Abstract
The structure of coagulation factor XII (Hageman factor), inferred from its DNA sequence, includes two epidermal growth factor (EGF)-homologous domains in its amino-terminal region. This suggests that factor XII may exhibit EGF-like activities. Reciprocal antigenic cross-reactivity between factor XII and EGF was shown by exposing purified human factor XII or mouse EGF to anti-mouse EGF or anti-human factor XII. Western blot analysis showed that anti-mouse EGF recognized intact factor XII at 80 kDa. Together, these results suggest that the EGF-homologous domains are accessible for anti-EGF binding in native factor XII. To determine whether factor XII has mitogenic activity, HepG2 or L cells (10(4) cells per well) were grown in serum-free medium in the presence or absence of factor XII or kaolin-activated factor XII (factor XIIa). Both factors XII and XIIa (6.0 micrograms/ml) enhanced cell proliferation by approximately 2-fold (P less than 0.001 and P less than 0.005, respectively). In contrast, L cells, which are not EGF target cells, were not affected by either factor XII or factor XIIa. Various doses of factor XII enhanced cell proliferation, [3H]thymidine incorporation, and [3H]leucine incorporation in HepG2 cells cultured under the same conditions. These data indicate that factor XII, like EGF, is a mitogen for HepG2 cells and suggest a possible autocrine role in the liver.
Full text
PDF

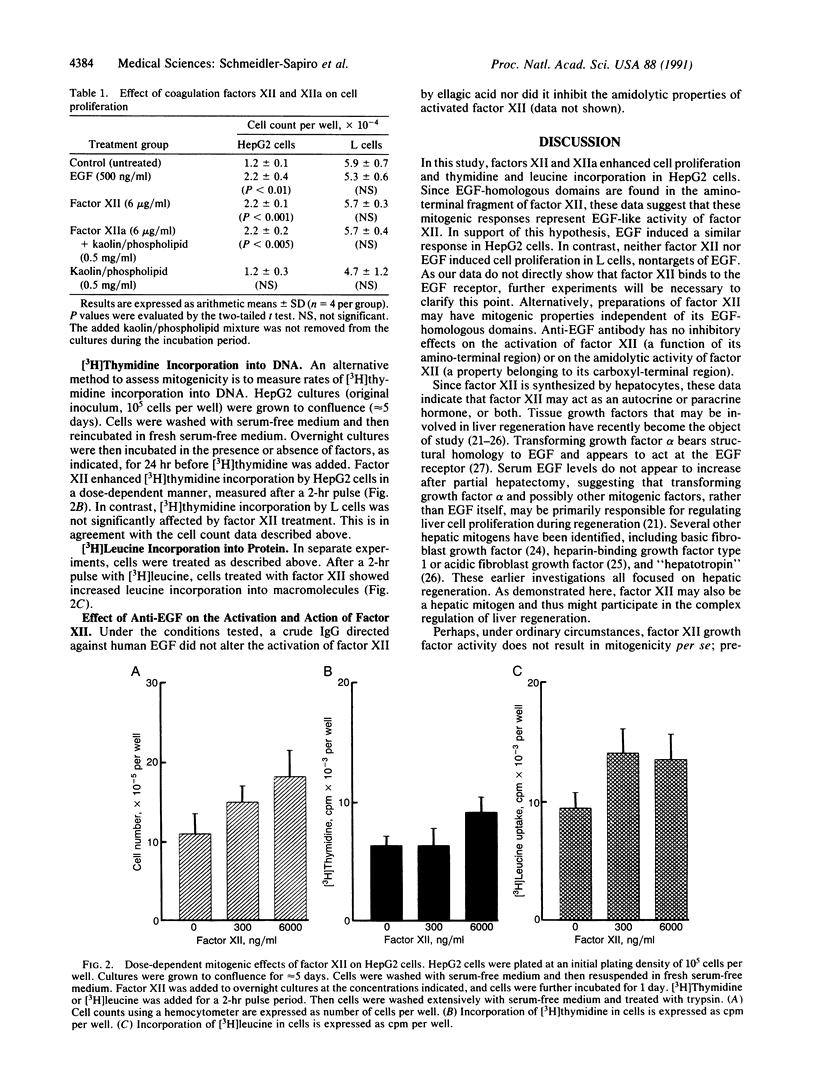

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun L., Mead J. E., Panzica M., Mikumo R., Bell G. I., Fausto N. Transforming growth factor beta mRNA increases during liver regeneration: a possible paracrine mechanism of growth regulation. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1539–1543. doi: 10.1073/pnas.85.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G. Receptors for epidermal growth factor and other polypeptide mitogens. Annu Rev Biochem. 1987;56:881–914. doi: 10.1146/annurev.bi.56.070187.004313. [DOI] [PubMed] [Google Scholar]
- Cohen S. Epidermal growth factor. In Vitro Cell Dev Biol. 1987 Apr;23(4):239–246. doi: 10.1007/BF02623704. [DOI] [PubMed] [Google Scholar]
- Cool D. E., Edgell C. J., Louie G. V., Zoller M. J., Brayer G. D., MacGillivray R. T. Characterization of human blood coagulation factor XII cDNA. Prediction of the primary structure of factor XII and the tertiary structure of beta-factor XIIa. J Biol Chem. 1985 Nov 5;260(25):13666–13676. [PubMed] [Google Scholar]
- Darlington G. J., Kelly J. H., Buffone G. J. Growth and hepatospecific gene expression of human hepatoma cells in a defined medium. In Vitro Cell Dev Biol. 1987 May;23(5):349–354. doi: 10.1007/BF02620991. [DOI] [PubMed] [Google Scholar]
- Fujikawa K., Davie E. W. Human factor XII (Hageman factor). Methods Enzymol. 1981;80(Pt 100):198–211. doi: 10.1016/s0076-6879(81)80018-3. [DOI] [PubMed] [Google Scholar]
- Fujikawa K., McMullen B. A. Amino acid sequence of human beta-factor XIIa. J Biol Chem. 1983 Sep 25;258(18):10924–10933. [PubMed] [Google Scholar]
- Gordon E. M., Douglas J. G., Ratnoff O. D., Arafah B. M. The influence of estrogen and prolactin on Hageman factor (factor XII) titer in ovariectomized and hypophysectomized rats. Blood. 1985 Sep;66(3):602–605. [PubMed] [Google Scholar]
- Gordon E. M., Douglas J., Ratnoff O. D. Influence of augmented Hageman factor (Factor XII) titers on the cryoactivation of plasma prorenin in women using oral contraceptive agents. J Clin Invest. 1983 Nov;72(5):1833–1838. doi: 10.1172/JCI111143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E. M., Gallagher C. A., Johnson T. R., Blossey B. K., Ilan J. Hepatocytes express blood coagulation factor XII (Hageman factor). J Lab Clin Med. 1990 Apr;115(4):463–469. [PubMed] [Google Scholar]
- Gordon E. M., Hellerstein H. K., Ratnoff O. D., Arafah B. M., Yamashita T. S. Augmented Hageman factor and prolactin titers, enhanced cold activation of factor VII, and spontaneous shortening of prothrombin time in survivors of myocardial infarction. J Lab Clin Med. 1987 Apr;109(4):409–413. [PubMed] [Google Scholar]
- Gordon E. M., Ratnoff O. D., Jones P. K. The role of augmented Hageman factor (factor XII) titers in the cold-promoted activation of factor VII and spontaneous shortening of the prothrombin time in women using oral contraceptives. J Lab Clin Med. 1982 Mar;99(3):363–369. [PubMed] [Google Scholar]
- Hoffmann B., Paul D. Basic fibroblast growth factor and transforming growth factor-alpha are hepatotrophic mitogens in vitro. J Cell Physiol. 1990 Jan;142(1):149–154. doi: 10.1002/jcp.1041420118. [DOI] [PubMed] [Google Scholar]
- Kan M., Huang J. S., Mansson P. E., Yasumitsu H., Carr B., McKeehan W. L. Heparin-binding growth factor type 1 (acidic fibroblast growth factor): a potential biphasic autocrine and paracrine regulator of hepatocyte regeneration. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7432–7436. doi: 10.1073/pnas.86.19.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt H., Hunkapiller M. W., Hood L. E., Todaro G. J. Rat transforming growth factor type 1: structure and relation to epidermal growth factor. Science. 1984 Mar 9;223(4640):1079–1082. doi: 10.1126/science.6320373. [DOI] [PubMed] [Google Scholar]
- McMullen B. A., Fujikawa K. Amino acid sequence of the heavy chain of human alpha-factor XIIa (activated Hageman factor). J Biol Chem. 1985 May 10;260(9):5328–5341. [PubMed] [Google Scholar]
- Mead J. E., Fausto N. Transforming growth factor alpha may be a physiological regulator of liver regeneration by means of an autocrine mechanism. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1558–1562. doi: 10.1073/pnas.86.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos G. K. Liver regeneration: molecular mechanisms of growth control. FASEB J. 1990 Feb 1;4(2):176–187. [PubMed] [Google Scholar]
- Que B. G., Davie E. W. Characterization of a cDNA coding for human factor XII (Hageman factor). Biochemistry. 1986 Apr 8;25(7):1525–1528. doi: 10.1021/bi00355a009. [DOI] [PubMed] [Google Scholar]
- Ratnoff O. D. Studies on the product of the reaction between activated Hageman factor (factor XII) and plasma thromboplastin antecedent (factor XI). J Lab Clin Med. 1972 Nov;80(5):704–710. [PubMed] [Google Scholar]
- Ratnoff O. D. The relative amidolytic activity of Hageman factor (factor XII) and its fragments. The effect of high-molecular-weight kininogen and kaolin. J Lab Clin Med. 1980 Aug;96(2):267–277. [PubMed] [Google Scholar]
- SANFORD K. K., EARLE W. R., EVANS V. J., WALTZ H. K., SHANNON J. E. The measurement of proliferation in tissue cultures by enumeration of cell nuclei. J Natl Cancer Inst. 1951 Feb;11(4):773–795. [PubMed] [Google Scholar]
- Saito H., Ratnoff O. D., Pensky J. Radioimmunoassay of human Hageman factor (factor XII). J Lab Clin Med. 1976 Sep;88(3):506–514. [PubMed] [Google Scholar]
- Selden C., Hodgson H. J. Further characterisation of 'hepatotropin', a high molecular weight hepatotrophic factor in rat serum. J Hepatol. 1989 Sep;9(2):167–176. doi: 10.1016/0168-8278(89)90047-0. [DOI] [PubMed] [Google Scholar]
- Steinbuch M., Audran R. The isolation of IgG from mammalian sera with the aid of caprylic acid. Arch Biochem Biophys. 1969 Nov;134(2):279–284. doi: 10.1016/0003-9861(69)90285-9. [DOI] [PubMed] [Google Scholar]




