SiglecF+Gr1hi eosinophils are distinct from the canonical SiglecF+Gr1- eosinophil population, and contain lymphocyte-targeting cytokines CXCL13 and IL-27.
Keywords: allergy, inflammation, flow cytometry, cytokines
Abstract
Although eosinophils as a group are readily identified by their unique morphology and staining properties, flow cytometry provides an important means for identification of subgroups based on differential expression of distinct surface Ags. Here, we characterize an eosinophil subpopulation defined by high levels of expression of the neutrophil Ag Gr1 (CD45+CD11c−SiglecF+Gr1hi). SiglecF+Gr1hi eosinophils, distinct from the canonical SiglecF+Gr1− eosinophil population, were detected in allergen-challenged wild-type and granule protein-deficient (EPX−/− and MBP-1−/−) mice, but not in the eosinophil-deficient ΔdblGATA strain. In contrast to Gr1+ neutrophils, which express both cross-reacting Ags Ly6C and Ly6G, SiglecF+Gr1hi eosinophils from allergen-challenged lung tissue are uniquely Ly6G+. Although indistinguishable from the more-numerous SiglecF+Gr1− eosinophils under light microscopy, FACS-isolated populations revealed prominent differences in cytokine contents. The lymphocyte-targeting cytokines CXCL13 and IL-27 were identified only in the SiglecF+Gr1hi eosinophil population (at 3.9 and 4.8 pg/106 cells, respectively), as was the prominent proinflammatory mediator IL-13 (72 pg/106 cells). Interestingly, bone marrow-derived (SiglecF+), cultured eosinophils include a more substantial Gr1+ subpopulation (∼50%); Gr1+ bmEos includes primarily a single Ly6C+ and a smaller, double-positive (Ly6C+Ly6G+) population. Taken together, our findings characterize a distinct SiglecF+Gr1hi eosinophil subset in lungs of allergen-challenged, wild-type and granule protein-deficient mice. SiglecF+Gr1hi eosinophils from wild-type mice maintain a distinct subset of cytokines, including those active on B and T lymphocytes. These cytokines may facilitate eosinophil-mediated immunomodulatory responses in the allergen-challenged lung as well as in other distinct microenvironments.
Introduction
Eosinophilic leukocytes are unique effector cells with complex roles in promoting health and homeostasis [1–3]. Until recently, eosinophils were considered end-stage cells with limited capacity for plasticity or flexibility after maturation in the bone marrow and release into systemic circulation. This perception has changed [4]. Among recent findings, eosinophils are now recognized for their prominent roles as resident cells, notably in the intestines, where they support gut homeostasis [5], and in adipose tissue, where they promote survival and function of alternatively activated macrophages [6]. At the same time, eosinophils may or may not provide host defense against helminth parasites [7]; in some circumstances, eosinophils provide factors necessary to support parasite survival in somatic tissues [8, 9]. Furthermore, in the lung, where eosinophil recruitment has been perceived as primarily detrimental, we and others [10, 11] have recognized that cytokine-induced eosinophil activation may be a component of local antiviral host defense.
Although eosinophils are readily detected in various leukocyte preparations because of their unique morphology and avidity for eosin-containing dyes [12, 13], flow cytometry provides an unsurpassed means for rapid identification and quantitative evaluation of cells isolated as components of mixed populations from organs and tissues. There are several specific Ag profiles used to identify eosinophils that permit separation and differentiation from other leukocyte lineages [14, 15]. Flow cytometric profiles for mouse eosinophils in lung tissue are typically based on the absence of the myeloid cell surface marker CD11c, together with the presence of unique markers, such Siglec F and CCR3; in the thymus and small intestines, CD11c+ eosinophils are more prevalent [16, 17]. Among the Abs used to differentiate between mouse granulocyte populations is the rat–anti-mouse monoclonal RB6-8C5, which detects the cell surface Ag Gr1 (reviewed in [18]). Mouse eosinophils are most often categorized as Gr1-negative or low [12]. Individual instances of Gr1+ eosinophils in peripheral tissues have been described [19, 20], but the unique characteristics and relationships between these cells and the predominant SiglecF+Gr1− eosinophil population have not been clearly identified.
Here, we characterize a distinct subset of SiglecF+Gr1hi cells in lung tissue of allergen-challenged mice with cytokine contents that distinguish them from their more-prominent SiglecF+Gr1− counterparts. Furthermore, we present evidence suggesting that Gr1 may be a maturation marker for the eosinophil lineage.
METHODS
Mice
C57BL/6 and BALB/c wild-type mice were from Charles River Laboratories (Frederick, MD, USA). EPX−/− [21], MBP-1−/− [22], and BALB/c ΔdblGATA [23] mice are maintained onsite. All mouse studies were approved by the U.S. National Institutes of Health National Institute of Allergy and Infectious Diseases and carried out in accordance with Animal Care and Use Committee guidelines. Sensitization and challenge with Af followed by infection with PVM, as previously described [11]; briefly, mice were sensitized via i.p. injection with 20 µg Af Ags (HollisterStier Allergy, Spokane, WA, USA) in Imject Alum (100 µl/ mouse; Thermo Fisher Scientific, Waltham, MA, USA) on d 0 and 14 and were challenged via intranasal inoculation with 25 µg Af in sterile tissue culture-grade PBS or PBS alone on d 25, 26, and 27 and PVM (2.0 TCID50 units for C57BL/6 mice, 0.2 TCID50 units for BALB/c mice) on d 28; outcomes were evaluated on d 30. Challenge with Aa (Stallergenes Greer, Lenoir, NC, USA) was also performed as previously described [24]; mice were inoculated intranasally with 50 µg of a filtrate of Aa on d 0, 3, and 6; outcomes were evaluated on d 10.
Single-cell suspensions, flow cytometry, and sort and cytospin
Single-cell suspensions from mouse lung tissue were prepared as previously described [25]. Cells were incubated with Live-Dead Fixable Aqua (Thermo Fisher Scientific) for 30 min before washing with PBS with 0.1% BSA (PBS/BSA) and then evaluated with fluorochrome-conjugated Abs against CD16/CD32, Siglec F, and Gr1 (BD Biosciences, Franklin Lakes, NJ, USA); CD45 and CD11c (eBioscience, San Diego, CA, USA); Ly6C and Ly6G (1A8; BioLegend, San Diego, CA, USA). After incubation with Abs for 30 min at 4°C, the cells were washed with 2 ml of PBS/BSA and then fixed in 4% paraformaldehyde. The samples were stored at 4°C in the dark until analyzed. A minimum of 100,000 events was collected on an LSRII flow cytometer (BD Biosciences), and data were analyzed in FlowJo (Tree Star, Ashland, OR, USA). For collections, samples were sorted using a FACSAria (BD Biosciences), and cytospins were prepared: 100,000 cells/slide, fixed and stained with Diff-Quik. Cells were scored visually with 100 cells counted/mouse/slide. Images shown were photographed with a Leica DMI 4000B microscope (Leica Microsystems, Wetzlar, Germany) and a Retiga model 2000R imaging system (Qimaging, Surrey, BC, Canada) at magnifications indicated in the figure legends.
Detection of cytokines in eosinophil subsets
Lysates were prepared per manufacturer’s instructions from paired samples of CD45+CD11c−SiglecF+Gr1− and CD45+CD11c−SiglecF+Gr1hi cells collected from 10 mice (6–9 × 105 cells), which were evaluated by Proteome Profiler Mouse Cytokine Array Kit, Panel A (R&D Systems, Minneapolis, MN, USA). Membranes were probed with 300 μg of each lysate as determined by microBCA assay (Thermo Fisher Scientific), washed, and incubated with streptavidin-linked-IR Dye 800CW (LI-COR Biosciences, Lincoln, NE, USA), followed by infrared detection also per the manufacturer’s instructions. Data were collected on a LI-COR Odyssey Model 9120 System and expressed as the percentage of the average of the reference spots on the sample membrane and read as signal intensity of the sample/signal intensity of the reference. Lysates prepared independently as described above were evaluated by ELISA (Quantikine and DuoSet; R&D Systems) per manufacturer’s instructions.
bmEOS
Eosinophils were generated from bone marrow cells of C57BL/6 mice, as previously described [26]. Cells were evaluated by flow cytometry as described above.
Statistics
All quantitative findings were from replicate data sets (n = 2 or more); flow plots shown are representative of typical results. Data were analyzed via appropriate algorithms within GraphPad Prism (GraphPad Software, La Jolla, CA, USA) as indicated in the figure legends.
RESULTS
SiglecF+Gr1hi eosinophils are an independent cell subset detected in lungs of wild-type C57BL/6 and EPX−/− mice
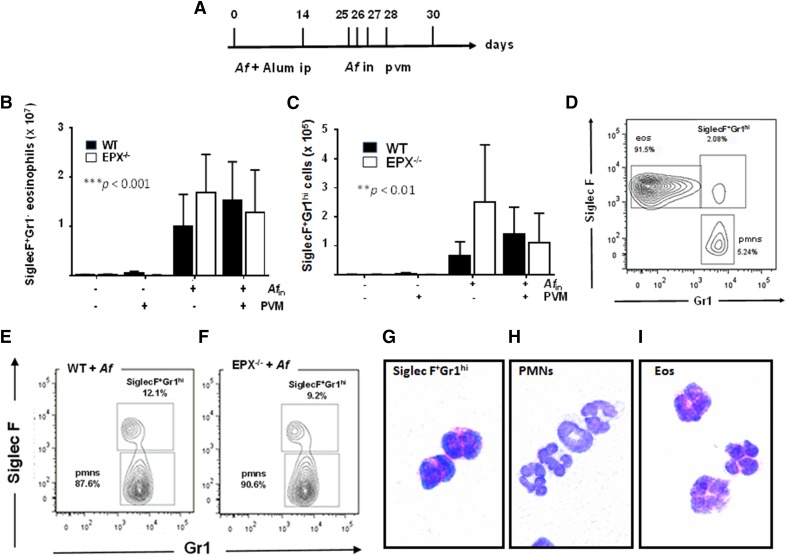
In earlier work, we explored the effect of virus infection superimposed on allergen sensitization and challenge as a model of exacerbation of allergic airway disease. As part of this study, we examined recruitment of eosinophils (CD45+CD11c−SiglecF+Gr1−) to the lungs in response to allergen challenge both with and without virus infection (Fig. 1A). As anticipated, allergen (Af) sensitization and challenge resulted in a substantial increase in eosinophil recruitment (***P < 0.001); virus infection had no effect on eosinophil recruitment in wild-type C57BL/6 mice or in mice devoid of the major granule protein (EPX−/−) (Fig. 1B). As a part of this study, we identified a small, discontinuous population of CD45+CD11c− cells that were Siglec F+Gr1hi. This population represented ∼2% of the total CD45+CD11c− granulocyte population in the lungs of Af-sensitized and -challenged mice (Figs. 1C and 1D) and ∼10% of the total Gr1hi cells in both wild-type and EPX−/− mice (Fig. 1E and 1F). CD45+CD11c−SiglecF+Gr1hi cells isolated by FACS maintain typical eosinophil morphology, most notably prominent cytoplasmic granules that stain red with modified Giemsa (Fig. 1G). These cells are clearly distinct from CD45+CD11c−SiglecF−Gr1hi lung neutrophils (Fig. 1H), although morphologically similar, if not indistinguishable from their more-numerous CD45+CD11c−SiglecF+Gr1− eosinophil counterparts at the light microscopic level (Fig. 1I).
Figure 1. SiglecF+Gr1hi eosinophils are an independent subpopulation detected in lungs of wild-type and eosinophil–peroxidase gene-deleted (EPX−/−) mice.
(A) Protocol I: Af sensitization on d 0 and d 14, followed by Af or PBS challenge (d 25, 26, 27). Mice were evaluated on d 30 as indicated. SiglecF+Gr1− eosinophils (CD45+CD11c−SiglecF+Gr1−) (B) and SiglecF+Gr1hi cells (CD45+CD11c−SiglecF+Gr1hi) (C) in single-cell lung suspensions from Af-sensitized and Af- or PBS-challenged mice as indicated in (A). ***P < 0.001, **P < 0.01 Af-sensitization and Af challenge vs. Af-sensitization and PBS challenge, 2-way ANOVA, n = 5–13 mice/group. (D) SiglecF+Gr1hi cells, as distinct from SiglecF+Gr1− eosinophils, represent ∼2% of the total CD45+CD11c− leukocyte population isolated from the lungs of allergen-sensitized and -challenged mice. (E and F) SiglecF+Gr1hi cells represent ∼10% of the CD45+CD11c−Gr1hi cells isolated from lungs of Af-sensitized and -challenged, wild-type mice and EPX−/− mice, respectively. CD45+CD11c−SiglecF+Gr1hi cells (G), CD45+CD11c−SiglecF−Gr1hi (polymorphonuclear neutrophils; neutrophils) (H), and CD45+CD11c−SiglecF+Gr1− (Eos; eosinophils) (I) isolated from lung single-cell suspensions from Af-sensitized and -challenged mice by flow cytometry and cell sorting (FACS); original magnification ×100.
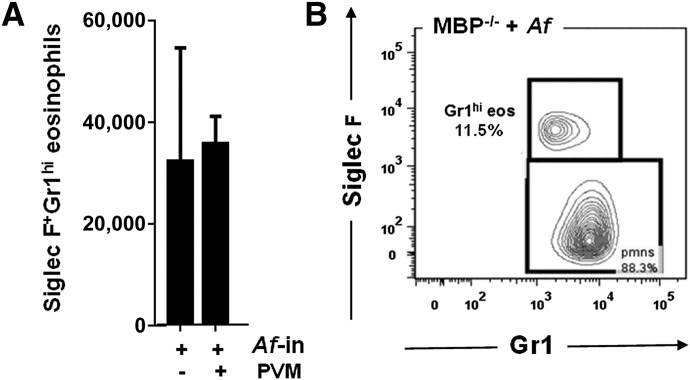
SiglecF+Gr1hi eosinophils are also detected lung cell suspensions from MBP-1−/− mice
In contrast to eosinophils from EPX−/− mice, which display relatively subtle alterations in the granule matrix [21], eosinophils from mice devoid of MBP-1 lack the electron-dense granule core [22]. Although eosinophils from MBP-1−/− mice cannot be readily identified by Giemsa stain, they can be identified by flow cytometry, based on expression of Siglec F [27]. We detected the SiglecF+Gr1hi eosinophils (12% of total granulocytes) in lung suspensions from Af-sensitized and -challenged MBP-1−/− mice (Fig. 2A and 2B).
Figure 2. SiglecF+Gr1hi eosinophils are detected in lungs of eosinophil MBP-1−/− mice.
(A) SiglecF+Gr1hi eosinophils in single-cell lung suspensions from Af-sensitized and -challenged MBP-1−/− mice both with and without subsequent virus infection; n = 5–6 mice/group. (B) SiglecF+Gr1hi eosinophils represent >10% of the CD45+CD11c−Gr1hi cells isolated from lungs of Af-sensitized and -challenged, wild-type mice and MBP-1−/− mice.
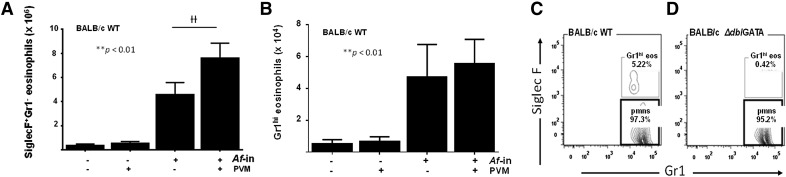
SiglecF+Gr1hi eosinophils are also detected in wild-type BALB/c mice but not in the eosinophil-deficient ΔdblGATA strain
A similar pattern of SiglecF+Gr1hi eosinophil recruitment was observed in wild-type BALB/c mice subjected to Af sensitization and challenge both with and without virus infection (Fig. 3A and 3B). Of note, our findings differ from those of Rose et al. [20] who reported that all eosinophils recruited to the lungs of ovalbumin-sensitized and -challenged BALB/c mice were Gr1+; it is not immediately clear what the source of this discrepancy might be. No SiglecF+Gr1hi cells were identified among lung cells of ΔdblGATA mice [22], which are devoid of eosinophils (Figs. 3C and 3D).
Figure 3. SiglecF+Gr1hi eosinophils are detected in response to Af-sensitization and challenge in wild-type (WT) BALB/c, but not eosinophil-deficient, ΔdblGATA mice.
SiglecF+Gr1− eosinophils (A) and SiglecF+Gr1hi eosinophils (B) in single-cell lung suspensions from Af-sensitized and -challenged mice, as described in Fig. 1; **P < 0.01 Af-sensitization and Af-challenge vs. Af-sensitization and PBS challenge, ††P < 0.01 PVM infected vs. diluent control, 2-way ANOVA, n = 3–5 mice/group. C. SiglecF+Gr1hi eosinophils are an independent population and represent ∼5% of the CD45+CD11c−Gr1+ cells isolated from lungs of Af-sensitized and -challenged BALB/c mice. (D) SiglecF+Gr1hi cells cannot be detected in Af-sensitized and -challenged, eosinophil-deficient ΔdblGATA mice.
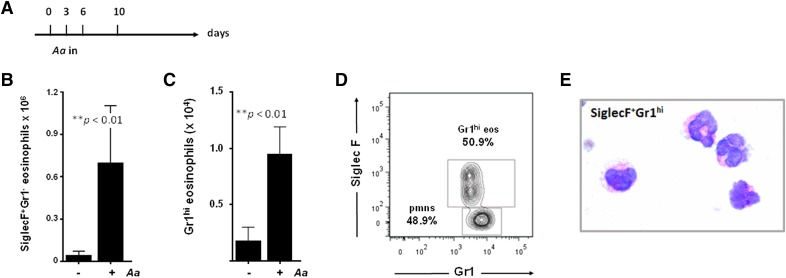
SiglecF+Gr1hi eosinophils have unique granule contents
Bartemes et al. [24] recently described eosinophil recruitment in response to inhalation of the fungal Ag Aa in mice that had not undergone systemic sensitization. Using this protocol (Fig. 4A), we detected eosinophil recruitment to the lungs (Fig. 4B) and, likewise, a significant number of SiglecF+Gr1hi eosinophils (Fig. 4C), which represented 50% of the total Gr1hi cells in the allergen-challenged lungs because of the relative paucity of neutrophils at this time point in this challenge model (Fig. 4D). As in the Af-sensitized and -challenged mice, the SiglecF+Gr1hi eosinophils appear morphologically normal at the light microscopic level (Fig. 4E). Nonetheless, the granule cytokine contents of these 2 populations of eosinophils are not identical (Table 1). Although cytokine release is a prominent property of both human and mouse eosinophils, the cytokine content of mouse eosinophils (as opposed to human eosinophils [12, 28, 29]) has not been addressed in any systematic fashion. Despite SiglecF+Gr1− and SiglecF+Gr1hi eosinophils being indistinguishable from one another at the light microscopic level, their cytokine contents are not identical. Of note, there are several mediators that were detected only in the SiglecF+Gr1hi eosinophil subset. Most notably among these, is IL-13, a prominent mediator that promotes tissue remodeling in association with allergic airway dysfunction (72 pg/106 cells). Similarly, the SiglecF+Gr1hi subset is the source of CXCL13 and IL-27 (4 and 5 pg/106 cells, respectively), which are both mediators that act on B lymphocytes.
Figure 4. SiglecF+Gr1hi eosinophils are detected in response to intranasal challenge with Alternaria alternata in wild-type BALB/c mice.
(A) Protocol II: Aa intranasal challenge on d 0, 3, and 6; mice were evaluated on d 10 as indicated. SiglecF+Gr1− eosinophils (B) and SiglecF+Gr1hi eosinophils (C) in single-cell lung suspensions from mice challenged with Aa, **P < 0.01 Mann-Whitney U test, n = 4–6 mice/group. (D) SiglecF+Gr1hi eosinophils represent 50% of the CD45+CD11c−Gr1+ cells isolated from lungs of the Aa-challenged BALB/c mice. (E) CD45+CD11c−SiglecF+Gr1hi eosinophils isolated from lung single-cell suspensions from Aa-challenged mice by flow cytometry; original magnification ×100.
Table 1.
Cytokines and chemokines detected in lysates of SiglecF+Gr1− vs. SiglecF+Gr1hi eosinophils from lungs of allergen-challenged mice
| Cytokines | Abbreviations and alternative names | In SiglecF+Gr1hi eosinophilsa | In SiglecF+Gr1− eosinophilsb | Change | |||
|---|---|---|---|---|---|---|---|
| CC chemokine ligand 1 | CCL1 | I-309 | − | + | ↑ | ||
| CC chemokine ligand 12 | CCL12 | + | + | − | |||
| CC chemokine ligand 17 | CCL17 | − | ++ | ↑ | |||
| CC chemokine ligand 2c | CCL2 | MCP-2 | − | 0 | + | 5.5 | ↑ |
| CC chemokine ligand 3 | CCL3 | MIP-1α | − | + | ↑ | ||
| CC chemokine ligand 4 | CCL4 | MIP-1β | − | + | ↑ | ||
| CXC chemokine ligand 10c | CXCL10 | IP-10 | − | 0 | ++ | 1680 | ↑ |
| CXC chemokine ligand 12 | CXCL12 | SDF-1 | + | ++ | ↑ | ||
| CXC chemokine ligand 13c | CXCL13 | BCA-1 | ++ | 3.9 | − | 0 | ↓ |
| Interferon-γ | IFN-γ | + | − | ↓ | |||
| Interleukin-10d | IL-10 | 322 | 1220 | ↑ | |||
| Interleukin-13c | IL-13 | + | 71.7 | − | 0 | ↓ | |
| Interleukin-16 | IL-16 | − | + | ↑ | |||
| Interleukin-17d | IL-17 | + | 34.2 | ++ | 47.1 | ↑/− | |
| Interleukin-1α | IL-1α | IL1-F1 | + | + | − | ||
| Interleukin-1β | IL-1β | IL1-F2 | + | + | − | ||
| Interleukin-2 | IL-2 | + | ++ | ↑ | |||
| Interleukin-23 | IL-23 | + | ++ | ↑ | |||
| Interleukin-27c | IL-27 | + | 4.8 | − | 0 | ↓ | |
| Interleukin-3 | IL-3 | +++ | ++ | ↓ | |||
| Interleukin-7 | IL-7 | + | + | − | |||
| Colony stimulating factor-1 | CSF-1 | M-CSF | + | + | − | ||
| Transforming growth fact βd | TGF-β | 0 | 0 | − | |||
| Tumor necrosis factor α | TNF-α | − | 29 | + | 19 | ↑/− | |
Eosinophils (CD45+CD11c−SiglecF+Gr1− and CD45+CD11c−SiglecF+Gr1hi) were isolated by FACS from single-cell suspensions from lung tissue of mice challenged with a filtrate from Aa (see Fig. 4A). Lysates were prepared as described in the “Methods” section, with 300 µg of each lysate used in simultaneous probes of Ab-embedded membranes for detection of total cytokine contents. Cytokines were detected on the profiler as the signal intensity of the sample/signal intensity of the reference. Cytokines included in the evaluation but not detected in either eosinophil lysate included G-CSF, CCL11, GM-CSF, IL-4, CXCL9, and CCL5. Values shown are in units of picograms/106 cells. −, no difference from reference; +, ++, and +++, serial multipliers over the reference standard.
Purity determined by back sorts at 97.5%.
Purity determined by back sorts at 99.7%.
Primary values for CCL2, CXCL10, CXCL13, IL-13, IL-17, and IL-27 determined by profiler were confirmed by ELISA.
Values for IL-10 and TGFβ were generated by ELISA.
Bone marrow-derived eosinophils are SiglecF+Gr1+
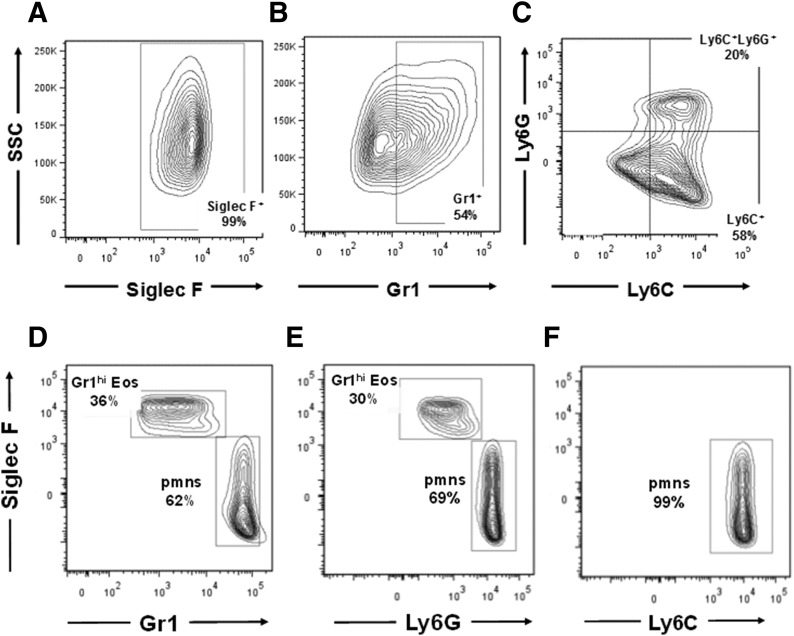
Unselected progenitors from the bone marrow of C57BL/6 mice were used to generate eosinophils as previously described [26]. Mature bmEos are SiglecF+ (Fig. 5A). However, in contrast to the lung of allergen-challenged mice, in which the SiglecF+Gr1hi eosinophil population represents a small fraction of the total eosinophils, >50% of the bmEos were Gr1+ (Fig. 5B). The Ag Gr1, now understood to be the cell surface Ag Ly6G, was initially defined by detection with rat anti-mouse mAb clone R6B-8C5 [18], although recent studies have detected cross-reactivity with the related surface Ag Ly6C. To define the surface Ags present on SiglecF+Gr1hi eosinophils, we examined the bmEos with anti-Ly6G (1A8) and anti-Ly6C, together with their respective isotype controls. As shown in Fig. 5C, SiglecF+Gr1+ bmEos were Ly6C+; a smaller but significant fraction of this population (∼30%) was both Ly6C+ and Ly6G+.
Figure 5. Ly6C/Ly6G Ag profiles of bmEos and Gr1+ eosinophils from allergen-challenged lung.
Eosinophils generated in culture from unselected bmEos from C57BL/6 mice are SiglecF+ (99%) (A), Gr1+ (54%) (B), and Ly6C+ or Ly6C+Ly6G+ (C) SiglecF+Gr1hi (D) eosinophils identified in lung suspensions from mice challenged with Aa (see Fig. 4A) are Ly6G+ (E) and Ly6C− (F).
SiglecF+Gr1hi lung eosinophils are Ly6G+
A similar evaluation was carried out for SiglecF+Gr1hi eosinophils identified in single-cell suspensions from lung tissue from mice challenged with Aa (see Fig. 4A). As in Fig. 4D, SiglecF+Gr1hi eosinophils represent a substantial portion of the Gr1+ cells in the lung, given the paucity of neutrophils (Fig. 5D). As shown in Fig. 5E and F, both Gr1hi eosinophils and neutrophils were Ly6G+. SiglecF+Gr1hi eosinophils were not detected with anti-Ly6C.
DISCUSSION
Although cell morphology remains the standard for identification of the major leukocyte lineages [30], flow cytometry is an important method for distinguishing between subsets of cells that may appear similar, if not identical, at the light microscopic level. Here, we have identified cells that express CD45 and SiglecF together with the cell-surface Ag Gr1; in our experiments, this profile identifies a distinct subset of eosinophils recruited to the lungs in response to allergen challenge. These cells were detected among eosinophils in mice of both C57BL/6 and BALB/c backgrounds and, likewise, in mice devoid of granule proteins EPX and MBP.
The Ab RB6-8C5 detects the Ag Gr1, defined as the 25 kDa, GPI-linked, cell surface molecule Ly6G, a member of the murine Ly6 family of leukocyte cell surface Ags (reviewed in Lee et al. [18]). Strict Ly6G expression has, until now, been limited to mature neutrophils and myeloid-derived suppressor cells. However, because the RB6-8C5 Ab lacks strict specificity, many cells defined as Gr1+ have been found to express the related (and cross-reacting) Ag Ly6C [18, 31]. Although newer monoclonal Abs permit specific detection of Ly6G, the term Gr1 and the use of the RB6-8C5 remains prominent in the literature. We show here that eosinophils generated in culture from unselected mouse bone marrow progenitors, all of which are SiglecF+ express primarily Ly6C, with a subset of the Ly6C+ population (∼30%) being double positive (Ly6C+Ly6G+). Most intriguing, the small population of Gr1hi eosinophils detected in lungs from allergen-challenged mice is Ly6G+ and expresses no Ly6C. Taken together, these results suggest that expression of Ly6C and Ly6G may be part of a maturation continuum. Although eosinophils are considered to be phenotypically complete upon exit from the bone marrow (albeit no longer “end-stage”), loss of Gr1 later in development (i.e., Ly6C to Ly6G to fully Ag-negative) may reflect a specific transition and/or a further maturation response characteristic of this leukocyte lineage. In support of this hypothesis, Iwasaki et al. [32] reported the isolation of phenotypically mature IL-5R+Gr1+ eosinophils from normal bone marrow. Similar to our findings with SiglecF+Gr1+ bmEos grown in tissue culture, de Bruin et al. [33] found that eosinophils induced to develop ex vivo from defined murine myeloid progenitors also expressed the Gr1 Ag. Likewise, Daley et al. [31] reported that eosinophils from mouse peripheral blood are Gr1int rather than Gr1−. Among points worthy of further consideration, we also considered the possibility that the SiglecF+Gr1hi cells in our study were derived from eosinophil progenitors found in the lung that undergo maturation in situ [34].
There has been a recent appreciation of the role of eosinophils as immunomodulatory effector cells [2, 4], actions promoted, in part, by release of granule proteins and cytokines. Spencer et al. [29] explored the cytokine contents of human eosinophils systematically and identified stores of both Th1 and Th2 types, together with numerous immunoregulatory cytokines; Lee et al. [12] performed a thorough and systematic review of the literature and assembled a comprehensive list of cytokine mediators released from both human and mouse eosinophils under numerous circumstances and experimental settings. Among canonical SiglecF+Gr− lung eosinophils, we have detected numerous CC and CXC chemokines and immunomodulatory mediators. Of particular note, we detected relatively high levels of the chemoattractant CXCL10, as well as the anti-inflammatory mediator, IL-10 (at 1680 and 1220 pg/106 cells, respectively). Furthermore, we found that SiglecF+Gr1hi eosinophil population maintains a unique subset of cytokines that were not detected in the dominant SiglecF+Gr− group. With this observation, we focused on one of the more intriguing of the newly described immunomodulatory functions, the role of eosinophils in promoting B cell homeostasis [5, 35, 36]. Among the cytokines detected only in the SiglecF+Gr1hi eosinophil subset is CXCL13, a selective chemoattractant for B cells [37]. Gr1hi eosinophils are also the unique eosinophil source of IL-27, another cytokine implicated in regulating the activity of B lymphocytes [38]. SiglecF+Gr1hi cells are a prominent eosinophil source of IL-13, a cytokine implicated in tissue remodeling associated with allergen provocation and allergic airway dysfunction [39]. Although this has not been characterized specifically in the lung microenvironment, IL-13 promotes B cell maturation and has a profound effect on IgE production [40]. In addition to further characterization of the role of this unique subset, including localization within the lungs of allergen-challenged mice, it will be important to understand what factors might promote cytokine release and whether Gr1hi eosinophils respond to activation agents in a manner similar to, or different from, the canonical Gr1− population [28].
Within the larger context of the eosinophil literature, the concept of eosinophil heterogeneity has been considered previously and remains of significant interest. As one prominent example, several groups have defined a subset of eosinophils characterized as hypodense; these eosinophils have smaller granules and thus greater buoyancy in Percoll gradients [41–43], although the role of these cells vis à vis the pathophysiology of eosinophil-associated diseases (asthma, allergies, hypereosinophilic syndromes) has not been clearly determined. More recently, Abdala Valencia et al. [44] identified eosinophil subsets that transition from SiglecFmed to SiglecFhi in association with recruitment to the airways. Voehringer et al. [45] reported an analogous increase in SiglecF expression in the lung cells using IL4/eGFP mice, and Griseri et al. [46] identified SiglecFhi as a marker of activated eosinophil subsets in a chronic colitis model. Although individual examples of Gr1+ eosinophils in peripheral tissues have been reported [19, 20], to our knowledge, this is the first report to document distinct subsets defined by this marker and, likewise, by their unique cytokine contents.
In conclusion, we have identified an independent population of eosinophils that are SiglecF+Gr1hi, representing 2% of the eosinophils in lungs of allergen-challenged, wild-type mice and granule protein-deficient mice (EPX−/− and MBP-1−/−). In addition to surface Ag expression, these subsets maintain unique cytokine contents, including those not previously identified as being associated with this leukocyte lineage. Notably, the SiglecF+Gr1hi population is the major source of eosinophil-derived IL-13 and maintains cytokines IL-27 and CXCL13, factors that may contribute to eosinophil-mediated B cell homeostasis in this unique microenvironment.
AUTHORSHIP
C.M.P. performed most of the experimental work, prepared the figures for publication, and reviewed and edited the manuscript. T.A.B., M.M., L.S.K., and R.M.A.H. assisted with specific experimental studies and analysis and reviewed the final manuscript. J.J.L. provided unique, gene-deleted mice and reviewed and edited the manuscript. H.F.R. designed the study, advised on experimental design and execution, and wrote the manuscript.
ACKNOWLEDGMENTS
This work was performed with support from the U.S. National Institutes of Health National Institute of Allergy and Infectious Diseases, Division of Intramural Research (Grant AI 000941) to H.F.R. We dedicate this work to our colleague, Dr. Kimberly Dyer, and send our wishes for her speedy recovery.
Glossary
- Aa
Alternaria alternata
- Af
Aspergillus fumigatus
- BAL
bronchoalveolar lavage
- bmEos
bone marrow-derived eosinophils
- EPX
eosinophil peroxidase
- MBP-1
major basic protein-1
- PVM
pneumonia virus of mice
- TCID50
50% tumor culture-infective dose
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Lee J. J., Rosenberg H. F.. 2013. Eosinophils in Health and Disease. Elsevier, Amsterdam. [Google Scholar]
- 2.Rosenberg H. F., Dyer K. D., Foster P. S. (2013) Eosinophils: changing perspectives in health and disease. Nat. Rev. Immunol. 13, 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobsen E. A., Lee N. A., Lee J. J. (2014) Re-defining the unique roles for eosinophils in allergic respiratory inflammation. Clin. Exp. Allergy 44, 1119–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee J. J., Jacobsen E. A., McGarry M. P., Schleimer R. P., Lee N. A. (2010) Eosinophils in health and disease: the LIAR hypothesis. Clin. Exp. Allergy 40, 563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu V. T., Beller A., Rausch S., Strandmark J., Zänker M., Arbach O., Kruglov A., Berek C. (2014) Eosinophils promote generation and maintenance of immunoglobulin-A-expressing plasma cells and contribute to gut immune homeostasis. Immunity 40, 582–593. [DOI] [PubMed] [Google Scholar]
- 6.Wu D., Molofsky A. B., Liang H. E., Ricardo-Gonzalez R. R., Jouihan H. A., Bando J. K., Chawla A., Locksley R. M. (2011) Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332, 243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makepeace B. L., Martin C., Turner J. D., Specht S. (2012) Granulocytes in helminth infection -- who is calling the shots? Curr. Med. Chem. 19, 1567–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang L., Gebreselassie N. G., Gagliardo L. F., Ruyechan M. C., Lee N. A., Lee J. J., Appleton J. A. (2014) Eosinophil-derived IL-10 supports chronic nematode infection. J. Immunol. 193, 4178–4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang L., Beiting D. P., Gebreselassie N. G., Gagliardo L. F., Ruyechan M. C., Lee N. A., Lee J. J., Appleton J. A. (2015) Eosinophils and IL-4 support nematode growth coincident with an innate response to tissue injury. PLoS Pathog. 11, e1005347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenberg H. F., Dyer K. D., Domachowske J. B. (2009) Respiratory viruses and eosinophils: exploring the connections. Antiviral Res. 83, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Percopo C. M., Dyer K. D., Ochkur S. I., Luo J. L., Fischer E. R., Lee J. J., Lee N. A., Domachowske J. B., Rosenberg H. F. (2014) Activated mouse eosinophils protect against lethal respiratory virus infection. Blood 123, 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J. J., Jacobsen E. A., Ochkur S. I., McGarry M. P., Condjella R. M., Doyle A. D., Luo H., Zellner K. R., Protheroe C. A., Willetts L., Lesuer W. E., Colbert D. C., Helmers R. A., Lacy P., Moqbel R., Lee N. A. (2012) Human versus mouse eosinophils: “that which we call an eosinophil, by any other name would stain as red.” J. Allergy Clin. Immunol. 130, 572–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyerholz D. K., Griffin M. A., Castilow E. M., Varga S. M. (2009) Comparison of histochemical methods for murine eosinophil detection in an RSV vaccine-enhanced inflammation model. Toxicol. Pathol. 37, 249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens W. W., Kim T. S., Pujanauski L. M., Hao X., Braciale T. J. (2007) Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry. J. Immunol. Methods 327, 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dyer K. D., Garcia-Crespo K. E., Killoran K. E., Rosenberg H. F. (2011) Antigen profiles for the quantitative assessment of eosinophils in mouse tissues by flow cytometry. J. Immunol. Methods 369, 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Throsby M., Herbelin A., Pléau J. M., Dardenne M. (2000) CD11c+ eosinophils in the murine thymus: developmental regulation and recruitment upon MHC class I-restricted thymocyte deletion. J. Immunol. 165, 1965–1975. [DOI] [PubMed] [Google Scholar]
- 17.Jung Y., Rothenberg M. E. (2014) Roles and regulation of gastrointestinal eosinophils in immunity and disease. J. Immunol. 193, 999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee P. Y., Wang J.-X., Parisini E., Dascher C. C., Nigrovic P. A. (2013) Ly6 family proteins in neutrophil biology. J. Leukoc. Biol. 94, 585–594. [DOI] [PubMed] [Google Scholar]
- 19.Wang H. B., Weller P. F. (2008) Pivotal advance: eosinophils mediate early alum adjuvant-elicited B cell priming and IgM production. J. Leukoc. Biol. 83, 817–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rose C. E. Jr.,Lannigan J. A., Kim P., Lee J. J., Fu S. M., Sung S. S. (2010) Murine lung eosinophil activation and chemokine production in allergic airway inflammation. Cell. Mol. Immunol. 7, 361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denzler K. L., Borchers M. T., Crosby J. R., Cieslewicz G., Hines E. M., Justice J. P., Cormier S. A., Lindenberger K. A., Song W., Wu W., Hazen S. L., Gleich G. J., Lee J. J., Lee N. A. (2001) Extensive eosinophil degranulation and peroxidase-mediated oxidation of airway proteins do not occur in a mouse ovalbumin-challenge model of pulmonary inflammation. J. Immunol. 167, 1672–1682. [DOI] [PubMed] [Google Scholar]
- 22.Denzler K. L., Farmer S. C., Crosby J. R., Borchers M., Cieslewicz G., Larson K. A., Cormier-Regard S., Lee N. A., Lee J. J. (2000) Eosinophil major basic protein-1 does not contribute to allergen-induced airway pathologies in mouse models of asthma. J. Immunol. 165, 5509–5517. [DOI] [PubMed] [Google Scholar]
- 23.Yu C., Cantor A. B., Yang H., Browne C., Wells R. A., Fujiwara Y., Orkin S. H. (2002) Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J. Exp. Med. 195, 1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartemes K. R., Iijima K., Kobayashi T., Kephart G. M., McKenzie A. N., Kita H. (2012) IL-33-responsive lineage- CD25+ CD44hi lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J. Immunol. 188, 1503–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gabryszewski S. J., Bachar O., Dyer K. D., Percopo C. M., Killoran K. E., Domachowske J. B., Rosenberg H. F. (2011) Lactobacillus-mediated priming of the respiratory mucosa protects against lethal pneumovirus infection. J. Immunol. 186, 1151–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dyer K. D., Moser J. M., Czapiga M., Siegel S. J., Percopo C. M., Rosenberg H. F. (2008) Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J. Immunol. 181, 4004–4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doyle A. D., Jacobsen E. A., Ochkur S. I., McGarry M. P., Shim K. G., Nguyen D. T., Protheroe C., Colbert D., Kloeber J., Neely J., Shim K. P., Dyer K. D., Rosenberg H. F., Lee J. J., Lee N. A. (2013) Expression of the secondary granule proteins major basic protein 1 (MBP-1) and eosinophil peroxidase (EPX) is required for eosinophilopoiesis in mice. Blood 122, 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee J. J., Lee N. A. (2005) Eosinophil degranulation: an evolutionary vestige or a universally destructive effector function? Clin. Exp. Allergy 35, 986–994. [DOI] [PubMed] [Google Scholar]
- 29.Spencer L. A., Szela C. T., Perez S. A., Kirchhoffer C. L., Neves J. S., Radke A. L., Weller P. F. (2009) Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J. Leukoc. Biol. 85, 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGarry M. P., Protheroe C. A., Lee J. J.. 2010. Mouse Hematology: A Laboratory Manual. Cold Spring Harbor Laboratory Press, New York. [Google Scholar]
- 31.Daley J. M., Thomay A. A., Connolly M. D., Reichner J. S., Albina J. E. (2008) Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 83, 64–70. [DOI] [PubMed] [Google Scholar]
- 32.Iwasaki H., Mizuno S., Mayfield R., Shigematsu H., Arinobu Y., Seed B., Gurish M. F., Takatsu K., Akashi K. (2005) Identification of eosinophil lineage-committed progenitors in the murine bone marrow. J. Exp. Med. 201, 1891–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Bruin A. M., Buitenhuis M., van der Sluijs K. F., van Gisbergen K. P., Boon L., Nolte M. A. (2010) Eosinophil differentiation in the bone marrow is inhibited by T cell-derived IFN-γ. Blood 116, 2559–2569. [DOI] [PubMed] [Google Scholar]
- 34.Rådinger M., Bossios A., Sjöstrand M., Lu Y., Malmhäll C., Dahlborn A. K., Lee J. J., Lötvall J. (2011) Local proliferation and mobilization of CCR3+ CD34+ eosinophil-lineage-committed cells in the lung. Immunology 132, 144–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chu V. T., Fröhlich A., Steinhauser G., Scheel T., Roch T., Fillatreau S., Lee J. J., Löhning M., Berek C. (2011) Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat. Immunol. 12, 151–159. [DOI] [PubMed] [Google Scholar]
- 36.Wong T. W., Doyle A. D., Lee J. J., Jelinek D. F. (2014) Eosinophils regulate peripheral B cell numbers in both mice and humans. J. Immunol. 192, 3548–3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foo S. Y., Phipps S. (2010) Regulation of inducible BALT formation and contribution to immunity and pathology. Mucosal Immunol. 3, 537–544. [DOI] [PubMed] [Google Scholar]
- 38.Hunter C. A., Kastelein R. (2012) Interleukin-27: balancing protective and pathological immunity. Immunity 37, 960–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wynn T. A. (2015) Type 2 cytokines: mechanisms and therapeutic strategies. Nat. Rev. Immunol. 15, 271–282. [DOI] [PubMed] [Google Scholar]
- 40.Wu L. C., Scheerens H. (2014) Targeting IgE production in mice and humans. Curr. Opin. Immunol. 31, 8–15. [DOI] [PubMed] [Google Scholar]
- 41.Peters M. S., Gleich G. J., Dunnette S. L., Fukuda T. (1988) Ultrastructural study of eosinophils from patients with the hypereosinophilic syndrome: a morphological basis of hypodense eosinophils. Blood 71, 780–785. [PubMed] [Google Scholar]
- 42.Shult P. A., Lega M., Jadidi S., Vrtis R., Warner T., Graziano F. M., Busse W. W. (1988) The presence of hypodense eosinophils and diminished chemiluminescence response in asthma. J. Allergy Clin. Immunol. 81, 429–437. [DOI] [PubMed] [Google Scholar]
- 43.Conesa A., Tassinari P., Rivera H., De Sanctis J. B., Bianco N., Aldrey O. (2002) Hypodense eosinophils: characterization of surface molecule expression. Allergy Asthma Proc. 23, 117–124. [PubMed] [Google Scholar]
- 44.Abdala Valencia H., Loffredo L. F., Misharin A. V., Berdnikovs S. (2016) Phenotypic plasticity and targeting of Siglec-Fhigh CD11clow eosinophils to the airway in a murine model of asthma. Allergy 71, 267–271. [DOI] [PubMed] [Google Scholar]
- 45.Voehringer D., van Rooijen N., Locksley R. M. (2007) Eosinophils develop in distinct stages and are recruited to peripheral sites by alternatively activated macrophages. J. Leukoc. Biol. 81, 1434–1444. [DOI] [PubMed] [Google Scholar]
- 46.Griseri T., Arnold I. C., Pearson C., Krausgruber T., Schiering C., Franchini F., Schulthess J., McKenzie B. S., Crocker P. R., Powrie F. (2015) Granulocyte macrophage colony-stimulating factor-activated eosinophils promote interleukin-23 driven chronic colitis. Immunity 43, 187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]