Abstract
Ion channels that give rise to the excitable properties of the neuronal plasma membrane are synthesized, transported, and degraded in cytoplasmic organelles. To determine whether plasma membrane ion channels from these organelles could be physiologically activated, we extruded axoplasm from squid giant axons, dissociated organelles from the cytoskeletal matrix, and fused the free organelles with planar lipid bilayers. Three classes of ion channels normally associated with the plasma membrane were identified based on conductance, selectivity, and gating properties determined from steady-state single-channel recordings: (i) voltage-dependent Na channels, (ii) voltage-dependent delayed rectifier K channels, and (iii) large, voltage-independent K channels. The identity of the delayed rectifier channels was confirmed by reconstructing the time course of activation from single-channel responses to depolarizing voltage steps applied across the bilayer. These observations suggest that several classes of plasma membrane ion channels are transported in cytoplasmic organelles in physiologically active forms.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augustine C. K., Bezanilla F. Phosphorylation modulates potassium conductance and gating current of perfused giant axons of squid. J Gen Physiol. 1990 Feb;95(2):245–271. doi: 10.1085/jgp.95.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens M. I., Oberhauser A., Bezanilla F., Latorre R. Batrachotoxin-modified sodium channels from squid optic nerve in planar bilayers. Ion conduction and gating properties. J Gen Physiol. 1989 Jan;93(1):23–41. doi: 10.1085/jgp.93.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brismar T., Gilly W. F. Synthesis of sodium channels in the cell bodies of squid giant axons. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1459–1463. doi: 10.1073/pnas.84.5.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. Structure and function of voltage-sensitive ion channels. Science. 1988 Oct 7;242(4875):50–61. doi: 10.1126/science.2459775. [DOI] [PubMed] [Google Scholar]
- Clay J. R. Slow inactivation and reactivation of the K+ channel in squid axons. A tail current analysis. Biophys J. 1989 Mar;55(3):407–414. doi: 10.1016/S0006-3495(89)82834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F., Neher E. Single channel recordings of K+ currents in squid axons. Nature. 1980 May 15;285(5761):140–143. doi: 10.1038/285140a0. [DOI] [PubMed] [Google Scholar]
- Fishman H. M., Tewari K. P., Stein P. G. Injury-induced vesiculation and membrane redistribution in squid giant axon. Biochim Biophys Acta. 1990 Apr 30;1023(3):421–435. doi: 10.1016/0005-2736(90)90135-b. [DOI] [PubMed] [Google Scholar]
- Grafstein B., Forman D. S. Intracellular transport in neurons. Physiol Rev. 1980 Oct;60(4):1167–1283. doi: 10.1152/physrev.1980.60.4.1167. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I., Bookman R. J. Ionic conductances of squid giant fiber lobe neurons. J Gen Physiol. 1986 Oct;88(4):543–569. doi: 10.1085/jgp.88.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahar J., Lukács G. L., Li Y., Hall S., Moczydlowski E. Pharmacological and biochemical properties of saxiphilin, a soluble saxitoxin-binding protein from the bullfrog (Rana catesbeiana). Toxicon. 1991;29(1):53–71. doi: 10.1016/0041-0101(91)90039-t. [DOI] [PubMed] [Google Scholar]
- Perozo E., Bezanilla F., Dipolo R. Modulation of K channels in dialyzed squid axons. ATP-mediated phosphorylation. J Gen Physiol. 1989 Jun;93(6):1195–1218. doi: 10.1085/jgp.93.6.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
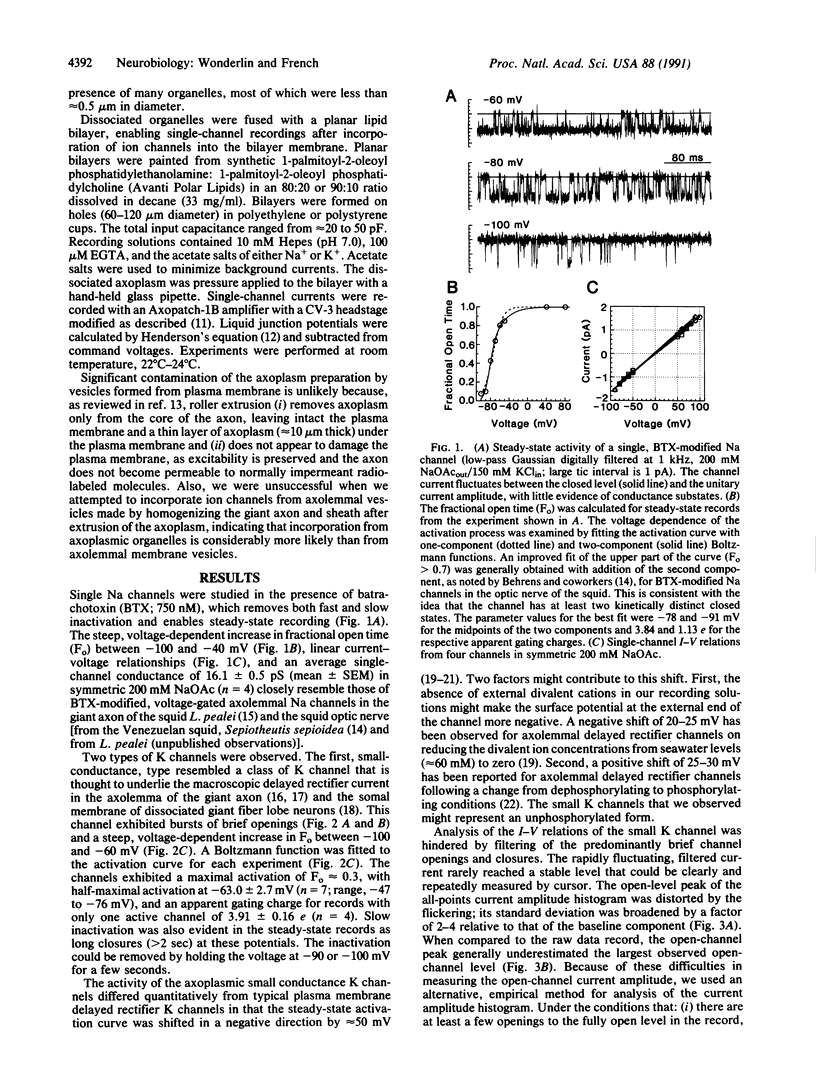
- Schmidt J., Rossie S., Catterall W. A. A large intracellular pool of inactive Na channel alpha subunits in developing rat brain. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4847–4851. doi: 10.1073/pnas.82.14.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer T. A., Schnapp B. J., Reese T. S., Sheetz M. P. The role of kinesin and other soluble factors in organelle movement along microtubules. J Cell Biol. 1988 Nov;107(5):1785–1792. doi: 10.1083/jcb.107.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D., Schnapp B. J., Reese T. S., Sheetz M. P. Movement of organelles along filaments dissociated from the axoplasm of the squid giant axon. Cell. 1985 Feb;40(2):449–454. doi: 10.1016/0092-8674(85)90159-x. [DOI] [PubMed] [Google Scholar]
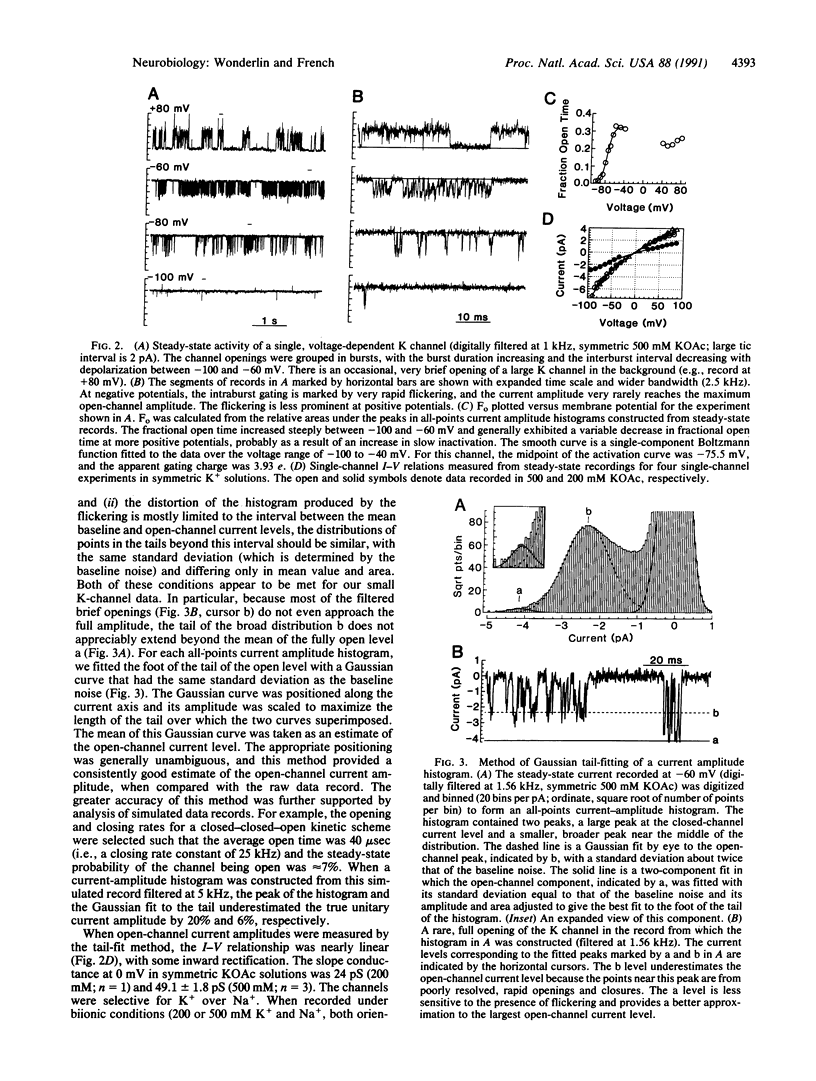
- Wonderlin W. F., Finkel A., French R. J. Optimizing planar lipid bilayer single-channel recordings for high resolution with rapid voltage steps. Biophys J. 1990 Aug;58(2):289–297. doi: 10.1016/S0006-3495(90)82376-6. [DOI] [PMC free article] [PubMed] [Google Scholar]



