Distinct myeloid cells play a role in regulating the death vs. the survival of Th17 cells during HIV infection.
Keywords: Th17, Th1, HIV envelope
Abstract
HIV infection leads to CD4 helper T cell (Th) loss, but not all Th cells are equally depleted. The contribution of other immune cells in the Th depletion also remains unclear. This study investigates HIV transmission from monocyte-derived dendritic cells (MDDCs) vs. monocytes to Th17 and Th1 cells using an allogeneic coculture model. The addition of HIV to MDDCs increased the expression of the negative regulatory molecule PD-L1 and decreased the expression of the activation markers HLA-DR and CD86, whereas the virus up-regulated HLA-DR and CD86, but not PD-L1, on monocytes. Coculturing of CD4+ T cells with MDDCs pretreated with HIV led to the decline of Th17, but not Th1, responses. In contrast, pretreatment of monocytes with HIV increased Th17 without affecting Th1 responses. The enhanced Th17 responses in the cocultures with HIV-treated monocytes were also accompanied by high numbers of virus-infected CD4+ T cells. The Th17 expansion arose from memory CD4+ T cells with minimal contribution from naïve CD4+ T cells. The Th17-enhancing activity was mediated by the HIV envelope and did not require productive virus infection. Comparison of MDDCs and monocytes further showed that, although HIV-treated MDDCs reduced Th proliferation and increased the activation of the apoptosis mediator caspase-3, HIV-treated monocytes enhanced Th proliferation without increasing the active caspase-3 levels. This study indicates the potential role of distinct myeloid cell populations in shaping Th17 responses during HIV infection.
Introduction
HIV infection causes severe destruction of various immune cells that leads to AIDS-associated opportunistic infections and malignancies. CD4+ T cells are the main cell type targeted by HIV and the most profoundly affected. However, not all CD4+ T cells are equally affected. Memory CD4+ T cells are more permissive to HIV than are naïve CD4+ T cells [1–4]. Among memory CD4+ T cells, central memory cells, transitional memory cells, and memory stem cells have been reported to harbor significant proportions of HIV proviral DNA [5–9]. Aside from the differential infection of CD4+ T cells at distinct maturation/differentiation stages, the functional polarization of CD4+ T cells also influences susceptibility to HIV and HIV-mediated depletion. In addition to long-studied Th1 and Th2 subsets [10], many other functional Th subsets have been reported, including Th17, Th9, Th22, and Treg cells [11–14]. The Th1 subset is characterized by IFN-γ synthesis and expression of the T bet transcription factor. Many of the Th1 cells secrete the CCR5 ligands MIP-1α, MIP-1β, and RANTES. These chemokines are potent inhibitors of R5-tropic HIV, and their autocrine production renders the Th1 cells more resistant to the virus [15–18]. The Th2 subset expresses the GATA-3 transcription factor and synthesizes cytokines, such as IL-4 and IL-13, important for B cell responses and immune defense against extracellular bacteria and parasites. The Th2 subset defined by the CCR4+ CCR6− phenotype expresses more CXCR4 than CCR5 and, thus, is relatively resistant to most HIV isolates that are R5-tropic [19–22].
Compared with Th1 and other Th subsets, Th17 cells are more susceptible to HIV and are severely depleted during infection [15, 23–26]. Th17 cells secrete the defining cytokine IL-17, serve as an important mediator of inflammation caused by bacterial and fungal pathogens, and are enriched in the gut-associated lymphoid and mucosal tissues [27, 28]. Th17 cells in the blood of HIV-infected patients have been found to harbor greater proportions of proviral DNA and HIV Gag-expressing cells as compared with the other Th subsets [20, 29, 30], although increased IL-17 production by Th and CD4− T cells has been reported in patients with HIV [31]. Th17 cells are also more permissive to HIV infection in vitro [15, 20, 23, 29]. The greater susceptibility of Th17 cells is associated with the higher expression of HIV receptors CD4, CCR5, CXCR4, and α4β7 and the lack of anti-HIV CC-chemokine production by these cells [15]. Lower expression of HIV-inhibitory RNases also renders Th17 cells more permissive to HIV infection [32]. Because of the critical role of IL-17 in maintaining epithelial tight junctions in gut mucosal tissues [33], the Th17 loss is thought to cause destruction of the gut mucosal barrier and increase microbial translocation that leads to HIV-associated immune hyperactivation [34–37]. Interestingly, the Th17 loss is more prominent in the gut specimens than in the peripheral blood or bronchoalveolar lavage from HIV-infected subjects [15]. Th17 cells are also depleted in the intestinal mucosal of macaques infected with pathogenic SIV [38, 39], and restoration of Th17 cells by administration of IL-21 alone or with probiotic therapy to the SIV-infected monkeys has been reported to alleviate immune hyperactivation and systemic bacterial translocation associated with disease progression [40, 41]. Nonetheless, the reasons for Th17 susceptibility to HIV-mediated depletion are not fully understood. A better understanding about the mechanisms and factors contributing to Th17 depletion and preservation is essential to protect this important but highly vulnerable Th subset from HIV.
In our earlier studies [15], we used an in vitro system to evaluate the effects of HIV infection on Th17 and Th1 cells within the same cultures and recapitulated the ex vivo observation of a greater loss of Th17 cells over Th1 cells during HIV infection. These cultures were established using peripheral CD4+ T cells that were stimulated with anti-CD3 and anti-CD28 Abs under the influence of Th17 differentiation cytokines IL-1β and IL-23. In the present study, we evaluated the contribution of allogeneic MDDCs vs. monocytes as APCs in stimulating Th17 and Th1 responses. Further, these APCs were treated with HIV and cocultured with CD4+ T cells to evaluate the effects of HIV transmission from MDDCs or monocytes to Th17 and Th1 cells within the same cultures. The data demonstrated the distinct effects of HIV transmission from MDDCs vs. monocytes on Th17 cells. Virus transmission from MDDCs led to Th17 decline, whereas infection from monocytes enhanced Th17 responses, even in the face of robust virus replication. These differential activities were associated with reduced Th proliferation and increased caspase-3 activation induced by MDDCs pretreated with HIV but not by HIV-pretreated monocytes. The data implicate the distinct roles of monocyte populations in regulating the Th17 responses during HIV infection.
MATERIALS AND METHODS
Cell isolation and culture
Human PBMCs were isolated from leukopaks from healthy donors by centrifugation through the Lymphocyte Separation Medium (Mediatech/Corning, Manassas, VA, USA). CD14+ monocytes were isolated from PBMCs by positive selection with anti-CD14-coated magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany). This protocol yielded a greater level of purity (99%) than did negative selection, and the cells displayed similar activity to those isolated by negative selection (Supplemental Fig. 1). MDDCs were generated by culturing CD14+ monocytes with GM-CSF (10 ng/ml; eBioscience, San Diego, CA, USA) and IL-4 (10 ng/ml; R&D Systems, Minneapolis, MN, USA) for 7 d. The surface phenotypes of the MDDCs and monocytes were assessed by Ab staining to CD14, HLA-DR, DC-SIGN, CD1a, CD1c, CD83, and CD86 (Supplemental Fig. 2A). To compare their APC functions, the MDDCs and monocytes were also tested for the ability to stimulate allogeneic T cell proliferation and elicit Th1 and Th17 responses to bacterial PGN (Supplemental Fig. 2B and C). Total CD4+ T cells were isolated from PBMCs using an EasySep Human CD4+ T Cell Enrichment Kit (StemCell Technologies, Vancouver, BC, Canada). Memory CCR6+ T cells were then isolated by sequential enrichment with a MagniSort Human CD4 Memory T cell Enrichment Kit (eBioscience) and a CCR6+ T cell isolation kit (StemCell Technologies). Naïve CD4+ T cells were enriched from total CD4+ T cells by a MagniSort Human CD4 Naïve T Cell Enrichment Kit (eBioscience).
Monocytes and MDDCs were treated with HIV or mock-treated and then cocultured with allogeneic CD4+ T cells at an APC/T cell ratio of 1:5 for the initial 3 d in RPMI 1640 medium (Lonza, Walkersville, MD, USA) containing penicillin, streptomycin (Lonza), and l-glutamine (Lonza) without serum or exogenous cytokines. The culture medium was changed on d 3 to complete RPMI 1640 containing 10% (vol/vol) FBS (HyClone; GE Healthcare, Little Chalfont, United Kingdom) and 20 U/ml of IL-2 (Roche Diagnostics, Indianapolis, IN, USA), and the cultures were maintained for an additional 10 d. On the designated days, cells expressing CD3, IL-17A, IFN-γ, and HIV Gag p24 were determined by Ab staining after 5 h stimulation with PMA and ionomycin, as previously described [15]. The frequencies of cells expressing IL-17A, IFN-γ, and HIV Gag p24 were determined from the gated CD3+ cells after removing dead cells stained by LIVE/DEAD Fixable Aqua Dead Cell Stain Kit for 405 nm excitation (Thermo Fisher Scientific, Waltham, MA, USA). Allophycocyanin-Cy7–labeled anti-human CD3 was purchased from eBioscience. FITC-labeled anti-human IFN-γ and allophycocyanin-labeled anti-human IL-17A were purchased from BD Biosciences (Franklin Lakes, NJ, USA). Anti-HIV Gag p24 (KC57) was purchased from Beckman Coulter, Inc. (Fullerton, CA, USA).
Virus stock preparation and virus treatment
HIV-1 SF162, JRFL, or MN strains were produced in PHA-stimulated PBMCs. Virus infection was done after the cells were washed to remove excess PHA. Supernatants from infected cultures were then harvested at d 3, 6, and 10, filtered through a 0.45-µm filter, aliquoted, and stored at −80°C. REJO was produced by transfecting 293T/17 cells with the full-length molecular clone, pREJO.c/2864, using jetPEI transfection reagent (Polyplus-transfection SA, Illkirch-Graffenstaden, France), and the supernatant was harvested 48 h later. Infectivity of each virus stock was determined in the MT-4 TMZ R5 cell line [42]. To evaluate the effect of HIV-treated MDDCs vs. monocytes on Th1 and Th17 cells, MDDCs or monocytes (1 × 105/well) were incubated with HIV (MOI of 0.5) at 37°C for 2 h, washed to remove cell-free virus, and cocultured with CD4+ T cells (5 × 105/well) in 96-well, U-bottomed culture plates, as described above. To distinguish the effect of residual PHA that might be present in the virus stock from that of HIV, monocytes treated with supernatants of PHA blasts containing SF162 or no virus were cultured with CD4+ T cells and tested for the ability to stimulate IL-17 and IFN-γ responses (Supplemental Fig 3). For comparison, CD4+ T cells (5 × 105/well) were also infected with cell-free virus at 37°C for 2 h and then cocultured with untreated monocytes. For some experiments, recombinant HIV envelope gp120 proteins (produced in 293 mammalian cells, from Immune Technology Corp., New York, NY, USA), anti-CD4 Ab (eBioscience), and recombinant IL-16 (R&D Systems) were used to treat the monocytes before coculturing with CD4+ T cells.
Cytokine secretion and surface marker expression by MDDCs and monocytes
MDDCs and monocytes that had been pretreated with HIV or left untreated were cocultured with CD4+ T cells for 3 d in Costar Ultra-Low attachment round-bottom 96-well plates (Corning, Corning, NY, USA). Cell-free supernatants were harvested on d 3 and assessed for IL-23, IL-1β, and IL-6 with ELISA or Luminex kits (Thermo Fisher Scientific). Cells were then stained with eVolve 605 (eBioscience)–labeled anti-HLA-DR Ab (BD Biosciences), PE-labeled anti-CD86 Ab (BioLegend, San Diego, CA, USA), allophycocyanin-labeled anti-PD-L1 (eBioscience), and allophycocyanin-Cy7 labeled anti-CD3 (eBioscience). The expression levels of HLA-DR, CD86, and PD-L1 were measured on MDDCs and monocytes after removing the CD3+ cells and the LIVE/DEAD Aqua+ dead cells.
T cell proliferation and caspase-3 activation
CD4+ T cells were labeled with CFSE using the CellTrace CFSE Cell Proliferation Kit (Thermo Fisher Scientific) and cocultured with HIV-treated or untreated MDDCs and monocytes. On d 13, the cells were treated with the CaspGLOW Fluorescein Active Caspase-3 Staining Kit (eBioscience) and then stained with allophycocyanin-Cy7–labeled anti-human CD3 and LIVE/DEAD Aqua. T cell proliferation and caspase-3 activation were analyzed in the gated CD3+ cells after removing the LIVE/DEAD Aqua+ dead cells.
Statistical analysis
The significance of the data was evaluated using the unpaired or paired 2-tailed t test using a GraphPad Prism software version 6.0 or 7.0 (GraphPad, La Jolla, CA, USA).
RESULTS
Monocytes stimulate Th17 responses better than MDDCs
Previous studies have shown the differential capacity of APCs, such as monocytes, conventional DCs, and MDDCs, to induce Th17 priming upon activation with various TLR ligands [43]. To assess the ability of the different APCs to induce Th17 and Th1 responses in the context of HIV, we established a coculture system in which allogeneic monocytes or DCs derived from monocytes after treatment with GM-CSF and IL-4 (MDDCs) were used to stimulate Th1 and Th17 responses in unfractionated CD4+ T cells from the peripheral blood of healthy donors. Before use in the cocultures, the MDDCs and monocytes were evaluated for surface expression of CD14, HLA-DR, DC-SIGN, CD1a, CD1c, CD83, and CD86 (Supplemental Fig. 1A). The CD14 expression was down-regulated on the MDDCs, whereas the expression levels of the other markers were up-regulated, consistent with the typical MDDC phenotypes reported previously [44, 45]. Similar to past findings [46], the MDDCs were also more potent APCs than were monocytes for stimulating allogeneic T cell proliferation. Moreover, in a short-term, 1-d culture, the MDDCs displayed the ability to elicit more robust Th1 and Th17 responses to Staphylococcus aureus PGN (Supplemental Fig. 1B and C).
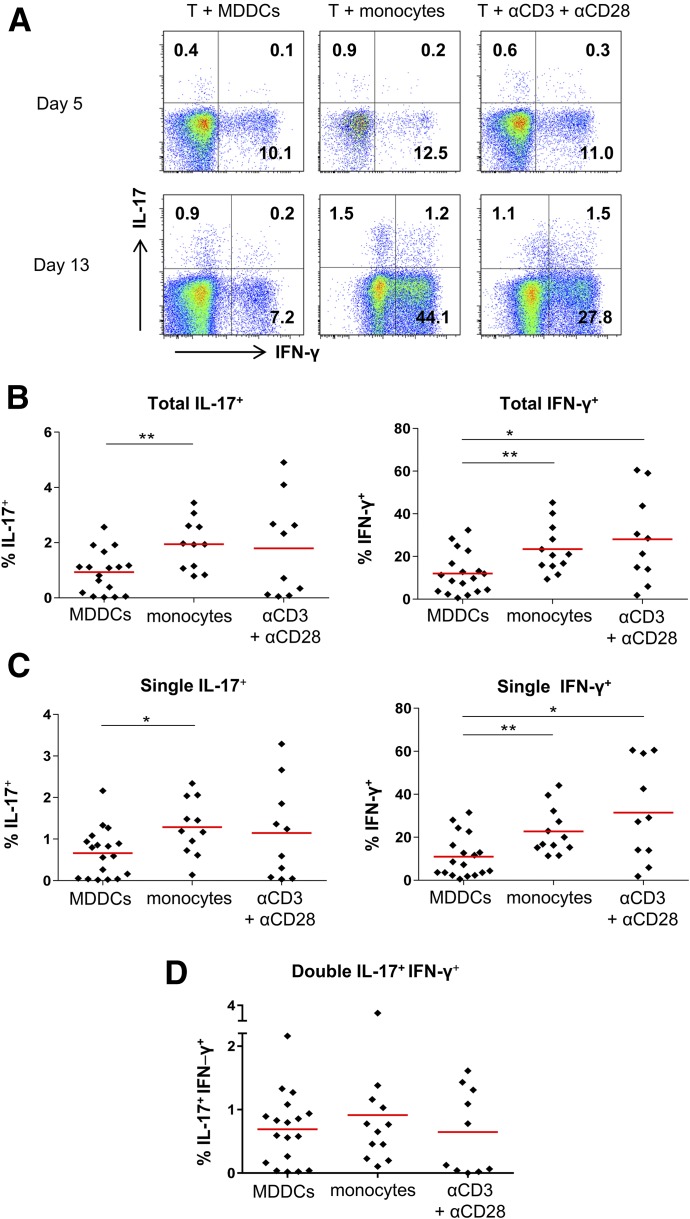
The 2 different APCs were then compared for the ability to induce Th17 and Th1 responses in the allogenic cultures from different donors (n = 10–17) in the absence of HIV. CD4+ T cells were cultured with monocytes or MDDCs in RPMI 1640 medium without serum and cytokines for 3 d to assess the capacity of these APCs to induce Th17 and Th1 responses without exogenous stimuli. CD4+ T cells were then expanded in RPMI 1640 medium with 10% FBS and IL-2 for an additional 10 d. As a positive control, CD4+ T cells were stimulated with a combination of anti-CD3 and anti-CD28 Abs. On d 5 and 13, the frequencies of Th17 and Th1 cells in the cultures were determined by intracellular staining of IL-17 and IFN-γ (Fig. 1).
Figure 1. IL-17 and IFN-γ responses in CD4+ T cell cultures with different stimuli.
Purified CD4+ T cells were cultured with allogeneic monocytes or MDDCs at a T cell/APC ratio of 5:1 or stimulated with a combination of anti-CD3 (2 µg/ml; eBioscience) and anti-CD28 (2 µg/ml; eBioscience) Abs. At d 5 and 13, these CD4+ T cells were stimulated with PMA and ionomycin, followed by intracellular staining with anti-IL-17 and IFN-γ Abs. The frequencies of IL-17+ cells and IFN-γ+ cells were determined by flow cytometry. (A) Dot plots from one representative subject showing IL-17 and IFN-γ expression in the CD4+ T cells. (B–D) Cumulative data showing the percentages of total IL-17+ (B, left panel) and IFN-γ+ (B, right panel), single-positive IL-17+ (C, left panel), single-positive IFN-γ+ (C, right panel), and double-positive IL-17+IFN-γ+ (D) cells out of CD4+ T cells in the cultures from different donors. The red bars represent means. P values were calculated using the unpaired t test. *P < 0.05, **P < 0.01.
On d 5, relatively low frequencies of IL-17+CD4+ T cells and IFN-γ+CD4+ T cells were detected in all cultures (Fig. 1A). On d 13, the frequencies of IL-17+ and IFN-γ+ cells were increased and a differential pattern became evident, notwithstanding the individual donor variability (Fig. 1A and B). Higher frequencies of IL-17+CD4+ T cells were present in the cocultures with monocytes than those with MDDCs. Indeed, IL-17+CD4+ T cells were detected in each of the monocyte cocultures from all donors tested, and the mean frequency was as high as that in the cultures stimulated with anti-CD3 and anti-CD28 Abs. The numbers of IFN-γ+CD4+ T cells in the monocyte cocultures were also higher than they were in the MDDC cocultures and were comparable to those in the anti-CD3– and anti-CD28–stimulated cultures. The differential effects of MDDCs vs. monocytes were evident on single-positive IL-17+IFN-γ− and IL-17−IFN-γ+ cells (Fig. 1C). The double-positive IL-17+IFN-γ+ cells were also detected, albeit at lower numbers than the single-positive populations, and their frequencies were comparable for the 3 culture conditions (Fig. 1D). Hence, in agreement with past studies [43], under the allogenic conditions tested here, and in the absence of additional stimuli, monocytes were better stimulators of IL-17+ Th17 and IFN-γ+ Th1 cells than MDDCs.
HIV induces differential up-regulation of HLA-DR, CD86, and PD-L1 on MDDCs vs. monocytes
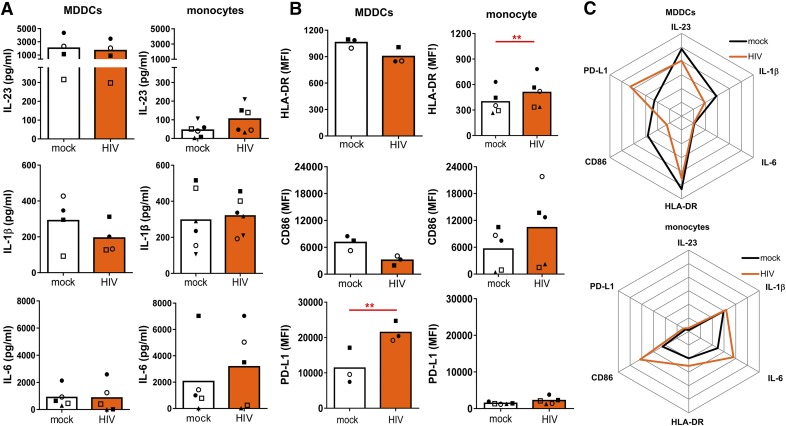
We next sought to evaluate how HIV exposure affects the ability of MDDCs and monocytes to stimulate Th17 vs. Th1 cells. Given that the Th17 cell polarization is influenced by multiple cytokines, including IL-1β, IL-6, IL-23, and TGF-β, we examined whether HIV alters cytokine production by MDDCs and monocytes during the initial 3 d of Th stimulation. IL-1β, IL-23, and IL-6 are 3 of the key cytokines shown to be required for Th17 differentiation and expansion in the context of different pathogens [43, 47–49]. The levels of IL-1β, IL-23, and IL-6 secreted by monocytes and MDDCs treated with HIV or mock-treated were determined by ELISA or Luminex (Fig. 2A). The data show that the amounts of IL-23 produced by MDDCs were much higher than those by monocytes. In contrast, MDDCs secreted lower amounts of IL-6 than monocytes did. However, HIV did not cause consistent changes to the secretion of IL-23 and IL-6 by MDDCs or monocytes. IL-1β secretion by monocytes and MDDCs was comparable and was also unaltered by HIV.
Figure 2. HIV-induced alterations of cytokine secretion and regulatory molecule expression in MDDCs vs. monocytes.
MDDCs or monocytes were treated with SF162 (MOI = 0.5) or left untreated and then cocultured with CD4+ T cells. (A) After 3 d, culture supernatants were assessed for IL-23, IL-1β, and IL-6. (B) The expression of HLA-DR, CD86, and PD-L1 on MDDCs or monocytes treated or not treated with HIV was also evaluated. P values were calculated using the paired t test. **P < 0.01. (C) Radar plots summarizing the differential responses of MDDCs vs. monocytes to HIV.
Subsequently, we analyzed whether HIV differentially affects the expression of the activation molecules HLA-DR and CD86 and the regulatory molecule PD-L1 on MDDCs vs. monocytes (Fig. 2B). A previous study [50] showed that HIV induces the PD-1/PD-L1 inhibitory pathway on MDDCs to impair the ability to stimulate T cell proliferation, but it is unknown whether monocytes are similarly affected. The expression levels of HLA-DR and PD-L1 were much higher in MDDCs than they were in monocytes, whereas CD86 expression was comparable in the 2 cell types. HIV increased HLA-DR on monocytes but induced a slight decline on MDDCs. A similar pattern was seen with CD86, although the differences were not significant. In contrast, HIV further enhanced the high PD-L1 expression on MDDCs and did not alter PD-L1 expression on monocytes. These data show that HIV has distinct effects on MDDCs as compared with monocytes (Fig. 2C). HIV induces up-regulation of the activation markers HLA-DR and CD86 on monocytes but not on MDDCs. In contrast, HIV dramatically increases the expression of the negative regulatory molecule PD-L1 on MDDCs but not on monocytes. These results suggest that HIV may differentially affect the ability of MDDCs and monocytes to stimulate functional Th responses.
HIV-treated MDDCs reduce Th17 responses, whereas HIV-treated monocytes enhance Th17 responses
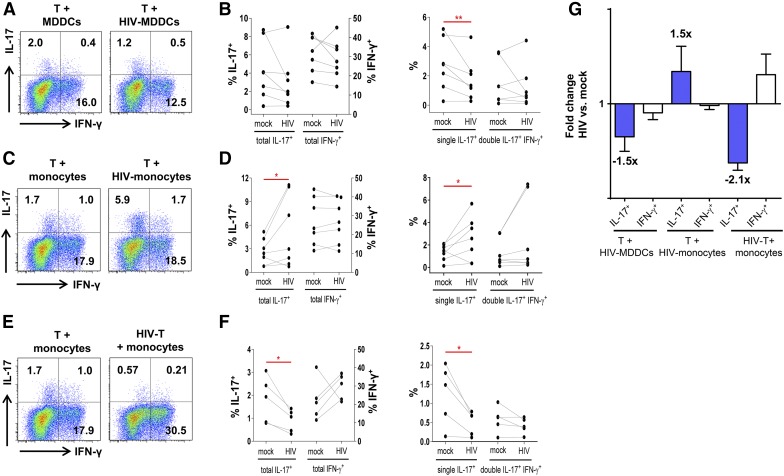
To compare the ability of MDDCs vs. monocytes pre-exposed to HIV to stimulate Th17 and Th1 responses, we incubated MDDCs and monocytes with HIV for 2 h, washed the cells to remove free virus, and cultured them with CD4+ T cells. CD4+ T cells were enriched from PBMCs of uninfected donors. MDDCs or monocytes were treated with HIV-1 R5 tropic SF162 (Fig. 3). Cultures of CD4+ T cells with mock-treated monocytes and MDDCs from the same donors were maintained in parallel for comparison. As in Fig. 1, the frequencies of IL-17+ and IFN-γ+CD4+ T cells were assessed on d 13 by intracellular staining.
Figure 3. Th17 and Th1 responses in CD4+ T cell cultures with HIV-treated MDDCs or monocytes.
CD4+ T cells were cultured with HIV-treated allogeneic MDDCs or monocytes at a T cell/APC ratio of 5:1 for 13 d. Mock-treated cocultures from the same donors were maintained in parallel. After PMA and ionomycin treatment, the cells were stained and analyzed for the frequencies of IL-17+ cells and IFN-γ+ cells by flow cytometry. (A–B) CD4+ T cells were cultured with mock-treated or HIV-1 SF162-treated MDDCs (MOI = 0.5). (A) Dot plots from one representative subject showing IL-17 and IFN-γ expression in the CD4+ T cells. (B) Cumulative data showing the percentages of total IL-17+ and IFN-γ+ (left panel), single-positive IL-17+, and double-positive IL-17+IFN-γ+ CD4+ T cells (right panel) in the mock vs. HIV-treated MDDCs cocultures from multiple donors. (C–D) CD4+ T cells were cultured with mock-treated or HIV-1 SF162-treated monocytes (MOI = 0.5). (C) Dot plots from one representative subject showing IL-17 and IFN-γ expression in the CD4+ T cells. (D) The percentages of total IL-17+ and IFN-γ+ (left panel), single-positive IL-17+, and double-positive IL-17+IFN-γ+ CD4+ T cells (right panel) in the monocytes cocultures from different donors. (E–F) CD4+ T cells were infected with cell-free HIV-1 SF162 at MOI = 0.1 and cocultured with untreated monocytes. (E) Dot plots from one representative subject showing the expression of IL-17 and IFN-γ in the CD4+ T cells. (F) The percentages of total IL-17+ and IFN-γ+ (left panel), single-positive IL-17+ and double-positive IL-17+IFN-γ+ CD4+ T cells (right panel) in cultures from different donors. P values were calculated using the paired t test. *P < 0.05, **P < 0.01 (G) Fold-changes in the frequencies of total IL-17+ and IFN-γ+CD4+ T cells in the cocultures of CD4+ T cells with HIV-1-treated MDDCs or monocytes over those of mock-treated controls. Means ± se from all donors tested are shown.
Lower frequencies of IL-17+CD4+ T cells, particularly the single-positive IL-17+IFN-γ− population, were induced in the presence of HIV-treated MDDCs as compared with the untreated MDDCs. IFN-γ+CD4+ T cells were not significantly reduced (Fig. 3A and B). This finding is consistent with the pattern of Th17 loss previously seen in HIV-infected CD4+ T cell cultures stimulated with anti-CD3 and anti-CD28 Abs [15, 23]. By contrast, the frequencies of IL-17+CD4+ T cells were increased in the cultures with monocytes pretreated with HIV as compared with mock-treated monocytes. The increase was observed with total IL-17+CD4+ T cells from all donors tested, except one, and was more evident with the single-positive IL-17+ cells than with the double-positive IL-17+IFN-γ+ cells (Fig. 3C and D). IFN-γ+CD4+ T cells were unaltered, indicating the specific effects on Th17 cells. Given that the virus stock was produced in PHA blasts, we wanted to rule out the potential effect of PHA on Th17 responses by treating monocytes with supernatants from PHA blasts containing virus or no virus before coculture with CD4+ T cells. Only HIV treatment of monocytes increased the frequencies of IL-17+CD4+ T cells (Supplemental Fig. 3).
To evaluate whether the addition of monocytes to infected CD4+ T cells would also enhance the Th17 responses, the CD4+ T cells were infected with cell-free virus and then cultured with untreated monocytes. Under this condition, no Th17 increase was observed. In fact, the Th17 responses declined, and the Th1 responses increased proportionally (Fig. 3E and F). To better visualize the changes in Th17 and Th1 responses under the different experimental conditions, the fold changes of Th17 or Th1 frequencies in cultures with HIV over those without HIV were calculated (Fig. 3G). The data clearly show the specific effects of HIV-treated MDDCs and cell-free virus infection in depleting Th17 responses and the unique activity of HIV-exposed monocytes in augmenting IL-17 responses.
Memory CD4+ T cells contribute to the enhancement of Th17 response by HIV-exposed monocytes
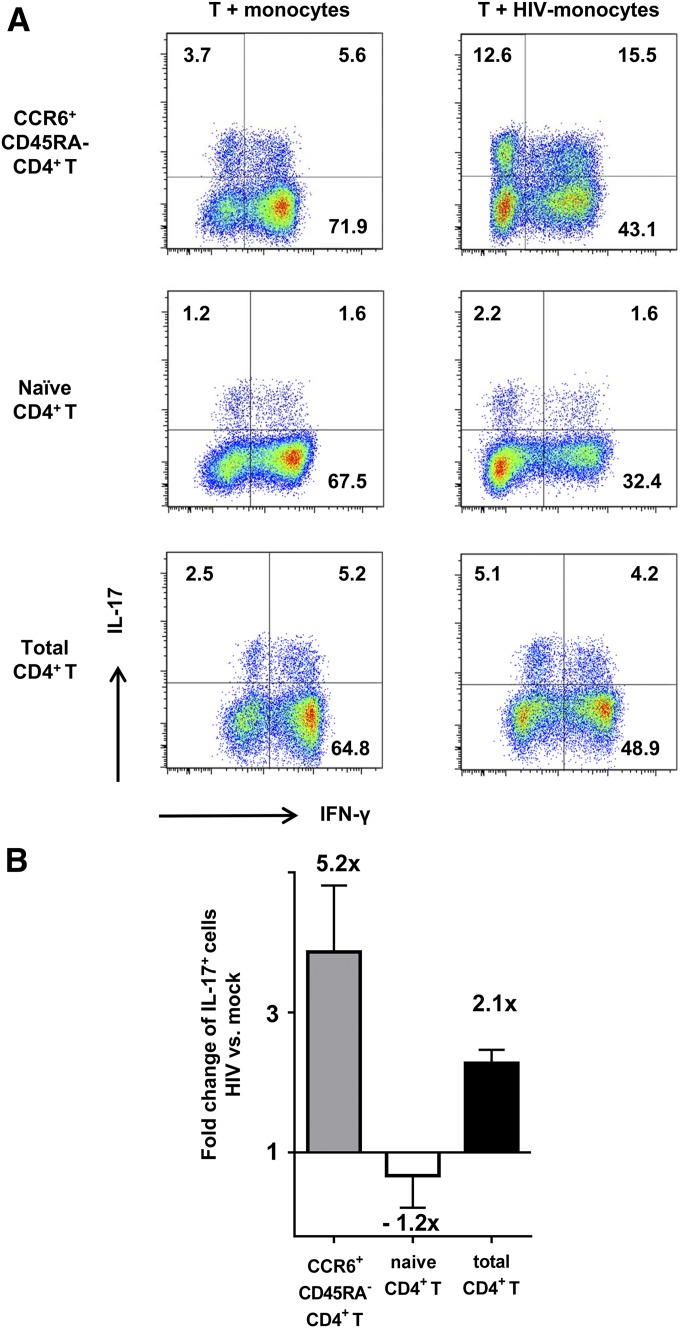
Given that Th17 cells are enriched in memory CCR6+ T cell population, we tested the ability of monocytes pretreated with HIV or no virus to stimulate Th17 responses in the memory CCR6+CD4+ T cells. CD45RA−CD4+ T cells were purified from PBMCs by negative selection, and, subsequently, the CCR6+ population was enriched by positive selection and cultured with HIV-treated or mock-treated monocytes. Unfractionated total CD4+ T cells were also tested in parallel. The frequency of IL-17+ cells was greatly enhanced (3.7 vs. 12.6% for IL-17+ and 5.6 vs. 15.5% for IL-17+IFN-γ+) when the memory CCR6+ T cells were cultured with HIV-treated monocytes as compared with mock-treated monocytes (Fig. 4A). In contrast, the single-positive IFN-γ+ cells proportionally declined.
Figure 4. IL-17 and IFN-γ responses induced in memory, naïve, and total CD4+ T cells cocultured with HIV-treated monocytes.
Memory CD45RA− CCR6+ CD4+ T cells, naïve CD45RA+ CD4+ T cells, and total CD4+ T cells were cocultured with allogenic HIV-treated monocytes at a T/APC ratio of 5 to 1 for 13 d. At d 13, the cells were stained with anti-IL-17 and IFN-γ Abs after PMA and ionomycin treatment and analyzed by flow cytometry. (A) Dot plots from one representative subject showing IL-17 and IFN-γ expression in the memory (top panels), naïve (middle panels), and total CD4+ T cells (lower panels). (B) Fold-changes of the IL-17+ CD4+ T cell frequencies in the cocultures with HIV-1-treated monocytes over those of mock-treated controls. Means ± se from 2–3 different donors are shown.
We next sought to evaluate whether HIV also enhanced the ability of monocytes to differentiate Th17 cells from naïve CD4+ T cells. Naïve CD4+ T cells were purified by negative selection and cocultured with HIV-treated or mock-treated monocytes. IL-17+ cells were induced by monocytes from naïve CD4+ T cells, albeit at a much lower extent than from memory CCR6+ and total CD4+ T cells (Fig. 4A). However, no change was observed in the percentages of IL-17+ cells in the naïve CD4+ T cell cultures with HIV-treated vs. mock-treated monocytes. This is in contrast to the enhanced frequencies of IL-17+ cells among memory CCR6+ and total CD4+ T cells cocultured with HIV-treated monocytes (Figs. 4 and 3C and D). Thereby, the average-fold changes of IL-17+ cells in the HIV-treated over mock-treated cultures of memory CCR6+, naïve, and total CD4+ T cells from multiple donors were 5.2, −1.2, and 2.1, respectively. These results indicate that the Th17-enhancing activity of HIV-exposed monocytes is mainly directed to memory CD4+ T cells and not naïve CD4+ T cells.
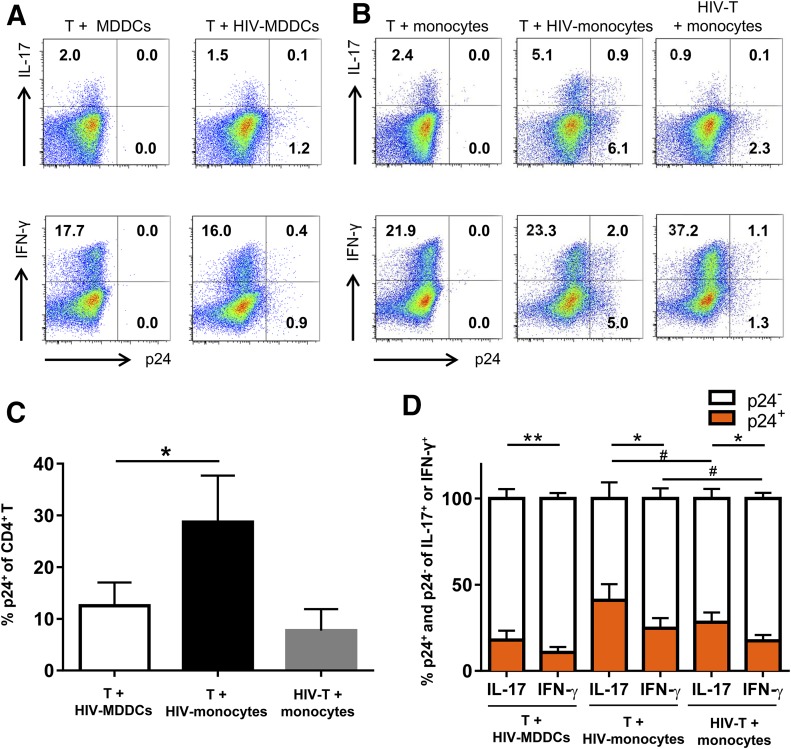
High levels of HIV-infected p24+ cells are present in the Th cultures with HIV-treated monocytes
Although MDDCs and monocytes are largely refractory to HIV infection, these cells can efficiently capture and transmit infectious HIV to Th cells [51–53]. To determine the extent of HIV trans-infection to Th cells in the different cocultures, the numbers of HIV Gag p24+CD4+CD3+ cells were measured by intracellular staining with the anti-p24 mAb KC57-RD1 and analyzed along with IL-17 and IFN-γ expression by flow cytometry (Fig. 5A and B). The frequency of total p24+ CD4 T cells was highest in the coculture with HIV-treated monocytes (7.0%) as compared with the other 2 cocultures (1.3 and 2.4%). This pattern was observed consistently in all cultures from multiple donors (Fig. 5C).
Figure 5. HIV infection in the CD4+ T cells cocultured with virus-treated MDDCs or monocytes.
(A) Dot plots from one representative subject showing p24+ and IL-17+ (top) or IFN-γ+ (bottom) CD4+ T cells in the cultures with virus-treated or untreated allogeneic MDDCs. (B) Dot plots from one representative subject showing p24+ and IL-17+ (top) or IFN-γ+ (bottom) CD4+ T cells in the cocultures without virus or with virus-treated monocytes. For comparison, CD4+T cells were also infected with cell-free virus and then cocultured with monocytes. (C) The frequencies of total HIV-1-infected (p24+) CD4+ T cells in the different cocultures from multiple donors (n = 6). (D) The proportions of Th17 and Th1 cells infected with HIV (p24+) in the different cocultures from multiple donors (n = 3-6). P values were calculated using paired t test, *P < 0.05, **P < 0.01 or by unpaired t test, #P < 0.05.
When we calculated the percentages of IL-17+ cells and IFN-γ+ cells that were infected with HIV, a greater proportion of IL-17+ cells was found to be p24+. Infection was detected in both single-positive IL-17+ and double-positive IL-17+IFN-γ+ cells (data not shown). The higher rates of infection in Th17 cells than in Th1 cells were observed whether the T cells were cocultured with virus-treated MDDCs or monocytes or were infected with cell-free virus (Fig. 5D).
Taken together with the data in Figs. 3 and 4, these results demonstrate that HIV-exposed monocytes enhanced and sustained Th17 responses, even when a significant fraction of the Th17 cells were infected by the virus. In contrast, Th17 cells declined upon virus trans-infection from MDDCs. Hence, HIV transmission from MDDCs, but not from monocytes, contributes to Th17 decline. These results implicate important differences between Th17 cells induced in the presence of MDDCs vs. monocytes that affect the survival of these Th cells during HIV infection.
Treatment with HIV envelope gp120 is sufficient to induce monocytes to augment Th17 responses
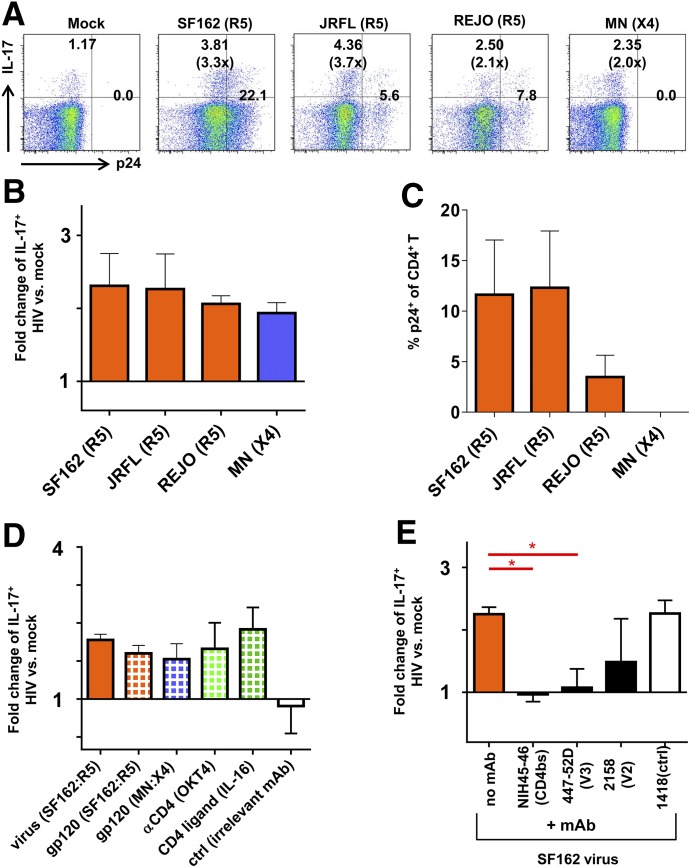
To evaluate whether the different strains of R5-tropic and X4-tropic HIV mediate the Th17-enhancing activity, monocytes were treated with R5-tropic viruses SF162, JRFL, and REJO, or an X4-tropic virus MN. As compared with mock-treated monocytes, monocytes treated with each of the R5-tropic viruses increased the frequencies of IL-17+CD4+ T cells (Fig. 6A and 6B), and a fraction of these cells were p24+ (Fig. 6C). Similar enhancement was seen with IL-17+ T cells in the cocultures with X4-tropic MN-treated monocytes, although p24+ cells were not detectable in these cultures. These data indicate that enhanced Th17 responses can be induced by monocytes upon R5-tropic or X4-tropic HIV exposure, and the enhancing activity is independent of virus infection.
Figure 6. Th17 responses in the CD4+ T cell cultures containing monocytes treated with different HIV-1 strains.
(A–C) CD4+ T cells were cocultured at a T cell/APC ratio of 5:1 with allogenic monocytes treated with SF162, JRFL, REJO, or MN viruses (MOI = 0.5). At d 13, the cells were stimulated with PMA and ionomycin, stained with anti–IL-17 and anti–IFN-γ Abs, and analyzed by flow cytometry. (A) Dot plots from one representative subject showing the expression of IL-17 and p24 in the CD4+ T cells. (B) Fold-changes in the percentages of IL-17+ cells in the cultures with virus-treated monocytes over those with mock-treated monocytes. (C) The frequencies of p24+CD4+ T cells in the cocultures with virus-treated monocytes. (D) Fold-changes in the frequencies of IL-17+ cells cultured with treated vs. untreated monocytes. Monocytes are treated with SF162 virus, viral gp120 proteins (SF162 and MN, 1 µg/ml), an anti-CD4 Ab (OKT4, 2 µg/ml), recombinant IL-16 (40 ng/ml), or an irrelevant Ab control (2 µg/ml). (E) Fold-changes in IL-17+ cell frequencies in the cultures with monocytes treated with virus ± Abs. SF162 virus was preincubated with mAbs against the CD4-binding site (NIH45-46), V3 (447-52D), V2 (2158) of the HIV envelope gp120 or with a control Ab (1418). Means ± se from at least 2 independent experiments are shown. P values were calculated using the paired t test. *P < 0.05.
We further showed that the treatment of monocytes with the HIV envelope gp120 proteins (R5-tropic SF162 or X4-tropic MN) was sufficient to enhance Th17 responses, and this activity was similarly induced by the anti-CD4 mAb OKT4 and the natural CD4 ligand IL-16 (Fig. 6D). Further, HIV-induced enhancement of Th17 responses could be blocked by anti-gp120 mAbs directed to the CD4 binding site (NIH45-46) and the V3 loop (447-52D) (Fig. 6E). The mAb to the V1V2 loop (2158) caused only partial blockage. These data indicate the importance of HIV gp120 interaction with CD4 and possibly the chemokine receptors in triggering the monocytes to augment the Th17 responses.
HIV-treated MDDCs reduce CD4+ T cell proliferation and increase apoptosis, whereas HIV-treated monocytes induce CD4+ T cell proliferation without increasing apoptosis
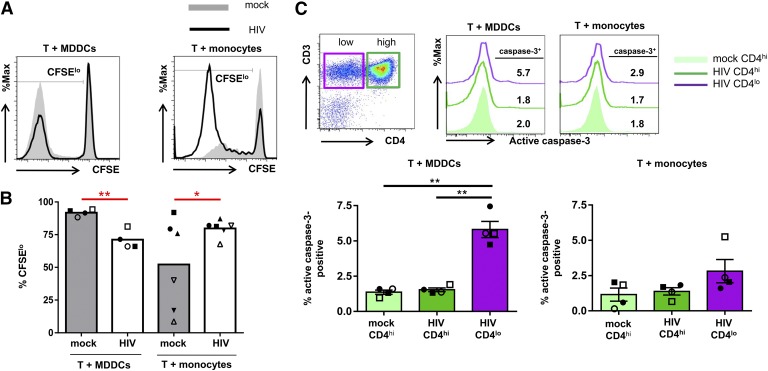
We postulate that the distinct effects of virus-treated MDDCs vs. virus-treated monocytes on the expansion of Th17 cells may be attributed to their differential capacity to induce T cell proliferation or cell death. To assess T cell proliferation, purified CD4+ T cells were labeled with CFSE and cocultured with MDDCs or monocytes that were pretreated with HIV or with no virus. After 13 d, the frequencies of proliferating cells were measured by flow cytometry. In the absence of HIV, MDDCs induced a greater proliferation of CD4+ T cells than monocytes did, as indicated by the higher percentages of CFSEloCD4+ T cells in the cocultures with untreated MDDCs vs. untreated monocytes. However, HIV exposure of MDDCs and monocytes had opposing effects. The CD4+ T cell proliferation in the cocultures with HIV-treated MDDCs was lower than that in the untreated MDDC cocultures (Fig. 7A and B). These results were consistent with previous findings showing inhibition of T cell proliferation by HIV-infected MDDCs is in part due to the virus Env and Tat activities [50, 54, 55]. In contrast, HIV-treated monocytes enhanced T cell proliferation as shown by higher percentages of CFSEloCD4+ T cells in the cultures with virus-treated monocytes as compared with those with mock-treated monocytes (Fig. 7A and B).
Figure 7. Proliferation and apoptosis of CD4+ T cells cocultured with MDDCs or monocytes.
CD4+ T cells were labeled with CFSE, cocultured with MDDCs or monocytes that were pretreated with or without HIV, and analyzed for proliferation and apoptosis. (A and B) The proliferation of CD4+ T cells cocultured with virus-treated or untreated MDDCs vs. monocytes. (A) Representative data showing the proliferating CFSElo CD4+ T cells in the cocultures with MDDCs (left panel) vs. monocytes (right panel) with or without HIV. (B) The frequencies of CFSEloCD4+ T cells in the cocultures from different donors. (C) The expression of active caspase-3 in CD4+ T cells cocultured with HIV-treated and mock-treated MDDCs vs. monocytes. (Top left panel) A representative dot plot showing the gating of CD4lo and CD4hiCD3+ T cells. (Top right panel) A representative histogram to show the up-regulation of active caspase-3 in the CD4loCD3+ T cells cocultured with HIV-treated MDDCs but not in the CD4hi cells with HIV- or mock-treated MDDCs. No increase in active caspase-3 staining was detected in CD4lo or CD4hiCD3+ T cells in the cultures with HIV- and mock-treated monocytes. (Bottom panels) The percentages of active caspase-3+ cells among the CD4lo and CD4hi T cell populations in the cultures from different donors. P values were calculated using the paired t test. *P < 0.05, **P < 0.01.
To examine whether apoptosis was also differentially induced in the CD4+ T cells cultured with virus-treated MDDCs vs. monocytes, we analyzed the relative levels of active caspase-3 expressed by the CD4+ T cells in the cocultures using the fluorescent caspase-3 inhibitor Z-DEVD-FMK (fluoromethyl ketone-derivatized peptide). Because CD4 expression is down-regulated after HIV infection, we measured the levels of active caspase-3 in CD4loCD3+ T cells and CD4hiCD3+ T cells from the infected cocultures as compared with those in CD4hiCD3+ T cells from mock-infected cultures (Fig. 7C). Active caspase-3 was up-regulated in a fraction of CD4lo T cells, but not in the CD4hi T cells present in the cocultures containing virus-treated MDDCs. However, no significant increase of active caspase-3 was detected in the CD4lo T cells from the cocultures with virus-bearing monocytes or in the CD4hi counterparts from the cocultures with or without HIV. These results demonstrate that HIV-treated monocytes stimulated CD4+ T cell proliferation without up-regulating caspase-3. In contrast, virus-treated MDDCs reduced CD4+ T cell proliferation and activated the caspase-3–mediated apoptosis pathway particularly in the CD4lo T cells that had acquired HIV from the MDDCs. The differential activities observed here corresponded with the distinctive capacity of these 2 myeloid cells to either decrease or expand the Th17 cells.
DISCUSSION
This study evaluated the effects of HIV on the ability of MDDCs and monocytes to stimulate Th17 and Th1 responses. As reported previously [43], our results confirmed that monocytes are more-potent APCs in stimulating Th17 responses than are MDDCs. More remarkably, however, this study demonstrated that HIV induced the monocytes to further enhance the Th17 responses. In contrast, HIV decreased the frequencies of Th17 cells, especially the single-positive IL-17+ cells, stimulated by MDDCs. The molecular basis for these differential activities is not fully understood; however, we observed distinct alterations in the expression of the regulatory molecules HLA-DR, CD86, and PD-L1 on MDDCs vs. monocytes upon HIV exposure. Thereby, HIV treatment of monocytes increased the stimulatory molecules HLA-DR and CD86, without up-regulating PD-L1. Correspondingly, virus treatment of monocytes enhanced CD4+ T cell proliferation without increasing caspase-3 activation. On the other hand, HIV induced MDDCs to up-regulate the negative-regulator PD-L1. HIV exposure also reduced the ability of MDDCs to stimulate CD4+ T cell proliferation and increased the activation of the apoptosis-inducing caspase-3. These data are in agreement with a previous study [50] showing that HIV compromises the ability of MDDCs to stimulate T cells, which is in part due to the induction of the PD-1/PD-L1 coinhibitory pathway by the HIV Tat protein.
The mechanisms by which HIV induces monocytes to confer the Th17-enhancing activity also remain unclear. The present study indicates that this activity does not require productive HIV replication and can be induced by the interaction of the virus envelope gp120 with its receptors. Th17 enhancement was triggered by R5-tropic virus that resulted in transmission to CD4+ T cells as well as by the nontransmitted X4-tropic virus. Similar to HIV, dead or live Streptococcus pneumonia, Candida albicans, Staphylococcus aureus, and microbial products, such as LPS and PGN, have been shown to augment the induction of Th17 responses [43, 49, 56]. The HIV single-strand RNAs and the HIV Tat protein have also been shown to engage TLR8 and TLR4 on myeloid cells, such as monocytes, macrophages, and MDDCs [50, 57]. However, our data demonstrated that the HIV-envelope glycoprotein gp120 was sufficient to induce the monocytes to augment Th17 responses. The CD4 engagement by an Ab or the ligand IL-16 was also able to enhance Th17 responses. Further, the Th17-enhancing activity was abrogated by pretreating the virus with anti-HIV–envelope Abs. Abs against the CD4-binding site and the V3 loop displayed an equally potent inhibitory activity, suggesting that the Th17-enhancing activity may be triggered via multiple receptors, including CD4 and the chemokine receptors.
In agreement with past in vitro and ex vivo data from our laboratory and others [15, 20, 58], the present study provided further supporting evidence for the preferential targeting of Th17 cells over Th1 cells by HIV. Notably, although Th1 cells were >10-fold more abundant in the cultures than were Th17 cells, higher rates of infection were seen among Th17 cells than among Th1 cells. The same results were observed whether the virus transmission occurred from monocytes or MDDCs or from cell-free virions. These findings are consistent with ex vivo data showing the greater levels of HIV proviral DNA burden among CCR6+ Th cells enriched with Th17 and Th1/Th17 lineages and the preferential loss of these Th populations in HIV-infected subjects on or off antiretroviral therapy [20, 30, 58, 59]. Our earlier studies showed that the preferential targeting of Th17 cells was determined at the entry stage of HIV infection and correlated with higher binding of the HIV envelope to CCR6+ Th17-enriched cells [15]. Indeed, the CCR6+ CD4+ T cells expressed higher levels of the virus receptors, including CD4, the chemokine receptors, and α4β7 than the CCR6− counterparts, and the enhanced virus envelope binding to the CCR6+CD4+ T cells was abrogated by Abs against β7. In addition, CCR5 ligands with potent HIV fusion-inhibiting activities were endogenously expressed by Th1 cells but not by Th17 cells, further contributing to the differential susceptibility of Th1 and Th17 to HIV [15, 58, 60].
Whether differences at postentry stages also have a role in promoting HIV replication in Th17 cells over Th1 cells remains controversial. Infection with HIV pseudotyped with the vesicular stomatitis virus G envelope in vitro resulted in higher numbers of infected cells among Th17- and Th1/Th17-polarized cells as compared with those polarized toward Th1 or Th2 [30]. However, the enhanced susceptibility was not as marked as that seen with HIV bearing the native R5- or X4-tropic envelopes [20, 30, 61]. As compared with Th1 cells, Th1/Th17 cells also exhibited a distinct transcriptional profile that may favor HIV replication, although one of the transcription factors expressed at higher levels in Th1/Th17 cells was the nuclear receptor peroxisome proliferator-activated receptor-γ [61], a negative regulator known to restrict HIV replication [61–63]. A recent study, however, shows that Th17-polarized cells have reduced expression of RNases with known anti-HIV activities [32].
Although HIV transmission from both monocytes and MDDCs resulted in infection of Th17 cells, the impact on the Th17 cells was distinct. HIV trans-infection from MDDCs led to the decline of Th17 responses similar to that observed in infection with cell-free virus [15], whereas transmission of virus from monocytes augmented Th17 responses. Expressing high levels of DC-SIGN, MDDCs are known to efficiently transmit HIV to Th cells. However, we did not examine specifically the relative efficiency of virus transfer from MDDCs vs. monocytes in the study. Virus transfer from MDDCs may indeed be more efficient but may also increase Th cell death. Indeed, HIV-treated MDDCs suppressed Th proliferation and induced apoptosis. Altogether, these factors are likely to contribute to the fewer infected Th cells (both Th1 and Th17) in the cocultures with MDDCs. It is also not understood how HIV induces monocytes, but not MDDCs, to enhance Th17 responses in the face of productive HIV infection. Adding monocytes to the T cells infected with cell-free virus did not recapitulate the Th17 enhancement. Further, the Th17 expansion was found to arise mainly from memory CD4+ T cells and not from naïve T cells, indicating that HIV-bearing monocytes did not drive Th17 differentiation from naïve T cells. The enhancing effect was not seen with Th1 cells present in the same cultures, indicating the specific activity of HIV-exposed monocytes to expand Th17 cells from memory CD4+ T cells.
The depletion of Th17 cells in the infected Th cell cultures established by stimulation with Abs to CD3 and CD28 plus the Th17-differentiation cytokines was associated with increased levels of activated caspase-3 and caspase-1 in the Th17-enriched cell population [15], indicating the induction of both apoptotic and pyroptotic programmed cell-death pathways. In this study, the reduction of Th17 cells in the cocultures with virus-treated MDDCs was found to correlate with a reduced Th cell proliferation and an increased activation of caspase-3 essential for triggering apoptosis. HIV-treated monocytes, on the other hand, heightened Th cell proliferation without increasing caspase-3 activation. HIV also induces the up-regulation of PD-L1 expression on MDDCs, whereas monocytes express much lower levels of PD-L1 and HIV does not increase PD-L1 on these cells. The contribution of the PD-L1/PD-1 inhibitory pathway to Th17 depletion during HIV infection needs to be investigated further. Moreover, monocytes should be made of heterogeneous populations with distinct phenotypes and functional properties. This study used total CD14+ monocytes, and the roles of the different monocyte subsets remain to be delineated. For examples, the CD16+ monocytes are a minor subset in the peripheral blood, but they transmit HIV to Th cells more efficiently than do the CD16− monocytes and promote virus infection by activating the Th cells via the secretion of CCR3 and CCR4 ligands [64]. Further studies are needed to evaluate whether HIV transmission from CD16+ monocytes to Th17 cells, many of which express the CCR4 receptors, may yield different outcomes from transmission from the more-abundant CD16− monocytes or the MDDCs studied here.
Notwithstanding the underlying mechanisms, the effects of HIV transmission from MDDCs and monocytes to Th17 cells suggests the distinct roles of these 2 cell types in determining Th17 responses during HIV infection. Given that Th17 cells predominate in the gut mucosal tissues and interact mostly with DCs in the tissues, the Th17 destruction is likely to prevail in the mucosa. However, Th17 cells may also encounter the occasional HIV-bearing blood monocytes, and our data indicate that such interactions would allow virus transmission without incurring Th17 loss. Indeed, the Th17 decline in the peripheral blood is less pronounced than it is in the gut mucosa [65]. The proportions of HIV DNA in the peripheral Th17 and Th1/Th17 populations have also been observed to increase overtime in patients on antiretroviral therapy [30]. Whether virus-exposed monocytes have a role in the maintenance of such cells remains to be determined. At this juncture, very little is known about the roles of monocytes, MDDCs, and other types of APCs in influencing HIV-infected Th17 cell survival vs. death. The study presented herein is a first step toward a better understanding about the interplays between the different types of APCs with Th17 and other Th subsets during HIV infection.
AUTHORSHIP
Y.M. designed and performed the experiments, analyzed the data, and prepared the manuscript. M.T. helped perform the experiments and reviewed the manuscript. C.E.H. was the principal investigator, who conceived the idea, helped design the experiments, and wrote the manuscript.
ACKNOWLEDGMENTS
This study was supported in part by the U.S. Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, the Department of Veterans Affairs Merit Review Award (C.E.H.) and by U.S. National Institutes of Health National Institute of Allergy and Infectious Diseases Grants AI093210, AI102740, and AI114520 (to C.E.H.). We thank Dr. Paul D. Bieniasz (Aaron Diamond AIDS Research Center) for providing the MT-4 TMZ R5 cell line, Dr. Susan Zolla-Pazner (Icahn School of Medicine at Mount Sinai) for providing monoclonal anti-HIV envelope Abs, and Ms. Radhika Wikramanayake for editing the manuscript.
Glossary
- DC
dendritic cell
- DC-SIGN
dendritic cell–specific intercellular adhesion molecule 3-grabbing non-integrin
- HLA-DR
human leukocyte antigen–DR
- MDDC
monocyte-derived dendritic cell
- MOI
multiplication of infection
- PGN
peptidoglycan
- R5
chemokine receptor CCR5
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Chun T. W., Chadwick K., Margolick J., Siliciano R. F. (1997) Differential susceptibility of naive and memory CD4+ T cells to the cytopathic effects of infection with human immunodeficiency virus type 1 strain LAI. J. Virol. 71, 4436–4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dai J., Agosto L. M., Baytop C., Yu J. J., Pace M. J., Liszewski M. K., O’Doherty U. (2009) Human immunodeficiency virus integrates directly into naive resting CD4+ T cells but enters naive cells less efficiently than memory cells. J. Virol. 83, 4528–4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambotte O., Demoustier A., de Goër M. G., Wallon C., Gasnault J., Goujard C., Delfraissy J. F., Taoufik Y. (2002) Persistence of replication-competent HIV in both memory and naive CD4 T cell subsets in patients on prolonged and effective HAART. AIDS 16, 2151–2157. [DOI] [PubMed] [Google Scholar]
- 4.Ostrowski M. A., Chun T. W., Justement S. J., Motola I., Spinelli M. A., Adelsberger J., Ehler L. A., Mizell S. B., Hallahan C. W., Fauci A. S. (1999) Both memory and CD45RA+/CD62L+ naive CD4+ T cells are infected in human immunodeficiency virus type 1-infected individuals. J. Virol. 73, 6430–6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bacchus C., Cheret A., Avettand-Fenoël V., Nembot G., Mélard A., Blanc C., Lascoux-Combe C., Slama L., Allegre T., Allavena C., Yazdanpanah Y., Duvivier C., Katlama C., Goujard C., Seksik B. C., Leplatois A., Molina J. M., Meyer L., Autran B., Rouzioux C.; OPTIPRIM ANRS 147 study group (2013) A single HIV-1 cluster and a skewed immune homeostasis drive the early spread of HIV among resting CD4+ cell subsets within one month post-infection. PLoS One 8, e64219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buzon M. J., Sun H., Li C., Shaw A., Seiss K., Ouyang Z., Martin-Gayo E., Leng J., Henrich T. J., Li J. Z., Pereyra F., Zurakowski R., Walker B. D., Rosenberg E. S., Yu X. G., Lichterfeld M. (2014) HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat. Med. 20, 139–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cashin K., Paukovics G., Jakobsen M. R., Østergaard L., Churchill M. J., Gorry P. R., Flynn J. K. (2014) Differences in coreceptor specificity contribute to alternative tropism of HIV-1 subtype C for CD4+ T-cell subsets, including stem cell memory T-cells. Retrovirology 11, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chomont N., El-Far M., Ancuta P., Trautmann L., Procopio F. A., Yassine-Diab B., Boucher G., Boulassel M. R., Ghattas G., Brenchley J. M., Schacker T. W., Hill B. J., Douek D. C., Routy J. P., Haddad E. K., Sékaly R. P. (2009) HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med. 15, 893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soriano-Sarabia N., Bateson R. E., Dahl N. P., Crooks A. M., Kuruc J. D., Margolis D. M., Archin N. M. (2014) Quantitation of replication-competent HIV-1 in populations of resting CD4+ T cells. J. Virol. 88, 14070–14077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romagnani S., Parronchi P., D’Elios M. M., Romagnani P., Annunziato F., Piccinni M. P., Manetti R., Sampognaro S., Mavilia C., De Carli M., Maggi E., Del Prete G. F. (1997) An update on human Th1 and Th2 cells. Int. Arch. Allergy Immunol. 113, 153–156. [DOI] [PubMed] [Google Scholar]
- 11.Annunziato F., Cosmi L., Santarlasci V., Maggi L., Liotta F., Mazzinghi B., Parente E., Filì L., Ferri S., Frosali F., Giudici F., Romagnani P., Parronchi P., Tonelli F., Maggi E., Romagnani S. (2007) Phenotypic and functional features of human Th17 cells. J. Exp. Med. 204, 1849–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duhen T., Geiger R., Jarrossay D., Lanzavecchia A., Sallusto F. (2009) Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat. Immunol. 10, 857–863. [DOI] [PubMed] [Google Scholar]
- 13.Fehérvari Z., Sakaguchi S. (2004) Development and function of CD25+CD4+ regulatory T cells. Curr. Opin. Immunol. 16, 203–208. [DOI] [PubMed] [Google Scholar]
- 14.Soroosh P., Doherty T. A. (2009) Th9 and allergic disease. Immunology 127, 450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvarez Y., Tuen M., Shen G., Nawaz F., Arthos J., Wolff M. J., Poles M. A., Hioe C. E. (2013) Preferential HIV infection of CCR6+ Th17 cells is associated with higher levels of virus receptor expression and lack of CCR5 ligands. J. Virol. 87, 10843–10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Annunziato F., Galli G., Nappi F., Cosmi L., Manetti R., Maggi E., Ensoli B., Romagnani S. (2000) Limited expression of R5-tropic HIV-1 in CCR5-positive type 1-polarized T cells explained by their ability to produce RANTES, MIP-1α, and MIP-1β. Blood 95, 1167–1174. [PubMed] [Google Scholar]
- 17.Kaur G., Tuen M., Virland D., Cohen S., Mehra N. K., Münz C., Abdelwahab S., Garzino-Demo A., Hioe C. E. (2007) Antigen stimulation induces HIV envelope gp120-specific CD4+ T cells to secrete CCR5 ligands and suppress HIV infection. Virology 369, 214–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ofori H., Jagodziński P. P. (2004) Increased in vitro replication of CC chemokine receptor 5-restricted human immunodeficiency virus type 1 primary isolates in Th2 lymphocytes may correlate with AIDS progression. Scand. J. Infect. Dis. 36, 46–51. [DOI] [PubMed] [Google Scholar]
- 19.Bonecchi R., Bianchi G., Bordignon P. P., D’Ambrosio D., Lang R., Borsatti A., Sozzani S., Allavena P., Gray P. A., Mantovani A., Sinigaglia F. (1998) Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 187, 129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gosselin A., Monteiro P., Chomont N., Diaz-Griffero F., Said E. A., Fonseca S., Wacleche V., El-Far M., Boulassel M. R., Routy J. P., Sekaly R. P., Ancuta P. (2010) Peripheral blood CCR4+CCR6+ and CXCR3+CCR6+CD4+ T cells are highly permissive to HIV-1 infection. J. Immunol. 184, 1604–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moonis M., Lee B., Bailer R. T., Luo Q., Montaner L. J. (2001) CCR5 and CXCR4 expression correlated with X4 and R5 HIV-1 infection yet not sustained replication in Th1 and Th2 cells. AIDS 15, 1941–1949. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki Y., Koyanagi Y., Tanaka Y., Murakami T., Misawa N., Maeda N., Kimura T., Shida H., Hoxie J. A., O’Brien W. A., Yamamoto N. (1999) Determinant in human immunodeficiency virus type 1 for efficient replication under cytokine-induced CD4+ T-helper 1 (Th1)- and Th2-type conditions. J. Virol. 73, 316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alvarez Y., Tuen M., Nàdas A., Hioe C. E. (2012) In vitro restoration of Th17 response during HIV infection with an antiretroviral drug and Th17 differentiation cytokines. AIDS Res. Hum. Retroviruses 28, 823–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brenchley J. M., Paiardini M., Knox K. S., Asher A. I., Cervasi B., Asher T. E., Scheinberg P., Price D. A., Hage C. A., Kholi L. M., Khoruts A., Frank I., Else J., Schacker T., Silvestri G., Douek D. C. (2008) Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood 112, 2826–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macal M., Sankaran S., Chun T. W., Reay E., Flamm J., Prindiville T. J., Dandekar S. (2008) Effective CD4+ T-cell restoration in gut-associated lymphoid tissue of HIV-infected patients is associated with enhanced Th17 cells and polyfunctional HIV-specific T-cell responses. Mucosal Immunol. 1, 475–488. [DOI] [PubMed] [Google Scholar]
- 26.Prendergast A., Prado J. G., Kang Y. H., Chen F., Riddell L. A., Luzzi G., Goulder P., Klenerman P. (2010) HIV-1 infection is characterized by profound depletion of CD161+ Th17 cells and gradual decline in regulatory T cells. AIDS 24, 491–502. [DOI] [PubMed] [Google Scholar]
- 27.Iwakura Y., Ishigame H., Saijo S., Nakae S. (2011) Functional specialization of interleukin-17 family members. Immunity 34, 149–162. [DOI] [PubMed] [Google Scholar]
- 28.McGeachy M. J., Cua D. J. (2008) Th17 cell differentiation: the long and winding road. Immunity 28, 445–453. [DOI] [PubMed] [Google Scholar]
- 29.Monteiro P., Gosselin A., Wacleche V. S., El-Far M., Said E. A., Kared H., Grandvaux N., Boulassel M. R., Routy J. P., Ancuta P. (2011) Memory CCR6+CD4+ T cells are preferential targets for productive HIV type 1 infection regardless of their expression of integrin β7. J. Immunol. 186, 4618–4630. [DOI] [PubMed] [Google Scholar]
- 30.Sun H., Kim D., Li X., Kiselinova M., Ouyang Z., Vandekerckhove L., Shang H., Rosenberg E. S., Yu X. G., Lichterfeld M. (2015) Th1/17 polarization of CD4 T cells supports HIV-1 DNA persistence during antiretroviral therapy. J. Virol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maek-A-Nantawat W., Buranapraditkun S., Klaewsongkram J., Ruxrungtham K. (2007) Increased interleukin-17 production both in helper T cell subset Th17 and CD4-negative T cells in human immunodeficiency virus infection [published correction in Viral Immunol. (2007) 20, 328.]. Viral Immunol. 20, 66–75. [DOI] [PubMed] [Google Scholar]
- 32.Christensen-Quick A., Lafferty M., Sun L., Marchionni L., DeVico A., Garzino-Demo A. (2016) Human Th17 cells lack HIV-inhibitory RNases and are highly permissive to productive HIV infection. J. Virol.. doi:10.1128/JVI.02869-15 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guglani L., Khader S. A. (2010) Th17 cytokines in mucosal immunity and inflammation. Curr. Opin. HIV AIDS 5, 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brenchley J. M., Price D. A., Schacker T. W., Asher T. E., Silvestri G., Rao S., Kazzaz Z., Bornstein E., Lambotte O., Altmann D., Blazar B. R., Rodriguez B., Teixeira-Johnson L., Landay A., Martin J. N., Hecht F. M., Picker L. J., Lederman M. M., Deeks S. G., Douek D. C. (2006) Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12, 1365–1371. [DOI] [PubMed] [Google Scholar]
- 35.Dandekar S., George M. D., Bäumler A. J. (2010) Th17 cells, HIV and the gut mucosal barrier. Curr. Opin. HIV AIDS 5, 173–178. [DOI] [PubMed] [Google Scholar]
- 36.Gordon S. N., Cervasi B., Odorizzi P., Silverman R., Aberra F., Ginsberg G., Estes J. D., Paiardini M., Frank I., Silvestri G. (2010) Disruption of intestinal CD4+ T cell homeostasis is a key marker of systemic CD4+ T cell activation in HIV-infected individuals. J. Immunol. 185, 5169–5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vyboh K., Jenabian M. A., Mehraj V., Routy J. P. (2015) HIV and the gut microbiota, partners in crime: breaking the vicious cycle to unearth new therapeutic targets. J. Immunol. Res. 2015, 614127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raffatellu M., Santos R. L., Verhoeven D. E., George M. D., Wilson R. P., Winter S. E., Godinez I., Sankaran S., Paixao T. A., Gordon M. A., Kolls J. K., Dandekar S., Bäumler A. J. (2008) Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat. Med. 14, 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu H., Wang X., Liu D. X., Moroney-Rasmussen T., Lackner A. A., Veazey R. S. (2012) IL-17-producing innate lymphoid cells are restricted to mucosal tissues and are depleted in SIV-infected macaques. Mucosal Immunol. 5, 658–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ortiz A. M., Klase Z. A., DiNapoli S. R., Vujkovic-Cvijin I., Carmack K., Perkins M. R., Calantone N., Vinton C. L., Riddick N. E., Gallagher J., Klatt N. R., McCune J. M., Estes J. D., Paiardini M., Brenchley J. M. (2016) IL-21 and probiotic therapy improve Th17 frequencies, microbial translocation, and microbiome in ARV-treated, SIV-infected macaques. Mucosal Immunol. 9, 458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pallikkuth S., Micci L., Ende Z. S., Iriele R. I., Cervasi B., Lawson B., McGary C. S., Rogers K. A., Else J. G., Silvestri G., Easley K., Estes J. D., Villinger F., Pahwa S., Paiardini M. (2013) Maintenance of intestinal Th17 cells and reduced microbial translocation in SIV-infected rhesus macaques treated with interleukin (IL)-21. PLoS Pathog. 9, e1003471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Busnadiego I., Kane M., Rihn S. J., Preugschas H. F., Hughes J., Blanco-Melo D., Strouvelle V. P., Zang T. M., Willett B. J., Boutell C., Bieniasz P. D., Wilson S. J. (2014) Host and viral determinants of Mx2 antiretroviral activity. J. Virol. 88, 7738–7752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Acosta-Rodriguez E. V., Napolitani G., Lanzavecchia A., Sallusto F. (2007) Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immunol. 8, 942–949. [DOI] [PubMed] [Google Scholar]
- 44.Chang C. C., Wright A., Punnonen J. (2000) Monocyte-derived CD1a+ and CD1a− dendritic cell subsets differ in their cytokine production profiles, susceptibilities to transfection, and capacities to direct Th cell differentiation. J. Immunol. 165, 3584–3591. [DOI] [PubMed] [Google Scholar]
- 45.Geijtenbeek T. B., Kwon D. S., Torensma R., van Vliet S. J., van Duijnhoven G. C., Middel J., Cornelissen I. L., Nottet H. S., KewalRamani V. N., Littman D. R., Figdor C. G., van Kooyk Y. (2000) DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100, 587–597. [DOI] [PubMed] [Google Scholar]
- 46.Kwajah M. M. S., Schwarz H. (2010) CD137 ligand signaling induces human monocyte to dendritic cell differentiation. Eur. J. Immunol. 40, 1938–1949. [DOI] [PubMed] [Google Scholar]
- 47.Barrie A., Khare A., Henkel M., Zhang Y., Barmada M. M., Duerr R., Ray A. (2011) Prostaglandin E2 and IL-23 plus IL-1β differentially regulate the Th1/Th17 immune response of human CD161+ CD4+ memory T cells. Clin. Transl. Sci. 4, 268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harrington L. E., Hatton R. D., Mangan P. R., Turner H., Murphy T. L., Murphy K. M., Weaver C. T. (2005) Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6, 1123–1132. [DOI] [PubMed] [Google Scholar]
- 49.Zielinski C. E., Mele F., Aschenbrenner D., Jarrossay D., Ronchi F., Gattorno M., Monticelli S., Lanzavecchia A., Sallusto F. (2012) Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature 484, 514–518. [DOI] [PubMed] [Google Scholar]
- 50.Planès R., BenMohamed L., Leghmari K., Delobel P., Izopet J., Bahraoui E. (2014) HIV-1 Tat protein induces PD-L1 (B7-H1) expression on dendritic cells through tumor necrosis factor alpha- and toll-like receptor 4-mediated mechanisms. J. Virol. 88, 6672–6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bergamaschi A., Pancino G. (2010) Host hindrance to HIV-1 replication in monocytes and macrophages. Retrovirology 7, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geijtenbeek T. B., van Kooyk Y. (2003) DC-SIGN: a novel HIV receptor on DCs that mediates HIV-1 transmission. Curr. Top. Microbiol. Immunol. 276, 31–54. [DOI] [PubMed] [Google Scholar]
- 53.Pino M., Erkizia I., Benet S., Erikson E., Fernández-Figueras M. T., Guerrero D., Dalmau J., Ouchi D., Rausell A., Ciuffi A., Keppler O. T., Telenti A., Kräusslich H. G., Martinez-Picado J., Izquierdo-Useros N. (2015) HIV-1 immune activation induces Siglec-1 expression and enhances viral trans-infection in blood and tissue myeloid cells. Retrovirology 12, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fantuzzi L., Purificato C., Donato K., Belardelli F., Gessani S. (2004) Human immunodeficiency virus type 1 gp120 induces abnormal maturation and functional alterations of dendritic cells: a novel mechanism for AIDS pathogenesis. J. Virol. 78, 9763–9772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Granelli-Piperno A., Golebiowska A., Trumpfheller C., Siegal F. P., Steinman R. M. (2004) HIV-1-infected monocyte-derived dendritic cells do not undergo maturation but can elicit IL-10 production and T cell regulation. Proc. Natl. Acad. Sci. USA 101, 7669–7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olliver M., Hiew J., Mellroth P., Henriques-Normark B., Bergman P. (2011) Human monocytes promote Th1 and Th17 responses to Streptococcus pneumoniae. Infect. Immun. 79, 4210–4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Donninelli G., Gessani S., Del Corno M. (2015) Interplay between HIV-1 and Toll-like receptors in human myeloid cells: friend or foe in HIV-1 pathogenesis? J. Leukoc. Biol. 99, 97–105. [DOI] [PubMed] [Google Scholar]
- 58.El Hed A., Khaitan A., Kozhaya L., Manel N., Daskalakis D., Borkowsky W., Valentine F., Littman D. R., Unutmaz D. (2010) Susceptibility of human Th17 cells to human immunodeficiency virus and their perturbation during infection. J. Infect. Dis. 201, 843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guillot-Delost M., Le Gouvello S., Mesel-Lemoine M., Cheraï M., Baillou C., Simon A., Levy Y., Weiss L., Louafi S., Chaput N., Berrehar F., Kerbrat S., Klatzmann D., Lemoine F. M. (2012) Human CD90 identifies Th17/Tc17 T cell subsets that are depleted in HIV-infected patients. J. Immunol. 188, 981–991. [DOI] [PubMed] [Google Scholar]
- 60.Nakayama K., Nakamura H., Koga M., Koibuchi T., Fujii T., Miura T., Iwamoto A., Kawana-Tachikawa A. (2012) Imbalanced production of cytokines by T cells associates with the activation/exhaustion status of memory T cells in chronic HIV type 1 infection. AIDS Res. Hum. Retroviruses 28, 702–714. [DOI] [PubMed] [Google Scholar]
- 61.Bernier A., Cleret-Buhot A., Zhang Y., Goulet J. P., Monteiro P., Gosselin A., DaFonseca S., Wacleche V. S., Jenabian M. A., Routy J. P., Tremblay C., Ancuta P. (2013) Transcriptional profiling reveals molecular signatures associated with HIV permissiveness in Th1Th17 cells and identifies peroxisome proliferator-activated receptor gamma as an intrinsic negative regulator of viral replication. Retrovirology 10, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Potula R., Ramirez S. H., Knipe B., Leibhart J., Schall K., Heilman D., Morsey B., Mercer A., Papugani A., Dou H., Persidsky Y. (2008) Peroxisome proliferator-activated receptor-γ activation suppresses HIV-1 replication in an animal model of encephalitis. AIDS 22, 1539–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Skolnik P. R., Rabbi M. F., Mathys J. M., Greenberg A. S. (2002) Stimulation of peroxisome proliferator-activated receptors α and γ blocks HIV-1 replication and TNFα production in acutely infected primary blood cells, chronically infected U1 cells, and alveolar macrophages from HIV-infected subjects. J. Acquir. Immune Defic. Syndr. 31, 1–10. [DOI] [PubMed] [Google Scholar]
- 64.Ancuta P., Autissier P., Wurcel A., Zaman T., Stone D., Gabuzda D. (2006) CD16+ monocyte-derived macrophages activate resting T cells for HIV infection by producing CCR3 and CCR4 ligands. J. Immunol. 176, 5760–5771. [DOI] [PubMed] [Google Scholar]
- 65.Brenchley J. M., Schacker T. W., Ruff L. E., Price D. A., Taylor J. H., Beilman G. J., Nguyen P. L., Khoruts A., Larson M., Haase A. T., Douek D. C. (2004) CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200, 749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]