Review of how exposure of bacteria to antibiotics can affect how the immune system recognizes microbes.
Keywords: peptidoglycan, Staphylococcus aureus, autolysis, β-lactam
Abstract
Antibiotics have proven to be enormously effective tools in combating infectious diseases. A common roadblock to the effective use of antibiotics is the development of antibiotic resistance. We have recently observed that the very mechanism by which methicillin-resistant Staphylococcus aureus (MRSA) becomes antibiotic resistant causes the organism to be more inflammatory to innate immune cells. In this review, we offer some thoughts on the ways in which antibiotics have been observed to influence immune responses to bacteria.
Introduction
Since the initial discovery of penicillin in 1928 and its development as a drug in the 1940s, antibiotics have served as one of the marvels of modern medicine. Antibiotics are used medically to kill bacteria, and they have proven remarkably effective and saved innumerable lives. However, with the use of antibiotics, we also impact how our immune system detects and responds to bacteria, which can have beneficial or harmful effects on the outcome of an infection.
Antibiotics in vivo work in conjunction with host immunity, and there is evidence that impact of antibiotic exposure is to allow for the immune system to recognize bacteria better. Indeed, antibiotics are often not as efficacious in immunocompromised individuals as might be expected, suggesting that part of the mechanism by which antibiotics work is through synergy with the immune system [1]. Likewise, Sakoulas and colleagues [2] have observed, using nafcillin-sensitized MRSA, that killing is ultimately mediated by human defense peptides produced by neutrophils and keratinocytes, although the underlying mechanism or this sensitivity is unclear. Muller et al. [3] have observed that treatment of MRSA with β-lactams reduces peptidoglycan cross-links and thereby, facilitates bacterial killing by the host lysozyme. Thus, how antibiotics affect immune responses to bacteria may have a direct impact on the efficacy of treatments.
Conversely, with increased inflammatory potential, antibiotics have the potential to induce significant immunopathology, as will be discussed below. We speculate that there are circumstances during which the benefits of inflammation outweigh the adverse effects. For example, for low inoculum infections and infections at sites where tissues regenerate readily (e.g., minor skin infection), enhanced immune clearance of the pathogen by antibiotics would be beneficial. Conversely, for prolonged or high inoculum infections where the increased host inflammation does not rapidly contribute to the clearance of the pathogen and causes substantial immunopathology (overwhelming sepsis) or for infections in host compartments where tissue regeneration is suboptimal (e.g., the brain), inflammation induced by antibiotics could lead to more harm than good.
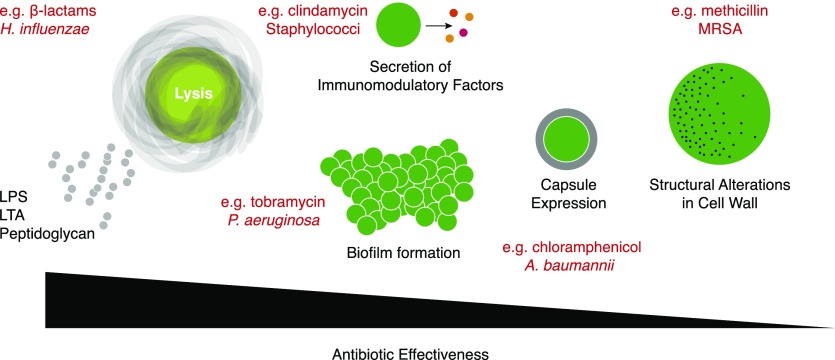
In this review, we reflect on some of the mechanisms by which antibiotics affect inflammatory responses during infection (Fig. 1). Specifically, we consider that when highly effective, antibiotics can kill bacteria, leading to the release of inflammatory molecules; when poorly effective (as, for example, upon exposure to subinhibitory concentrations), antibiotics can induce changes in bacterial gene expression and growth properties that influence immune responses; and when antibiotic resistance develops, the mechanisms of resistance can influence inflammatory responses.
Figure 1. Effects of antibiotics on bacteria that can influence inflammatory responses.
When highly effective, antibiotics can kill bacteria, leading to release of soluble PAMPS. When less effective, bacteria can respond to the presence of antibiotics by increasing production of virulence factors or changing growth properties (e.g., form biofilms), responses that can either dampen or exacerbate inflammatory responses to immune cell sensing. When antibiotics fail to block bacterial proliferation (as in the development of antibiotic resistance), structural changes in the cell wall may allow stronger inflammatory responses to immune cell engagement. Specific antibiotics involved in examples of these themes that are noted in the text are indicated in red. H. influenzae, Haemophilus influenzae; P. aeruginosa, Pseudomonas aeruginosa; A. baumannii, Acinetobacter baumannii.
The list of available antibiotics and their many derivatives is extensive. Broadly, antibiotics can be grouped into 3 main classes based on their mode of action: 1) cell-wall synthesis inhibitors, 2) protein synthesis inhibitors, and 3) nucleic acid synthesis inhibitors. Table 1, although by no means complete, provides a list of some the common antibiotics in each of these groups and their modes of action. Antibiotic resistance can develop through acquisition of enzymes that detoxify antibiotics, acquisition or mutation of enzymes that are altered so as not to be targets of antibiotics, acquisition of mechanisms to efflux antibiotics, or changes in cellular properties that make bacteria resistant to penetration by antibiotics [4].
TABLE 1.
Common antibiotics
| Target | Antibiotic class | Examples | Mechanisms |
|---|---|---|---|
| Cell-wall synthesis | β-Lactams | Inhibits PBPs involved in cell-wall synthesis | |
| Penicillins | Penicillin G | ||
| Cephalosporins | Ceftriaxone | ||
| Ceftazidime | |||
| Carbapenems | Meropenem | ||
| Glycopeptides | Vancomycin | ||
| Polymyxin B | Binds to LPS and negatively charged cell membrane to alter membrane permeability | ||
| Protein synthesis | Aminoglycosides | Gentamicin | Inhibits 30S subunit |
| Tobramycin | |||
| Tetracyclines | Tetracycline | ||
| Macrolides | Erythromycin | Inhibits 50S subunit | |
| Azithromycin | |||
| Chloramphenicol | |||
| Oxazolidinones | Linezolid | ||
| Lincosamides | Clindamycin | ||
| Nucleic acid synthesis | Quinolones | Ciprofloxacin | Inhibits DNA gyrase |
| Aminocoumarin | Novobiocin | ||
| Ansamycins | Rifampin | Inhibits RNA polymerase | |
| Nitroimidazoles | Metronidazole | Creates free radicals | |
| Folic acid synthesis | DHFR inhibitors | Trimethoprim | Inhibits DHFR |
| Sulfonamides | Sulfamethoxazole | Inhibits dihydropteroate synthase |
DHFR, Dihydrofolate reductase.
This review will focus on antibiotic-induced changes in bacteria that influence how the immune system responds to them. There is evidence that antibiotics may directly interact with host cells to influence their function and gene expression [5], although this will not be considered further. Additionally, antibiotics can alter host microbial communities in the gut and on other mucosal surfaces, which indirectly affects host immunity and inflammation (see other reviews for discussion of this topic [6, 7]).
BACTERIAL KILLING AND LYSIS
Naturally, when an antibiotic is effective in a clinical setting, bacteria are cleared. The process by which bacteria die under these circumstances can be highly inflammatory. Cell-wall synthesis inhibiting β-lactams interfere with PBPs, blocking peptidoglycan synthesis and causing the activation of a series of autolytic wall enzymes, leading the bacteria to undergo autolysis in which the cell wall is degraded and compromised catastrophically [8]. This leads to the release of highly inflammatory products, such as LPS, LTA, and peptidoglycan, collectively referred to as PAMPs, which are detected by innate immune receptors on many cell types. For example, exposure of wild-type Streptococcus pneumoniae (but not an autolysin-deficient mutant) to penicillin enhances TLR2-mediated inflammatory signaling [9]. This might not be so bad (or might even be beneficial) if a small number of bacteria are affected, but if it occurs in a sensitive location (such as the brain) or if a large number of bacteria are lysed rapidly, it may be a life-threatening problem. Typically, antibiotics that inhibit protein synthesis cause less release of bacterial products as they kill. For example, whereas treatment of S. aureus with the cell wall activate antibiotic vancomycin enhances its ability to stimulate cytokine production by macrophages and via TLR2, exposure to the protein synthesis inhibitor antibiotic linezolid suppresses these responses [10, 11].The exact reason for this observation is not completely clear. It could be decreased PAMP synthesis or the lack of bacterial lysis. Protein synthesis inhibitors are more likely to be bacteriostatic and may rely more on host cells to clear organisms.
Perhaps the most well-discussed instance of antibiotic-induced exacerbation of inflammatory responses is in the case of Gram-negative bacterial sepsis. There has been interest since the 1960s and 1970s, with the idea that many antibiotics induce shedding of LPS from the cell walls of Gram-negative bacteria [12]. LPS is sensed very efficiently by the innate immune system via a complex of myeloid differentiation protein 2 and TLR4 expressed by many cell types, including circulating myeloid cells, and triggers a potent inflammatory response [13]. It has been known for over a century that LPS is sufficient to induce septic shock. Whereas there have been many reports supporting in vitro the idea that various antibiotics can induce the release of LPS from bacterial cell walls [12], relatively few reports truly document this phenomenon in vivo. In one early study, 10 of 10 patients with sepsis associated with detectable blood LPS developed increased levels of free LPS in the blood upon initiation of treatment with a variety of different antibiotics [14]. The authors noted some correlation between which antibiotics induced the greatest LPS release in vivo and in vitro. Still, this type of study offers minimal insight into the net contribution of such changes to morbidity and mortality. Indeed, a study reporting an increase in serum LPS levels upon antibiotic treatment in patients with Burkholderia pseudomallei bacteremia noted no associated changes in symptoms [15]. Likewise, in an Escherichia coli sepsis model in rabbits, an antibiotic treatment that elevated serum LPS levels 10-fold did not impact survival relative to parallel treatments that did not elevate LPS [16]. As LPS is detected by the innate immune system at phenomenally low levels and as sensing of LPS induces “tolerance” to challenge with LPS further [17], it may be that increases in LPS associated with antibiotic treatment often have minimal net overall impact on disease. Still, much has evolved in our understanding of the mechanisms by which LPS is detected and promotes inflammation, such that the time might be right to revisit some of these questions with a more advanced set of tools and understanding.
The case for significant consequences associated with release of inflammatory bacterial products upon antibiotic-induced lysis might be clearer during certain types of infection, such as S. pneumoniae and H. influenzae meningitis [18]. In vitro treatment of S. pneumoniae with β-lactam antibiotics induces lysis and release of molecules, likely including peptidoglycan, LTA, RNA, DNA, and many others that together, activate inflammatory responses [9, 19]. In a mouse model of S. pneumoniae meningitis, treatment with a cell-wall synthesis-inhibiting β-lactam is associated with higher mortality than an antibiotic that inhibits protein synthesis and triggers less release of inflammatory molecules in vitro [20]. Treatment of patients or laboratory animals with corticosteroids before or concurrent with β-lactam antibiotics leads to improved neurologic outcome in cases of S. pneumoniae or H. influenzae meningitis [21, 22] and is a standard recommendation for treatment of select bacterial meningitis [23].
Whereas studies have documented correlations between inflammatory pathology induced by antibiotics in vivo with the degree of bacterial inflammatory products released during in vitro assays, surprisingly little seems to be known about the detailed immunologic mechanisms that are most relevant. Which released factor(s) are most important in this context and how they are recognized have not been clearly defined.
ANTIBIOTIC EFFECTS AT SUBINHIBITORY CONCENTRATIONS
Although antibiotics are designed to be administered at concentrations that effectively clear pathogens, optimal antibiotic concentrations are frequently not achieved in infected patients. Poor patient compliance could lead to partial antibiotic dosing. Furthermore, certain antibiotics penetrate host compartments or host cells poorly or are relatively inactive in select environments [24, 25]. For example, vancomycin is a bulky molecule that poorly penetrates the lung or the brain [26], and aminoglycosides are ineffective in low pH environments, such as abscesses or lysosomal compartments [24]. Additionally, many bacteria generate biofilms that resist antibiotic killing or harbor resistance elements that can reduce the efficacy of an antibiotic [25]. As a consequence, exposure to subtherapeutic or subinhibitory concentrations of antibiotics likely occurs more frequently than is realized in treatment of clinical infections.
Antibiotics administered at subtherapeutic or subinhibitory concentrations can have prolonged and profound effects on immunopathology. In the presence of sublytic antibiotic doses, microbes can initiate de novo synthesis of proinflammatory and virulence factors [27] and undergo various cellular modifications, including cell-wall changes [28, 29]. These microbial responses can expose the host to increased proinflammatory stimuli. Additionally, induction of virulence determinants has the effect of promoting persistence of the microbes, further prolonging the inflammatory state. Generally, studies that have investigated host inflammatory responses to microbe–antibiotic interactions have focused on release of specific toxins and virulence factors. The effects of releasing these factors on inflammation are frequently inferred, not studied directly. Fewer studies have investigated which specific mechanisms are most significant in the context of coordination of resulting inflammatory responses.
One predictable outcome of microbial exposure to sublytic antibiotic doses is the initiation of a microbial stress response that leads to microbial adaptations, including the induction of various virulence and proinflammatory programs. These responses promote repair of cellular damage and prepare the microbe for a potentially hostile environment. Consistent with this idea, treatment of Listeria monocytogenes with subinhibitory doses of β-lactam antibiotics or vancomycin (cell-wall synthesis inhibitors) leads to up-regulation of a number of stress-related genes and virulence-related genes, including the pore-forming listeriolysin O, whose biologic activities include the induction of cellular cytotoxicity, apoptosis, and proinflammatory cytokines [30, 31]. In P. aeruginosa, exposure to subinhibitory concentrations of antibiotics, including ciprofloxacin (DNA gyrase inhibitor) and tetracycline (protein synthesis inhibitor) triggers changes in expression of over 5% of the organism’s genes [32], including inducing virulence systems, such as the type III secretion system that is directly implicated in modulating macrophage phagocytic and inflammatory responses to the organism [33, 34].
Quite different from the idea that antibiotics simply activate microbial stress responses, a provocative, new idea is that subinhibitory antibiotics might mimic signaling molecules that naturally regulate microbial cellular processes and virulence gene expression [35]. This hypothesis arose from the observation that many microbial signaling molecules have antibiotic properties and play important roles in virulence gene expression. For example, in the well-characterized S. aureus agr system, the secreted, autoinducing peptide exhibits antimicrobial activity by inhibiting growth of related and unrelated bacteria in the community and additionally, coordinates expression of various cytolytic and proinflammatory toxins in these bacteria [36]. Consistent with the signaling hypothesis, the antimicrobial peptide LL-37 has been shown to bind the CsrS component of the CovRS 2-component system in Group A Streptococcus and at subinhibitory concentrations, was shown to induce up-regulation of several virulence genes, including those that control the transition from localized infection to invasive disease [37, 38]. Furthermore, from the LL-37 sequence, investigators have been able to isolate a 10-residue fragment lacking antimicrobial activity that still signals through CovRS [37]. Therefore, antibiotics can directly induce bacterial signaling to modify the inflammatory outcome of infections.
Although protein synthesis inhibitors are generally thought of as inhibitors of virulence factor expression and inflammation, under selective circumstance, this class of antibiotic can be proinflammatory. Published studies provide many examples of protein synthesis inhibitors suppressing toxin and virulence factor expression at sublytic and subinhibitory concentrations. For example, exposure of staphylococci or streptococci to subinhibitory doses of clindamycin has been shown to cause reduction in expression of the Staphylococcal toxic shock syndrome toxin 1 [39], the NLRP3 inflammasome activating α-toxin [40, 41], and the superantigen Streptococcal pyrogenic exotoxin A [42, 43]. Clindamycin and linezolid also inhibit transcription of S. aureus Panton-Valentine leucocidin, which induces inflammation via the NLRP3 inflammasome in human monocytes [44] and neutrophil cell death [45, 46]. However, effects of protein synthesis inhibition can be both gene and antibiotic dependent. A transcriptional study of P. aeruginosa demonstrated up-regulation as well as down-regulation of many genes by subinhibitory concentrations of tobramycin and tetracycline [32]. Among genes up-regulated, tetracycline induced expression of the type III secretion system. This has the subsequent effect of increasing the cytolytic activity of the microbe against macrophages [32]. In S. aureus, tetracycline and clindamycin induce expression of the proinflammatory and cytolytic phenol-soluble modulin toxin (PSMα) [47] via a mechanism that involves activation of the previously mentioned S. aureus virulence regulator agr, but how the protein synthesis inhibitors induce agr is less clear [48, 49].
Many bacteria synthesize protective capsules that offer protection from the environment and immune responses [50]. Exposure to subinhibitory doses of antibiotics can induce or suppress production of capsules. A. baumannii is a Gram-negative bacterium that often infects patients receiving antibiotics. Geisinger and Isberg [51] recently noted that subinhibitory doses of protein synthesis-inhibiting antibiotics chloramphenicol or erythromycin strongly induce synthesis of the microbe’s exopolysaccharide capsule, which had been demonstrated to protect against activation of serum immune defenses and to increase virulence. They observed that this response additionally contributes to the microbe’s resistance to peptide antibiotics. Likewise, exposure of Klebsiella pneumonia to polymyxin B induces expression of capsule polysaccharides, thereby increasing its resistance to antimicrobial peptides [52]. In contrast, Bacteriodes fragilis exposed to subinhibitory doses of clindamycin has been observed to lose capsule expression and become more susceptible to complement and phagocytosis by immune cells [53]. Furthermore, Pasteurella multocida responds to subinhibitory doses of nucleic acid synthesis-inhibiting novobiocin or trimethoprim by reducing expression of capsular genes, resulting in a loss of the capsular layer [54].
Another reported response of several types of bacteria to subinhibitory concentrations of particular antibiotics is the induction of biofilm formation [55]. For example, P. aeruginosa has been shown to form biofilms readily in response to subinhibitory doses of tobramycin, an aminoglycoside antibiotic, but not to many other types of antibiotics [56]. E. coli responds to subinhibitory doses of a broad range of translation-inhibiting antibiotics by forming biofilms [57]. Interestingly, the mechanisms by which these 2 bacteria respond are related but opposite. P. aeruginosa responds to tobramycin by degrading cyclic di-GMP, a signal that seems necessary to promote biofilm formation [56]. In contrast, E. coli respond to subinhibitory doses of specific antibiotics by increasing production of cyclic di-GMP [57]. Cyclic di-GMP is sensed by the innate immune system via the stimulator of IFN genes pathway, although it is not clear whether this type of regulation of cyclic dinucleotide production would affect inflammatory immune responses [58]. Certainly, biofilm formation itself influences how the immune system interacts with bacteria. Macrophages and neutrophils are less able to ingest and clear bacteria in biofilms, and biofilm bacteria may produce factors that kill immune cells or provide evasion from sensing mechanisms. Biofilm formation often leads to enhanced resistance of bacteria to attack by the immune system, including reducing phagocytic efficiency of macrophages and neutrophils and promoting death of phagocytes, either by secretion of lethal factors or through manipulation of the environment to be toxic to immune cells. In addition, biofilm-associated bacteria often develop remarkable resistance to host antimicrobial peptides and resistance to diverse antibiotics [59–61].
Overall, much remains to be learned about how antibiotics affect the proinflammatory state of microbes. Although it is not always easy to predict how an antibiotic would affect expression of a particular bacterial factor, especially at subinhibitory concentrations, a few patterns for how certain antibiotic classes affect inflammation have emerged. Cell-wall active bactericidal antibiotics, such as β-lactams, are largely proinflammatory, as they induce release of the highest amount of bacterial products through cell lysis or induce proinflammatory products at subinhibitory doses. In comparison, protein synthesis inhibitors, such as erythromycin or clindamycin, routinely block production of toxins and virulence factors and are thought of as anti-inflammatory. These findings, supported by significant preclinical data, have led to the clinical practice of coupling protein synthesis inhibitors to β-lactam antibiotics in treatment of severe infections, for example, the combined use of β-lactams and clindamycin for the treatment of S. aureus- or Group A Streptococcus-induced severe bloodstream infection or toxic shock syndrome [62].
ANTIBIOTIC RESISTANCE AND CELL-WALL DEGRADATION
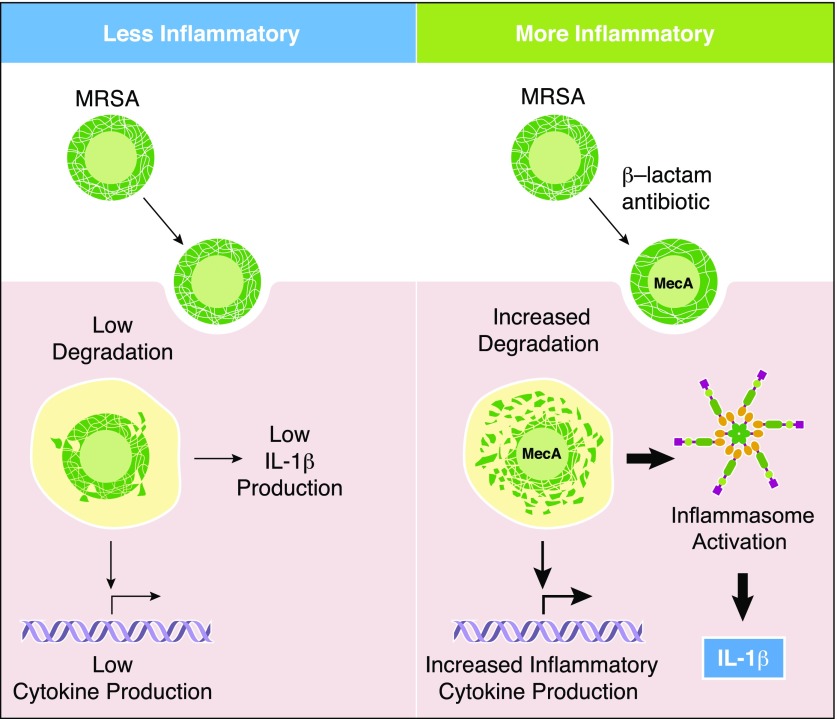
Until recently, the preponderance of work regarding antibiotic-induced changes in inflammatory responses to bacteria has focused on antibiotics that inhibit cell-wall synthesis and are often bactericidal. As discussed above, such work primarily focuses on the PAMPs released into culture supernatants; however, this work rarely takes into account the importance of the remaining bacterial particles in the inflammatory processes. The role of immune responses to the whole bacterium after exposure to antibiotics has taken on new significance with recent work on MRSA by Muller et al. [3]. β-Lactam antibiotics block peptidoglycan cell-wall synthesis by binding to and inactivating bacterial PBPs, which are necessary for the final steps in peptidoglycan synthesis and cross-linking [63]. Resistant strains of S. aureus have acquired an additional gene, MecA, encoding PBP-2a, which is resistant to β-lactam antibiotics [64]. HPLC analysis revealed that although resistant to the antibiotics, the resulting cell-wall peptidoglycan is inefficiently cross-linked. This results in a looser, more degradation-sensitive cell wall [3, 65]. We had previously noted that phagocytosis and degradation are necessary for peptidoglycan to activate NLRP3-dependent inflammatory responses in macrophages [66]. Indeed, when β-lactam-treated MRSA is seen by human and mouse phagocytic cells, the immune response is characterized by increased IL-1β production (Fig. 2). As a consequence, β-lactam-treated MRSA causes more severe immunopathology in a mouse model, as measured by skin lesion size [3]. The importance of the degree of peptidoglycan cross-linking was further supported by genetic mutants lacking PBP4, a condition that causes low peptidoglycan cross-linking and also induced more proinflammatory IL-1β and immunopathology during infection. In contrast, mutants lacking the caseinolytic protease have increased cell-wall thickness and peptidoglycan cross-linking, and this not only makes MRSA more resistant to β-lactam antibiotics, but it also reduces the amount of IL-1β produced by macrophages.
Figure 2. β-Lactam-treated MRSA are more sensitive to degradation leading to increased inflammatory responses.
Following exposure to β-lactams, MRSA up-regulates expression of MecA, which less efficiently cross-links the peptidoglycan in the bacterial cell wall. The bacteria are antibiotic resistant and do not die but are more readily degraded by lysosomal enzymes than untreated MRSA. The resulting degradation products from β-lactam-treated MRSA activate the inflammasome and IL-1β production by phagocytes.
Recently, there have been several studies noting the importance of bacterial degradation in eliciting inflammatory responses by phagocytes. In addition, it has been observed that several bacteria modify their cell walls to protect from degradation, and this has profound consequences for the inflammatory responses [67]. Degradation of Gram-positive bacteria in phagosomes has been shown to increase the production of inflammatory mediators, such as IL-1β [66], TNF-α, IL-6, NO [11, 68], and IFN-β [69], and the degree of inflammation can be linked directly to the sensitivity of the cell-wall peptidoglycan to degradation. The nature of the enhanced inflammatory response is not a result of only sensing of the antibiotic-altered peptidoglycan, but it is also associated with changes in availability of other PAMPs, such as LTA and bacterial DNA and RNA when peptidoglycan is degraded. Exposure to antibiotics can affect the sensitivity of bacteria to degradation. Exposure of antibiotic-sensitive S. aureus to subinhibitory doses of the cell-wall active antibiotic vancomycin increased the inflammatory response of macrophages challenged with the whole bacteria, but bacteria similarly exposed to the protein synthesis inhibitor linezolid did not [11]. Given the enhanced, overall inflammation associated with increased sensitivity to degradation, it is likely that the greater inflammatory responses induced by antibiotic-exposed MRSA, described by Muller et al. [3], are not limited to IL-1β production.
MecA expression is not the only mechanism influencing antibiotic resistance in MRSA. MurT and GatG expression has been shown to affect peptidoglycan amidation, β-lactam resistance, and lysozyme resistance [70]. How the expression of these genes might affect the inflammatory process remains unclear. The resistance of MRSA strains to most β-lactam antibiotics and the rise in MRSA incidence have led to the use of vancomycin as a primary alternate drug of choice to treat MRSA infections. However, as with use of other antibiotics, resistance develops. In this case, resistance has been linked to agr group II polymorphisms associated with an increase in cell-wall thickness of 105% and decreased autolysis [71–73]. Associated changes in inflammatory sensing in these contexts have not been investigated, but given the increased cell-wall thickness, it would be interesting to know if this adaptation decreases PAMP release or affects susceptibility to degradation.
The Gram-positive bacterium L. monocytogenes is intrinsically resistant to cephalosporin antibiotics. However, in response to penicillin G, it up-regulates expression of the surface protein Lmo1941 that appears to alter cell-wall structure in such a manner that the bacterium becomes significantly more sensitive to cephalosporins, a different type of cell-wall synthesis inhibitors [74]. Lmo1941 contains a LysM domain and is presumed to be noncovalently bound to peptidoglycan. Whether Lmo1941-influenced changes in peptidoglycan structure affect sensitivity to degradation or inflammatory responses of phagocytes has not yet been determined, but it illustrates the concept that there are many types of antibiotic-related perturbations that may affect wall structure and access to inflammatory PAMPs and thus, might warrant investigation for inflammatory sequelae.
CONCLUDING REMARKS
In the context of antibiotic therapy, efficacy can be influenced both by a drug’s direct effect on an infectious microbe, as well as by how the drug might alter the mechanisms by which our immune systems respond to the organism. A clearer understanding of how antibiotics affect immune recognition of microbes should contribute to new and rational approaches to antibiotic therapy. Simple rules might be hard to come by; even subclasses of β-lactam antibiotics affecting different PBPs have differing effects on bacteria. For example, P. aeruginosa releases up to 40 times more LPS when treated with PBP-3-specific ceftazidime than PBP-2-specific imipenem [75]. Aminoglycosides, such as gentamicin, block protein synthesis and thus, might be expected to be less inflammatory, but the drugs also stimulate P. aeruginosa to release vesicles packed with inflammatory factors, such as LPS and outer-membrane proteins [76].
A deeper understanding of the mechanisms by which antibiotics influence inflammatory responses may inspire rationally developed, new approaches to using antibiotics. There is increasing evidence that combining cell-wall synthesis inhibitors and protein synthesis inhibitors may decrease some of associated inflammation for certain infections. Even more interesting is the possibility that cell-wall synthesis inhibitors previously thought to be ineffective at killing bacteria on their own may be useful in weakening bacterial cell walls so that other drugs, such as antimicrobial peptides—LL-37 or daptomycin—can kill bacteria more effectively, thus opening up a whole new avenue of possible therapies [2]. We may find that antibiotics that are minimally effective in vitro are highly effective through rendering organisms susceptible to natural host defenses. Finally, the knowledge of how antibiotics alter inflammatory responses triggered by immune cells may permit selection of therapies that both kill the bacteria and skew the resulting inflammation to promote long-term protection.
AUTHORSHIP
A.J.W., G.Y.L., and D.M.U. researched and wrote the manuscript.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health (NIH) National Institute of General Medical Sciences Grant GM085796 and NIH National Institute of Allergy and Infectious Disease Grant AI097741.
Glossary
- agr
accessory gene regulator
- CovRS
cluster of virulence responder/sensor
- cyclic di-GMP
cyclic diguanylate
- LTA
lipoteichoic acid
- MRSA
methicillin-resistant Staphylococcus aureus
- NLRP3
nucleotide-binding oligomerization domain-like receptor family, pyrin domain-containing 3
- PAMP
pathogen-associated molecular pattern
- PBP
penicillin-binding protein
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Periti P., Mazzei T. (1985) Infections in immunocompromised patients. II. Established therapy and its limitations. Clin. Ther. 8, 100–117. [PubMed] [Google Scholar]
- 2.Sakoulas G., Okumura C. Y., Thienphrapa W., Olson J., Nonejuie P., Dam Q., Dhand A., Pogliano J., Yeaman M. R., Hensler M. E., Bayer A. S., Nizet V. (2014) Nafcillin enhances innate immune-mediated killing of methicillin-resistant Staphylococcus aureus. J. Mol. Med. 92, 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Müller S., Wolf A. J., Iliev I. D., Berg B. L., Underhill D. M., Liu G. Y. (2015) Poorly cross-linked peptidoglycan in MRSA due to MecA induction activates the inflammasome and exacerbates immunopathology. Cell Host Microbe 18, 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blair J. M., Webber M. A., Baylay A. J., Ogbolu D. O., Piddock L. J. (2015) Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 13, 42–51. [DOI] [PubMed] [Google Scholar]
- 5.Tamaoki J. (2004) The effects of macrolides on inflammatory cells. Chest 125 (2 Suppl) 41S–50S, quiz 51S. [DOI] [PubMed] [Google Scholar]
- 6.Nishio J., Honda K. (2012) Immunoregulation by the gut microbiota. Cell. Mol. Life Sci. 69, 3635–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez-Chanona E., Trinchieri G. (2016) The role of microbiota in cancer therapy. Curr. Opin. Immunol. 39, 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ginsburg I. (2002) The role of bacteriolysis in the pathophysiology of inflammation, infection and post-infectious sequelae. APMIS 110, 753–770. [DOI] [PubMed] [Google Scholar]
- 9.Moore L. J., Pridmore A. C., Dower S. K., Read R. C. (2003) Penicillin enhances the Toll-like receptor 2-mediated proinflammatory activity of Streptococcus pneumoniae. J. Infect. Dis. 188, 1040–1048. [DOI] [PubMed] [Google Scholar]
- 10.Hilmi D., Parcina M., Stollewerk D., Ostrop J., Josten M., Meilaender A., Zaehringer U., Wichelhaus T. A., Bierbaum G., Heeg K., Wolz C., Bekeredjian-Ding I. (2014) Heterogeneity of host TLR2 stimulation by Staphylocoocus aureus isolates. PLoS One 9, e96416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolf A. J., Arruda A., Reyes C. N., Kaplan A. T., Shimada T., Shimada K., Arditi M., Liu G., Underhill D. M. (2011) Phagosomal degradation increases TLR access to bacterial ligands and enhances macrophage sensitivity to bacteria. J. Immunol. 187, 6002–6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holzheimer R. G. (2001) Antibiotic induced endotoxin release and clinical sepsis: a review. J. Chemother. 13, 159–172. [DOI] [PubMed] [Google Scholar]
- 13.Munford R. S. (2008) Sensing gram-negative bacterial lipopolysaccharides: a human disease determinant? Infect. Immun. 76, 454–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dofferhoff A. S., Nijland J. H., de Vries-Hospers H. G., Mulder P. O., Weits J., Bom V. J. (1991) Effects of different types and combinations of antimicrobial agents on endotoxin release from gram-negative bacteria: an in-vitro and in-vivo study. Scand. J. Infect. Dis. 23, 745–754. [DOI] [PubMed] [Google Scholar]
- 15.Simpson A. J., Opal S. M., Angus B. J., Prins J. M., Palardy J. E., Parejo N. A., Chaowagul W., White N. J. (2000) Differential antibiotic-induced endotoxin release in severe melioidosis. J. Infect. Dis. 181, 1014–1019. [DOI] [PubMed] [Google Scholar]
- 16.Shenep J. L., Barton R. P., Mogan K. A. (1985) Role of antibiotic class in the rate of liberation of endotoxin during therapy for experimental gram-negative bacterial sepsis. J. Infect. Dis. 151, 1012–1018. [DOI] [PubMed] [Google Scholar]
- 17.Seeley J. J., Ghosh S. (2013) Tolerization of inflammatory gene expression. Cold Spring Harb. Symp. Quant. Biol. 78, 69–79. [DOI] [PubMed] [Google Scholar]
- 18.English B. K. (2014) Limitations of beta-lactam therapy for infections caused by susceptible Gram-positive bacteria. J. Infect. 69 (Suppl 1), S5–S9. [DOI] [PubMed] [Google Scholar]
- 19.Tuomanen E., Liu H., Hengstler B., Zak O., Tomasz A. (1985) The induction of meningeal inflammation by components of the pneumococcal cell wall. J. Infect. Dis. 151, 859–868. [DOI] [PubMed] [Google Scholar]
- 20.Nau R., Wellmer A., Soto A., Koch K., Schneider O., Schmidt H., Gerber J., Michel U., Brück W. (1999) Rifampin reduces early mortality in experimental Streptococcus pneumoniae meningitis. J. Infect. Dis. 179, 1557–1560. [DOI] [PubMed] [Google Scholar]
- 21.Mustafa M. M., Ramilo O., Mertsola J., Risser R. C., Beutler B., Hansen E. J., McCracken G. H. Jr (1989) Modulation of inflammation and cachectin activity in relation to treatment of experimental Hemophilus influenzae type b meningitis. J. Infect. Dis. 160, 818–825. [DOI] [PubMed] [Google Scholar]
- 22.Kadurugamuwa J. L., Hengstler B., Zak O. (1989) Cerebrospinal fluid protein profile in experimental pneumococcal meningitis and its alteration by ampicillin and anti-inflammatory agents. J. Infect. Dis. 159, 26–34. [DOI] [PubMed] [Google Scholar]
- 23.Chávez-Bueno S., McCracken G. H. Jr (2005) Bacterial meningitis in children. Pediatr. Clin. North Am. 52, 795–810, vii. [DOI] [PubMed] [Google Scholar]
- 24.Baudoux P., Bles N., Lemaire S., Mingeot-Leclercq M. P., Tulkens P. M., Van Bambeke F. (2007) Combined effect of pH and concentration on the activities of gentamicin and oxacillin against Staphylococcus aureus in pharmacodynamic models of extracellular and intracellular infections. J. Antimicrob. Chemother. 59, 246–253. [DOI] [PubMed] [Google Scholar]
- 25.Høiby N., Bjarnsholt T., Givskov M., Molin S., Ciofu O. (2010) Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 35, 322–332. [DOI] [PubMed] [Google Scholar]
- 26.Rybak M. J. (2006) The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin. Infect. Dis. 42 (Suppl 1), S35–S39. [DOI] [PubMed] [Google Scholar]
- 27.Andersson D. I., Hughes D. (2014) Microbiological effects of sublethal levels of antibiotics. Nat. Rev. Microbiol. 12, 465–478. [DOI] [PubMed] [Google Scholar]
- 28.Keller N., Raponi G., Hoepelman I. M., Overbeek B. P., Rozenberg-Arska M., Verhoef J. (1991) Effect of sub-minimal inhibitory concentrations of ciprofloxacin and fleroxacin on the bacterial capsular antigen and opsonophagocytosis by human polymorphonuclear leukocytes. Zentralbl. Bakteriol. 274, 519–526. [DOI] [PubMed] [Google Scholar]
- 29.Gemmell C. G., O’Dowd A. (1983) Regulation of protein A biosynthesis in Staphylococcus aureus by certain antibiotics: its effect on phagocytosis by leukocytes. J. Antimicrob. Chemother. 12, 587–597. [DOI] [PubMed] [Google Scholar]
- 30.Knudsen G. M., Holch A., Gram L. (2012) Subinhibitory concentrations of antibiotics affect stress and virulence gene expression in Listeria monocytogenes and cause enhanced stress sensitivity but do not affect Caco-2 cell invasion. J. Appl. Microbiol. 113, 1273–1286. [DOI] [PubMed] [Google Scholar]
- 31.Hernández-Flores K. G., Vivanco-Cid H. (2015) Biological effects of listeriolysin O: implications for vaccination. BioMed Res. Int. 2015, 360741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linares J. F., Gustafsson I., Baquero F., Martinez J. L. (2006) Antibiotics as intermicrobial signaling agents instead of weapons. Proc. Natl. Acad. Sci. USA 103, 19484–19489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rangel S. M., Logan L. K., Hauser A. R. (2014) The ADP-ribosyltransferase domain of the effector protein ExoS inhibits phagocytosis of Pseudomonas aeruginosa during pneumonia. MBio 5, e01080–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sutterwala F. S., Mijares L. A., Li L., Ogura Y., Kazmierczak B. I., Flavell R. A. (2007) Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J. Exp. Med. 204, 3235–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aminov R. I. (2009) The role of antibiotics and antibiotic resistance in nature. Environ. Microbiol. 11, 2970–2988. [DOI] [PubMed] [Google Scholar]
- 36.Ji G., Beavis R., Novick R. P. (1997) Bacterial interference caused by autoinducing peptide variants. Science 276, 2027–2030. [DOI] [PubMed] [Google Scholar]
- 37.Velarde J. J., Ashbaugh M., Wessels M. R. (2014) The human antimicrobial peptide LL-37 binds directly to CsrS, a sensor histidine kinase of group A Streptococcus, to activate expression of virulence factors. J. Biol. Chem. 289, 36315–36324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker M. J., Hollands A., Sanderson-Smith M. L., Cole J. N., Kirk J. K., Henningham A., McArthur J. D., Dinkla K., Aziz R. K., Kansal R. G., Simpson A. J., Buchanan J. T., Chhatwal G. S., Kotb M., Nizet V. (2007) DNase Sda1 provides selection pressure for a switch to invasive group A streptococcal infection. Nat. Med. 13, 981–985. [DOI] [PubMed] [Google Scholar]
- 39.Schlievert P. M., Kelly J. A. (1984) Clindamycin-induced suppression of toxic-shock syndrome—associated exotoxin production. J. Infect. Dis. 149, 471. [DOI] [PubMed] [Google Scholar]
- 40.Ohlsen K., Ziebuhr W., Koller K. P., Hell W., Wichelhaus T. A., Hacker J. (1998) Effects of subinhibitory concentrations of antibiotics on alpha-toxin (hla) gene expression of methicillin-sensitive and methicillin-resistant Staphylococcus aureus isolates. Antimicrob. Agents Chemother. 42, 2817–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kebaier C., Chamberland R. R., Allen I. C., Gao X., Broglie P. M., Hall J. D., Jania C., Doerschuk C. M., Tilley S. L., Duncan J. A. (2012) Staphylococcus aureus α-hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. J. Infect. Dis. 205, 807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sriskandan S., McKee A., Hall L., Cohen J. (1997) Comparative effects of clindamycin and ampicillin on superantigenic activity of Streptococcus pyogenes. J. Antimicrob. Chemother. 40, 275–277. [DOI] [PubMed] [Google Scholar]
- 43.Braun M. A., Gerlach D., Hartwig U. F., Ozegowski J. H., Romagné F., Carrel S., Köhler W., Fleischer B. (1993) Stimulation of human T cells by streptococcal “superantigen” erythrogenic toxins (scarlet fever toxins). J. Immunol. 150, 2457–2466. [PubMed] [Google Scholar]
- 44.Holzinger D., Gieldon L., Mysore V., Nippe N., Taxman D. J., Duncan J. A., Broglie P. M., Marketon K., Austermann J., Vogl T., Foell D., Niemann S., Peters G., Roth J., Löffler B. (2012) Staphylococcus aureus Panton-Valentine leukocidin induces an inflammatory response in human phagocytes via the NLRP3 inflammasome. J. Leukoc. Biol. 92, 1069–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Otto M. P., Martin E., Badiou C., Lebrun S., Bes M., Vandenesch F., Etienne J., Lina G., Dumitrescu O. (2013) Effects of subinhibitory concentrations of antibiotics on virulence factor expression by community-acquired methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 68, 1524–1532. [DOI] [PubMed] [Google Scholar]
- 46.Löffler B., Hussain M., Grundmeier M., Brück M., Holzinger D., Varga G., Roth J., Kahl B. C., Proctor R. A., Peters G. (2010) Staphylococcus aureus Panton-Valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog. 6, e1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang R., Braughton K. R., Kretschmer D., Bach T. H., Queck S. Y., Li M., Kennedy A. D., Dorward D. W., Klebanoff S. J., Peschel A., DeLeo F. R., Otto M. (2007) Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 13, 1510–1514. [DOI] [PubMed] [Google Scholar]
- 48.Joo H. S., Chan J. L., Cheung G. Y., Otto M. (2010) Subinhibitory concentrations of protein synthesis-inhibiting antibiotics promote increased expression of the agr virulence regulator and production of phenol-soluble modulin cytolysins in community-associated methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 54, 4942–4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Otto M., O’Mahoney D. S., Guina T., Klebanoff S. J. (2004) Activity of Staphylococcus epidermidis phenol-soluble modulin peptides expressed in Staphylococcus carnosus. J. Infect. Dis. 190, 748–755. [DOI] [PubMed] [Google Scholar]
- 50.Geno K. A., Gilbert G. L., Song J. Y., Skovsted I. C., Klugman K. P., Jones C., Konradsen H. B., Nahm M. H. (2015) Pneumococcal capsules and their types: past, present, and future. Clin. Microbiol. Rev. 28, 871–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Geisinger E., Isberg R. R. (2015) Antibiotic modulation of capsular exopolysaccharide and virulence in Acinetobacter baumannii. PLoS Pathog. 11, e1004691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Llobet E., Campos M. A., Giménez P., Moranta D., Bengoechea J. A. (2011) Analysis of the networks controlling the antimicrobial-peptide-dependent induction of Klebsiella pneumoniae virulence factors. Infect. Immun. 79, 3718–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gemmell C. G., Peterson P. K., Schmeling D., Mathews J., Quie P. G. (1983) Antibiotic-induced modification of Bacteroides fragilis and its susceptibility to phagocytosis by human polymorphonuclear leukocytes. Eur. J. Clin. Microbiol. 2, 327–334. [DOI] [PubMed] [Google Scholar]
- 54.Melnikow E., Schoenfeld C., Spehr V., Warrass R., Gunkel N., Duszenko M., Selzer P. M., Ullrich H. J. (2008) A compendium of antibiotic-induced transcription profiles reveals broad regulation of Pasteurella multocida virulence genes. Vet. Microbiol. 131, 277–292. [DOI] [PubMed] [Google Scholar]
- 55.Kaplan J. B. (2011) Antibiotic-induced biofilm formation. Int. J. Artif. Organs 34, 737–751. [DOI] [PubMed] [Google Scholar]
- 56.Hoffman L. R., D’Argenio D. A., MacCoss M. J., Zhang Z., Jones R. A., Miller S. I. (2005) Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 436, 1171–1175. [DOI] [PubMed] [Google Scholar]
- 57.Boehm A., Steiner S., Zaehringer F., Casanova A., Hamburger F., Ritz D., Keck W., Ackermann M., Schirmer T., Jenal U. (2009) Second messenger signalling governs Escherichia coli biofilm induction upon ribosomal stress. Mol. Microbiol. 72, 1500–1516. [DOI] [PubMed] [Google Scholar]
- 58.Burdette D. L., Vance R. E. (2013) STING and the innate immune response to nucleic acids in the cytosol. Nat. Immunol. 14, 19–26. [DOI] [PubMed] [Google Scholar]
- 59.Roilides E., Simitsopoulou M., Katragkou A., Walsh T. J. (2015) How biofilms evade host defenses. Microbiol. Spectr. 3, 1–10. [DOI] [PubMed] [Google Scholar]
- 60.Rybtke M., Hultqvist L. D., Givskov M., Tolker-Nielsen T. (2015) Pseudomonas aeruginosa biofilm infections: community structure, antimicrobial tolerance and immune response. J. Mol. Biol. 427, 3628–3645. [DOI] [PubMed] [Google Scholar]
- 61.Scherr T. D., Heim C. E., Morrison J. M., Kielian T. (2014) Hiding in plain sight: interplay between Staphylococcal biofilms and host immunity. Front. Immunol. 5, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lappin E., Ferguson A. J. (2009) Gram-positive toxic shock syndromes. Lancet Infect. Dis. 9, 281–290. [DOI] [PubMed] [Google Scholar]
- 63.Sauvage E., Kerff F., Terrak M., Ayala J. A., Charlier P. (2008) The penicillin-binding proteins: structure and role in peptidoglycan biosynthesis. FEMS Microbiol. Rev. 32, 234–258. [DOI] [PubMed] [Google Scholar]
- 64.Beck W. D., Berger-Bächi B., Kayser F. H. (1986) Additional DNA in methicillin-resistant Staphylococcus aureus and molecular cloning of Mec-specific DNA. J. Bacteriol. 165, 373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Jonge B. L., Tomasz A. (1993) Abnormal peptidoglycan produced in a methicillin-resistant strain of Staphylococcus aureus grown in the presence of methicillin: functional role for penicillin-binding protein 2A in cell wall synthesis. Antimicrob. Agents Chemother. 37, 342–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shimada T., Park B. G., Wolf A. J., Brikos C., Goodridge H. S., Becker C. A., Reyes C. N., Miao E. A., Aderem A., Götz F., Liu G. Y., Underhill D. M. (2010) Staphylococcus aureus evades lysozyme-based peptidoglycan digestion that links phagocytosis, inflammasome activation, and IL-1beta secretion. Cell Host Microbe 7, 38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davis K. M., Weiser J. N. (2011) Modifications to the peptidoglycan backbone help bacteria to establish infection. Infect. Immun. 79, 562–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ip W. K., Sokolovska A., Charriere G. M., Boyer L., Dejardin S., Cappillino M. P., Yantosca L. M., Takahashi K., Moore K. J., Lacy-Hulbert A., Stuart L. M. (2010) Phagocytosis and phagosome acidification are required for pathogen processing and MyD88-dependent responses to Staphylococcus aureus. J. Immunol. 184, 7071–7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaplan A., Ma J., Kyme P., Wolf A. J., Becker C. A., Tseng C. W., Liu G. Y., Underhill D. M. (2012) Failure to induce IFN-β production during Staphylococcus aureus infection contributes to pathogenicity. J. Immunol. 189, 4537–4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Figueiredo T. A., Ludovice A. M., Sobral R. G. (2014) Contribution of peptidoglycan amidation to beta-lactam and lysozyme resistance in different genetic lineages of Staphylococcus aureus. Microb. Drug Resist. 20, 238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cázares-Domínguez V., Cruz-Córdova A., Ochoa S. A., Escalona G., Arellano-Galindo J., Rodríguez-Leviz A., Hernández-Castro R., López-Villegas E. O., Xicohtencatl-Cortes J. (2015) Vancomycin tolerant, methicillin-resistant Staphylococcus aureus reveals the effects of vancomycin on cell wall thickening. PLoS One 10, e0118791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cui L., Iwamoto A., Lian J. Q., Neoh H. M., Maruyama T., Horikawa Y., Hiramatsu K. (2006) Novel mechanism of antibiotic resistance originating in vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 50, 428–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cui L., Ma X., Sato K., Okuma K., Tenover F. C., Mamizuka E. M., Gemmell C. G., Kim M. N., Ploy M. C., El-Solh N., Ferraz V., Hiramatsu K. (2003) Cell wall thickening is a common feature of vancomycin resistance in Staphylococcus aureus. J. Clin. Microbiol. 41, 5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krawczyk-Balska A., Korsak D., Popowska M. (2014) The surface protein Lmo1941 with LysM domain influences cell wall structure and susceptibility of Listeria monocytogenes to cephalosporins. FEMS Microbiol. Lett. 357, 175–183. [DOI] [PubMed] [Google Scholar]
- 75.Jackson J. J., Kropp H. (1992) beta-Lactam antibiotic-induced release of free endotoxin: in vitro comparison of penicillin-binding protein (PBP) 2-specific imipenem and PBP 3-specific ceftazidime. J. Infect. Dis. 165, 1033–1041. [DOI] [PubMed] [Google Scholar]
- 76.Kadurugamuwa J. L., Beveridge T. J. (1997) Natural release of virulence factors in membrane vesicles by Pseudomonas aeruginosa and the effect of aminoglycoside antibiotics on their release. J. Antimicrob. Chemother. 40, 615–621. [DOI] [PubMed] [Google Scholar]