanoenzyme, that is, by constricting the vesicle neck via a conformational change coupled to its catalytic action (Takei et al, 1995; Sweitzer and Hinshaw, 1998; Marks et al, 2001; Chen et al, 2004). Another model proposes that GTP-bound dynamin functions by activating a still unidentified downstream effector (Sever et al, 1999; Song and Schmid, 2003). The GTPase module of dynamin is located at the N-terminus of the protein. This domain is followed by the evolutionary conserved middle domain, a plecstrin homology (PH) domain, and the C-terminal proline/arginine-rich domain (PRD). The region between the PH domain and the PRD is referred to as the GTPase effector domain (GED), because it binds the GTPase module of adjacent dynamin molecules within dynamin polymers and may function as a GTPase activating protein (GAP) (Muhlberg et al, 1997). The PH domain binds phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2), while the PRD binds a variety of src-homology 3 (SH3) domain-containing proteins. These interactions also have been reported to stimulate dynamin GTPase activity (Gout et al, 1993; Zheng et al, 1996; Barylko et al, 1998). Dynamin 1 is the best-characterized dynamin isoform and is enriched at synapses.
Amphiphysin, which is present in brain primarily as a dimer of two similar isoforms, amphiphysin 1 and 2, is a major binding partner of dynamin 1 in the vertebrate nervous system. Amphiphysin binds the PRD of dynamin via a C-terminal SH3 domain and co-assembles with dynamin into rings either in solution or on lipid tubules (Takei et al, 1999). This interaction is expected to be physiologically important in nerve terminals, because acute disruption of amphiphysin by peptide microinjection leads to a block of synaptic vesicle endocytosis (Shupliakov et al, 1997). The N-terminal portion of amphiphysin contains a BAR (BIN/amphiphysin/Rvs) domain that mediates homo- and heterodimerization (Wigge et al, 1997; Ramjaun et al, 1999). Via this domain, amphiphysin, like dynamin, binds to and tubulates liposomes (Takei et al, 1999), and can also generate narrow plasma membrane tubules in living cells when overexpressed (Peter et al, 2004).
The structure of the BAR domain of amphiphysin was recently determined by crystallographic analysis and found to represent an evolutionary conserved protein module present in a variety of proteins (Habermann, 2004; Peter et al, 2004). It is represented by a triple helix arranged in an antiparallel dimer to form a crescent-like structure. It was proposed that lipid interactions mediated by the concave portion of this module, in cooperation with an amphipathic N-terminal helix (Farsad et al, 2001), may be responsible for its property to curve the bilayer (Peter et al, 2004). In addition, lipid-binding studies revealed preferential binding to small liposomes, suggesting that the BAR domain may also function as a curvature sensor, thus facilitating the recruitment of amphiphysin to pre-existing high curvature membranes (Peter et al, 2004). Curvature-sensitive properties of proteins may have an important role in orchestrating vesicle formation and fission, with the sequential recruitment and dissociation of cytosolic factor (Bigay et al, 2003). The central region of brain amphiphysin 1 and 2 contains binding sites for clathrin and for the clathrin adopter protein 2 (AP-2) (Ramjaun and McPherson, 1998; Slepnev et al, 2000; Miele et al, 2004). Via this multiplicity of interactions, which are regulated by an intramolecular binding (Farsad et al, 2003), cyclin-dependent kinase 5-dependent phosphorylation (Floyd et al, 2001; Tomizawa et al, 2003) and calcineurin-dependent dephosphorylation (Bauerfeind et al, 1997), amphiphysin is thought to play an important role in coordinating vesicle budding and fission (Farsad et al, 2003). Accordingly, defects in synaptic vesicle recycling were demonstrated in amphiphysin 1 knockout mice (Di Paolo et al, 2002). In these mice, not only expression of amphiphysin 1 is suppressed, but also the level of amphiphysin 2 is severely reduced in brain, probably due to the stabilizing effect of the amphiphysin heterodimer on the turnover of amphiphysin 2.
Although these studies support the importance of the amphiphysin–dynamin interaction in endocytosis, it remains unclear whether amphiphysin is directly implicated together with dynamin in the fission reaction. The reported lack of significant amphiphysin expression in Drosophila neurons (Leventis et al, 2001; Razzaq et al, 2001; Zelhof et al, 2001) and of a major synaptic vesicle recycling defect in amphiphysin 1 knockout mice raise questions on the importance of this partnership. However, other proteins with similar domain structure and enriched at synapses, for example, endophilin and intersectin/pacsin, may functionally replace amphiphysin (Simpson et al, 1999; Modregger et al, 2000; Qualmann and Kelly, 2000; Farsad et al, 2001). In this study, we demonstrate the importance of amphiphysin for the vesicle-generating properties of dynamin 1 within the context of mouse brain cytosol in vitro. We also show a striking stimulatory effect of amphiphysin 1 on the GTPase activity of dynamin in the presence of liposomes, which is dependent on the size of liposomes.
Results
Amphiphysin potentiates vesicle formation by dynamin
Dynamin-dependent vesicle formation can be reconstituted in vitro by incubation of large unilamellar liposomes with brain cytosol in the presence of nucleotides, and monitored quantitatively by dynamic light scattering (DLS) (Kinuta et al, 2002). To establish the importance of amphiphysin in vesicle formation, we compared brain cytosol from wild-type and amphiphysin 1 knockout mice in this assay. The brain cytosol of the knockout mice is also nearly completely devoid of amphiphysin 2. When incubated with large unilamellar liposomes (1779.5±461.7 nm in diameter) (Figure 1A), wild-type cytosol induced a massive formation of small vesicles (112.3±19.7 nm in diameter) (Figure 1B), while knockout mice brain cytosol was very inefficient in this process (Figure 1C). At the end of the incubation, small vesicles represented 82.9±11.7% of the total vesicle number in the wild-type cytosol sample, but only 6.8±5.5% in the knockout cytosol sample (Figure 1B and C). Addition of purified amphiphysin 1 (0.5 μg/ml) to the knockout cytosol rescued vesicle formation, with small vesicles (176.8±33.9 nm in diameter) representing 82.1±4.3% of the total vesicles at the end of the reaction (Figure 1E). No vesicle formation was observed when liposomes were incubated with amphiphysin 1 alone (Figure 1D). Results of these assays are summarized in Figure 1F.
Figure 1.
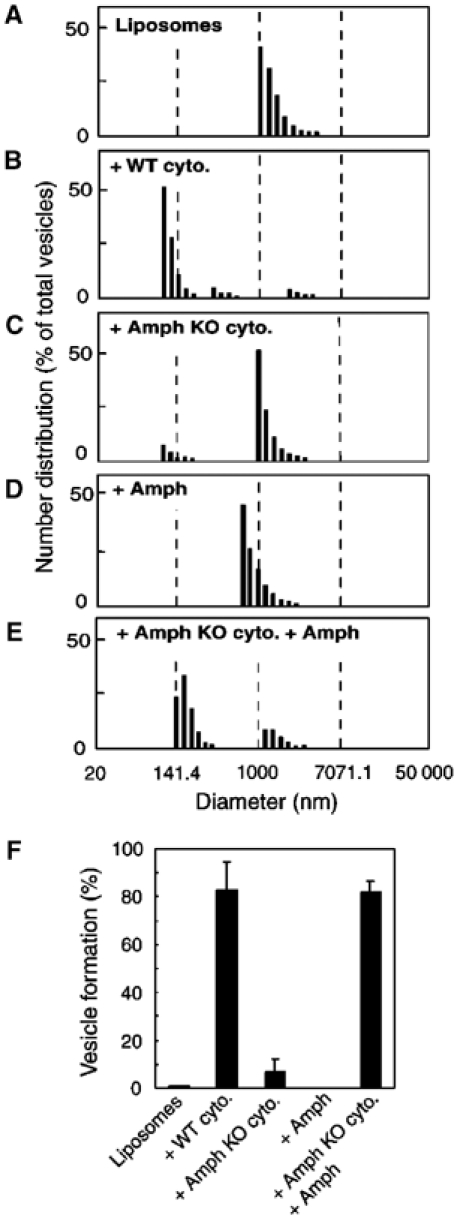
Requirement of amphiphysin 1 for vesicle formation by brain cytosol. (A–E) Vesicle formation quantified by the DLS assay. Data represented are the relative distribution in numbers of formed vesicles. Large unilamellar liposomes were incubated in the presence of 200 μM GTP and 2 mM ATP under the conditions indicated. (F) Percentage of small vesicles formed in each incubation condition. Vesicles smaller than 200 nm in diameter were defined as small vesicles. Values represent the mean±s.d. for more than three independent measurements. Amph=amphiphysin 1; Amph KO cyto.=amphiphysin knockout mice brain cytosol; WT cyto.=wild-type mice brain cytosol.
We next examined the effect of purified amphiphysin 1 on vesicle formation by purified dynamin. Large unilamellar liposomes were incubated with dynamin alone or a mixture of dynamin plus amphiphysin 1 in the presence of GTP. At the end of the reaction, small vesicles represented 15.7±12.7% of the total vesicles (Figure 2B and D) in the sample containing dynamin 1 alone, and 71.1±4.5% of the total in the samples containing both proteins (Figure 2C and D), thus indicating a potent action of amphiphysin 1 on the vesicle-generating properties of dynamin. Massive fragmentation of liposomes into small vesicles following incubations with dynamin and amphiphysin 1 was confirmed by negative staining electron microscopy (EM) (Figure 2E).
Figure 2.
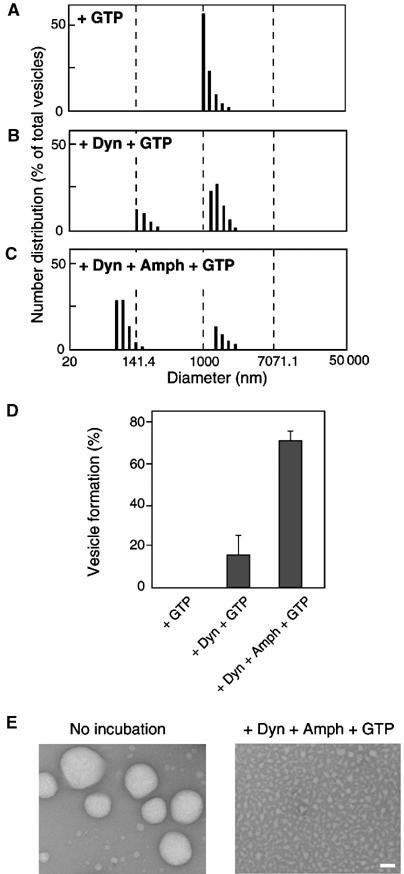
Quantitative analysis of vesicle formation from liposomes by dynamin and amphiphysin 1. (A–C) Vesicle formation by purified proteins was quantified by DLS assay, and the relative number distribution was represented. Large unilamellar liposomes were incubated in the presence of 1 mM GTP under the conditions indicated. The ratio of dynamin (Dyn) to amphiphysin 1 (Amph) was 1:2.67 (mol/mol), a ratio of 1:2 (w/w). (D) Percentage of small vesicles formed in each incubation condition. Values represent the mean±s.d. for more than three measurements. (E) Negative-staining EM of large unilamellar liposomes before incubation (left), and vesicles formed by incubation with dynamin and amphiphysin 1 (right). Bar=100 nm.
Stimulation of dynamin GTPase activity by amphiphysin is affected by liposome size
To gain some insight into the mechanisms through which amphiphysin 1 stimulates dynamin-dependent vesicle formation, we examined whether amphiphysin 1 enhanced dynamin GTPase activity. Addition of increasing amount of amphiphysin 1 to a reaction mixture containing dynamin, [γ-32P]GTP and large unilamellar liposomes (1779.5±461.7 nm in diameter) composed of a brain lipid extract (Folch fraction type 1, FF) (74%, w/w), cholesterol (20%) and PtdIns(4,5)P2 (6%) produced a prominent increase in the GTPase activity. The activity was maximal when the molar ratio of dynamin to amphiphysin 1 was in the 1:1–2 range (Figure 3A). Such effect may be explained in part by enhanced recruitment of dynamin, because amphiphysin also increased the recruitment of dynamin to large liposomes (approximately two-fold) (Figure 3D).
Figure 3.
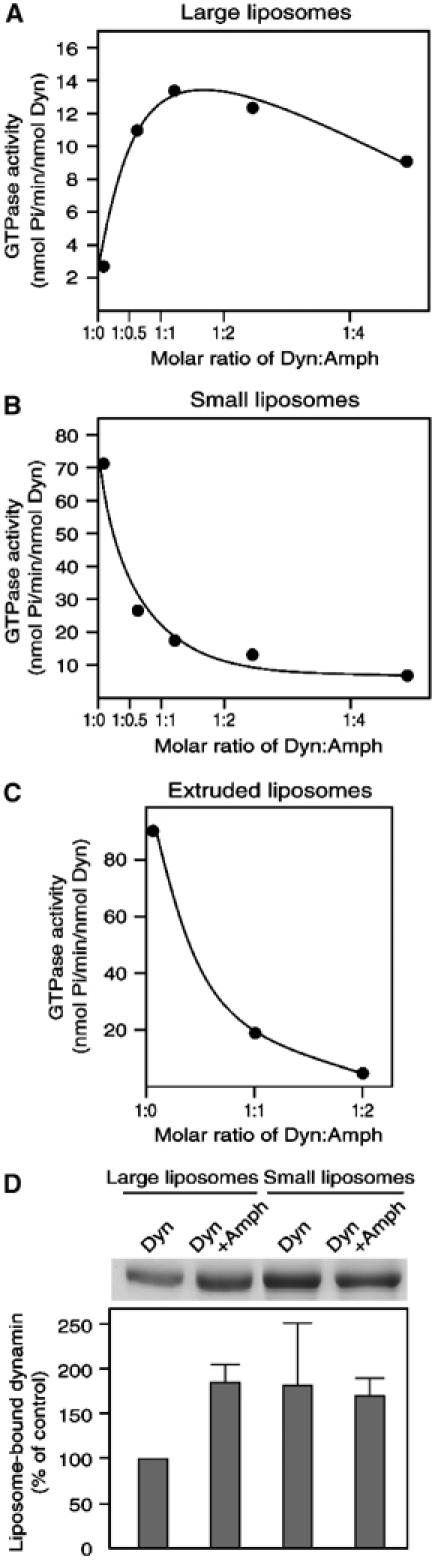
Stimulation of dynamin GTPase activity by amphiphysin 1. Dynamin (0.2 μM) and increasing amount of amphiphysin 1 were incubated either with 2 μg of large unilamellar liposomes (A) or with 2 μg of sonicated small liposomes (B), in the presence of 1 mM GTP containing [γ-32P]GTP (0.8 μCi). After incubation, 32Pi released from the radiolabeled GTP was measured. Data represent the mean value of triplicate measurements. (C) GTPase activity assayed using extruded small liposomes. (D) Recruitment of dynamin to large unilamellar liposomes (left two lanes) or to sonicated small liposomes (right two lanes) in the presence or absence of amphiphysin 1. Results shown are the average of three assays±s.d.
Since small liposomes were often utilized in previous studies on dynamin GTPase activity (Tuma et al, 1993; Barylko et al, 1998), we also tested small liposomes prepared by sonication (80.7±10.8 nm in diameter) in the GTPase assay. In the absence of amphiphysin, the GTPase activity of dynamin was much higher with small liposomes than with large liposomes, possibly due to a more efficient oligomerization of dynamin on small liposomes. Surprisingly, addition of increasing amount of amphiphysin 1 led to a drastic decrease in the GTPase activity in the presence of the small liposomes. The molar ratio that generated maximal stimulation in the presence of large liposomes produced a 75% inhibition under these conditions (Figure 3B). A similar inhibitory effect was observed also with small liposomes prepared by extrusion (143.8±31.7 nm in diameter) (Figure 3C), ruling out a possible effect of sonication on the properties of the bilayer. Thus, the effect of amphiphysin 1 on dynamin GTPase activity is strongly affected by the size of the liposomes.
An attractive possibility is that the stimulation of dynamin GTPase activity is related to the property of amphiphysin and dynamin to co-oligomerize into ring structures around membrane tubules. While large liposomes allow massive tubulation (Takei et al, 1999), the size of the small liposomes may be incompatible with tubulation and constriction, explaining the different results obtained with large and small liposomes.
Stimulatory action of amphiphysin is affected by lipid component
If the effect of amphiphysin on dynamin GTPase activity is linked to its property to co-oligomerize with dynamin on tubular membranes, the effect should be strongly dependent on the presence of liposomes. Therefore, we examined whether amphiphysin stimulates dynamin GTPase activity in the presence or absence of liposomes. In the absence of liposomes, the GTPase activity of dynamin was low, and amphiphysin 1 stimulated this activity only slightly, approximately 1.2-fold (compare lanes 1 and 2 in Figure 4A). The presence of large unilamellar liposomes (FF (74%, w/w), cholesterol (20%) and PtdIns(4,5)P2 (6%)) produced a 2.6-fold increase in dynamin GTPase activity (compare lanes 1 and 3 in Figure 4A), and the additional presence of amphiphysin 1 produced a further 3.1-fold stimulation (compare lanes 3 and 4 in Figure 4A). Replacement of PtdIns(4,5)P2 with L-α-phosphatidylcholine (PC) in the liposomes reduced the stimulation produced by the liposomes (compare lanes 3 and 5 in Figure 4A), consistent with the known role of PtdIns(4,5)P2 in dynamin recruitment and activation. However, the stimulatory effect of amphiphysin 1 on the GTPase activity of dynamin was nearly unaffected (approximately 3.4-fold) (compare lanes 5 and 6 in Figure 4A).
Figure 4.
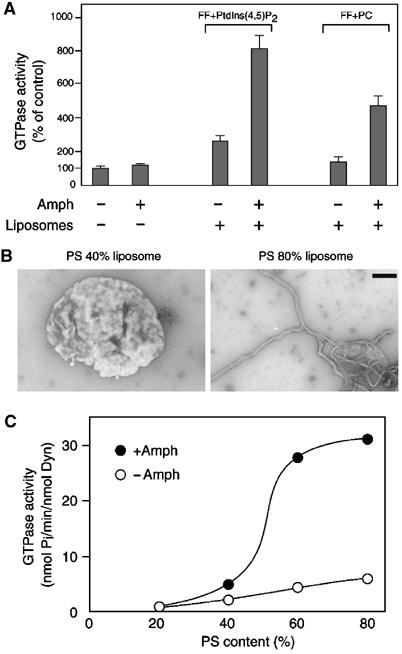
Effect of the lipid composition on stimulation of dynamin GTPase activity by amphiphysin 1. (A) Dynamin GTPase activity measured with or without amphiphysin 1, and in the absence or presence of liposomes. The lipid composition of FF+PtdIns(4,5)P2 and FF+PC liposomes are cholesterol/FF/PtdIns(4,5)P2=20:74:6% (w/w) and cholesterol/FF/PC=20:74:6% (w/w), respectively. Results were normalized to the average of control value (1.06 nmol Pi/min/nmol Dyn). (B) Negative-staining electron micrographs of liposomes incubated with amphiphysin 1. Left, a single liposome containing 40% PS (cholesterol/PS/PC=20:40:40%, w/w) generated irregular surface, but little tubulation was observed. Right, a single liposome containing 80% PS (cholesterol/PS=20:80%, w/w) formed massive tubules. Bar=500 nm. (C) Dynamin GTPase activity measured with (closed circles) or without (open circles) amphiphysin 1, in the presence of liposomes containing various amounts of PS, PC and 20% cholesterol. Data represent the mean value of triplicate measurements.
Another acidic phospholipid was tested. L-α-Phosphatidylserine (PS) was reported to support the binding and polymerization of both dynamin and amphiphysin 1 and to stimulate the GTPase activity of dynamin (Figure 4B, Tuma et al, 1993; Sweitzer and Hinshaw, 1998; Takei et al, 1999). Thus, liposomes containing variable amounts of PS (from 20 to 80%) were used in the tubulation and GTPase assays. Amphiphysin 1 tubulated very efficiently liposomes containing the higher concentrations of PS (Figure 4B, Takei et al, 1999). The GTPase activity of dynamin in the absence of amphiphysin 1 increased linearly in parallel with the concentration of PS (Figure 4C, open circles). The presence of amphiphysin 1 produced an additional powerful stimulation of the GTPase activity, which was highly synergistic as the amount of PS was increased. The sigmoidal shape of the stimulation curve indicated a strong positive cooperativity when the amount of PS was between 40 and 60% (Figure 4C, closed circles), possibly reflecting gathering of PS and preferential assembly of dynamin–amphiphysin polymers on PS-rich microdomains.
Both BAR domain and SH3 domain of amphiphysin 1 are required for the stimulation of dynamin GTPase activity
The effect of truncated amphiphysin 1 molecules (Figure 5A) was investigated to identify the domains of the protein responsible for its stimulatory action on the catalytic activity of dynamin. These fragments were tested in the presence of large liposomes composed of FF (74%), cholesterol (20%) and PtdIns(4,5)P2 (6%). Under this assay condition, full-length amphiphysin 1 stimulated the GTPase activity about three-fold (Figure 5B, see also lanes 3 and 4 in Figure 4A). A roughly similar stimulation was produced by Amph 1–226, which comprises the BAR domain only, and by Amph 1–306, which contains an additional flanking region at the C-terminal side of this domain. Strikingly, Amph Δ248–601, which contains both BAR and SH3 domains, but lacks the central region, produced a 26-fold stimulation of the GTPase activity. As the proline-rich stretch binds intramolecularly to the SH3 domain and regulates negatively the interaction of amphiphysin with dynamin (Farsad et al, 2003), we also tested Amph Δ248–315, a mutant lacking the proline-rich stretch. This mutant stimulated the GTPase activity about three-fold over the value obtained with full-length amphiphysin 1. Amph 545–695 alone, corresponding to the C-terminal SH3 domain, had no effect (Figure 5B). Furthermore, stimulation of the GTPase activity by the BAR domain was not enhanced by the presence of the free SH3 domain (data not shown). These results suggested that presence of both the BAR domain and the SH3 domain in a single molecule was critical for the stimulation of dynamin GTPase activity.
Figure 5.
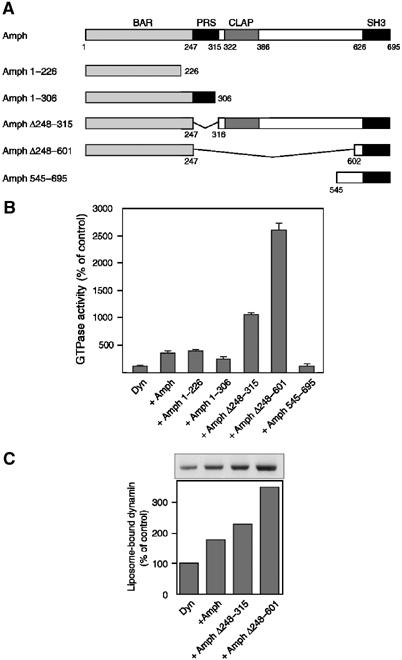
Amphiphysin 1 domains required for the stimulation of dynamin GTPase activity. (A) Domain structure of amphiphysin 1 constructs used for assays. Numbers indicate amino-acid residues of full-length amphiphysin 1. (B) Dynamin GTPase activity in the presence of each construct and of large unilamellar liposomes containing 74% FF, 20% cholesterol and 6% PtdIns(4,5)P2. Dyn:Amph=1:2 (mol/mol). Data represent the mean value of triplicate measurements. Results were normalized to the average of control value (2.86 nmol Pi/min/nmol Dyn). (C) Dynamin binding to large liposomes in the presence of amphiphysin or truncated mutants was checked by the sedimentation assay. BAR=BIN/amphiphysin/Rvs; PRS=proline-rich stretch; CLAP=clathrin-AP-2-binding domain; SH3=src-homology 3.
The observed effects of the amphiphysin deletion constructs on the GTPase activity of dynamin correlated qualitatively with their property to enhance dynamin recruitment to large liposomes, with Amph Δ248–601 producing a more than three-fold increase in recruitment (Figure 5C).
Both BAR domain and SH3 domain of amphiphysin 1 are sufficient for the formation of rings
The incubation of dynamin and amphiphysin 1 in physiological buffer results in the formation of ring structures, similar in size to dynamin-containing collars of endocytic pits (Takei et al, 1999). These collars are thought to represent a transient intermediate in the fission reaction. We used negative staining EM to determine whether these rings were present following incubation of dynamin with the truncated amphiphysins. Rings were observed after incubation of dynamin with full-length amphiphysin 1, with Amph Δ248–315, or with Amph Δ248–601, but not with the other amphiphysin 1 fragment (Figure 6). The diameter of rings with Amph Δ248–601 (41.2±3.0 nm of outer diameter and 22.3±2.6 nm of inner diameter) was slightly shorter than that of rings with amphiphysin 1 (outer diameter 51.7±4.3 nm and inner diameter 27.5±3.3 nm).
Figure 6.
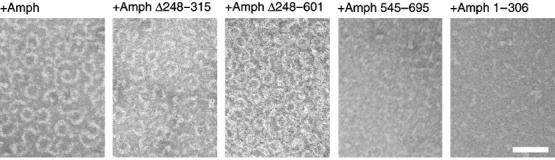
Negative staining EM of reaction mixtures containing dynamin and each construct indicated. Note the massive ring formation with full-length amphiphysin 1, Amph Δ248–315 or with Amph Δ248–601. Bar=100 nm.
Discussion
We provide strong evidence for the physiological role of amphiphysin in the regulation of dynamin function and new insights concerning the mechanisms through which amphiphysin achieves this effect. Several previous reports, including studies in living cells and cell-free studies, had suggested an important partnership of amphiphysin and dynamin in the fission reaction of endocytic vesicles, in particular in the fission of recycling synaptic vesicles at synapses (Shupliakov et al, 1997; Takei et al, 1999). Yet, the importance of this interaction has been challenged by studies in Drosophila, where amphiphysin is expressed primarily outside the nervous system (Leventis et al, 2001; Razzaq et al, 2001; Zelhof et al, 2001), and by the relatively mild neurotransmission defects observed in amphiphysin 1 knockout mice (Di Paolo et al, 2002), which lack both amphiphysin 1 and 2 in the neuronal cytosplasm. In contrast with these findings, we have now found that the property of brain cytosol to vesiculate large liposomes is drastically impaired in the cytosol of amphiphysin 1 knockout brain (Figure 1). Since this property was previously shown to be mediated mainly by dynamin (Takei et al, 1998; Kinuta et al, 2002), our findings point to a role of amphiphysin in the regulation of dynamin. Addition of amphiphysin 1 is sufficient to rescue the defect of the knockout brain cytosol, indicating that in this assay the amphiphysin 1 homodimer and the amphiphysin 1/2 heterodimer have similar properties. The discrepancy between these in vitro results and in vivo data may reflect the compensatory action in vivo of other BAR domain-containing proteins (Peter et al, 2004), such as endophilin and syndapin/pacsin, that have a domain structure similar to amphiphysin (Qualmann and Kelly, 2000; Habermann, 2004; Peter et al, 2004) and are also present at synapses. The importance of amphiphysin relative to other proteins with overlapping function may be greater in in vitro assays than in situ because of differential compartmentalization and/or regulation in living nerve terminals. Furthermore, yet unidentified molecules present in cellular membranes, and absent in our cell-free assays, may compensate for the absence of amphiphysin.
We have found that the property of amphiphysin to stimulate the GTPase activity of dynamin is critically influenced by the presence of liposomes and that this effect, in turn, is strongly affected by the composition and size of liposomes (Figures 3 and 4). The presence of acidic phospholipids is required, as expected. Amphiphysin stimulated the GTPase activity of dynamin and its binding on large unilamellar liposomes. Large liposomes, which can be evaginated to narrow tubules, may allow dynamin and amphiphysin to co-oligomerize into a stack of rings, and the co-oligomers bound to lipids may represent the configuration with the maximal GTPase activity. In contrast, amphiphysin inhibited the GTPase activity, with little change in the dynamin recruitment in the presence of small liposomes. These results suggest that amphiphysin–dynamin rings may not assemble on small liposomes, whose size may prevent the formation of narrow tubular membranes. In this context, amphiphysin may in fact perturb dynamin polymerization. The effect of amphiphysin 1 on the GTPase activity of dynamin was analyzed previously (Wigge et al, 1997; Takei et al, 1999). We have reported that amphiphysin 1 stimulates dynamin GTPase activity in the absence of liposomes, and that in the presence of liposomes addition of amphiphysin 1 did not further stimulate this activity (Takei et al, 1999). A stimulatory effect of amphiphysin 1 and 2 on the catalytic activity of dynamin was also reported by another group (Wigge et al, 1997). As clearly indicated in this study, the activity of dynamin is dependent on the experimental conditions, thus explaining the inconsistency between the results reported here and previous studies.
As for the molecular mechanisms by which amphiphysin stimulates dynamin GTPase activity, some clues are provided by experiments using amphiphysin deletion mutants (Figures 5 and 6). Surprisingly, BAR domain-containing constructs that miss the dynamin-binding domain (the SH3 domain) can stimulate the GTPase activity of dynamin in the presence of liposomes (Figure 5B). Most likely, the powerful liposome-tubulating activity of these constructs enhances the GTPase activity by providing a template for dynamin polymerization. Although dynamin alone can tubulate liposomes, it may assemble more efficiently on previously formed lipid tubules. For example, dynamin has been reported to assemble efficiently on preformed lipid tubules (Stowell et al, 1999) and microtubules (Shpetner and Vallee, 1989). The amphiphysin construct missing the central region, or a portion of it comprising the proline-rich stretch, stimulated dynamin GTPase activity more potently than full-length amphiphysin. This is consistent with the occurrence of an autoinhibitory intramolecular interaction in amphiphysin (Farsad et al, 2003). The SH3 domain alone had no effect on the GTPase activity of dynamin in the presence of liposomes. This indicates that the importance of the SH3 domain relates to its ability to recruit dynamin to the membrane via the BAR domain. Collectively, these results provide new support for the hypothesis that amphiphysin functions together with dynamin in fission (Takei et al, 1999), both by helping the generation of membrane curvature and by facilitating the recruitment and oligomerization of dynamin.
The precise orientation of dynamin and amphiphysin in the rings remains to be elucidated. The amphiphysin–dynamin rings are similar in diameter to the rings formed by dynamin alone. This observation suggests a model in which amphiphysin and dynamin are arranged side by side along the ring. Such arrangement would be consistent with the greater thickness of the hybrid amphiphysin–dynamin ring (Takei et al, 1999). Cryo-EM studies have shown that dynamin rings generated by purified dynamin 1 around tubular liposomes comprise about 13–15 dynamin dimers (Zhang and Hinshaw, 2001; Chen et al, 2004). Considering the curvature of the BAR domain, a ring could not accommodate an equivalent number of crescent-shaped BAR domains (Peter et al, 2004). Thus, BAR domains may be arranged obliquely, with their positively charged edges juxtaposed to the negatively charged middle portion of the adjacent BAR domain (Figure 7).
Figure 7.
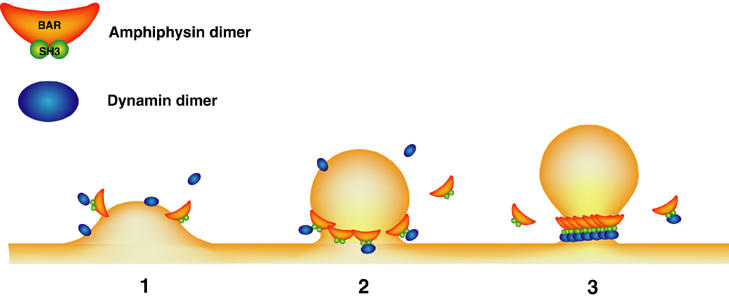
Possible function of amphiphysin in the fission process of endocytic pit. (1) Amphiphysin is recruited to the membrane initiating invagination either via its BAR domain or via clathrin/AP-2-binding domain. (2) The BAR domain may help generating the bud neck of the appropriate dimensions. (3) As the pit has been matured, amphiphysin could be more efficiently recruited to the neck due to its curvature-sensing properties. Dynamin also assembles to form a ring at the bud neck, and its GTPase is fully activated so that vesicle fission is achieved. Coat components are omitted.
Irrespective of the precise stoichiometry of amphiphysin and dynamin around tubular lipid templates, these results add new strong support to a model implicating amphiphysin in the regulation of clathrin-mediated endocytosis (Figure 7). At early stages of clathrin-coated pit formation, the curvature-generating properties of amphiphysin may help maturation of the bud into a deeply invaginated pit. In addition, via a positive feedback mechanism, preference of its BAR domain for a curved membrane with radius in the size range of bud neck may enhance its concentration at the neck as the coated pit forms. This concentration could facilitate dynamin oligomerization into a ring. Dynamin recruitment may be further facilitated by the binding of the central domain of amphiphysin to clathrin and AP-2, which results in the release of the SH3 domain from the autoinhibitory intramolecular interaction (Farsad et al, 2003). In conclusion, our results suggest a model in which narrowing of the stalk of an endocytic pits is coupled to activation of dynamin GTPase activity, thus explaining how the deep invagination of the pit may lead to fission.
Materials and methods
Materials
Bovine brain lipid extract (Folch fraction type I, FF) and PtdIns(4,5)P2 from bovine brain were purchased from Sigma (St Louis, MO). PS and PC were from Avanti Polar Lipids (Alabaster, AL). Glutathione sepharose 4B beads, pGEX-6P vector, PreScission protease and thrombin were from Amersham Bioscience (Piscataway, NJ). [γ-32P]GTP (5 kCi/mol) was from the Institute of Isotopes Co. Ltd (Hungary).
Preparation of brain cytosol and dynamin 1
Brain cytosol from wild-type mice and amphiphysin 1 knockout mice (Di Paolo et al, 2002) was prepared essentially by the previous method and used (Kinuta et al, 2002). Dynamin 1 was purified from bovine brains by the method of Liu et al (1994). The dynamin solution was concentrated using Centriplus YM50 (Millipore, MA), and stored at −80°C. The protein solution (0.6 mg/ml protein) was thawed at 37°C before use.
Preparation of amphiphysin 1 and its truncation constructs
The cDNAs encoding full-length human amphiphysin 1 and its truncation constructs were prepared by PCR amplification using specific primers. Full-length amphiphysin 1, Amph 1–226 and 1–306 were subcloned into pGEX-6P vector as BamHI–EcoRI fragments. To prepare Amph Δ248–315, Amph 1–247aa was subcloned into pGEX-6P vector as a BamHI–EcoRI fragment. EcoRI–EcoRI fragment containing Amph 316–695aa was then inserted into the EcoRI site. As for Amph Δ248–601, BAR domain was subcloned into pGEX-6P vector as a BamHI–NcoI fragment, and inserted at the BamHI–NcoI site of full-length amphiphysin 1 plasmid described above. The SH3 domain was subcloned into pGEX-2T vector. The nucleotide sequences of the constructs were verified using a DNA sequence analyzer. The expression of GST-fusion proteins was induced by 0.1 mM isopropyl-1-thio-β-D-galactopyranoside at 37°C for 3–6 h in LB medium supplemented with 100 μg/ml ampicillin at A600=0.8. The purification of GST-fusion proteins was performed as described previously (Slepnev et al, 2000), and the cleavage of the GST with PreScission protease was carried out according to the manufacturer's instruction. Finally, it was purified on MonoQ column equilibrated in 20 mM Tris–HCl (pH 7.7) and 0.2 M NaCl. The protein solution (1 mg/ml protein) was stored at −80°C, and thawed at 37°C before use.
Preparation of liposomes
Large unilamellar liposomes in 0.3 M sucrose (1 mg/ml) were prepared as described (Takei et al, 2001). The size of large unilamellar liposomes measured using DLS was 1779.5±461.7 nm in diameter. Small liposomes were made from large unilamellar liposomes by sonication (80.7±10.8 nm in diameter). Extruded liposomes were prepared by extrusion of large liposomes through polycarbonate membranes with pores of 100 nm for five times (143.8±31.7 nm in diameter).
Formation of small vesicles from liposomes
Large unilamellar liposomes composed of 80% (w/w) FF and 20% cholesterol in 0.3 M sucrose (1 mg/ml) were prepared as described previously (Takei et al, 1999). Vesicle formation was performed by the method of Kinuta et al (2002), with minor modification. A reaction mixture (500 μl) containing cytosolic buffer (25 mM Hepes–KOH (pH 7.2), 25 mM KCl, 2.5 mM magnecium acetate, 100 mM potassium glutamate), 100 μg of liposomes, brain cytosol from either wild-type or amphiphysin knockout mice (500 μg/ml protein), 2 mM ATP and 200 μM GTP was incubated at 37°C for 15 min. As for vesicle formation by purified proteins, 25 μg dynamin 1 and 50 μg of amphiphysin 1 were made to react. The size and relative numbers in each size of the formed vesicles were measured by DLS assay (Kinuta et al, 2002; Tomizawa et al, 2003). Formed vesicles smaller than 200 nm in diameter were defined as small vesicles.
Liposome sedimentation assay
For liposome sedimentation assay, in vitro incubation was performed under the similar protein lipid concentration as used for GTPase assay. Dynamin (0.2 μM) and amphiphysin (0.4 μM) or amphiphysin truncation construct (0.4 μM) were incubated with 10 μg of liposomes, composed of 74% (w/w) FF, 20% cholesterol and 6% PtdIns(4,5)P2, in the cytosolic buffer at 37°C for 15 min. The reaction mixture was then centrifuged at 20 600 g for 10 min. The proteins in the pellet were analyzed by SDS–PAGE, subsequently checked by Coomassie blue staining. The quantification of binding protein was performed by scanning the gel and analyzing the scanned images with the NIH image program.
Dynamin GTPase assay
GTPase activity was measured under similar condition as DLS assay, with little modification of liposome composition, 74% (w/w) FF, 20% cholesterol and 6% PtdIns(4,5)P2. Other lipid compositions of liposomes are described in figure legends. Small liposomes were prepared by sonication of the large unilamellar liposomes. Assay was performed essentially as described (Barylko et al, 2001). A reaction mixture (100 μl) containing the cytosolic buffer, 0.2 μM dynamin, 2 μg liposomes and either amphiphysin 1 or 0.4 μM truncated amphiphysins was incubated at 37°C for 5 min. Reactions were initiated by the addition of [γ-32P]GTP. After incubation, 32Pi from [γ-32P]GTP was extracted with benzene in the presence of silicotungstic acid and (NH4)6Mo7O24·4H2O, and measured by a liquid scintillation counter using Packard 2260XL (Hewlett Packard, Houston, TX) at the Department of Radiation Research, Shikata Laboratory Advanced Science Research Center Okayama University.
Electron microscopy (EM)
To test the tubulation of large unilamellar liposomes, 1.5 μg of amphiphysin 1 was incubated with 4 μg of liposomes at the same condition. To test the formation of dynamin–amphiphysin ring structures, mixtures of dynamin 1 and either amphiphysin 1 or truncation constructs (molar ratio=1:1.5) in the cytosolic buffer were incubated at 37°C for 15 min. The reaction mixture is adsorbed to carbon-coated EM grids that had been glow-discharged just prior to use, and negatively stained with 2% aqueous uranyl acetate. The grids were observed under a Hitachi H-7100 transmission electron microscope (Japan) at the Central Research Laboratory at Okayama University Medical School.
Acknowledgments
We thank Dr Kazuhito Tomizawa (Okayama University, Japan) for critical technical advice. This work was supported by a grant-in-aid from the Ministry of Education, Science, Sports, and Culture of Japan (to M Kinuta and K Takei), by US–Japan Brain Research Collaborative Program (BRCP) (to K Takei), and by the National Institutes of Health NS 36251 and CA46128 (to P De Camilli).
References
- Barylko B, Binns DD, Albanesi JP (2001) Activation of dynamin GTPase activity by phosphoinositides and SH3 domain-containing proteins. Methods Enzymol 329: 486–496 [DOI] [PubMed] [Google Scholar]
- Barylko B, Binns DD, Lin K-M, Atkinson MAL, Jameson DM, Yin HL, Albanesi JP (1998) Synergistic activation of dynamin GTPase by Grb2 and phosphoinositides. J Biol Chem 273: 3791–3797 [DOI] [PubMed] [Google Scholar]
- Bauerfeind R, Takei K, De Camilli P (1997) Amphiphysin I is associated with coated endocytic intermediates and undergoes stimulation-dependent dephosphorylation in nerve terminals. J Biol Chem 272: 30984–30992 [DOI] [PubMed] [Google Scholar]
- Bigay J, Gounon P, Robineau S, Antonny B (2003) Lipid packing sensed by ArfGAP1 couples COPI coat disassembly to membrane bilayer curvature. Nature 426: 563–566 [DOI] [PubMed] [Google Scholar]
- Chen YJ, Zhang P, Egelman EH, Hinshaw JE (2004) The stalk region of dynamin drives the constriction of dynamin tubes. Nat Struct Mol Biol 11: 574–575 [DOI] [PubMed] [Google Scholar]
- Di Paolo G, Sankaranarayanan S, Wenk MR, Daniell L, Perucco E, Caldarone BJ, Flavell R, Picciotto MR, Ryan TA, Cremona O, De Camilli P (2002) Decreased synaptic vesicle recycling efficiency and cognitive deficits in amphiphysin 1 knockout mice. Neuron 33: 789–804 [DOI] [PubMed] [Google Scholar]
- Farsad K, Ringstad N, Takei K, Floyd SR, Rose K, De Camilli P (2001) Generation of high curvature membranes mediated by direct endophilin bilayer interactions. J Cell Biol 155: 193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsad K, Slepnev V, Ochoa G, Daniell L, Haucke V, De Camilli P (2003) A putative role for intramolecular regulatory mechanisms in the adaptor function of amphiphysin in endocytosis. Neuropharmacology 45: 787–796 [DOI] [PubMed] [Google Scholar]
- Floyd SR, Porro EB, Slepnev VI, Ochoa G-C, Tsai L-H, De Camilli P (2001) Amphiphysin 1 binds the cyclin-dependent kinase (cdk) 5 regulatory subunit p35 and is phosphorylated by cdk5 and cdc2. J Biol Chem 276: 8104–8110 [DOI] [PubMed] [Google Scholar]
- Gout I, Dhand R, Hiles ID, Fry MJ, Panayotou G, Das P, Truong O, Totty NF, Hsuan J, Booker GW, Campbell ID, Waterfield MD (1993) The GTPase dynamin binds to and is activated by a subset of SH3 domains. Cell 75: 25–36 [PubMed] [Google Scholar]
- Habermann B (2004) The BAR-domain family of proteins: a case of bending and binding? EMBO Rep 5: 250–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinuta M, Yamada H, Abe T, Watanabe M, Li S-A, Kamitani A, Yasuda T, Matsukawa T, Kumon H, Takei K (2002) Phosphatidylinositol 4,5-bisphosphate stimulates vesicle formation from liposomes by brain cytosol. Proc Natl Acad Sci USA 99: 2842–2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventis PA, Chow BM, Stewart BA, Iyengar B, Campos AR, Boulianne GL (2001) Drosophila amphiphysin is a post-synaptic protein required for normal locomotion but not endocytosis. Traffic 2: 839–850 [DOI] [PubMed] [Google Scholar]
- Liu J-P, Powell KA, Südhof TC, Robinson PJ (1994) Dynamin I is a Ca2+-sensitive phospholipid-binding protein with very high affinity for protein kinase C. J Biol Chem 269: 21043–21050 [PubMed] [Google Scholar]
- Marks B, Stowell MHB, Vallis Y, Mills IG, Gibson A, Hopkins CR, McMahon HT (2001) GTPase activity of dynamin and resulting conformation change are essential for endocytosis. Nature 410: 231–235 [DOI] [PubMed] [Google Scholar]
- Miele AE, Watson PJ, Evans PR, Traub LM, Owen DJ (2004) Two distinct interaction motifs in amphiphysin bind two independent sites on the clathrin terminal domain β-propeller. Nat Struct Mol Biol 11: 242–248 [DOI] [PubMed] [Google Scholar]
- Modregger J, Ritter B, Witter B, Paulsson M, Plomann M (2000) All three PACSIN isoforms bind to endocytic proteins and inhibit endocytosis. J Cell Sci 113: 4511–4521 [DOI] [PubMed] [Google Scholar]
- Muhlberg AB, Warnock DE, Schmid SL (1997) Domain structure and intramolecular regulation of dynamin GTPase. EMBO J 16: 6676–6683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJG, Evans PR, McMahon HT (2004) BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 303: 495–499 [DOI] [PubMed] [Google Scholar]
- Qualmann B, Kelly RB (2000) Syndapin isoforms participate in receptor-mediated endocytosis and actin organization. J Cell Biol 148: 1047–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramjaun AR, McPherson PS (1998) Multiple amphiphysin II splice variants display differential clathrin binding: identification of two distinct clathrin-binding sites. J Neurochem 70: 2369–2376 [DOI] [PubMed] [Google Scholar]
- Ramjaun AR, Philie J, De Heuvel E, McPherson PS (1999) The N terminus of amphiphysin II mediates dimerization and plasma membrane targeting. J Biol Chem 274: 19785–19791 [DOI] [PubMed] [Google Scholar]
- Razzaq A, Robinson IM, McMahon HT, Skepper JN, Su Y, Zelhof AC, Jackson AP, Gay NJ, O'Kane CJ (2001) Amphiphysin is necessary for organization of the excitation–contraction coupling machinery of muscles, but not for synaptic vesicle endocytosis in Drosophila. Genes Dev 15: 2967–2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sever S, Muhlberg AB, Schmid SL (1999) Impairment of dynamin's GAP domain stimulates receptor-mediated endocytosis. Nature 398: 481–486 [DOI] [PubMed] [Google Scholar]
- Shpetner HS, Vallee RB (1989) Identification of dynamin, a novel mechanochemical enzyme that mediates interactions between microtubules. Cell 59: 421–432 [DOI] [PubMed] [Google Scholar]
- Shupliakov O, Löw P, Grabs D, Gad H, Chen H, David C, Takei K, De Camilli P, Brodin L (1997) Synaptic vesicle endocytosis impaired by disruption of dynamin-SH3 domain interactions. Science 276: 259–263 [DOI] [PubMed] [Google Scholar]
- Simpson F, Hussain NK, Qualmann B, Kelly RB, Kay BK, McPherson PS, Schmid SL (1999) SH3-domain-containing proteins function at distinct steps in clathrin-coated vesicle formation. Nat Cell Biol 1: 119–124 [DOI] [PubMed] [Google Scholar]
- Slepnev VI, De Camilli P (2000) Accessory factors in clathrin-dependent synaptic vesicle endocytosis. Nat Rev Neurosci 1: 161–172 [DOI] [PubMed] [Google Scholar]
- Slepnev VI, Ochoa G-C, Butler MH, De Camilli P (2000) Tandem arrangement of the clathrin and AP-2 binding domains in amphiphysin 1 and disruption of clathrin coat function by amphiphysin fragments comprising these sites. J Biol Chem 275: 17583–17589 [DOI] [PubMed] [Google Scholar]
- Song BD, Schmid SL (2003) A molecular motor or a regulator? Dynamin's in a class of its own. Biochemistry 42: 1369–1376 [DOI] [PubMed] [Google Scholar]
- Stowell MHB, Marks B, Wigge P, McMahon HT (1999) Nucleotide-dependent conformational changes in dynamin: evidence for a mechanochemical molecular spring. Nat Cell Biol 1: 27–32 [DOI] [PubMed] [Google Scholar]
- Sweitzer SM, Hinshaw JE (1998) Dynamin undergoes a GTP-dependent conformational change causing vesiculation. Cell 93: 1021–1029 [DOI] [PubMed] [Google Scholar]
- Takei K, Haucke V (2001) Clathrin-mediated endocytosis: membrane factors pull the trigger. Trends Cell Biol 11: 385–391 [DOI] [PubMed] [Google Scholar]
- Takei K, Haucke V, Slepnev V, Farsad K, Salazar M, Chen H, De Camilli P (1998) Generation of coated intermediates of clathrin-mediated endocytosis on protein-free liposomes. Cell 94: 131–141 [DOI] [PubMed] [Google Scholar]
- Takei K, McPherson PS, Schmid SL, De Camilli P (1995) Tubular membrane invaginations coated by dynamin rings are induced by GTP-gamma S in nerve terminals. Nature 374: 186–190 [DOI] [PubMed] [Google Scholar]
- Takei K, Slepnev VI, De Camilli P (2001) Interactions of dynamin and amphiphysin with liposomes. Methods Enzymol 329: 478–486 [DOI] [PubMed] [Google Scholar]
- Takei K, Slepnev VI, Haucke V, De Camilli P (1999) Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nat Cell Biol 1: 33–39 [DOI] [PubMed] [Google Scholar]
- Tomizawa K, Sunada S, Lu Y-F, Oda Y, Kinuta M, Ohshima T, Saito T, Wei F-Y, Matsushita M, Li S-T, Tsutsui K, Hisanaga S, Mikoshiba K, Takei K, Matsui H (2003) Cophosphorylation of amphiphysin I and dynamin I by Cdk5 regulates clathrin-mediated endocytosis of synaptic vesicles. J Cell Biol 163: 813–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuma PL, Stachniak MC, Collins CA (1993) Activation of dynamin GTPase by acidic phospholipids and endogenous rat brain vesicles. J Biol Chem 268: 17240–17246 [PubMed] [Google Scholar]
- Wigge P, Köhler K, Vallis Y, Doyle CA, Owen D, Hunt SP, McMahon HT (1997) Amphiphysin heterodimers: potential role in clathrin-mediated endocytosis. Mol Biol Cell 8: 2003–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelhof AC, Bao H, Hardy RW, Razzaq A, Zhang B, Doe CQ (2001) Drosophila Amphiphysin is implicated in protein localization and membrane morphogenesis but not in synaptic vesicle endocytosis. Development 128: 5005–5015 [DOI] [PubMed] [Google Scholar]
- Zhang P, Hinshaw JE (2001) Three-dimensional reconstruction of dynamin in the constricted state. Nat Cell Biol 3: 922–926 [DOI] [PubMed] [Google Scholar]
- Zheng J, Cahill SM, Lemmon MA, Fushman D, Schlessinger J, Cowburn D (1996) Identification of the binding site for acidic phospholipids on the PH domain of dynamin: implications for stimulation of GTPase activity. J Mol Biol 255: 14–21 [DOI] [PubMed] [Google Scholar]


