ArrB1/Rap2 complex is necessary for chemotactic polarization and migration in neutrophils.
Keywords: GPCR, cell migration, GTPase
Abstract
β-Arrestins have emerged as key regulators of cytoskeletal rearrangement that are required for directed cell migration. Whereas it is known that β-arrestins are required for formyl-Met-Leu-Phe receptor (FPR) recycling, less is known about their role in regulating FPR-mediated neutrophil chemotaxis. Here, we show that β-arrestin 1 (ArrB1) coaccumulated with F-actin within the leading edge of neutrophil-like HL-60 cells during chemotaxis, and its knockdown resulted in markedly reduced migration within fMLP gradients. The small GTPase Ras-related protein 2 (Rap2) was found to bind ArrB1 under resting conditions but dissociated upon fMLP stimulation. The FPR-dependent activation of Rap2 required ArrB1 but was independent of Gαi activity. Significantly, depletion of either ArrB1 or Rap2 resulted in reduced chemotaxis and defects in cellular repolarization within fMLP gradients. These data strongly suggest a model in which FPR is able to direct ArrB1 and other bound proteins that are required for lamellipodial extension to the leading edge in migrating neutrophils, thereby orientating and directing cell migration.
Introduction
During infections by pathogens, such as bacteria and fungi, neutrophils migrate toward these microbes to eliminate them. These microbes shed molecules, including endotoxins and fMLP, forming chemoattractive gradients that guide neutrophils to migrate toward them. fMLP binds to the GPCR family member FPRs [1, 2]. Activation of FPRs induces a number of inflammatory cellular responses, including chemotaxis, degranulation, superoxide production, and phagocytosis [3, 4]. In the resting state, GPCRs bind to heterotrimeric G proteins composed of α, β, and γ subunits. Ligand binding to the extracellular pocket of GPCRs induces heterotrimeric G-protein dissociation into Gα and βγ subunits, which in turn, induce rapid production of a variety of second messengers. A number of these messengers are necessary for the rearrangement of the F-actin cytoskeleton that drives cell migration [5–10].
GRKs phosphorylate activated GPCRs and create binding sites for β-arrestins, which primarily deactivate these GPCRs [11]. β-Arrestins can also regulate signaling events that are G-protein independent, such as Erk1/2 and c-Src activation [12–14]. Over the past few years, β-arrestins have emerged as essential regulators of actin cytoskeleton and also as components involved in directed cell migration in various cancer and immune cells [15–19]. However, the mechanism by which β-arrestins regulate neutrophil chemotaxis is not yet well understood.
β-Arrestins have been shown to act as adaptor/scaffolding proteins that spatially control F-actin nucleation proteins and their upstream regulators for the GPCRs, such as PAR2 and an AT1AR [20–24]. For example, activation of PAR2 promotes the interaction between β-arrestins and the LIM kinase that associates with 2 phosphatases: slingshot and chronophin. These 2 phosphatases dephosphorylate P-cofilin proteins and thus, regulate the activity of cofilin that controls F-actin dynamics and filament reorganization [20, 21]. Rearrangement of F-actin at the leading edge is also controlled by the activity of small GTPases, such as RhoA, Cdc42, or Rac, which all play important roles in cell migration [25–29]. β-Arrestins can regulate small GTPases by modulating the activity of GEFs or GAPs or through activating PI3K to affect F-actin cytoskeleton rearrangement [25, 30, 31].
The interaction between ArrB1 and the Ral-GEF, Ral-GDS, was first identified by yeast two-hybrid screening and subsequently confirmed by coimmunoprecipitation experiments. In HEK293 cells cotransfected with ArrB1 and Ral-GDS, fMLP binding to the FPR promoted Ral activation by Ral-GDS [30], which also associates with members of the Rap family [32, 33]. Rap1 and Rap2 ubiquitously are expressed family members that share 60% identity. Rap signaling is involved in a broad number of cellular processes, including cell proliferation, cell adhesion, cell polarity, cytoskeleton dynamics, and cell motility [31, 34, 35]. Furthermore, in the past decade, a role for Rap proteins in cancer metastasis has emerged [36–38]. Rap1 and Rap2 share the common binding region through which Ras family proteins are thought to exert their biologic effects. However, in spite of their great similarity, the half-life of GTP-Rap2 is significantly longer than that of GTP-Rap1, and both proteins have been found to possess distinct physiologic properties [39–43]. Indeed, it has been shown that in epithelial cells, Rap1 and Rap2 counter-regulates barrier resistance [44]. However, unlike Rap1, Rap2 cannot reverse Ras-induced transformation of NIH3T3 cells [39, 45, 46]. Reports have suggested that Rap1 activation occurs through GPCR activation [47–49]. However, little is known about the function and regulation of Rap2 in these systems.
In this study, we describe a novel role for ArrB1 in fMLP-driven neutrophil chemotaxis. We identified a complex of ArrB1 and Rap2, but not Rap1, which forms under basal conditions. fMLP stimulation induces complex dissociation. fMLP stimulation activates Rap2, this activation required ArrB1 and is independent of heterotrimeric G-proteins. We also showed that ArrB1 and Rap2 are required for regulating cell adhesion and chemotaxis. Our results also shed a light onto how cell polarity is generated to support directional cell migration in a chemoattractant gradient. Therefore, we propose that FPR phosphorylation leads to ArrB1 accumulation at the plasma membrane closest to the source of fMLP. As ArrB1 is bound to Rap2, it thus directs it to the forming leading edge. Once there, it is activated and released to promote firm adhesion and cytoskeletal changes that are necessary for lamellipodial extension.
MATERIALS AND METHODS
Reagents and plasmids
fMLP, phenylmethanesulfonyl fluoride, polybrene, gelatin, fibronectin, and anti-FLAG antibodies were purchased from Sigma-Aldrich (St. Louis, MO, USA). Lipofectamine and G418 were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Complete protease inhibitor cocktail, phosphatase inhibitors tablets, and mouse antibodies against GFP were from Roche Diagnostics (Indianapolis, IN, USA). PTX was purchased from EMD Millipore (Billerica, MA, USA). Mouse antibodies against Rap2 and SHP-1 were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). β-Arrestin and CD11b PE antibody were purchased from BD Biosciences (San Jose, CA, USA). FPR1 and FPR-like 1 antibodies were purchased from Abcam (Cambridge, MA, USA). The ArrB1-eYFP was purchased from OriGene Technologies (Rockville, MD, USA), and the Flag pJLM1 Rap2A clone was purchased from AddGene (Cambridge, MA, USA). pcDNA3-eGFP-Ral-GDS, pMSCVneo and pVSVG, and actin-mCherry were generous gifts from Mark Philips (New York University, New York, NY, USA), Carole A. Parent [National Institutes of Health (NIH), Bethesda, MD, USA], and Youhong Wang (U.S. NIH), respectively.
Cell culture and differentiation
The cell line was obtained from American Type Culture Collection (Manassas, VA, USA) and cultured according to the supplied protocol. HL-60 cells were maintained in an undifferentiated state in RPMI 1640 medium containing 10% FBS and 25 mM HEPES at 37°C in a humidified 5% CO2 atmosphere. HL-60 cells were differentiated in culture media containing 1.3% DMSO for 5 d before experiment.
Purification of PMN
Whole blood was drawn from healthy volunteers at the blood bank of U.S. NIH. Coagulation was prevented by heparin. The majority of the RBCs was removed by dextran (0.2 g/l; GE Healthcare, Pittsburgh, PA, USA) sedimentation (30 min, room temperature). Upper-phase-containing WBCs were collected and washed twice in PBS. Cells were suspended in PBS and were layered on the top of a 5-step Percoll gradient (65, 70, 75, 80, and 85%; Sigma-Aldrich) in 15 ml. After centrifugation (800 g, 20 min at room temperature), the 70–75–80% Percoll layers containing granulocytes were collected and washed twice in PBS. Collected neutrophils were resuspended in PBS, cell concentration was determined, and cells were kept at room temperature until use. The purity of preparations was determined by Wright-Giemsa staining and yielded >95% neutrophil granulocytes.
Construct and transfection of HL-60 cells
Retroviral approach
The ArrB1-eYFP was amplified from the construct, cloned in pMSCVneo vector, and confirmed by sequencing. The expression plasmids were transfected into Phoenix packaging cell lines using lipofectamine. The transiently produced virus was harvested after 72 h. HL-60 cells were infected with these virus particles in with fresh RPMI 1640 culture medium containing 15 µg/ml polybrene and incubated for an additional 48 h. Cells stably expressing the genes were selected in media containing 1 mg/ml G418. Stable clonal populations were generated after 14–21 d and maintained in selection media. Stable ArrB1 KD cells were generated using RNA interference technology.
Lentiviral approach
Undifferentiated HL-60 cells were infected with pLKO.1 lentiviruses (Sigma-Aldrich) carrying the ArrrB1 hairpin sequence 5′-CCGGAGATCTCAGTGCGCCAGTATGCTCGAGCATACTGGCGCACTGAGATCTTTTTTG-3′; the Rap2A hairpin sequence 5′-CCGGGTATGAGAAAGTGCCAGTCATCTCGAGATGACTGGCACTTTCTCATACTTTTTG-3′; and the nontarget mammalian hairpin sequence 5′-CCGGCAACAAGATGAAGAGCACCAACTCGAGTTGGTGCTCTTCATCTTGTTGTTTTT-3′ and selected in media containing puromycin (0.6 µg/ml) for 14–21 d.
The transient transfection with eGFP-Ral-GDS and Flag pJLM1 Rap2A (1:2 molar ratio) was performed by using the Amaxa Nucleofector kit (Solution V and Program Y-001; Lonza, Basel, Switzerland).
Coimmunoprecipitation and immunoblots
In brief, cells (1×107/ml) were preincubated with serum-free RPMI medium supplemented with 25 mM HEPES and 0.1% FBS at 37°C for overnight. Resuspended at 2 × 107 cells/ml fresh serum-free medium and stimulated with 1 µM fMLP or control without fMLP at 37°C for 7 min, cells were lysed with 1% Nonidet P-40 (EMD Millipore) lysis buffer, precleared with protein G magnetic beads (Thermo Fisher Scientific), and immunoprecipitated with either control- or specific antibody-bound protein G magnetic beads at 4°C overnight. After gentle rotation, beads were washed 4 times with lysis buffer, and bound protein was eluted with Laemmli buffer, resolved by SDS-PAGE on a precast gel (4–15% Tris-HCl; Bio-Rad Laboratories, Hercules, CA, USA), and transferred electrophoretically to polyvinylidene difluoride membranes. Filters were probed with specific antibodies, diluted in 20 mM Tris-HCl, pH 8, 150 mM NaCl, and 0.05% Tween 20 (TBST). After washing in TBST, the membranes were incubated with HRP-labeled goat anti- rabbit or goat anti-mouse IgG (The Jackson Laboratory, Bar Harbor, ME, USA), and immunoreactivity was visualized using the ECL system (Thermo Fisher Scientific). Densitometric analysis of the films was performed using NIH ImageJ software.
Rap2 activation assay
A Rap2 activation assay was performed, according to the manufacturer’s instructions (Cell Biolabs, San Diego, CA, USA). Cells were serum starved overnight, with or without PTX, and active Rap2 was pulled down from lysates using agarose beads conjugated to Ral-GDS-RBD and detected by Western blot analysis using mouse anti-Rap2 antibodies.
Confocal imaging
For live cell imaging, neutrophil-like differentiated cells were plated in 1- or 4-well glass-bottom chambers (Nalge Nunc International, Naperville, IL, USA), coated with 0.2% gelatin at 37°C for 1 h. Chambers were washed, and cells were plated. Cells were stimulated at the 1 µM concentration of fMLP. The difference in intracellular fluorescent proteins was directly imaged using a confocal microscope. Images were exported and analyzed with ZEN software (Zeiss, Jena, Germany).
Micropipette chemotaxis assay
Differentiated cells were plated on a single-well chamber with a glass bottom (Nalge Nunc International), which was coated with fibronectin (10 µg/ml), and a chemotactic gradient was generated using an Eppendorf microinjector with Femtotips (Eppendorf, Hamburg, Germany), loaded with 1 µm fMLP.
EZ-TAXIScan chemotaxis assay
Cell migration was recorded every 15 s for 30 min at 37°C in a humidified environmental EZ-TAXIScan chamber (Effector Cell Institute, Tokyo, Japan). Coverslips and chips used in the chamber were coated with 1% BSA at room temperature for 30 min. Cell migration analysis was conducted with DIAS software (DIAS Infrared Systems, Dresden, Germany).
Cell adhesion assay
An adhesion assay was performed as described by Liu et al. [50], with little modification. In brief, 96-well plates were coated with 10 µg/ml fibronectin overnight at 4°C. Wells were washed and blocked with 1% BSA in PBS for 1 h. Cells (2 × 106) were seeded on a precoated well and stimulated for 10 min at 37°C with 1 µM fMLP. Unbound cell were removed by shaking the plate for 10 s and washed 3 times with washing buffer (0.1% BSA, 1% aprotinin in PBS). The remaining cells were fixed at 4% paraformaldehyde and stained with crystal violet (5 mg/ml in 2% ethanol; Sigma-Aldrich), which was extracted with 1% SDS, and absorbance was measured at 570 nm. Values are presented as fold over differentiated HL-60 cells.
Calcium assay
Differentiated HL-60 cells, treated with or without 100 ng/ml PTX, incubated with Fluo-4 (Thermo Fisher Scientific) at a final concentration of 1 µg/ml for 30 min. To remove Fluo-4, cells were washed once and incubated in starving media containing PTX and then washed once. After that, cells were suspended in starving medium and seeded on a gelatin-coated chamber and were subjected to experiment.
Statistical analysis
Data were tested and analyzed by 1-way ANOVA and Student’s t test. Statistical evaluations were performed using GraphPad Prism (GraphPad Software, La Jolla, CA, USA). The difference with a P < 0.05 was considered statistically significant.
RESULTS
ArrB1 redistributed to the leading edge of neutrophil-like HL-60 cells during chemotaxis
The pluripotent hematopoietic cell line HL-60 can be differentiated into neutrophil-like cells using 1.3% DMSO and express chemoattractant GPCRs, including FPRs. These cells are highly chemotactic toward fMLP [51]. We found ArrB1 and ArrB2 levels were unaltered during differentiation (Supplemental Fig. 1A). Differentiation of cells was confirmed by FACS analysis using antibodies against CD11b (Supplemental Fig. 1B).
Actin polymerization is strongly directed toward the leading edge of cells undergoing chemotaxis. The spatiotemporal regulation of actin–cytoskeleton is required for maintaining cell shape and directing cell movement [52, 53]. Activation of FPRs stimulates multiple signaling pathways that control the actin cytoskeleton, which drives extension of lamellipodia for cell migration [50, 54, 55]. Previous studies reported that β-arrestins interact with various proteins that are involved in the formation of the F-actin network required for lamellipodial extension [20, 21, 23]. To study the roles of β-arrestins in neutrophil chemotaxis, we first determined the localization of ArrB1 in HL-60 cells upon fMLP stimulation. We transiently expressed actin-mCherry and also stably expressed eYFP-tagged ArrB1 in HL-60 cells (Supplemental Fig. 1C). In both undifferentiated and differentiated neutrophil-like HL-60 cells, ArrB1-eYFP and actin-mCherry were mainly localized within the cytosol in the resting state (Fig. 1A). Upon uniformly applied fMLP stimulation, the neutrophil-like HL-60 cells became polarized in response to the global stimulation, and ArrB1-eYFP and actin-mCherry were enriched within their leading lamellar edges (Fig. 1A) [50, 56]. We next imaged the cells migrating toward the fMLP-filled micropipette by confocal fluorescence microscopy. We found that ArrB1-eYFP coaccumulated with F-actin at the leading edge of the chemotaxing cells (Fig. 1B). These results suggest that ArrB1 plays a role in lamellipodia formation in response to fMLP stimulation.
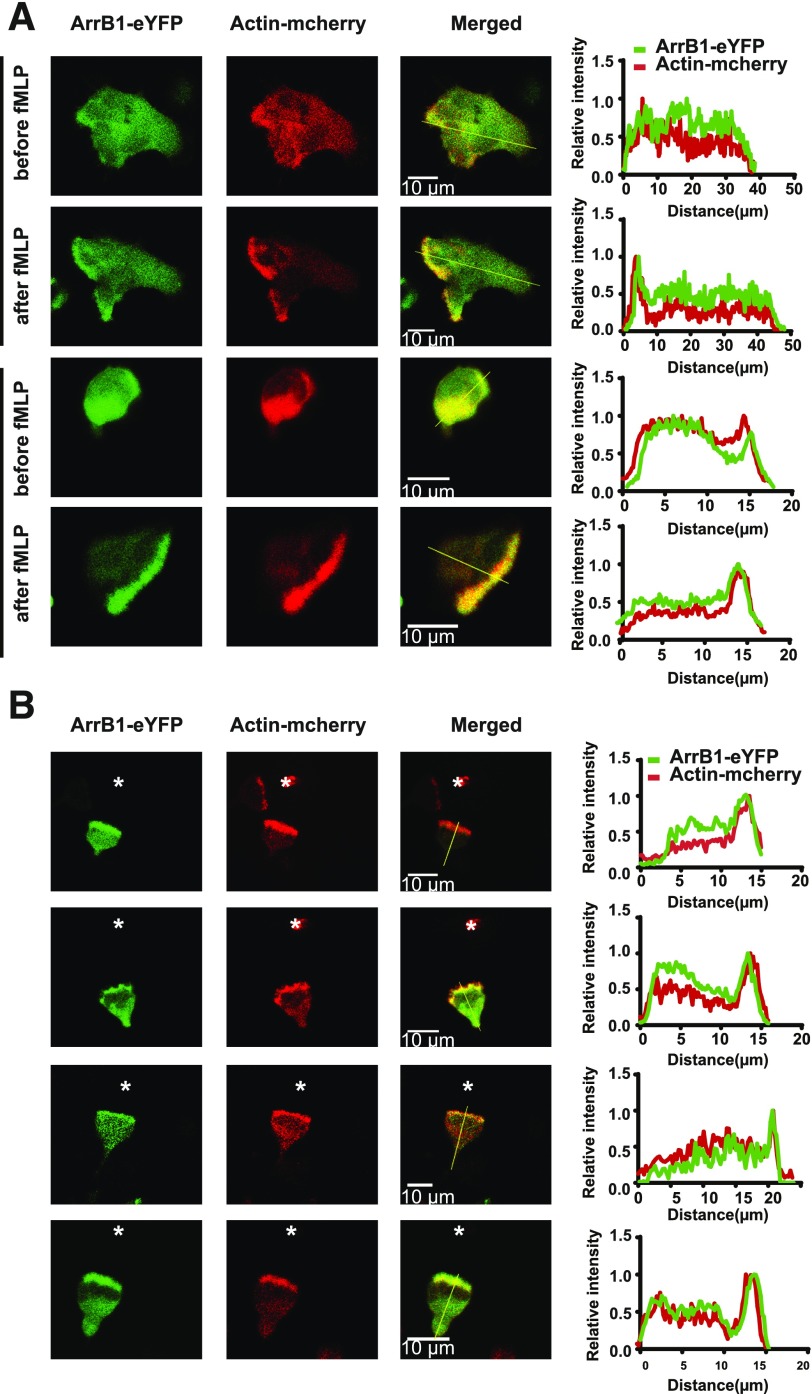
Figure 1. ArrB1 colocalized with F-actin under global or gradient fMLP stimulation.
(A) ArrB1-eYFP and actin-mCherry coaccumulated within the leading edge upon bath stimulation by fMLP (n = 6). (B) ArrB1-eYFP and actin-mCherry coaccumulated at the leading edge of cells (n = 5) during chemotaxis driven by the fMLP gradient generated by a needle source of fMLP (*). The distribution of ArrB1-eYFP and F-actin (actin-mCherry) is shown in green and red, respectively.
ArrB1 is necessary for fMLP-mediated chemotaxis in neutrophil-like HL-60 cells
To examine further the function of ArrB1 in neutrophils, we first generated an ArrB1-knockdown cell line using a lentivirus encoding an shRNA to deplete ArrB1. In ArrB1shRNA-expressing cells, ArrB1 protein levels decreased to ∼10% of the level in WT or NTshRNA cells (Fig. 2A). To test whether ArrB1 plays a role in chemotaxis, we recorded cell movement in an fMLP gradient and determined chemotactic parameters for WT, NTshRNA, ArrB1shRNA, and ArrB1shRNA reconstituted with ArrB1-eYFP cells (ArrB1shRNA + ArrB1-eYFP), using an EZ-TAXIScan analyses. We found that cells with depleted ArrB1 (ArrB1shRNA) displayed a clear defect in directional cell migration (Fig. 2B). Our analyses showed that ArrB1shRNA cells exhibited significantly shorted net path length, lower speed, and reduced directionality and roundness compared with WT or NTshRNA cells (Fig. 2C). In addition, we found that ArrB1shRNA cells displayed a stronger adhesion phenotype, which leads to the chemotaxis defects. Our analyses showed that >50% of ArrB1shRNA cells exhibited retarded migration as a result of uropod retraction, whereas only 1–2% of WT or NTshRNA cells had similar uropod morphology during chemotaxis (Fig. 2D and Supplemental Fig. 2A). These chemotaxis defects were rescued by re-expression of ArrB1-eYFP in ArrB1shRNA cells, and rescued cells were able to reconstitute cell polarization and migration (Fig. 2B and C and Supplemental Fig. 2B). Therefore, these data show that ArrB1 is required for proper cell polarization and efficient chemotaxis in HL-60 cells.
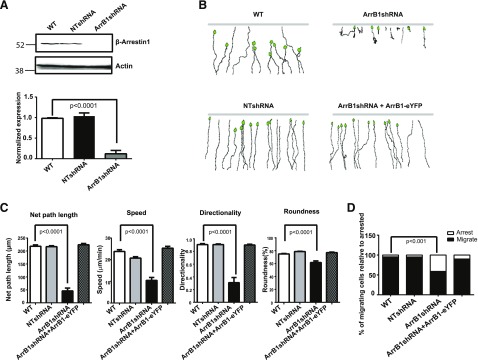
Figure 2. ArrB1 is required for fMLP-mediated chemotaxis.
(A) ArrB1 was depleted using ArrB1-targeting, shRNA-containing virus particles (upper, right lane; lower, right bar). Results are representative of 3 independent experiments. Quantification analysis of the ArrB1 expression level in uninfected, NTshRNA, and ArrB1shRNA cells; means ± sd (n = 3). (B) Tracings of chemotaxis paths toward fMLP of parental cells (WT), ArrB1-depleted cells, nontargeted control (NTshRNA), and ArrB1-depleted cells reconstituted with ArrB1-eYFP. Data are representative of 5 independent experiments. (C) Four different parameters of chemotactic behaviors in these cells were determined: net path length, speed (defined as the distance that the cell’s centroid moves as a function of time); directionality (0 represents random movements, and 1 represents movement in a straight line to the micropipette); and roundness (an indication of cell polarization; a higher value indicates less polarization). (D) Percentage of migrating cells relative to arrested in parental cells, nontargeted control, ArrB1-depleted cells, and ArrB1-depleted cells reconstituted with ArrB1-eYFP during migration in an EZ-TAXIScan chamber assay.
Chemoattractant-induced ArrB1 membrane redistribution is independent of Gαi signaling
In neutrophils, FPR is linked to the PTX-sensitive Gαi subunit for the activation of downstream signaling cascades. To determine whether fMLP-induced membrane redistribution of ArrB1 is dependent on Gαi signaling, cells were pretreated with PTX, a drug to block Gαi signaling. As expected [57, 58], the fMLP-stimulated Ca2+ response (Supplemental Fig. 3A) and Erk1/2 activation, both of which are mediated by Gαi signaling, were abolished (Fig. 3C). However, PTX pretreatment failed to block the fMLP-stimulated membrane redistribution of ArrB1-eYFP, albeit with slight delayed kinetics (Fig. 3A and B). However, with the use of EZ-TAXIScan analyses, we found that migration of all of these cell types was significantly compromised in linear fMLP gradients by PTX treatment (Supplemental Fig. 3B–D). This is consistent with the idea that neutrophil chemotaxis toward fMLP requires GPCR-mediated Gαi signaling [59]. Thus, the binding of fMLP to its receptor induces Gαi-dependent and -independent signaling events. It is known that FPR phosphorylation induced by fMLP is independent of Gαi signaling [58]. Therefore, our results suggest that fMLP-induced membrane redistribution of ArrB1 still proceeds in the absence of Gαi signaling, and ArrB1 plays an important role in fMLP-mediated chemotaxis in neutrophils.
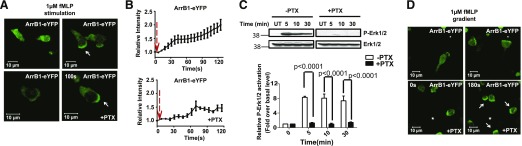
Figure 3. Chemoattractant-induced translocation of ArrB1 in a Gαi-independent fashion.
(A) Overexpressing ArrB1-eYFP HL-60 cells were differentiated into a neutrophil-like state and plated on 0.2% gelatin-coated coverslips. They were then bath stimulated with fMLP (1 μM) and imaged at 37°C. fMLP induced translocation of ArrB1 from the cytoplasm to the cell periphery, developing into polarized accumulation of ArrB1 at the leading edge in both the presence and absence of PTX (100 ng/ml for 3 h). (B) Measurement of membrane translocation of ArrB1-eYFP in response to fMLP stimulation, with or without PTX normalized to unstimulated ArrB1-eYFP distribution (n = 5). (C) fMLP-stimulated Erk1/2 activation was inhibited by similar pretreatment of PTX. Measurement of phosphorylated (P)-Erk1/2 (upper, first row) was normalized with total Erk1/2 (upper, second row). UT, Untreated. (D) Images show the leading-edge localization of ArrB1-eYFP in cells exposed to a 1 µm fMLP gradient. *Positions of the fMLP-containing micropipette. Arrows indicate the leading edge.
The small GTPase Rap2 is associated with ArrB1 in resting cells and dissociates with fMLP stimulation
We next examined the potential role of ArrB1 in a fMLP-driven, leading-edge formation. Previous studies showed that the Ral-GDS is an interacting partner of β-arrestin by the yeast two-hybrid screening and coimmunoprecipitation from human polymorphonuclear cells [30]. Ral-GDS binds to activated forms of Rap1 and Ras in vitro, preferentially to Rap1A rather than Ras [32, 60, 61]. However, the GTP-bound form of Rap2 (active Rap2) also interacts with full-length Ral-GEFs in yeast two-hybrid systems, as well as in vitro [33]. Given the interaction between ArrB1 and Ral-GDS, we decided to investigate if ArrB1 physically interacts with Rap1 and/or Rap2.
With the use of coimmunoprecipitation experiments with anti-GFP antibodies to pull down exogenous ArrB1 from lysates of cells stably overexpressing ArrB1-eYFP, we found that Rap2 bound to ArrB1 in unstimulated cells but dissociated following 7 min of fMLP stimulation (Fig. 4A and B). We chose the 7 min time point, as ArrB1-eYFP starts to localize to endosomes in stably expressing ArrB1-eYFP in differentiating cells, and stimulation was validated by Erk1/2 activation (Supplemental Movie 1 and Supplemental Fig. 4A and B), and >70% of FPR is also internalized near this time point [62, 63]. We also detected the interaction between ArrB1 and FPRs after 7 min of fMLP stimulation (Supplemental Fig. 4C). Interestingly, under the same assay conditions, we were unable to detect an interaction between ArrB1 and either the Rap1A or -B isoform, suggesting that the observed interaction between ArrB1 and Rap2 was specific to this family member (Fig. 4A). The phosphatase SHP-1 and β-arrestin are known to associate constitutively in NK cells [64]. Therefore, we probed the same immunoprecipitates for SHP-1 and found that this interaction also occurs in neutrophils, such as HL-60 cells (Fig. 4A and B). In reverse coimmunoprecipitation experiments using Rap2-specific antibodies, we again detected an interaction between Rap2 and ArrB1 before but significantly reduced following fMLP stimulation (Fig. 4C and D). In addition, we detected SHP-1 in these Rap2 immunoprecipitates. Together, these data demonstrated that Rap2, ArrB1, and SHP-1 form a complex, where both SHP-1 and Rap2 are bound to ArrB1 under resting conditions, and fMLP stimulation induced the release of Rap2 from the complex. The fMLP-induced dissociation of the complex of ArrB1/SHP-1/Rap2 was also confirmed by coimmunoprecipitation experiments using SHP-1-specific antibodies (Fig. 4E–G). However, it is unclear from these data if any given interaction within the complex is direct or indirect.
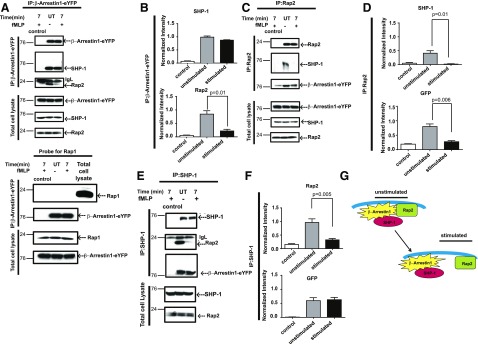
Figure 4. Chemoattractant-dependent dissociation of ArrB1-Rap2 protein complexes.
(A) ArrB1-eYFP-overexpressing neutrophil-like HL-60 cells were stimulated with fMLP (1 µM) for 7 min at 37°C. Lysates were subjected to immunoprecipitation (IP) using GFP-specific mAb, and immunoprecipitated proteins were resolved by SDS-PAGE. Western blots of the resulting gels were probed with specific antibodies against SHP-1, Rap2, and GFP. Rap1 was unaffected in these immunoprecipitates. IgL, . (B) The immunoblots were normalized to the total amount of immunoprecipitated YFP. (C) The Western blots of coimmunoprecipitates protein using control IgG2a and Rap2-specific antibody were probed with antibodies against SHP-1, Rap2, and GFP. (D) The Western blots were quantified and normalized to total amount of Rap2. (E) The Western blots of coimmunoprecipitates protein using control IgG2a and SHP-1-specific antibody were probed with antibodies against SHP-1, Rap2, and GFP. (F) The Western blots were quantified and normalized to the total amount of SHP-1. (G) Model for the formation and disassembly of ArrB1 proteins in complex. IgL, Ig light chain.
fMLP-triggered Rap2 activation is dependent on ArrB1 but not Gαi
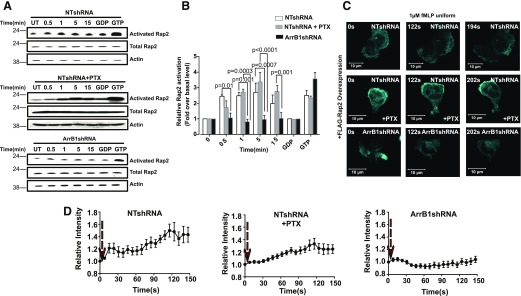
Our observation that fMLP stimulation forces the dissociation of Rap2 from ArrB1 suggested a role for ArrB1 in the regulation of Rap2 signaling. We measured fMLP-induced Rap2 activation in cells of NTshRNA, NTshRNA treated with PTX, and ArrB1shRNA cells. We determined the fMLP-induced levels of Rap2 (GTP-bound) activation by its binding ability to the Ral-GDS-RBD (Fig. 5A). Active Rap proteins bound to the Ral-GDS-RBD were detected by Western blot analysis using Rap2-specific antibodies. In NTshRNA cells, fMLP induced a clear increase in active Rap2 (Fig. 5A and B). To check Rap2 activation in the experiments, we treated NTshRNA cell lysates with GDP to inhibit or GTP-γS to activate Rap2 as negative and positive controls, respectively (Fig. 5A and B). When NTshRNA cells were pretreated with PTX, Rap2 activation was unaltered, indicating that Gαi signaling is not required for fMLP-induced Rap2 activation (Fig. 5A and B). However, fMLP stimulation failed to activate Rap2 in ArrB1shRNA cells, suggesting that fMLP-stimulated Rap2 activation is dependent on ArrB1.
Figure 5. Rap2 activation is independent of Gαi but is dependent on ArrB1.
(A) NTshRNA and NTshRNA-infected cells were pretreated with PTX and ArrB1shRNA stimulated with 1 µM fMLP for the indicated periods of time. Activated Rap2 (Rap2-GTP) was isolated by association with Ral-GDS-RBD immobilized on agarose beads, and captured proteins were detected by Western blots using anti-Rap2-specific antibodies (n = 3). (B) The resulting Western blots were quantified and normalized to Rap2. (C) Activated Rap2 translocated to plasma membrane under fMLP bath stimulation (1 µM) in an ArrB1-dependent manner. Panels show extracted frames from time-lapse confocal microscopy revealing the translocation of eGFP-Ral-GDS from the cytosol to the plasma membrane lamellar edge (7 s intervals). (D) The relative membrane translocation of eGFP-Ral-GDS upon fMLP stimulation in NTshRNA-treated (top; n = 6), NTshRNA + PTX-treated (middle; n = 4), or ArrB1shRNA-treated (bottom; n = 5), differentiated cells.
that ArrB1 is necessary for Rap2 activation, we next tested whether ArrB1 plays a role in its cellular localization. To detect the localization of active Rap2 in live cells, we coexpressed GFP-Ral-GDS, a fluorescence probe for active Rap2, and FLAG-tagged Rap2 as a result of an insufficient level of endogenous Rap2 to support detection [65, 66]. With the use of fluorescence microscopy, we imaged a response to fMLP stimulation in cells cotransfected with FLAG-tagged Rap2 and GFP-Ral-GDS (Fig. 5C and Supplemental Fig. 4D). We found that fMLP stimulation induced the membrane redistribution of GFP-Ral-GDS in both NTshRNA cells and NTshRNA cells treated with PTX. This indicates that fMLP stimulation promotes Rap2 activation that is GαiI independent in proximity to the membrane (Fig. 5C and D). However, the fMLP-induced membrane redistribution of GFP-Ral-GDS was markedly attenuated in ArrB1shRNA cells (Fig. 4C and quantified in Fig. 4D and Supplemental Movies 2–4). As a negative control, we used cells that only expressed eGFP-Ral-GDS (Supplemental Fig. 4E and F). Together, these findings demonstrate that the fMLP-induced activation of Rap2 is mediated through an ArrB1-dependent mechanism that is independent of Gαi activity.
Rap2-GTP regulates chemotaxis in neutrophil-like HL-60 cells
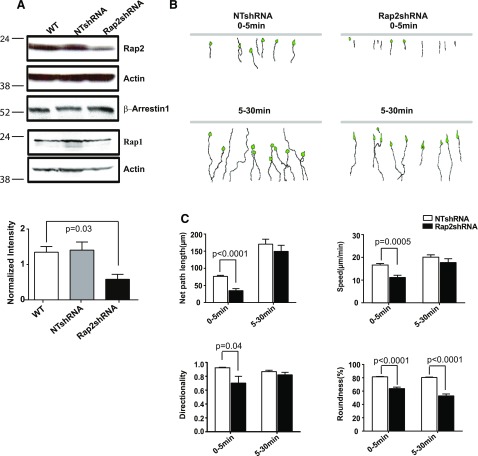
To test the potential role of Rap2 in fMLP-driven neutrophil chemotaxis, we examined chemotaxis of Rap2-depleted HL-60 cells. To establish Rap2-knockdown cells, we designed a Rap2-specific shRNA that was directed toward a common sequence to both Rap2A and -2B isoforms and obtained cells with Rap2 levels decreased by ∼50% (Fig. 6A). Western blot analysis also showed that the expression of ArrB1 and Rap1 was unaffected (Fig. 6A). In EZ-TAXIScan chamber assays, NTshRNA cells displayed normal chemotaxis (Fig. 6B compared with Fig. 2B); therefore, we used NTshRNA cells as the control for this analysis. In sharp contrast, Rap2shRNA cells displayed a dramatically elongated, spindle-shape phenotype over the first 5 min of exposure to the fMLP gradient. Quantification of cellular behavior revealed that Rap2shRNA cells exhibited a significant decrease in net migratory path length, directionality, and speed during the first 5 min of their migration. However, when Rap2shRNA cells were exposed to the fMLP gradient for a longer time, they behaved more like NTshRNA cells. At later timepoints, between 5 and 30 min, both Rap2shRNA cells and control cells moved in a similar manner (Fig. 6C). However, in contrast to other parameters, the roundness of Rap2shRNA cells, an indicator of cell polarization, decreased even further over time, as the cells appeared to stretch as a result of a defect in uropodal detachment (Fig. 6C and Supplemental Fig. 5A). Together, these observations suggested that Rap2 plays a role in uropod retraction in neutrophil chemotaxis, which results in the observed stretching of cell posterior end.
Figure 6. Rap2 KD in HL-60 cells exhibit chemotaxis defects.
(A) Rap2 was knocked down by using Rap2shRNA targeting virus particles (n = 3). Neither ArrB1 nor Rap1 expression was affected by Rap2shRNA. Quantification of Rap2 KD was normalized to actin. (B) Montage showing the path of chemotaxing NTshRNA and Rap2shRNA-infected cells was monitored by EZ-TAXIScan for 0–5 min (upper) or 5–30 min (lower) to show that Rap2shRNA-infected cells exhibited an early migratory defect. (C) Chemotaxis parameters, net path length, migration speed, directionality, and roundness were determined during the first 5 and 5–30 min and quantified using DIAS software.
Rap2 GTPase is required for mediating neutrophil adhesion
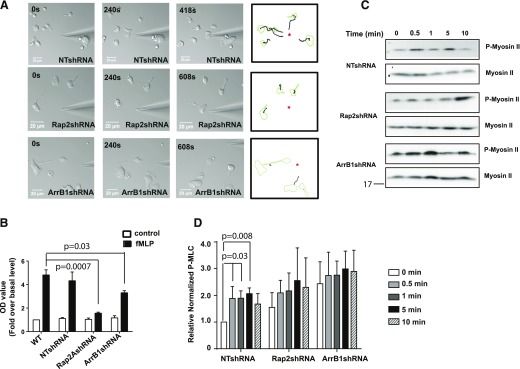
To examine the role of Rap2 in uropod retraction, we performed micropipette migration assays to look at individual cell behavior at higher magnification during fMLP-driven chemotaxis. Whereas NTshRNA cells formed a well-defined leading and trailing edge and migrated with a high chemotactic index toward the micropipette filled with fMLP (Fig. 7A), Rap2shRNA cells failed to migrate effectively and displayed spindle-shaped morphology (Fig. 7A). Furthermore, we found that ArrB1- and Rap2-depleted cells showed multiple similar defects, including those in tail retraction, in directionality toward fMLP (Fig. 7A), and in mobility (Fig. 7A, Supplemental Fig. 5B, and Supplemental Movies 5–7). Together, our observations showed that depletion of either Rap2 or ArrB1 resulted in similar chemotactic phenotypes.
Figure 7. Rap2 GTPase required for neutrophil adhesion.
(A) Rap2- and ArrB1-depleted cells showed defected migration in response to the chemotactic gradient shown in the middle and bottom panels, respectively, when compared with NTshRNA-infected cells (top). Tracings (right) show the migratory paths traveled by cells, as revealed by time-lapse microscopy. (B) Both Rap2- and ArrB1-depleted cells exhibited less fMLP-stimulated adhesion then their uninfected and NTshRNA-infected counterparts. Values are presented as fold over basal level in uninfected cell (n = 3). (C) Rap2- and ArrB1-depleted cells showed defective fMLP-stimulated myosin II activation by using p-MLC-specific antibodies on lysates by Western blot analysis. (D) Quantification revealed no significant stimulation of p-MLC II in Rap2- and ArrB1-depleted cells compared with NTshRNA when normalized to total myosin II on parallel blots. Asterisks indicate needle positions.
During chemotaxis, filopodia protrusions are stabilized by cell-surface receptor interaction with the extracellular matrix. These interactions are necessary to generate the forces required to move the cell forward and also play a role in uropod retraction [67]. Thus, we reasoned that our observed chemotaxis defects might be caused by an alteration in cell adhesion. Therefore, measured the attachment of fMLP-stimulated cells to a fibronectin-coated surface (Fig. 7B). We found that fMLP-stimulated adhesion to fibronectin was significantly decreased in both Rap2- and ArrB1-depleted cells compared with WT and NTshRNA cells (Fig. 7B). Thus, these findings suggested that both Rap2 and ArrB1 play a significant role in the interaction with the extracellular matrix.
Others [68] reported that AT1AR-mediated p-MLC, which is required for cell contraction and migration, is dependent on β-arrestins. Therefore, we examined whether ArrB1 and/or Rap2 play a role in fMLP-stimulated p-MLC II. In NTshRNA cells, fMLP stimulation triggered a rapid increase in p-MLC II level, reaching the peak levels between 1 and 5 min and then decreasing before reaching an apparent equilibrium. However, in both Rap2shRNA and ArrB1shRNA cells, we found that the p-MLC II level was not increased further by fMLP stimulation (Fig. 7C and D). As myosin II supports uropod attachment, this defect in myosin regulation may underlie the defect in uropod detachment seen in cells lacking either ArrB1 or Rap2.
DISCUSSION
Neutrophils constitute the majority of circulating WBCs and play a major role in immunity against bacterial and fungal infections. Chemoattractants shed by invasive organisms, including fMLP, act on GPCRs to guide the migration of neutrophils toward the site of infection. fMLP binding to its receptor, FPR, triggers the dissociation of the Gαi/Gβγ heterotrimer, generating free Gαi and Gβγ dimers [69]. Whereas Gαi mainly mediates the control of ion channels downstream of FPR stimulation, the free Gβγ dimer activates several signaling effectors, including PI3K [70]. The relevance of PI3K function in promoting chemotactic behavior has been studied in various model systems and primary neutrophils. The use of neutrophils from PI3Kγ null mice and the PI3K pan inhibitor, wortmannin, and a PI3Kγ-specific inhibitor, AS-252424 demonstrated a role for PIP3 production during chemotaxis [71]. However, whereas PIP3 plays a role in maintaining efficient migration, its generation cannot be the sole factor determining directionality [72]. Indeed, PIP3 controls pseudopodia production rate but not its direction [73]. β-Arrestins, which have been thought to function primarily in FPR inactivation [11], have also been found to interact with Ral-GDS in a yeast two-hybrid screening. This was confirmed further by coimmunoprecipitation of ArrB1 and Ral-GDS from PMNs [30]. However, the residue responsible for the binding between these 2 proteins remains unclear. In the current paper, we present evidence that Rap2, a Ral-GDS target, also binds ArrB1. This complex was found to exist in unstimulated neutrophil-like HL-60 cells but rapidly dissociated upon fMLP stimulation. These data strongly suggest a mechanism whereby ArrB1 is able to direct the activation of Rap2 in response to fMLP, independent of Gαi signaling. As Rap2 regulates stable adhesion upon its activation, our data suggest that FPR activation recruits this preformed ArrB1/Rap2 complex, and the integrity of this complex is required for lamellipodial stabilization during fMLP-stimulated migration.
β-Arrestins have emerged as important regulators of the actin cytoskeleton that drives the migration of mammalian cells [15]. They have been reported to be involved in regulating various F-actin assembly proteins, including LIM kinase, chronophin, and slingshot. Activation of GPCRs, PAR2 and AT1AR, drive the establishment of the leading edge of the cell by regulating cofilin functions [20, 21, 74]. In our study, we provide evidence for a direct role of ArrB1 on controlling change in the F-actin network during chemotaxis. First, we demonstrated that ArrB1 is localized to lamellipodia, where it coaccumulated with the actin network polarized toward the source of fMLP (Fig. 1). Second, depletion of ArrB1 resulted in chemotaxis defects that included reduced directionality, lower average speed, shorter net path length, and abnormal cell polarization (Fig. 2 and Supplemental Fig. 2). Third, ArrB1 forms a complex with the small GTPase Rap2 that dissociated from ArrB1 upon fMLP stimulation (Fig. 4 and Supplemental Fig. 4), and fMLP-stimulated activation of Rap2 required ArrB1 (Fig. 5). Fourth, Rap2 is also required for cell migration and cell polarity as Rap2-depleted cells also showed some overlapping chemotaxis defects similar to ArrB1-depleted cells (Fig. 6 and Supplemental Fig. 5). Significantly, both ArrB1- and Rap2-depleted cells also showed a defect in p-MLC II, which controls the retraction at the trailing ends of migrating cells (Fig. 7).
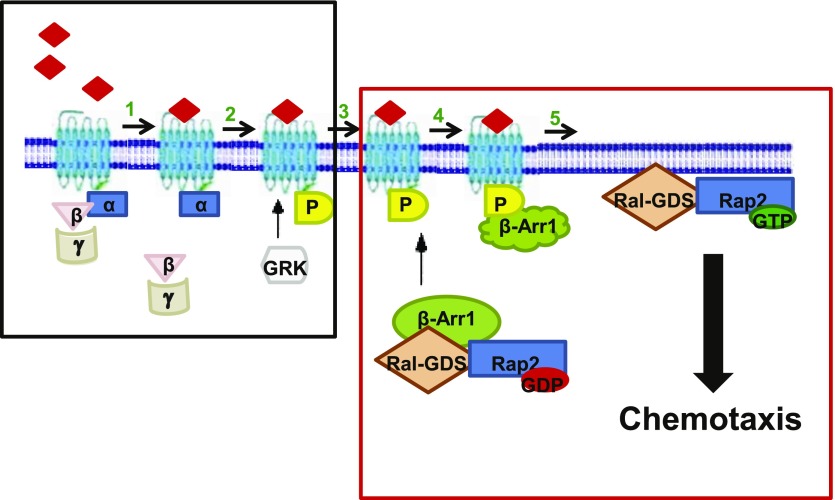
ArrB1 interacts with Ral-GDS [30] that activate Ras, Rap1, and Rap2 small G proteins in PMNs [33, 75, 76]. Similar to the case reported here for Rap2, the β-arrestin/Ral-GDS protein interaction is also disrupted upon FPR stimulation [30]. Thus, combined with our data, we propose that activation of FPR promotes the association between ArrB1 and the phosphorylated FPR receptor. The association of the complex to the FPR and/or membrane induces the release of both ArrB1-associated proteins required for lamellipodial stability and extension (Fig. 8). It is clear from our knockdown data that the integrity of this complex is required for the migration of cells toward the source of chemoattractant.
Figure 8. A schematic diagram illustrating the regulation of Rap2GTPase by ArrB1 in neutrophil-like HL-60 cells.
FPR activation leads to the dissociation of the active Rap2 GTPase-Ral-GDS complex from ArrB1. fMLP binds to the FPR at the plasma membrane. (1) Heterotrimeric G protein dissociates from the FPR after agonist binding. (2) GRK phosphorylates the receptor and creates a high-affinity binding site for ArrB1. (3) The translocation of the ArrB1-Ral-GDS-Rap2 GDP complex to the membrane. (4) ArrB1 binding to the receptor leads to dissociation of the Ral-GDS and Rap2-GDP complex, and Ral-GDS acts as a GEF for Rap2, which leads to activation of Rap2. (5) Required for cell adhesion and migration in response to fMLP stimulation.
Our study also provides evidence that the fMLP-stimulated membrane redistribution of ArrB1 is independent of Gαi. These results are consistent with previous data that showed that PTX treatment failed to affect phosphorylation of the FPR and the translocation of ArrB1/2 to the membrane [58]. However, we observe a time delay for the translocation of ArrB1-eYFP to the lamellipodia following PTX treatment. This slower kinetic of membrane translocation may be explained by a delay of FPR phosphorylation as a result of a direct steric hindrance of the kinases by the PTX-inactivated heterotrimeric G-protein complex. Therefore, these data demonstrate that the ArrB1 complex is required for fMLP-directed migration and does not require heterotrimeric G protein dissociation.
Both Rap1 and -2 have been implicated in a variety of biologic processes that are involved in cytoskeleton rearrangement, cell polarity, and adhesion [77–79]. The means by which the various GEFs and GAPs coordinate the spatiotemporal regulation of Rap signaling in response to fMLP stimulation in migrating cells are not well understood. Our study provides evidence that ArrB1 controls the localization for active Rap2 that acts during the polarization and migration of neutrophils. We show that ArrB1 is necessary for fMLP-induced Rap2 activation, suggesting that the ArrB1-associated pool constitutes a large fraction of the FPR-activated Rap2 in these cells. In addition, we showed that a majority of the activated Ral-GDS is localized in fMLP-stimulated membrane ruffles in cells overexpressing Rap2. Significantly, depletion of ArrB1 in these cells resulted in a failure of Ral-GDS redistribution to membrane ruffles upon fMLP stimulation. Therefore, these data show that the ArrB1-associated pool of Rap2 and Ral-GDS both require ArrB1 for their proper fMLP-stimulated intracellular localization. This is supported further by previous results showing that the expression of the N-terminal domain of ArrB1 blocked the membrane translocation of GFP-Ral-GDS in HEK293 cells [30].
Cells depleted of ArrB1 or Rap2 exhibited defects in uropod retraction during chemotaxis (Fig. 7 and Supplemental Figs. 3 and 6). Similar defects have been reported in cells lacking Rap1 or cells overexpressing Rap1-GAP [80, 81]. Rap1A-depleted neutrophils were unable to form tight adhesion at their leading edge, resulting in a spindle-shaped morphology with slowed uropod retraction. However Liu et al. [81] suggest that Rap1 controls uropod retraction through the Ras association and DIL domain protein (Radil), which is a regulator of β2-integrin-mediated adhesion. We found that Rap1 levels were unaffected in Rap2-depleted cells, and ArrB1 binds Rap2 not Rap1. In addition, our data revealed that adhesion to fibronectin-coated surfaces was decreased in both ArrB1 and Rap2 KD cells. Therefore, taken together, we postulate that both Rap GTPases ultimately regulate the pathways that govern adhesion and retraction of the trailing ends of chemotaxing cells. As Rap2-depleted cells were still capable of moving toward fMLP, we believe Rap1 and Rap2 may operate in separate pathways that are both necessary but individually insufficient to drive these processes. However, ArrB1-knockdown cells exhibited a verity of additional chemotactic defects, including speed, directionality, and net path length. As the ArrB1 complex likely controls effector functions, in addition to Rap2, it would be expected that the phenotype of a Rap2 KD is likely to constitute only a subset of the phenotypic effect caused by the KD of ArrB1. We also observed dysregulation of myosin phosphorylation in these cells, which may be related to observed defects in uropod retraction and migration [68].
In summary, data presented here revealed a novel signaling mechanism that allows the FPR to govern the directed cell migration toward the source of its ligand. The ArrB1 complex illuminated here contains several factors with known regulatory and effector functions to promote adhesion and lamellipodial stabilization during cell migration. As ArrB1 binds to FPR, phosphorylated downstream of its activation, it would be able to coordinate the recruitment of these proteins to the leading edge of the cells defined by an fMLP gradient. Thus, fMLP-induced ArrB1/Rap2 signaling regulates repolarization of the actin cytoskeleton for chemotaxis directly downstream of the FPR. Additional studies will be required to define further the regulatory interplay among the members of this ArrB1 complex, including Ral-GDS, Rap2, and SHP-1.
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program of the U.S. National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases Grant AI001156 (to Tian Jin); and NIH National Heart, Lung, and Blood Institute Grant R01-HL116327 (to David E. Golan). The authors thank David E. Golan for his support of N.G.
Glossary
- ArrB1/2
β-arrestin 1/2
- AT1AR
angiotensin II type 1A receptor
- eYFP
enhanced yellow fluorescent protein
- FPR
formyl-Met-Leu-Phe receptor
- GAP
GTPase-activating protein
- Ral-GDS
Ras-like GTPase GDP dissociation stimulator
- GEF
guanine nucleotide exchange factor
- GPCR
G protein-coupled receptor
- GRK
G protein-coupled receptor kinase
- HEK293
human embryonic kidney 293
- KD
knock down
- NIH
National Institutes of Health
- NTshRNA
nontarget (control) small hairpin RNA
- p-MLC
phosphorylated myosin light-chain
- PAR2
protease-activated receptor 2
- PIP3
phosphatidylinositol 3,4,5-trisphosphate
- PMN
polymorphonuclear neutrophil
- PTX
pertussis toxin
- Ral
Ras-like GTPase
- Rap
Ras-related protein
- RBD
Ras-like GTPase guanine nucleotide dissociation stimulator-binding domain
- SHP-1
Src homology region 2 domain-containing phosphatase 1
- shRNA
small hairpin RNA
- WT
wild-type
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Prossnitz E. R., Ye R. D. (1997) The N-formyl peptide receptor: a model for the study of chemoattractant receptor structure and function. Pharmacol. Ther. 74, 73–102. [DOI] [PubMed] [Google Scholar]
- 2.Selvatici R., Falzarano S., Mollica A., Spisani S. (2006) Signal transduction pathways triggered by selective formylpeptide analogues in human neutrophils. Eur. J. Pharmacol. 534, 1–11. [DOI] [PubMed] [Google Scholar]
- 3.Schiffmann E., Corcoran B. A., Wahl S. M. (1975) N-Formylmethionyl peptides as chemoattractants for leucocytes. Proc. Natl. Acad. Sci. USA 72, 1059–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haslett C., Savill J. S., Meagher L. (1989) The neutrophil. Curr. Opin. Immunol. 2, 10–18. [DOI] [PubMed] [Google Scholar]
- 5.Bagorda A., Mihaylov V. A., Parent C. A. (2006) Chemotaxis: moving forward and holding on to the past. Thromb. Haemost. 95, 12–21. [PubMed] [Google Scholar]
- 6.Bagorda A., Parent C. A. (2008) Eukaryotic chemotaxis at a glance. J. Cell Sci. 121, 2621–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall R. A., Premont R. T., Lefkowitz R. J. (1999) Heptahelical receptor signaling: beyond the G protein paradigm. J. Cell Biol. 145, 927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janetopoulos C., Jin T., Devreotes P. (2001) Receptor-mediated activation of heterotrimeric G-proteins in living cells. Science 291, 2408–2411. [DOI] [PubMed] [Google Scholar]
- 9.Jin T., Xu X., Hereld D. (2008) Chemotaxis, chemokine receptors and human disease. Cytokine 44, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parent C. A. (2004) Making all the right moves: chemotaxis in neutrophils and Dictyostelium. Curr. Opin. Cell Biol. 16, 4–13. [DOI] [PubMed] [Google Scholar]
- 11.Bennett T. A., Maestas D. C., Prossnitz E. R. (2000) Arrestin binding to the G protein-coupled N-formyl peptide receptor is regulated by the conserved “DRY” sequence. J. Biol. Chem. 275, 24590–24594. [DOI] [PubMed] [Google Scholar]
- 12.Lohse M. J., Benovic J. L., Codina J., Caron M. G., Lefkowitz R. J. (1990) beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science 248, 1547–1550. [DOI] [PubMed] [Google Scholar]
- 13.Li B., Wang C., Zhou Z., Zhao J., Pei G. (2013) β-Arrestin-1 directly interacts with Gαs and regulates its function. FEBS Lett. 587, 410–416. [DOI] [PubMed] [Google Scholar]
- 14.Luttrell L. M., Ferguson S. S., Daaka Y., Miller W. E., Maudsley S., Della Rocca G. J., Lin F., Kawakatsu H., Owada K., Luttrell D. K., Caron M. G., Lefkowitz R. J. (1999) Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science 283, 655–661. [DOI] [PubMed] [Google Scholar]
- 15.Min J., Defea K. (2011) β-Arrestin-dependent actin reorganization: bringing the right players together at the leading edge. Mol. Pharmacol. 80, 760–768. [DOI] [PubMed] [Google Scholar]
- 16.Ge L., Shenoy S. K., Lefkowitz R. J., DeFea K. (2004) Constitutive protease-activated receptor-2-mediated migration of MDA MB-231 breast cancer cells requires both beta-arrestin-1 and -2. J. Biol. Chem. 279, 55419–55424. [DOI] [PubMed] [Google Scholar]
- 17.Barnes W. G., Reiter E., Violin J. D., Ren X. R., Milligan G., Lefkowitz R. J. (2005) Beta-arrestin 1 and Galphaq/11 coordinately activate RhoA and stress fiber formation following receptor stimulation. J. Biol. Chem. 280, 8041–8050. [DOI] [PubMed] [Google Scholar]
- 18.Hunton D. L., Barnes W. G., Kim J., Ren X. R., Violin J. D., Reiter E., Milligan G., Patel D. D., Lefkowitz R. J. (2005) Beta-arrestin 2-dependent angiotensin II type 1A receptor-mediated pathway of chemotaxis. Mol. Pharmacol. 67, 1229–1236. [DOI] [PubMed] [Google Scholar]
- 19.DeWire S. M., Ahn S., Lefkowitz R. J., Shenoy S. K. (2007) Beta-arrestins and cell signaling. Annu. Rev. Physiol. 69, 483–510. [DOI] [PubMed] [Google Scholar]
- 20.Zoudilova M., Kumar P., Ge L., Wang P., Bokoch G. M., DeFea K. A. (2007) Beta-arrestin-dependent regulation of the cofilin pathway downstream of protease-activated receptor-2. J. Biol. Chem. 282, 20634–20646. [DOI] [PubMed] [Google Scholar]
- 21.Zoudilova M., Min J., Richards H. L., Carter D., Huang T., DeFea K. A. (2010) Beta-arrestins scaffold cofilin with chronophin to direct localized actin filament severing and membrane protrusions downstream of protease-activated receptor-2. J. Biol. Chem. 285, 14318–14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao K., McClatchy D. B., Shukla A. K., Zhao Y., Chen M., Shenoy S. K., Yates J. R. III, Lefkowitz R. J. (2007) Functional specialization of beta-arrestin interactions revealed by proteomic analysis. Proc. Natl. Acad. Sci. USA 104, 12011–12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao K., Sun J., Kim J., Rajagopal S., Zhai B., Villén J., Haas W., Kovacs J. J., Shukla A. K., Hara M. R., Hernandez M., Lachmann A., Zhao S., Lin Y., Cheng Y., Mizuno K., Ma’ayan A., Gygi S. P., Lefkowitz R. J. (2010) Global phosphorylation analysis of beta-arrestin-mediated signaling downstream of a seven transmembrane receptor (7TMR). Proc. Natl. Acad. Sci. USA 107, 15299–15304 [Erratum 2012 Aug 14;109, 13464]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Firat-Karalar E. N., Welch M. D. (2011) New mechanisms and functions of actin nucleation. Curr. Opin. Cell Biol. 23, 4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mythreye K., Blobe G. C. (2009) The type III TGF-beta receptor regulates epithelial and cancer cell migration through beta-arrestin2-mediated activation of Cdc42. Proc. Natl. Acad. Sci. USA 106, 8221–8226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anthony D. F., Sin Y. Y., Vadrevu S., Advant N., Day J. P., Byrne A. M., Lynch M. J., Milligan G., Houslay M. D., Baillie G. S. (2011) β-Arrestin 1 inhibits the GTPase-activating protein function of ARHGAP21, promoting activation of RhoA following angiotensin II type 1A receptor stimulation. Mol. Cell. Biol. 31, 1066–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lodeiro M., Alén B. O., Mosteiro C. S., Beiroa D., Nogueiras R., Theodoropoulou M., Pardo M., Gallego R., Pazos Y., Casanueva F. F., Camiña J. P. (2011) The SHP-1 protein tyrosine phosphatase negatively modulates Akt signaling in the ghrelin/GHSR1a system. Mol. Biol. Cell 22, 4182–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Povsic T. J., Kohout T. A., Lefkowitz R. J. (2003) Beta-arrestin1 mediates insulin-like growth factor 1 (IGF-1) activation of phosphatidylinositol 3-kinase (PI3K) and anti-apoptosis. J. Biol. Chem. 278, 51334–51339. [DOI] [PubMed] [Google Scholar]
- 29.Elsaesser R., Kalra D., Li R., Montell C. (2010) Light-induced translocation of Drosophila visual Arrestin2 depends on Rac2. Proc. Natl. Acad. Sci. USA 107, 4740–4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhattacharya M., Anborgh P. H., Babwah A. V., Dale L. B., Dobransky T., Benovic J. L., Feldman R. D., Verdi J. M., Rylett R. J., Ferguson S. S. (2002) Beta-arrestins regulate a Ral-GDS Ral effector pathway that mediates cytoskeletal reorganization. Nat. Cell Biol. 4, 547–555. [DOI] [PubMed] [Google Scholar]
- 31.Alemayehu M., Dragan M., Pape C., Siddiqui I., Sacks D. B., Di Guglielmo G. M., Babwah A. V., Bhattacharya M. (2013) β-Arrestin2 regulates lysophosphatidic acid-induced human breast tumor cell migration and invasion via Rap1 and IQGAP1. PLoS One 8, e56174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herrmann C., Horn G., Spaargaren M., Wittinghofer A. (1996) Differential interaction of the ras family GTP-binding proteins H-Ras, Rap1A, and R-Ras with the putative effector molecules Raf kinase and Ral-guanine nucleotide exchange factor. J. Biol. Chem. 271, 6794–6800. [DOI] [PubMed] [Google Scholar]
- 33.Nancy V., Wolthuis R. M., de Tand M. F., Janoueix-Lerosey I., Bos J. L., de Gunzburg J. (1999) Identification and characterization of potential effector molecules of the Ras-related GTPase Rap2. J. Biol. Chem. 274, 8737–8745. [DOI] [PubMed] [Google Scholar]
- 34.Bos J. L. (2005) Linking Rap to cell adhesion. Curr. Opin. Cell Biol. 17, 123–128. [DOI] [PubMed] [Google Scholar]
- 35.Gérard A., Mertens A. E., van der Kammen R. A., Collard J. G. (2007) The Par polarity complex regulates Rap1- and chemokine-induced T cell polarization. J. Cell Biol. 176, 863–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin K. B., Tan P., Freeman S. A., Lam M., McNagny K. M., Gold M. R. (2010) The Rap GTPases regulate the migration, invasiveness and in vivo dissemination of B-cell lymphomas. Oncogene 29, 608–615. [DOI] [PubMed] [Google Scholar]
- 37.Furstenau D. K., Mitra N., Wan F., Lewis R., Feldman M. D., Fraker D. L., Guvakova M. A. (2011) Ras-related protein 1 and the insulin-like growth factor type I receptor are associated with risk of progression in patients diagnosed with carcinoma in situ. Breast Cancer Res. Treat. 129, 361–372. [DOI] [PubMed] [Google Scholar]
- 38.Itoh M., Nelson C. M., Myers C. A., Bissell M. J. (2007) Rap1 integrates tissue polarity, lumen formation, and tumorigenic potential in human breast epithelial cells. Cancer Res. 67, 4759–4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kitayama H., Sugimoto Y., Matsuzaki T., Ikawa Y., Noda M. (1989) A ras-related gene with transformation suppressor activity. Cell 56, 77–84. [DOI] [PubMed] [Google Scholar]
- 40.Janoueix-Lerosey I., Polakis P., Tavitian A., de Gunzburg J. (1992) Regulation of the GTPase activity of the ras-related rap2 protein. Biochem. Biophys. Res. Commun. 189, 455–464. [DOI] [PubMed] [Google Scholar]
- 41.Kawata M., Matsui Y., Kondo J., Hishida T., Teranishi Y., Takai Y. (1988) A novel small molecular weight GTP-binding protein with the same putative effector domain as the ras proteins in bovine brain membranes. Purification, determination of primary structure, and characterization. J. Biol. Chem. 263, 18965–18971. [PubMed] [Google Scholar]
- 42.Pizon V., Chardin P., Lerosey I., Olofsson B., Tavitian A. (1988) Human cDNAs rap1 and rap2 homologous to the Drosophila gene Dras3 encode proteins closely related to ras in the ‘effector’ region. Oncogene 3, 201–204. [PubMed] [Google Scholar]
- 43.Pizon V., Lerosey I., Chardin P., Tavitian A. (1988) Nucleotide sequence of a human cDNA encoding a ras-related protein (rap1B). Nucleic Acids Res. 16, 7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pannekoek W. J., Linnemann J. R., Brouwer P. M., Bos J. L., Rehmann H. (2013) Rap1 and Rap2 antagonistically control endothelial barrier resistance. PLoS One 8, e57903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jimenez B., Pizon V., Lerosey I., Beranger F., Tavitian A., de Gunzburg J. (1991) Effects of the ras-related rap2 protein on cellular proliferation. Int. J. Cancer 49, 471–479. [DOI] [PubMed] [Google Scholar]
- 46.Ohba Y., Mochizuki N., Matsuo K., Yamashita S., Nakaya M., Hashimoto Y., Hamaguchi M., Kurata T., Nagashima K., Matsuda M. (2000) Rap2 as a slowly responding molecular switch in the Rap1 signaling cascade. Mol. Cell. Biol. 20, 6074–6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelley G. G., Reks S. E., Smrcka A. V. (2004) Hormonal regulation of phospholipase Cepsilon through distinct and overlapping pathways involving G12 and Ras family G-proteins. Biochem. J. 378, 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohtsuka T., Shimizu K., Yamamori B., Kuroda S., Takai Y. (1996) Activation of brain B-Raf protein kinase by Rap1B small GTP-binding protein. J. Biol. Chem. 271, 1258–1261. [DOI] [PubMed] [Google Scholar]
- 49.Weissman J. T., Ma J. N., Essex A., Gao Y., Burstein E. S. (2004) G-Protein-coupled receptor-mediated activation of rap GTPases: characterization of a novel Galphai regulated pathway. Oncogene 23, 241–249. [DOI] [PubMed] [Google Scholar]
- 50.Liu L., Das S., Losert W., Parent C. A. (2010) mTORC2 regulates neutrophil chemotaxis in a cAMP- and RhoA-dependent fashion. Dev. Cell 19, 845–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Millius A., Weiner O. D. (2009) Chemotaxis in neutrophil-like HL-60 cells. Methods Mol. Biol. 571, 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watanabe N., Mitchison T. J. (2002) Single-molecule speckle analysis of actin filament turnover in lamellipodia. Science 295, 1083–1086. [DOI] [PubMed] [Google Scholar]
- 53.Miyoshi T., Tsuji T., Higashida C., Hertzog M., Fujita A., Narumiya S., Scita G., Watanabe N. (2006) Actin turnover-dependent fast dissociation of capping protein in the dendritic nucleation actin network: evidence of frequent filament severing. J. Cell Biol. 175, 947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Millius A., Watanabe N., Weiner O. D. (2012) Diffusion, capture and recycling of SCAR/WAVE and Arp2/3 complexes observed in cells by single-molecule imaging. J. Cell Sci. 125, 1165–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weiner O. D., Marganski W. A., Wu L. F., Altschuler S. J., Kirschner M. W. (2007) An actin-based wave generator organizes cell motility. PLoS Biol. 5, e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weiner O. D., Rentel M. C., Ott A., Brown G. E., Jedrychowski M., Yaffe M. B., Gygi S. P., Cantley L. C., Bourne H. R., Kirschner M. W. (2006) Hem-1 complexes are essential for Rac activation, actin polymerization, and myosin regulation during neutrophil chemotaxis. PLoS Biol. 4, e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gripentrog J. M., Miettinen H. M. (2005) Activation and nuclear translocation of ERK1/2 by the formyl peptide receptor is regulated by G protein and is not dependent on beta-arrestin translocation or receptor endocytosis. Cell. Signal. 17, 1300–1311. [DOI] [PubMed] [Google Scholar]
- 58.Gripentrog J. M., Miettinen H. M. (2008) Formyl peptide receptor-mediated ERK1/2 activation occurs through G(i) and is not dependent on beta-arrestin1/2. Cell. Signal. 20, 424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu J., Wang F., Van Keymeulen A., Herzmark P., Straight A., Kelly K., Takuwa Y., Sugimoto N., Mitchison T., Bourne H. R. (2003) Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell 114, 201–214. [DOI] [PubMed] [Google Scholar]
- 60.Urano T., Emkey R., Feig L. A. (1996) Ral-GTPases mediate a distinct downstream signaling pathway from Ras that facilitates cellular transformation. EMBO J. 15, 810–816. [PMC free article] [PubMed] [Google Scholar]
- 61.Kishida S., Koyama S., Matsubara K., Kishida M., Matsuura Y., Kikuchi A. (1997) Colocalization of Ras and Ral on the membrane is required for Ras-dependent Ral activation through Ral GDP dissociation stimulator. Oncogene 15, 2899–2907. [DOI] [PubMed] [Google Scholar]
- 62.Maestes D. C., Potter R. M., Prossnitz E. R. (1999) Differential phosphorylation paradigms dictate desensitization and internalization of the N-formyl peptide receptor. J. Biol. Chem. 274, 29791–29795. [DOI] [PubMed] [Google Scholar]
- 63.Potter R. M., Maestas D. C., Cimino D. F., Prossnitz E. R. (2006) Regulation of N-formyl peptide receptor signaling and trafficking by individual carboxyl-terminal serine and threonine residues. J. Immunol. 176, 5418–5425. [DOI] [PubMed] [Google Scholar]
- 64.Yu M. C., Su L. L., Zou L., Liu Y., Wu N., Kong L., Zhuang Z. H., Sun L., Liu H. P., Hu J. H., Li D., Strominger J. L., Zang J. W., Pei G., Ge B. X. (2008) An essential function for beta-arrestin 2 in the inhibitory signaling of natural killer cells. Nat. Immunol. 9, 898–907. [DOI] [PubMed] [Google Scholar]
- 65.Bivona T. G., Philips M. R. (2005) Analysis of Ras and Rap activation in living cells using fluorescent Ras binding domains. Methods 37, 138–145. [DOI] [PubMed] [Google Scholar]
- 66.Bivona T. G., Quatela S., Philips M. R. (2006) Analysis of Ras activation in living cells with GFP-RBD. Methods Enzymol. 407, 128–143. [DOI] [PubMed] [Google Scholar]
- 67.Lefort C. T., Kim M. (2010) Human T lymphocyte isolation, culture and analysis of migration in vitro. J. Vis. Exp. 40, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simard E., Kovacs J. J., Miller W. E., Kim J., Grandbois M., Lefkowitz R. J. (2013) β-Arrestin regulation of myosin light chain phosphorylation promotes AT1aR-mediated cell contraction and migration. PLoS One 8, e80532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Futosi K., Fodor S., Mócsai A. (2013) Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int. Immunopharmacol. 17, 638–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Neer E. J. (1995) Heterotrimeric G proteins: organizers of transmembrane signals. Cell 80, 249–257. [DOI] [PubMed] [Google Scholar]
- 71.Ferguson G. J., Milne L., Kulkarni S., Sasaki T., Walker S., Andrews S., Crabbe T., Finan P., Jones G., Jackson S., Camps M., Rommel C., Wymann M., Hirsch E., Hawkins P., Stephens L. (2007) PI(3)Kgamma has an important context-dependent role in neutrophil chemokinesis. Nat. Cell Biol. 9, 86–91. [DOI] [PubMed] [Google Scholar]
- 72.King J. S., Insall R. H. (2009) Chemotaxis: finding the way forward with Dictyostelium. Trends Cell Biol. 19, 523–530. [DOI] [PubMed] [Google Scholar]
- 73.Andrew N., Insall R. H. (2007) Chemotaxis in shallow gradients is mediated independently of PtdIns 3-kinase by biased choices between random protrusions. Nat. Cell Biol. 9, 193–200. [DOI] [PubMed] [Google Scholar]
- 74.DeFea K. A. (2011) Beta-arrestins as regulators of signal termination and transduction: how do they determine what to scaffold? Cell. Signal. 23, 621–629. [DOI] [PubMed] [Google Scholar]
- 75.Ikeda M., Koyama S., Okazaki M., Dohi K., Kikuchi A. (1995) rap1 p21 regulates the interaction of ras p21 with RGL, a new effector protein of ras p21. FEBS Lett. 375, 37–40. [DOI] [PubMed] [Google Scholar]
- 76.Peterson J. E., Kulik G., Jelinek T., Reuter C. W., Shannon J. A., Weber M. J. (1996) Src phosphorylates the insulin-like growth factor type I receptor on the autophosphorylation sites. Requirement for transformation by src. J. Biol. Chem. 271, 31562–31571. [DOI] [PubMed] [Google Scholar]
- 77.Kooistra M. R., Dubé N., Bos J. L. (2007) Rap1: a key regulator in cell-cell junction formation. J. Cell Sci. 120, 17–22. [DOI] [PubMed] [Google Scholar]
- 78.Kortholt A., van Haastert P. J. (2008) Highlighting the role of Ras and Rap during Dictyostelium chemotaxis. Cell. Signal. 20, 1415–1422. [DOI] [PubMed] [Google Scholar]
- 79.Raaijmakers J. H., Bos J. L. (2009) Specificity in Ras and Rap signaling. J. Biol. Chem. 284, 10995–10999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.He Y., Kapoor A., Cook S., Liu S., Xiang Y., Rao C. V., Kenis P. J., Wang F. (2011) The non-receptor tyrosine kinase Lyn controls neutrophil adhesion by recruiting the CrkL-C3G complex and activating Rap1 at the leading edge. J. Cell Sci. 124, 2153–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu L., Aerbajinai W., Ahmed S. M., Rodgers G. P., Angers S., Parent C. A. (2012) Radil controls neutrophil adhesion and motility through β2-integrin activation. Mol. Biol. Cell 23, 4751–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]