Review of cytosolic and endosomal NA sensors in autoantibody production and systemic autoimmunity.
Keywords: Toll-like receptor, cGAS, systemic lupus erythematosus, autoantibody
Abstract
Both endosomal and cytosolic-nucleic acid–sensing receptors can detect endogenous ligands and promote autoimmunity and autoinflammation. These responses involve a complex interplay among and between the cytosolic and endosomal sensors involving both hematopoietic and radioresistant cells. Cytosolic sensors directly promote inflammatory responses through the production of type I IFNs and proinflammatory cytokines. Inflammation-associated tissue damage can further promote autoimmune responses indirectly, as receptor-mediated internalization of the resulting cell debris can activate endosomal Toll-like receptors (TLR). Both endosomal and cytosolic receptors can also negatively regulate inflammatory responses. A better understanding of the factors and pathways that promote and constrain autoimmune diseases will have important implications for the development of agonists and antagonists that modulate these pathways.
Studies over the past decade have revealed a central role for innate immune sensors in both the initiation and amplification of autoimmune and autoinflammatory diseases. SLE, a multisystem disease characterized by the overproduction of pathogenic autoantibodies and immune complex–driven inflammation [1, 2], is promoted by the activation of NA-sensing endosomal TLRs [3, 4]. TLR7-deficient and TLR7/TLR9 double-deficient autoimmune-prone mice develop much less severe clinical phenotypes and exhibit markedly prolonged survival [5, 6]. As predicted, overexpression of TLR7, as a result of the Yaa gene duplication or TLR7 transgene expression, confers a much more severe disease profile than that of SLE-prone mice [7, 8]. However, paradoxically, through mechanisms that remain unresolved, essentially all SLE-prone mice that fail to express only TLR9 develop more severe disease [9–11]. It has been proposed that TLR9-deficiency simply reflects overactivation of TLR7 or other TLRs, which more effectively engage Unc93B1 in the absence of TLR9 [12]. Alternatively, TLR9 may directly limit the duration or quality of an immune response through the induction of negative regulators of the TLR7 signaling cascade or other mechanisms that limit the duration of TLR7 activation. Either way, these data point to the importance of investigating both proinflammatory and anti-inflammatory activities of TLR9.
NA-sensing receptors are also present in the cytosol [13] and include DNA sensors, such as cGAS, that promote the production of type I IFNs and NFκB-dependent proinflammatory cytokines, as well as DNA sensors such as AIM2 that trigger inflammasome assembly and caspase-1-dependent maturation of IL-1β or -18 [14, 15]. Interferogenic cytosolic DNA sensors signal via an ER-associated protein, STING [16–18]. The role of the STING pathway in immunity to DNA viruses and bacterial infection is well established [19]. More recent studies have now further implicated STING in the pathogenesis of autoinflammatory diseases resulting from the excessive accrual of endogenous DNA or DNA/RNA hybrids in the cytosol of patients and mice that lack requisite nucleases [20, 21]. Examples include diseases resulting from hypoactive TREX1 (DNaseIII), RNase H2 subunits, or DNA repair enzymes (AGS and forms of SLE) [20, 22]. Autoinflammation also occurs in individuals with STING gain-of-function mutations such as children afflicted with SAVI [23, 24]. Despite the genetic associations linking cytosolic sensors and autoinflammation, numerous questions remain as far as the ligands, target tissues, and mechanisms responsible for the diverse clinical phenotypes associated with hyperactivation of STING, and in particular how pathways downstream of the endosomal and cytosolic receptors interface with one another.
ENDOGENOUS LIGANDS OF NA SENSORS
The endosomal TLRs detect NA-associated macromolecules released from damaged or dying cells, and thereby promote the activation of plasmacytoid dendritic cells, autoreactive B cells, neutrophils, and other immunoregulatory cell types [25], and the ensuing production of type I IFNs, proinflammatory cytokines, and pathogenic autoantibodies. In all cases, self-ligands need to gain access to the TLR-containing endolysosomal compartments, either through receptor-mediated or autophagosome-related processes. For B cells, the most relevant receptor is the antigen receptor, and the specificity of these activated B cells is reflected by the autoantibodies they produce. Many of these autoantibodies are referred to as ANAs, because they react with dsDNA or chromatin (homogeneous nuclear staining pattern), or with subcellular RNA-associated macromolecules such as spliceosomes or nucleoli (speckled nuclear staining pattern), reflecting the activation of TLR9 and -7/8, respectively. Other autoantibodies recognize nuclear and cytoplasmic structures involved in DNA repair, RNA transcription, or even generation of miRNAs [26–29]. The endogenous ligands for the cytosolic NA sensors include nucleic acids released from saturated phagolysosomes [30], but also retroelement intermediates, damaged DNA extruded from the nucleus, mitochondrial or oxidized DNA, and aberrant replication intermediates [31–35]. Therefore endogenous ligands in the cytosol are derived from both extrinsic (phagosomes) and intrinsic (retroelements) sources and may, or may not, be dependent on receptor-mediated internalization.
THE ROLES OF MULTIPLE CELL TYPES AND MORE THAN ONE RECEPTOR IN STING-ASSOCIATED INFLAMMATION
A wide range of hematopoietic and nonhematopoietic cells express STING. Nevertheless, diseases associated with hyperactive STING have unique tissue specific clinical manifestations. AGS presents as an early-onset neuroinflammatory condition, and SAVI is thought to begin as vasculitis. To better understand where and how nuclease deficiencies can trigger inflammation, Trex1, RNase H2, and DNaseII loss of function mutations have been evaluated in murine models [30, 31, 36]. In all cases, the gene-targeted mice developed type I IFN-driven systemic auto-inflammation that was either completely or partially prevented by STING deficiency. However, in contrast to the prominent neuroinflammation associated with AGS patient populations, Trex1−/− mice initially exhibit myocarditis and then subsequently develop inflammation of the skeletal muscle, tongue, skin, and the glandular stomach, but not the brain [37]. Stetson and colleagues [37] further explored the initiating events in the TREX1−/− mice using an IFN reporter line and found that STING appeared to first trigger inflammation in cardiac endothelial cells. Inflammation then spread to other tissues and resulted in the indirect activation of T and B cells along with the production of ANAs. They further demonstrated that Trex1 deficiency in hematopoietic cells was not sufficient for inflammation by comparing radiation chimeras where either the donor or radioresistant host cells were Trex1 deficient. Using a similar chimeric protocol, Barber and colleagues [38] found that that Trex1−/− macrophages and dendritic cells were inherently primed for elevated cytokine production. Together, these data point to a pivotal role for both hematopoietic and nonhematopoietic cells in STING-driven inflammation resulting from excessive accrual of endogenous DNA ligands. How these data pertain to target tissues in AGS and SAVI is an open question.
DNaseII is a lysosomal nuclease active at low pH that is essential for the degradation of cellular DNA taken up by phagocytosis. In the absence of DNaseII, excessive endolysosomal DNA that cannot be degraded is thought to escape from the phagocytic compartment and enter the cytosol, where it can then activate cytosolic DNA sensors [30]. DNaseII−/− mice die as embryos as a result of IFN-induced anemia, but can be rescued by intercrossing with mice that lack the type I IFNaR. The resulting [DNaseII × IFNaR] DKO mice eventually develop inflammatory arthritis that is associated with overproduction of TNFα, IL-1β, and IL-6, along with other features of systemic autoimmunity. STING-dependent pathways play a key role in this model as [STING × DNaseII × IFNaR] TKO mice do not develop arthritis. However [AIM2 × DNaseII × IFNaR], TKO mice have markedly attenuated joint inflammation [39, 40]. Therefore, both AIM2 and cGAS/STING pathways contribute to the inflammatory arthritis resulting from DNA accrual in DKO mice (summarized in Table 1).
TABLE 1.
Cytosolic and endosomal DNA sensors contribute to disease manifestations of DNaseII−/− IFNaR−/− mice
| Targeted genes | Abbreviation | Arthritis | ANA | Splenomegaly |
|---|---|---|---|---|
| IFNaR−/− DNaseII+/− | Het | − | − | − |
| IFNaR−/− DNaseII−/− | DKO | ++++ | ++++ | ++++ |
| IFNaR−/− DNaseII−/− STING−/− | STING TKO | − | ++++ | +++ |
| IFNaR−/− DNaseII−/− AIM2−/− | AIM2 TKO | ++ | ++++ | ++ |
| IFNaR−/− DNaseII−/− Unc93b13d/3d | Unc93b1 TKO | +++ | − | + |
Relative severity of clinical manifestations is indicated as a scale from the Het phenotype (−) to the DKO phenotype (++++).
Through the use of radiation chimeras, we further showed that both DNaseII−/− hematopoietic and DNaseII−/− radioresistant cells are absolutely required for the development of arthritis in this model, as neither DKO→Het nor Het→DKO chimeras show any clinical or histologic evidence of joint inflammation, whereas DKO→DKO chimeras completely replicate the original DKO clinical phenotype [41] (summarized in Table 2). The nature of the radioresistant cell(s) and the relevant sensor in each of these cell types (STING, AIM2, or additional receptors) remain to be determined, but these studies demonstrate synergy between hematopoietic and nonhematopoietic cells in NA sensor–induced inflammation.
TABLE 2.
Summary of bone marrow chimera clinical manifestations
| Donor | Recipient | Arthritis | ANA | Splenomegaly |
|---|---|---|---|---|
| Het | Het | − | − | − |
| Het | DKO | − | ++ | − |
| DKO | Het | − | + | − |
| DKO | DKO | ++++ | ++++ | ++++ |
| DKO | Unc93b1 TKO | +++ | ++++ | ++++ |
| Unc93b1 TKO | DKO | +++ | − | + |
| Unc93b1 TKO | Unc93b1 TKO | ++ | − | + |
Relative severity of clinical manifestations is indicated as a scale from the Het phenotype (−) to the DKO phenotype (++++).
B CELL EXPRESSION OF ENDOSOMAL, BUT NOT CYTOSOLIC, NA SENSORS IS NECESSARY FOR AUTOANTIBODY PRODUCTION
Endosomal TLRs clearly play a role in the activation of autoreactive B cells in the context of SLE as TLR9- and TLR7-deficient SLE-prone mice fail to make autoantibodies against dsDNA-associated autoantigens or RNA-associated autoantigens, respectively [5, 42]. STING is also expressed in B cells and Beutler and colleagues [43] reported a key role for STING in B-cell responses to T independent type II antigens, where extensive cross-linking of the BCR leads to the induction of retroelement transcription. This observation raised the possibility that autoantigen complexes could sufficiently cross-link the BCR to cause STING activation and thereby provide a “second signal” in place of TLRs. We have explored the potential contribution of STING to autoantibody production in [DNaseII × IFNaR] DKO mice. In addition to their arthritic phenotype [DNaseII × IFNaR], DKO mice also make ANAs. Autoantibody production in DNase II-deficient mice was initially attributed to STING, based on the observation that [DNaseII × STING] DKO mice failed to make anti-DNA autoantibodies. However, the positive control for this study was MRL/lpr sera and not [DNaseII × IFNaR] DKO mice [44]. By comparison, we have found that [DNaseII × IFNaR] DKO mice [DNaseII × IFNaR × STING] TKO mice, and [DNaseII × IFNaR × AIM2] TKO mice make ANAs, as detected by both autoantigen arrays and ANA immunofluorescent staining of HEp2 cells [39, 45]. ANA production was nevertheless completely abolished in [DNaseII × IFNaR × Unc93b13d/3d] TKO mice that cannot express functional Unc93b1, an ER-associated protein required for TLR-trafficking to endolysosomal compartments (Table 1). These data clearly demonstrated that endosomal TLRs, and not cytosolic DNA sensors, are required for autoantibody production even in models of STING-associated inflammation.
Although it may be assumed that ANA production in these mice would be driven by BCR/TLR9 coengagement after BCR-mediated internalization of undegraded DNA, we were surprised to find that the HEp2 staining pattern of [DNaseII × IFNaR] DKO and [DNaseII × IFNaR × STING] TKO sera were either speckled nuclear or cytoplasmic [45], not homogeneous nuclear, as typically found in Trex1−/− mice [37, 38]. These staining patterns are indicative of autoantibodies elicited in BCR/TLR7/3/8 activated cells. Even though DKO mice express functional TLR9 and can respond to CpG-rich oligodeoxynucleotides, these mice fail to make anti-dsDNA autoantibodies because DNaseII degradation of endogenous dsDNA is necessary to generate DNA fragments sufficiently small to engage TLR9 [45, 46].
As described above, DKO→Het radiation chimeras fail to develop arthritis; they also almost always fail to make ANAs [41]. However, DKO→DKO chimeras as well as DKO→Unc93b1 TKO chimeras do make ANAs, whereas Unc93b1 TKO→DKO chimeras do not [41] (and unpublished data). These studies indicate that inflammation triggered by radioresistant DNaseII-deficient cells promotes cell death and the generation of cell debris that can then be taken up by self-reactive B cells to drive autoantibody production through a TLR-dependent mechanism. Since DNaseII is required to generate endogenous TLR9 ligands, DNA/chromatin-specific B cells are not activated. Only RNA-reactive TLRs can be activated by the cell debris resulting in the activation of B cells specific for RNA or RNA-associated autoantigens.
DKO mice develop additional manifestations of autoimmunity, including splenomegaly. This splenomegaly is associated with increased Ter119+ cells and therefore extramedullary hematopoiesis, and is linked to the disruption of the erythropoietic niche in the bone marrow. DKO erythroid island macrophages cannot degrade extruded reticulocyte nuclei and therefore accrue excessive amounts of DNA, which is presumed to cause an inflammatory response that impairs normal erythropoiesis. Remarkably, DKO splenomegaly is highly dependent on a bone marrow–derived Unc93b1-sufficient cell and only modestly dependent on STING; DKO→DKO BM chimeras and DKO→Unc93b1 develop splenomegaly, but DKO→Het and Unc93b1 TKO→DKO chimeras do not (Table 2). Thus, splenomegaly also depends on the interplay between donor and host NA sensors.
A ROLE FOR STING IN MURINE SLE
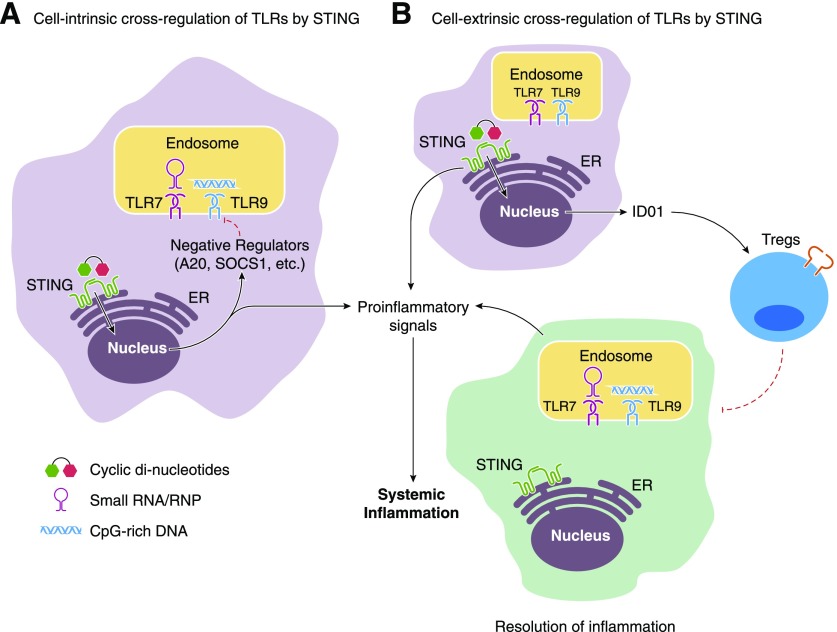
STING definitively promotes inflammation in Trex1−/−, RNaseH2−/−, and DNaseII−/− mice, all examples of overtly excessive DNA accrual. Genetic associations between TREX1 mutations and SLE in patient populations [47, 48] potentially implicate STING in additional instances of systemic autoimmunity. Endosomal TLRs have also been implicated in psoriasis, systemic sclerosis, Sjögren’s disease, and other rheumatologic disorders characterized by ANA production [43, 49–53]. These observations raised the question as to how cytosolic sensors may contribute to more common forms of SLE. To better understand the impact of cytosolic DNA sensing pathways in systemic autoimmune disease, we intercrossed STING−/− mice with the autoimmune-prone, Fas-deficient strains MRL.Faslpr and B6.Faslpr. Contrary to our expectation that STING-dependent pathways would synergize with the TLR-driven inflammation in SLE-prone mice, we found that STING suppressed disease as STING-deficient mice developed exacerbated clinical manifestations of disease compared to littermate controls and had a shorter lifespan [54]. Furthermore, 2,6,10,14-tetramethylpentadecane-mediated inflammation/lupus was similarly aggravated in B6 STING-deficient mice compared to STING-sufficient controls [54]. These studies revealed a previously unanticipated role for STING in the negative regulation of TLR-dependent systemic autoimmunity, and paralleled the paradoxical phenotype of TLR9-deficient SLE-prone mice. The exact mechanism(s) responsible for this apparently suppressive activity remains to be determined, but may include the activation of negative regulators of TLR signaling, such as SOCS1 or A20, or more subtle changes in the microbiome or gene transcription in the absence of STING-elicited pathways (Fig. 1).
Figure 1. Potential mechanisms for STING-mediated regulation of TLR activation in myeloid-derived cells or tissue-resident sentinels.
(A) A cell-intrinsic mechanism where STING activation not only initiates proinflammatory loops but also maintains critical levels of negative regulators that blunt TLR signaling within the same cell type. (B) A cell-extrinsic mechanism where STING-dependent signaling in one cell leads to the induction of both proinflammatory cytokines and tolerogenic cytokines like IDO-1. IDO-1 then recruits and activates T-regulatory cells (Tregs) that curb TLR-drive inflammation in other cells.
We are not the only group to show that STING can negatively regulate immune responses. McGaha and colleagues [55, 56] have shown that AT-rich DNA-coated nanoparticles and apoptotic debris trigger myeloid cell production of IDO, and the subsequent expansion of Tregs, through a STING-dependent mechanism. Together, these observations define the capacity of both endosomal and cytosolic DNA sensors to promote inflammation and elicit critical negative regulatory effects [54] and provide intriguing evidence for a more complex role for STING in autoimmunity than previously anticipated (Fig. 1).
As in the case of the cytosolic DNA-sensing receptors, it is intriguing to note that similar cross regulatory pathways have been identified between cytosolic RNA-sensing pathways and TLRs [57]. Indeed, Negishi et al. [57] showed that IRF3 activation downstream of RIG-I-like receptor signaling interferes with TLR-driven IL-12 transcriptional events. These data suggest multiple avenues for cross-regulation in the innate immune system that are likely to play a key role in attenuating the strength of inflammatory signals.
CONCLUDING REMARKS
The recent identification of the cGAS enzyme and the ability of STING to respond to cyclic dinucleotides [58, 59], make cGAS and STING attractive targets for small-molecule therapy for autoinflammatory conditions, such as AGS, SAVI, and perhaps even SLE. Moreover, these studies form a basis for directed GWAS analyses on linkage between known SLE polymorphisms and STING. However, NA sensors such as STING and TLR9 clearly serve as both positive and negative regulators of inflammation. Further exploration and characterization of the relevant pathways and cell types is warranted to avoid potentially unanticipated dangerous outcomes in patients predisposed to the development of SLE or other TLR-driven autoimmune diseases.
AUTHORSHIP
S.S., K.A.F., E.M.G. and A.M.-R. designed the research, S.P., S.S., R.B., K.N. and P.B. carried out the research and analyzed the data, S.P., S.S., K.A.F. and A.M.-R. wrote the manuscript.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health, National Institute for arthritis and Musculoskeletal and Skin Disease Grant AR066808 (to A.M.-R.) and AR067394 (to E.M.G) and National Institute of Allergy and Infectious Diseases Grants AI093752 and AI083713 (to K.A.F.) and by the Alliance for Lupus Research (to A.M.-R.)
Glossary
- AGS
Aicardi Goutières syndrome
- ANAs
anti-nuclear antibodies
- BCR
B-cell receptor
- BM
bone marrow
- cGAS
cyclic GMP-AMP synthase
- DKO
DNaseII−/−IFNaR−/−double knockout
- dsDNA
double-stranded DNA
- Het
DNaseII−/+ IFNaR−/−
- IFNaR
type I IFN receptor
- IDO
indoleamine 2,3-dioxygenase
- NA
nucleic acid
- SAVI
STING-associated vasculopathy with onset in infancy
- STING
stimulator of type I IFN genes
- TKO
triple knockout
DISCLOSURE
The authors declare no competing financial interests.
REFERENCES
- 1.Baccala R., Hoebe K., Kono D. H., Beutler B., Theofilopoulos A. N. (2007) TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat. Med. 13, 543–551. [DOI] [PubMed] [Google Scholar]
- 2.Craft J. E. (2011) Dissecting the immune cell mayhem that drives lupus pathogenesis. Sci. Transl. Med. 3, 73ps9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deane J. A., Pisitkun P., Barrett R. S., Feigenbaum L., Town T., Ward J. M., Flavell R. A., Bolland S. (2007) Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity 27, 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nickerson K. M., Christensen S. R., Shupe J., Kashgarian M., Kim D., Elkon K., Shlomchik M. J. (2010) TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J. Immunol. 184, 1840–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen S. R., Shupe J., Nickerson K., Kashgarian M., Flavell R. A., Shlomchik M. J. (2006) Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity 25, 417–428. [DOI] [PubMed] [Google Scholar]
- 6.Kono D. H., Haraldsson M. K., Lawson B. R., Pollard K. M., Koh Y. T., Du X., Arnold C. N., Baccala R., Silverman G. J., Beutler B. A., Theofilopoulos A. N. (2009) Endosomal TLR signaling is required for anti-nucleic acid and rheumatoid factor autoantibodies in lupus. Proc. Natl. Acad. Sci. USA 106, 12061–12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pisitkun P., Deane J. A., Difilippantonio M. J., Tarasenko T., Satterthwaite A. B., Bolland S. (2006) Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science 312, 1669–1672. [DOI] [PubMed] [Google Scholar]
- 8.Subramanian S., Tus K., Li Q. Z., Wang A., Tian X. H., Zhou J., Liang C., Bartov G., McDaniel L. D., Zhou X. J., Schultz R. A., Wakeland E. K. (2006) A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc. Natl. Acad. Sci. USA 103, 9970–9975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu P., Wellmann U., Kunder S., Quintanilla-Martinez L., Jennen L., Dear N., Amann K., Bauer S., Winkler T. H., Wagner H. (2006) Toll-like receptor 9-independent aggravation of glomerulonephritis in a novel model of SLE. Int. Immunol. 18, 1211–1219. [DOI] [PubMed] [Google Scholar]
- 10.Nickerson K. M., Christensen S. R., Cullen J. L., Meng W., Luning Prak E. T., Shlomchik M. J. (2013) TLR9 promotes tolerance by restricting survival of anergic anti-DNA B cells, yet is also required for their activation. J. Immunol. 190, 1447–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson S. W., Scharping N. E., Kolhatkar N. S., Khim S., Schwartz M. A., Li Q. Z., Hudkins K. L., Alpers C. E., Liggitt D., Rawlings D. J. (2014) Opposing impact of B cell-intrinsic TLR7 and TLR9 signals on autoantibody repertoire and systemic inflammation. J. Immunol. 192, 4525–4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukui R., Saitoh S., Kanno A., Onji M., Shibata T., Ito A., Onji M., Matsumoto M., Akira S., Yoshida N., Miyake K. (2011) Unc93B1 restricts systemic lethal inflammation by orchestrating Toll-like receptor 7 and 9 trafficking. Immunity 35, 69–81. [DOI] [PubMed] [Google Scholar]
- 13.Sharma S., DeOliveira R. B., Kalantari P., Parroche P., Goutagny N., Jiang Z., Chan J., Bartholomeu D. C., Lauw F., Hall J. P., Barber G. N., Gazzinelli R. T., Fitzgerald K. A., Golenbock D. T. (2011) Innate immune recognition of an AT-rich stem-loop DNA motif in the Plasmodium falciparum genome. Immunity 35, 194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rathinam V. A., Jiang Z., Waggoner S. N., Sharma S., Cole L. E., Waggoner L., Vanaja S. K., Monks B. G., Ganesan S., Latz E., Hornung V., Vogel S. N., Szomolanyi-Tsuda E., Fitzgerald K. A. (2010) The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 11, 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma S., Fitzgerald K. A. (2011) Innate immune sensing of DNA. PLoS Pathog. 7, e1001310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishikawa H., Barber G. N. (2008) STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455, 674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishikawa H., Barber G. N. (2011) The STING pathway and regulation of innate immune signaling in response to DNA pathogens. Cell. Mol. Life Sci. 68, 1157–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishikawa H., Ma Z., Barber G. N. (2009) STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461, 788–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson M. R., Kaminski J. J., Kurt-Jones E. A., Fitzgerald K. A. (2011) Pattern recognition receptors and the innate immune response to viral infection. Viruses 3, 920–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crow Y. J., Hayward B. E., Parmar R., Robins P., Leitch A., Ali M., Black D. N., van Bokhoven H., Brunner H. G., Hamel B. C., Corry P. C., Cowan F. M., Frints S. G., Klepper J., Livingston J. H., Lynch S. A., Massey R. F., Meritet J. F., Michaud J. L., Ponsot G., Voit T., Lebon P., Bonthron D. T., Jackson A. P., Barnes D. E., Lindahl T. (2006) Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 cause Aicardi-Goutières syndrome at the AGS1 locus. Nat. Genet. 38, 917–920. [DOI] [PubMed] [Google Scholar]
- 21.Crow Y. J., Manel N. (2015) Aicardi-Goutières syndrome and the type I interferonopathies. Nat. Rev. Immunol. 15, 429–440. [DOI] [PubMed] [Google Scholar]
- 22.Rice G. I., Reijns M. A., Coffin S. R., Forte G. M., Anderson B. H., Szynkiewicz M., Gornall H., Gent D., Leitch A., Botella M. P., Fazzi E., Gener B., Lagae L., Olivieri I., Orcesi S., Swoboda K. J., Perrino F. W., Jackson A. P., Crow Y. J. (2013) Synonymous mutations in RNASEH2A create cryptic splice sites impairing RNase H2 enzyme function in Aicardi-Goutières syndrome. Hum. Mutat. 34, 1066–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y., Jesus A. A., Marrero B., Yang D., Ramsey S. E., Montealegre Sanchez G. A., Tenbrock K., Wittkowski H., Jones O. Y., Kuehn H. S., Lee C. C., DiMattia M. A., Cowen E. W., Gonzalez B., Palmer I., DiGiovanna J. J., Biancotto A., Kim H., Tsai W. L., Trier A. M., Huang Y., Stone D. L., Hill S., Kim H. J., St Hilaire C., Gurprasad S., Plass N., Chapelle D., Horkayne-Szakaly I., Foell D., Barysenka A., Candotti F., Holland S. M., Hughes J. D., Mehmet H., Issekutz A. C., Raffeld M., McElwee J., Fontana J. R., Minniti C. P., Moir S., Kastner D. L., Gadina M., Steven A. C., Wingfield P. T., Brooks S. R., Rosenzweig S. D., Fleisher T. A., Deng Z., Boehm M., Paller A. S., Goldbach-Mansky R. (2014) Activated STING in a vascular and pulmonary syndrome. N. Engl. J. Med. 371, 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeremiah N., Neven B., Gentili M., Callebaut I., Maschalidi S., Stolzenberg M. C., Goudin N., Frémond M. L., Nitschke P., Molina T. J., Blanche S., Picard C., Rice G. I., Crow Y. J., Manel N., Fischer A., Bader-Meunier B., Rieux-Laucat F. (2014) Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J. Clin. Invest. 124, 5516–5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marshak-Rothstein A. (2006) Toll-like receptors in systemic autoimmune disease. Nat. Rev. Immunol. 6, 823–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plotz P. H. (2003) The autoantibody repertoire: searching for order. Nat. Rev. Immunol. 3, 73–78. [DOI] [PubMed] [Google Scholar]
- 27.Ikeda K., Satoh M., Pauley K. M., Fritzler M. J., Reeves W. H., Chan E. K. (2006) Detection of the argonaute protein Ago2 and microRNAs in the RNA induced silencing complex (RISC) using a monoclonal antibody. J. Immunol. Methods 317, 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casciola-Rosen L. A., Pluta A. F., Plotz P. H., Cox A. E., Morris S., Wigley F. M., Petri M., Gelber A. C., Rosen A. (2001) The DNA mismatch repair enzyme PMS1 is a myositis-specific autoantigen. Arthritis Rheum. 44, 389–396. [DOI] [PubMed] [Google Scholar]
- 29.Casciola-Rosen L., Andrade F., Ulanet D., Wong W. B., Rosen A. (1999) Cleavage by granzyme B is strongly predictive of autoantigen status: implications for initiation of autoimmunity. J. Exp. Med. 190, 815–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawane K., Ohtani M., Miwa K., Kizawa T., Kanbara Y., Yoshioka Y., Yoshikawa H., Nagata S. (2006) Chronic polyarthritis caused by mammalian DNA that escapes from degradation in macrophages. Nature 443, 998–1002. [DOI] [PubMed] [Google Scholar]
- 31.Stetson D. B., Ko J. S., Heidmann T., Medzhitov R. (2008) Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell 134, 587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lan Y. Y., Londoño D., Bouley R., Rooney M. S., Hacohen N. (2014) Dnase2a deficiency uncovers lysosomal clearance of damaged nuclear DNA via autophagy. Cell Reports 9, 180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.West A. P., Khoury-Hanold W., Staron M., Tal M. C., Pineda C. M., Lang S. M., Bestwick M., Duguay B. A., Raimundo N., MacDuff D. A., Kaech S. M., Smiley J. R., Means R. E., Iwasaki A., Shadel G. S. (2015) Mitochondrial DNA stress primes the antiviral innate immune response. Nature 520, 553–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gehrke N., Mertens C., Zillinger T., Wenzel J., Bald T., Zahn S., Tüting T., Hartmann G., Barchet W. (2013) Oxidative damage of DNA confers resistance to cytosolic nuclease TREX1 degradation and potentiates STING-dependent immune sensing. Immunity 39, 482–495. [DOI] [PubMed] [Google Scholar]
- 35.Yang Y. G., Lindahl T., Barnes D. E. (2007) Trex1 exonuclease degrades ssDNA to prevent chronic checkpoint activation and autoimmune disease. Cell 131, 873–886. [DOI] [PubMed] [Google Scholar]
- 36.Pokatayev V., Hasin N., Chon H., Cerritelli S. M., Sakhuja K., Ward J. M., Morris H. D., Yan N., Crouch R. J. (2016) RNase H2 catalytic core Aicardi-Goutières syndrome-related mutant invokes cGAS-STING innate immune-sensing pathway in mice. J. Exp. Med. 213, 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gall A., Treuting P., Elkon K. B., Loo Y. M., Gale M. Jr., Barber G. N., Stetson D. B. (2012) Autoimmunity initiates in nonhematopoietic cells and progresses via lymphocytes in an interferon-dependent autoimmune disease. Immunity 36, 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahn J., Ruiz P., Barber G. N. (2014) Intrinsic self-DNA triggers inflammatory disease dependent on STING. J. Immunol. 193, 4634–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baum R., Sharma S., Carpenter S., Li Q. Z., Busto P., Fitzgerald K. A., Marshak-Rothstein A., Gravallese E. M. (2015) Cutting edge: AIM2 and endosomal TLRs differentially regulate arthritis and autoantibody production in DNase II-deficient mice. J. Immunol. 194, 873–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jakobs C., Perner S., Hornung V. (2015) AIM2 drives joint inflammation in a Self-DNA triggered model of chronic polyarthritis. PLoS One 10, e0131702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baum R., Nündel K., Pawaria S., Sharma S., Busto P., Fitzgerald K. A., Gravallese E. M., Marshak-Rothstein A. (2016) Synergy between hematopoietic and radioresistant stromal cells is required for autoimmune manifestations of DNase II−/−IFNaR−/− mice. J. Immunol. 196, 1348–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Green N. M., Marshak-Rothstein A. (2011) Toll-like receptor driven B cell activation in the induction of systemic autoimmunity. Semin. Immunol. 23, 106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeng M., Hu Z., Shi X., Li X., Zhan X., Li X. D., Wang J., Choi J. H., Wang K. W., Purrington T., Tang M., Fina M., DeBerardinis R. J., Moresco E. M., Pedersen G., McInerney G. M., Karlsson Hedestam G. B., Chen Z. J., Beutler B. (2014) MAVS, cGAS, and endogenous retroviruses in T-independent B cell responses. Science 346, 1486–1492. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Ahn J., Gutman D., Saijo S., Barber G. N. (2012) STING manifests self DNA-dependent inflammatory disease. Proc. Natl. Acad. Sci. USA 109, 19386–19391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pawaria S., Moody K., Busto P., Nündel K., Choi C. H., Ghayur T., Marshak-Rothstein A. (2015) Cutting edge: DNase II deficiency prevents activation of autoreactive B cells by double-stranded DNA endogenous ligands. J. Immunol. 194, 1403–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chan M. P., Onji M., Fukui R., Kawane K., Shibata T., Saitoh S., Ohto U., Shimizu T., Barber G. N., Miyake K. (2015) DNase II-dependent DNA digestion is required for DNA sensing by TLR9. Nat. Commun. 6, 5853. [DOI] [PubMed] [Google Scholar]
- 47.Lee-Kirsch M. A., Gong M., Chowdhury D., Senenko L., Engel K., Lee Y. A., de Silva U., Bailey S. L., Witte T., Vyse T. J., Kere J., Pfeiffer C., Harvey S., Wong A., Koskenmies S., Hummel O., Rohde K., Schmidt R. E., Dominiczak A. F., Gahr M., Hollis T., Perrino F. W., Lieberman J., Hübner N. (2007) Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 are associated with systemic lupus erythematosus. Nat. Genet. 39, 1065–1067. [DOI] [PubMed] [Google Scholar]
- 48.Ellyard J. I., Jerjen R., Martin J. L., Lee A. Y., Field M. A., Jiang S. H., Cappello J., Naumann S. K., Andrews T. D., Scott H. S., Casarotto M. G., Goodnow C. C., Chaitow J., Pascual V., Hertzog P., Alexander S. I., Cook M. C., Vinuesa C. G. (2014) Identification of a pathogenic variant in TREX1 in early-onset cerebral systemic lupus erythematosus by whole-exome sequencing. Arthritis Rheumatol. 66, 3382–3386. [DOI] [PubMed] [Google Scholar]
- 49.Morizane S., Yamasaki K., Mühleisen B., Kotol P. F., Murakami M., Aoyama Y., Iwatsuki K., Hata T., Gallo R. L. (2012) Cathelicidin antimicrobial peptide LL-37 in psoriasis enables keratinocyte reactivity against TLR9 ligands. J. Invest. Dermatol. 132, 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lafyatis R., Farina A. (2012) New insights into the mechanisms of innate immune receptor signalling in fibrosis. Open Rheumatol. J. 6, 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Low H. Z., Witte T. (2011) Aspects of innate immunity in Sjögren’s syndrome. Arthritis Res. Ther. 13, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kramer J. M. (2014) Early events in Sjögren’s Syndrome pathogenesis: the importance of innate immunity in disease initiation. Cytokine 67, 92–101. [DOI] [PubMed] [Google Scholar]
- 53.Karlsen M., Jonsson R., Brun J. G., Appel S., Hansen T. (2015) TLR-7 and -9 stimulation of peripheral blood B cells indicate altered TLR signalling in primary Sjögren’s syndrome patients by increased secretion of cytokines. Scand. J. Immunol. 82, 523–531. [DOI] [PubMed] [Google Scholar]
- 54.Sharma S., Campbell A. M., Chan J., Schattgen S. A., Orlowski G. M., Nayar R., Huyler A. H., Nündel K., Mohan C., Berg L. J., Shlomchik M. J., Marshak-Rothstein A., Fitzgerald K. A. (2015) Suppression of systemic autoimmunity by the innate immune adaptor STING. Proc. Natl. Acad. Sci. USA 112, E710–E717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang L., Li L., Lemos H., Chandler P. R., Pacholczyk G., Baban B., Barber G. N., Hayakawa Y., McGaha T. L., Ravishankar B., Munn D. H., Mellor A. L. (2013) Cutting edge: DNA sensing via the STING adaptor in myeloid dendritic cells induces potent tolerogenic responses. J. Immunol. 191, 3509–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lemos H., Huang L., McGaha T. L., Mellor A. L. (2014) Cytosolic DNA sensing via the stimulator of interferon genes adaptor: Yin and Yang of immune responses to DNA. Eur. J. Immunol. 44, 2847–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Negishi H., Yanai H., Nakajima A., Koshiba R., Atarashi K., Matsuda A., Matsuki K., Miki S., Doi T., Aderem A., Nishio J., Smale S. T., Honda K., Taniguchi T. (2012) Cross-interference of RLR and TLR signaling pathways modulates antibacterial T cell responses. Nat. Immunol. 13, 659–666. [DOI] [PubMed] [Google Scholar]
- 58.Wu J., Sun L., Chen X., Du F., Shi H., Chen C., Chen Z. J. (2013) Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339, 826–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun L., Wu J., Du F., Chen X., Chen Z. J. (2013) Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–791. [DOI] [PMC free article] [PubMed] [Google Scholar]