In this review, Gomez-Navarro and Miller summarize the principles of cargo sorting by the vesicle traffic machinery and consider the diverse mechanisms by which cargo proteins are selected and captured into different transport vesicles.
Abstract
Protein traffic is of critical importance for normal cellular physiology. In eukaryotes, spherical transport vesicles move proteins and lipids from one internal membrane-bound compartment to another within the secretory pathway. The process of directing each individual protein to a specific destination (known as protein sorting) is a crucial event that is intrinsically linked to vesicle biogenesis. In this review, we summarize the principles of cargo sorting by the vesicle traffic machinery and consider the diverse mechanisms by which cargo proteins are selected and captured into different transport vesicles. We focus on the first two compartments of the secretory pathway: the endoplasmic reticulum and Golgi. We provide an overview of the complexity and diversity of cargo adaptor function and regulation, focusing on recent mechanistic discoveries that have revealed insight into protein sorting in cells.
Introduction
Eukaryotic cells are highly compartmentalized, with separate organelles each characterized by specific protein and lipid compositions. Yet, within the connected compartments of the secretory pathway, this material continuously exchanges as membranes and cargo proteins undergo dynamic traffic. Between 20% and 30% of the cell’s proteome is destined for either the extracellular environment or the internal endomembrane system. ER-to-Golgi transport is the first step in the secretory pathway. At the ER, proteins destined for the extracellular space or to organelles along the route are packaged into vesicles that transport them to the Golgi apparatus. At this point, cells seem to distinguish between native and nonnative proteins, ensuring that only appropriately folded and assembled cargo protein undergo forward transport. Many secretory proteins are actively sorted during ER export. However, traffic can also occur in a nonselective manner called bulk flow. Finally, retrieval from the Golgi to the ER ensures that immature cargoes or escaped ER resident proteins are efficiently transported back to the ER. Here, we consider how cells satisfy the sorting needs of the diverse set of proteins that navigate the ER–Golgi interface, an impressive feat considering the extent of cargo protein heterogeneity.
Principles of selective capture into transport vesicles
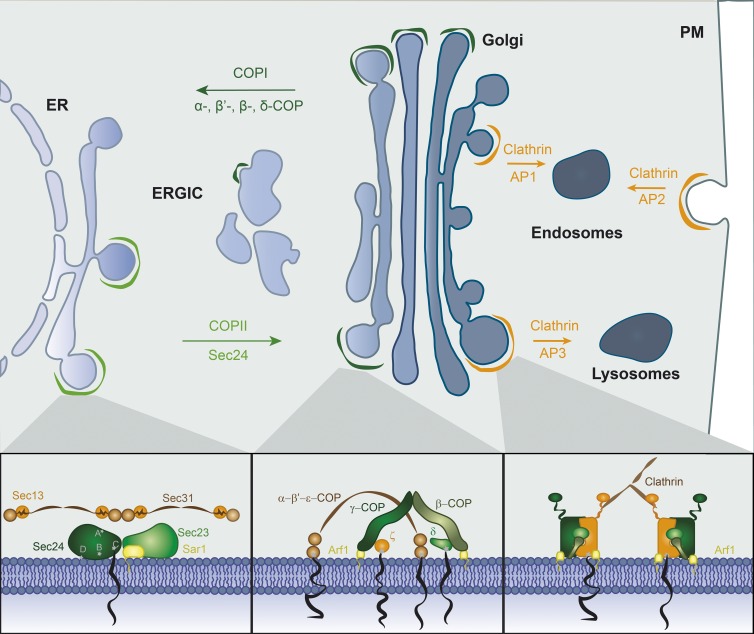
Transport of proteins between organelles within the secretory pathway occurs via spherical membrane-bounded vesicles that bud from a donor organelle and fuse with an acceptor in another part of the cell. This fission and fusion transport strategy allows secretory proteins to cross membrane barriers without perturbing the functional segregation conferred by organelles. Conserved sets of cytoplasmic proteins generate distinct classes of transport vesicles, which are largely classified by the protein coats that drive their formation. The three main vesicular frameworks found across eukaryotic life (clathrin, COPI, and COPII) come from evolutionarily related coat proteins. COPII-coated vesicles transport cargo proteins from the ER to the Golgi; COPI-coated vesicles transport cargo in the retrograde direction (from the cis-Golgi back to the ER) and between Golgi cisternae; and clathrin-coated vesicles form from the plasma membrane and the TGN to fuse with endosomes or lysosomes (Fig. 1). Vesicle coats perform two central functions: deforming the membrane into a spherical vesicle and populating the vesicle with specific cargo. By coupling cargo selection to vesicle formation, cells can achieve efficient protein sorting as an in-built outcome of the transport pathway itself.
Figure 1.
Overview of intracellular transport pathways. Schematic view of the secretory pathway and representation of the major coat proteins that mediate protein sorting at different cellular compartments. Secretory cargoes are trafficked in an anterograde direction from the ER to the Golgi in COPII-coated vesicles. Sec24 is the cargo adaptor that contains multiple cargo binding sites (marked A–D in the inset) to drive capture of a diverse set of cargo proteins. The COPI coat mediates retrograde transport from the Golgi to the ER and between Golgi compartments. The cargo-binding subunits of COPI vesicles form an arch-like structure that contacts the membrane through the N-terminal domains that interact with cargo proteins. Clathrin-coated vesicles bud from multiple organelles and transport proteins between the TGN, endosomes, and plasma membrane (PM). Different cargo adaptors function at the different donor membranes (AP1, AP2, and AP3). The general structure of the AP complexes consist of a discretely folded domain comprising the trunk domains of the two large subunits, which interact with the membrane and cargo proteins, and two unstructured sequence motifs, which bind clathrin and other accessory proteins.
Coat adaptors recognize sorting signals
Studies on the internalization of cell surface receptors via clathrin-mediated endocytosis first established the principle that specific protein-based signals mediate capture of cargo into vesicles. Subsequent biochemical, structural, and genetic dissection of clathrin and other vesicle systems has defined how these different coat assemblies couple cargo sorting with the general formation of vesicles. Central to the appropriate sorting of cargo, specific coat subunits (known as cargo adaptors) contain binding surfaces that recognize sorting signals present in the cytoplasmic domains of cargo proteins. Interaction between coat and signal is responsible for capture of cargo into the forming vesicles. Most binary cargo–coat interactions measured in vitro are relatively low affinity, which may be important in the context of coat dynamics during traffic. During the lifetime of the vesicle, coat proteins are shed from the vesicle surface to expose fusion machinery; therefore, interactions between coat and vesicle components must be reversible. However, cargo adaptors also often have affinity for lipids, which can contribute both to the specificity of recruitment and to the global affinity of the adaptor for a donor organelle. Finally, adaptors bind directly to the structural scaffolding elements of the various coats, thus bridging the membrane, cargo, and membrane deformation machinery. Understanding how local cooperative effects of interactions among cargo, lipid, adaptor, and scaffold components mutually influence vesicle formation remains a key question in many vesicle transport events.
Cargo receptors span the membrane to bridge cargo and coat
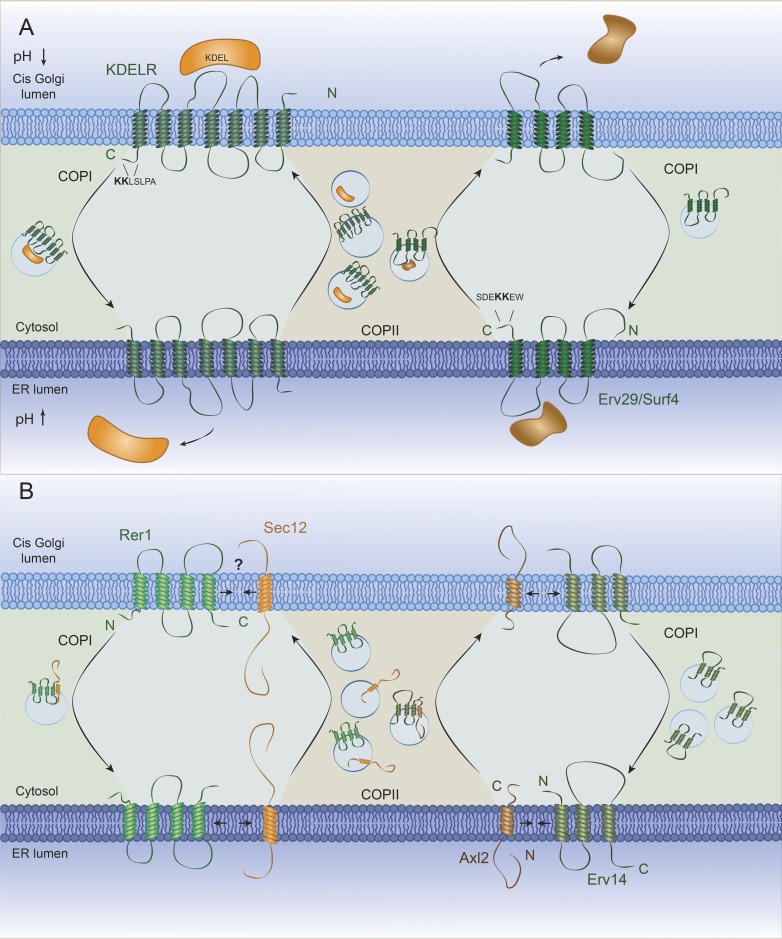
One limitation of the signal-mediated sorting of coat-bound cargo adaptors is access to a signal; the many soluble secreted and lysosomal proteins are precluded from interacting directly with the vesicle coat subunits by the barrier formed by the lipid bilayer. Sorting of these cargo proteins occurs by receptor-mediated transport. Cargo receptors are thus defined as proteins that span the membrane, binding simultaneously to cargo proteins and coat adaptors, to efficiently recruit soluble proteins to nascent vesicles (for recent comprehensive reviews, see Dancourt and Barlowe, 2010; Geva and Schuldiner, 2014; Guo et al., 2014; Barlowe and Helenius, 2016). Central to the function of cargo receptors is the ability to bind cargo in one compartment and release it upon fusion with the downstream organelle. In this way, the cargo receptor can be returned to the donor compartment for another trafficking event. Cargo receptors are thus long-lived proteins that mediate multiple individual rounds of transport. It remains unclear how most cargo receptors impose vectorial movement between compartments. Perhaps the best-characterized example is the KDEL receptor (named for the Lys-Asp-Glu-Leu ER retrieval signal that it recognizes), which cycles between the ER and Golgi to return escaped ER residents. Here, binding and release is pH dependent, such that in the lower-pH environment of the cis-Golgi, the receptor binds the retrieval motif, but in the neutral pH of the ER lumen, this interaction is reversed (Fig. 2). For other receptor–cargo pairs, additional lumenal conditions, like ion concentration, may also influence conditional binding. Moreover, there is evidence for cargo-driven modulation of KDEL receptor localization (Lewis and Pelham, 1992), suggesting that the receptor is not simply an inert platform but can respond to cargo sorting needs, perhaps by allosteric effects of cargo binding.
Figure 2.
Cartoon showing how cargo receptors mediate traffic by cycling between the ER and Golgi. (A) The KDEL receptor acts in retrograde transport of secretory proteins. It interacts with soluble cargo proteins at the lower pH of the Golgi and it is directed to the ER via COPI vesicles. At the neutral pH of the ER, the receptor releases the cargo protein to the lumen. In the opposite direction, Erv29 (Surf4 in mammals) transports cargo proteins from the ER to the Golgi. The cytoplasmic C-terminal sorting sequences from the human receptor proteins are shown. (B) Rer1 acts in the retrieval transport of transmembrane cargo proteins. The cargo receptor interacts with the transmembrane domain of the cargo protein, often via polar residues embedded within the membrane. Then, through its cytosolic domain, it recruits the COPI coat, which delivers the cargo proteins to the ER. Similarly, but in the opposite direction, Erv14 binds to transmembrane domains in the ER and concentrates them to ERES from where they are efficiently transported to the Golgi via COPII vesicles. Rer1 and Erv14 bind their clients in a regulated manner, capturing cargoes in one compartment and releasing them in another before they return to their original location, although the exact mechanisms involved still remain unknown.
Although integral membrane cargo proteins can in principle interact directly with coat adaptors and therefore should not need cargo receptors, examples of such proteins using cargo receptors have been described in multiple pathways. These membrane cargo receptors may simply provide a sorting signal that is absent from the cargo, but they may also play additional roles in chaperoning transmembrane domains (e.g., Erv14/CNIH) and recognizing or retrieving unassembled components of oligomeric complexes (e.g., Rer1). How recognition and release of membrane-embedded interactions is modulated according to cellular compartment remains poorly understood.
Mechanisms of sorting: Coat adaptors at the ER–Golgi interface
Cargo recruitment into COPII vesicles: Initiating ER export
Entry of a nascent protein into the secretory pathway is initiated by the selective incorporation of correctly folded and assembled secretory and membrane proteins into vesicles formed by the cytoplasmic COPII coat. The coat comprises five subunits: Sar1–GTP, dimeric Sec23/Sec24, and tetrameric Sec13/Sec31 (Barlowe et al., 1994). Budding of COPII vesicles is initiated when Sar1–GTP recruits Sec23/24 to the ER to form a “prebudding complex,” to which Sec13/31 is recruited via interaction between Sec23/Sar1 and Sec31 (Bi et al., 2007). Sec13/31 drives membrane bending and forms a cage-like outer coat on the nascent vesicle (Stagg et al., 2006; Fath et al., 2007; Bhattacharya et al., 2012), whereas Sec23/24 selects the cargo proteins. Assembly of COPII coat proteins occurs at membrane regions known as ER exit sites (ERESs; Lee et al., 2004; Sato and Nakano, 2007). Cargo proteins transiently accumulate at ERESs, before or concomitant with capture into a nascent vesicle. Some level of protein quality control exists in ERESs such that ER residents and misfolded cargo, like the thermosensitive vesicular stomatitis virus G-protein, are excluded from these regions (Mezzacasa and Helenius, 2002).
The Sec24 subunit is the cargo adaptor protein of the COPII coat (Miller et al., 2002). Genetic, biochemical, and structural studies have defined a set of cargo–Sec24 interactions revealing the basis for cargo specificity in COPII vesicle formation (Miller et al., 2003; Mossessova et al., 2003; Mancias and Goldberg, 2007, 2008; Pagant et al., 2015). Yeast Sec24 has at least four distinct cargo-binding sites that each recognize discrete sorting signals, thereby diversifying the spectrum of cargo that can be exported by the same kind of vesicle (Fig. 1). Mammalian Sec24 contains an additional structurally characterized cargo-binding site that recognizes yet another signal (Mancias and Goldberg, 2008). Further cargo diversity is achieved by expression of multiple isoforms of Sec24 subunits. For example, yeasts express two (Iss1/Sfb2 and Lst1/Sfb3) and mammals express four (Sec24A/B and Sec24C/D) isoforms of Sec24, each with distinct cargo specificities. In addition to simply expanding the cargo repertoire, different Sec24 isoforms might also impact structural aspects of vesicle formation; the yeast Sec24 paralog, Lst1/Sfb3, is specifically required for packaging of the large oligomeric protein, Pma1, and also appears to generate vesicles that are larger in size than Sec24-only vesicles (Roberg et al., 1999; Kurihara et al., 2000; Shimoni et al., 2000). Thus, vesicles might be preprogrammed to achieve a certain geometry based on the specific combinations of coat adaptors and corresponding clients. Or, from the context of the cargo protein, by recruiting the appropriate coat adaptor variant, the cargo itself can influence the geometry of the vesicle formed.
The sorting signals that Sec24 recognizes encompass both simple amino acid motifs (e.g., the DxE or LxxL/M/E motifs yeast Bet1, Sys1, and Sed5) and folded epitopes (e.g., the longin/SNARE domain of Sec22; Mossessova et al., 2003; Liu et al., 2004; Mancias and Goldberg, 2007). It remains unclear how many distinct sorting signals an individual Sec24 molecule can bind at one time. The majority of the structural information on cargo binding by Sec24 comes from cocrystals of coat proteins bound to synthetic peptides that encompass the sorting signal. Thus, the full footprint that each intact cargo protein occupies on the surface of Sec24 remains unclear. Sec24 itself seems to be an inert cargo-binding platform in that it does not undergo allosteric conformational changes upon cargo binding. In this regard, the COPII system is unlike the clathrin cargo adaptor complexes, which undergo conformational change upon cargo binding to trigger vesicle formation. Instead, the timing of COPII vesicle production is likely modulated by accessory proteins that are not intrinsic components of the coat (discussed further in the section on regulation of cargo sorting).
Cargo recruitment into COPI vesicles: Golgi-to-ER retrieval
COPI vesicles mediate the retrieval of escaped ER resident proteins and transport machinery that cycles continuously between the ER and Golgi. The COPI complex, also known as coatomer, consists of seven subunits (α, β, β', γ, δ, ε, and ζ-COP), which are recruited as an intact complex to membranes (Hara-Kuge et al., 1994). Coatomer can be biochemically divided into two subcomplexes: a trimer composed of α, β', and ε, named the B-subcomplex; and a tetramer, called the F-subcomplex, composed of β, γ, δ, and ζ (Fig. 1; Waters et al., 1991; Eugster et al., 2000). Bioinformatic and crystallographic analyses of the COPI coat show structural motifs similar to those in the clathrin and COPII coats, including extended α-helical solenoid domains coupled with β-propeller structures (Devos et al., 2004; Lee and Goldberg, 2010). However, the arrangement of these different structural elements is distinct for each coat system.
Although the tetrameric F-subcomplex shows similarity to the cargo-binding clathrin adaptor–protein (AP) complexes, structural analysis has clearly delineated the β-propeller domains of α-COPI and β′-COP as sites of interaction with dilysine motifs that are the predominant retrieval signal for ER resident membrane proteins (Jackson et al., 2012). However, the F-subcomplex may yet function in cargo binding: the β- and δ-subunits are important for sorting of Arg-based retrieval signals (Michelsen et al., 2007), and aromatic amino acids on the small cytoplasmic tails of the p24 family of proteins bind directly to the F-subcomplex (Fiedler et al., 1996). Furthermore, δ-COP binds a WxW motif of a cytosolic accessory protein, Dsl1, although this interaction occurs at a site that is likely distal to the membrane surface (Suckling et al., 2015). Nonetheless, the possibility remains that additional cargo-binding surfaces remain to be identified on the F-subcomplex. Alternatively, protein–protein interaction sites on the COPI AP-like complex may have evolved specialized functions in coat regulation other than cargo binding.
Insight into the arrangement of COPI subunits within the assembled coat comes from cryoelectron tomography of COPI-coated vesicles and global fitting of crystal structures into the EM density (Dodonova et al., 2015). The cargo-binding surfaces of an α- and β′-COP dimer were oriented close to the membrane, with their α-solenoid domains forming an arch-like structure pointing away from the membrane, likely contributing to membrane scaffolding once linked to each other via the F-subcomplex. A tetramer of γ-ζ-COP and β-δ-COP dimers also formed a hinge-like structure, analogous to that of clathrin AP complexes, albeit in a more extended conformation. The significance of the difference in structure between the COPI F-subcomplex and the AP complexes remains to be fully understood, but it is perhaps important in the context of cargo coupling to vesicle formation. Clathrin adaptors are thought to adopt a “closed” conformation in the cytosol that switches to an “open” conformation upon membrane binding, which exposes clathrin-binding sites to trigger vesicle formation (Jackson et al., 2010; Kelly et al., 2014). Thus, in the clathrin system, vesicle formation is mechanistically linked to cargo loading; only when an adaptor complex is bound to its ligand will vesicle formation progress. In contrast, the COPI structure, which was formed in the absence of cargo proteins but the presence of a lipid bilayer, is already in a “hyperopen” conformation (Dodonova et al., 2015). How these putative structural rearrangements might integrate cargo binding, membrane binding, and coat assembly remain to be fully understood, but it seems likely that allosteric effects influence COPI assembly.
Mechanisms of sorting: Diverse roles of cargo receptors
Cargo receptors in ER export
There is large diversity of cargo proteins that traverse the ER as soluble proteins and thus probably rely on cargo receptors to interact with the COPII coat (Fig. 2 A). Recent comprehensive reviews of known ER export receptors delineate the diversity of these important trafficking factors (Dancourt and Barlowe, 2010; Geva and Schuldiner, 2014; Barlowe and Helenius, 2016). One feature of receptor-mediated ER export is that the diversity of cargo proteins that can be selectively recruited into vesicles is amplified by the use of a hierarchy of adaptors, receptors, and accessory factors (Geva and Schuldiner, 2014). A second important aspect of receptor-mediated export is the potential for regulation of transport in the context of protein folding. If a cargo receptor only binds its ligand when the client protein is correctly folded, then only mature secretory proteins will leave the chaperone-rich environment of the ER lumen. Mechanistic insight into how specific cargo receptors recognize their clients is still accumulating, and we know little about whether client binding induces allosteric changes that might trigger coat binding, thereby ensuring that only cargo-laden receptors enter into the traffic system.
Perhaps the best-characterized mammalian ER export receptor is ERGIC-53/LMAN1. ERGIC-53 is a single-pass transmembrane protein (Schweizer et al., 1988; Schindler et al., 1993) with a large N-terminal lumenal lectin domain that binds glycosylated cargo proteins (Appenzeller et al., 1999). The cathepsin Z–related glycoprotein is captured by ERGIC-53 in the ER to be released moments later in the ERGIC using a well-established pH- and calcium ion–driven process (Appenzeller-Herzog et al., 2004). ERGIC-53 also traffics secreted serum coagulation factors; human blood clotting disorders are associated with mutations in ERGIC-53 (Nichols et al., 1998). ERGL, VIPL, and VIP36 are related lectins with distinct intracellular distributions within the secretory pathway but that also interact with glycoproteins in a Ca2+-, pH-, and sugar-dependent manner (Kamiya et al., 2008). An additional role for ERGIC-53 in protein assembly has been suggested from studies on oligomerization of IgM molecules. ERGIC-53 acts as an export receptor for assembled IgM “monomers” consisting of two heavy and two light chains held together by disulfide bonds (Anelli et al., 2007). Oligomerization of these subunits into polymeric IgM is facilitated by ERGIC-53, perhaps driven by the oligomeric state of ERGIC-53 itself, which forms a hexamer. In mediating traffic of diverse sets of unrelated glycoproteins, ERGIC-53 seems to make use of accessory proteins that likely also contribute to client specificity; ERp44 assists in export of IgM, whereas MCFD2 facilitates interaction with coagulation factors (Cortini and Sitia, 2010). By using such accessory factors, the diversity of clients shepherded by an individual cargo receptor can be amplified.
Cargo receptors can also mediate interaction between transmembrane cargo proteins and coat proteins (Fig. 2 B). In some cases, these transmembrane cargoes simply lack their own ER export motif, necessitating a receptor to couple to the coat. This is the case for lipid-anchored proteins that face the ER lumen. Glycosylphosphatidylinositol (GPI)-anchored proteins become embedded in the ER membrane by a lipid moiety after their synthesis and integration into the ER. They thus lack cytosolic domains with which to interact with the COPII coat and rely instead on a family of receptors known as the p24 family. These abundant oligomeric proteins cycle between the ER and ERGIC/cis-Golgi through efficient incorporation into both COPI and COPII vesicles (Wada et al., 1991; Stamnes et al., 1995; Belden and Barlowe, 1996; Blum et al., 1996; Emery et al., 2000; Gommel et al., 2001; Langhans et al., 2008). The p24 proteins are conserved across all eukaryotes and share a stereotypical domain organization: a lumenal N-terminal GOLD (for Golgi dynamics) domain, a coiled-coil region, a single transmembrane domain, and a short cytoplasmic tail that binds and promotes nucleation of both COPI and COPII components (Fiedler et al., 1996; Goldberg, 2000; Belden and Barlowe, 2001; Anantharaman and Aravind, 2002; Strating et al., 2009). Although the molecular details of cargo recognition remain unclear, p24 proteins seem to function as a lectin in recognizing the carbohydrate moiety of the GPI anchor (Manzano-Lopez et al., 2015). Furthermore, because the cargo only engages with the receptor complex once the GPI linkage has been appropriately remodeled, the p24 family also seems to function as a readout for protein maturation.
Some plasma membrane proteins require a cargo receptor despite containing their own ER export motifs. In this case, efficient ER export requires multiple signals: one signal is present in the cargo protein itself, and a second one comes from a cargo receptor. The best-characterized example of this is yeast Erv14 (cornichon in flies; CNIH in mammals), which functions as a receptor for numerous plasma membrane resident proteins (Fig. 2 B; Powers and Barlowe, 1998, 2002; Nakanishi et al., 2007; Herzig et al., 2012; Pagant et al., 2015). Erv14 likely serves multiple functions in its role as a cargo receptor. First, it provides affinity for the COPII coat via a cytoplasmic loop; this function is required for all of its cargo clients. In several cases, this Erv14 signal augments the interaction between Sec24 and endogenous sorting signals of cargo proteins, thereby increasing the affinity of the coat for Erv14-bound cargo (Powers and Barlowe, 2002; Pagant et al., 2015). Second, Erv14 may chaperone long transmembrane domains that are characteristic of plasma membrane proteins; Erv14 dependence for ER export is linked to transmembrane domain length (Herzig et al., 2012). Finally, because Erv14 interacts with a subset of cargo via a binding site embedded within the lipid bilayer, it may act as a quality control factor such that it binds only when the client transmembrane domains are properly assembled, thereby coupling COPII recruitment with folding (Pagant et al., 2015).
Cargo receptors in retrograde COPI traffic
The receptor that mediates Golgi–ER retrieval of ER resident proteins was one of the first cargo-binding trafficking receptors characterized. Yeast Erd2 binds the tetrapeptide motif, KDEL or HDEL (His-Asp-Glu-Leu), found on ER residents (Semenza et al., 1990). Humans have three homologues of the receptor, ERD21, ERD22, and ERD23, which appear to have slightly distinct substrate preferences (Raykhel et al., 2007). All are proteins with seven transmembrane domains that directly interact with both cargo and COPI components (Majoul et al., 2001). Cargo binding and release by KDEL receptors is regulated by differences in the luminal pH within the Golgi and the ER; lower pH environments promote binding to the cargo, and increased pH favors release (Scheel and Pelham, 1996). A second soluble cargo receptor complex that retrieves ER resident proteins that lack known sorting signals is the Erv41–Erv46 complex. Erv41 and Erv46 are related proteins with two transmembrane domains and a large lumenal domain each. The Erv41–Erv46 complex interacts with cargo proteins, such as Fpr2 and Gls1, in a pH-dependent manner similar to that of KDEL receptors (Shibuya et al., 2015).
As in the anterograde pathway, not all membrane proteins that require retrieval bear their own sorting signal. The ER retrieval receptor Rer1 mediates Golgi–ER traffic of a diverse group of ER membrane proteins that contain neither KKXX nor K/HDEL signals (Sato et al., 1997). In yeast, these cargoes include resident ER machinery (Sec12, Sed4, Mns1, Sec71, and Sec63), as well as monomeric subunits of complexes that generally require assembly before forward traffic (Sato et al., 2003, 2004; Kaether et al., 2007; Valkova et al., 2011). In this latter case, Rer1 recognizes a polar residue embedded within the transmembrane domain that would normally be masked in the assembled complex (Sato et al., 2003). Human Rer1 also has a similar function as an ER retrieval receptor (Füllekrug et al., 1997).
Finally, ER retrieval also uses accessory proteins to facilitate and/or expand cargo binding. Vps74 is a soluble cytoplasmic protein that acts as a vesicle loader for glycosyl transferases by binding a semiconserved motif (F/L-L/V/I-X-X-RK) in the cytosolic tail of these proteins and the COPI coat (Tu et al., 2008, 2012). ERp44 acts as both a quality control effector and retrieval adaptor in the thiol-dependent retention of misfolded or unassembled IgM heavy and light chains (Anelli et al., 2003). ERp44 shuttles between the ER and the Golgi, executing its quality control cycle in a pH-regulated manner (Anelli et al., 2007; Cortini and Sitia, 2010; Vavassori et al., 2013). In the ER (pH 7.1), ERp44 is in a closed conformation and unable to interact with cargo clients or the KDEL receptor. In the cis-Golgi (pH 6.7), ERp44 undergoes a conformational change favoring client proteins with unpaired cysteines and exposing a KDEL motif that is otherwise buried (Cortini and Sitia, 2010; Vavassori et al., 2013; Sannino et al., 2014). Bound to the KDEL receptor, ERp44 is transported back to the ER, where the client protein is released and the chaperone made ready for additional rounds of quality control.
Regulation of cargo sorting: Can cargo direct the traffic?
One of the emerging questions in the field is how vesicle biogenesis is regulated to accommodate the physiological needs of each individual cell. Are vesicles initiated locally only upon cargo recruitment? Can vesicles be tailored to suit a specific cargo load? One key to answering these questions is to understand mechanistic aspects of coat assembly in the context of different cargo proteins. The clathrin system clearly incorporates large-range movements by adaptor complexes upon cargo binding to trigger scaffold recruitment. Whether similar localized activation occurs in the COPI and COPII systems remains to be seen. Structural similarities between the COPI and clathrin AP complexes hints that allosteric changes are likely to regulate COPI assembly (Dodonova et al., 2015), but further insight into how the different coat subcomplexes interact with each other is required before this question will be answered. In contrast, the COPII system appears to lack allosteric changes upon cargo binding, but cargo-mediated modulation of coat assembly can instead come from regulation of coat stability. In this case, coat and accessory proteins can both modulate the GTP cycle of the coat and thus influence the residence time of the coat on the membrane. Sec16 is a peripheral ER protein that competes for binding on Sec23/Sec24 with Sec31, thereby delaying Sec31 recruitment and preventing it from fully activating the GTPase activity of Sar1 (Kung et al., 2012; Yorimitsu and Sato, 2012). Mutations in Sec24 that abrogate this effect offer a suggestion that this process might be influenced by cargo occupancy on Sec24 (Kung et al., 2012), although this remains to be demonstrated. Although there is clearly much to still be learned on how cargo might influence vesicle formation, multiple lines of evidence are emerging that vesicle formation may not be stereotyped and rigid but is flexible enough to accommodate distinct cellular needs.
Specialized cases of ER export: Large carriers required
Both yeast and mammalian cells secrete unusually large proteins that may pose a barrier to the efficient formation of small, spherical transport vesicles. Yeast Pma1 is an abundant oligomeric protein of ∼1 MD that has a specific requirement for the Sec24 paralog Lst1/Sfb1, which generates vesicles slightly larger than those made with Sec24 alone (Shimoni et al., 2000; Miller et al., 2002). Similarly, GPI-APs require Lst1 for their efficient ER export; binding of GPI-APs induces the p24 complex to specifically recruit Lst1 (Castillon et al., 2009, 2011; Manzano-Lopez et al., 2015). GPI-APs probably require special accommodations because of the nature of their asymmetric distribution across the membrane, which likely antagonizes vesicle formation (Copic et al., 2012). In both examples, by recruiting a coat component (Lst1) with the capacity to generate larger vesicles, the cargo itself ensures that it enters into a vesicle that can accommodate it.
In mammals, traffic of fibrillar procollagens poses a similar physical problem: the cargo is too large to fit into a canonical COPII vesicle. In this case, cargo transport depends on transmembrane accessory factors, MIA3/TANGO1 and cTAGE5, that are themselves not captured into vesicles (Malhotra and Erlmann, 2011, 2015). Like a traditional cargo receptor, TANGO1 interacts with collagen via an SH3 domain facilitating the loading of collagens into vesicles (Saito et al., 2009, 2011). However, it has been recently suggested that SH3 alone is not capable of directing collagens into COPII vesicles. Instead, Hsp47 seems to as an anchor molecule between TANGO1 and multiple different types of collagens (Ishikawa et al., 2016). cTAGE5 is a TANGO1-related protein that binds Sec12, the nucleotide exchange factor that triggers COPII coat assembly by loading GTP onto Sar1. By locally coupling Sar1 activation to a specific cargo, TANGO1/cTAGE5 is thought to modulate recruitment of sufficient quantities of the COPII coat to generate a large “megacarrier” capable of accommodating procollagen. In addition to simply stimulating COPII recruitment, however, TANGO1/cTAGE5 may also directly influence coat geometry. Both proteins contain within their cytoplasmic domains multiple interspersed polyproline motifs that bind to Sec23 (Ma and Goldberg, 2016). By simultaneously recruiting multiple Sec23/Sec24 dimers, TANGO1/cTAGE5 might position these coat elements in a lattice-like arrangement that has been observed on COPII-coated tubular structures (Zanetti et al., 2013). Another aspect of polyproline binding by Sec23 is that Sec31 also contains these interspersed motifs, which can compete for binding with those of TANGO1/cTAGE5 (Ma and Goldberg, 2016). A model thus emerges whereby as Sec31 is recruited to the incipient site of vesicle formation, it displaces the “receptor” that has delivered the cargo, which is captured as the coat builds locally. In this case, cargo–coat interactions might dictate both the geometry of the coat and the timing of coat turnover, thereby directly influencing the ultimate architecture of the carrier. Additional layers of regulation also seem to participate in modulating COPII coat activity during procollagen export; ubiquitination of Sec31 is required for collagen traffic (Jin et al., 2012), and modulation of Sar1 GTPase activity by a TANGO1-associated protein, sedlin, is also important (Venditti et al., 2012). How these factors might directly or indirectly influence formation of megacarriers remains to be determined.
Cargo-mediated signaling in the cis-Golgi
A role for cargo in directly stimulating COPI vesicle formation has been suggested by multiple independent avenues. The p24 family of proteins contains COPI-binding signals that can alter the catalytic activity of the coat, perhaps stabilizing it during assembly and thus promoting vesicle formation (Goldberg, 2000). Similar effects have been proposed for a family of proteins that cycle between the ER and Golgi in yeast; overexpression of Mst27/Mst28 suppresses lethality associated with COPI mutants, suggesting that by increasing COPI-binding events, vesicle formation can be stimulated (Sandmann et al., 2003). More directly, binding of a KDEL-containing ligand to the KDEL receptor induces oligomerization (as detected by fluorescence resonance energy transfer) and stimulates COPI association with Golgi membranes (Majoul et al., 2001). How these various cargo-mediated effects are implemented mechanistically remains to be fully determined. A second aspect of cargo-mediated modulation of traffic is suggested from recent work on Src-mediated cytoplasmic signaling events that follow engagement of the KDEL receptor (Pulvirenti et al., 2008). Client binding within the Golgi is thought to stimulate Src-family kinases that in turn influence membrane trafficking. How this signaling pathway might directly modulate COPI function remains to be further investigated, although more direct tests of retrograde traffic suggest a general effect on Golgi traffic rather than specific retrograde induction (Bard et al., 2003).
Outstanding questions remain: Sorting out dynamics and regulation
With many recent advances in structural aspects of coat assembly and cargo–coat interaction, we are gaining a detailed understanding of the mechanisms of protein sorting. However, insight into cellular regulation of these processes is still emerging and understanding cargo–coat allostery (or other forms of modulation) will be key to defining how trafficking can be tailored to specific cellular conditions. Further advances derive from the study of noncanonical trafficking, like procollagen secretion, where we are only beginning to appreciate the plasticity of known systems and the functional relevance of new specific factors. New examples of such distinct sorting events are likely to continue to push the field forward.
One example of “noncanonical” transport is actually the revival of an old model: nonselective bulk flow. Some cargoes exit the ER without concentration into vesicles or interaction with the vesicle coat, although probably still in a COPII-dependent manner (Wieland et al., 1987). Given that the known cargo receptors traffic only a fraction of the secretome, stochastic export via bulk flow must be re-evaluated as a significant mechanism of cargo transport. Indeed, a recent quantification of bulk flow in mammalian cells using a novel inert and rapidly folding marker suggests that there is significant flux of liquid and protein through the secretory pathway in a nonselective manner (Thor et al., 2009). Whether this holds true for other cell types and species remains to be seen. Similarly, whether bulk flow of fluid and membrane also occurs in retrograde vesicles has not been tested and is likely to be technically difficult to dissect from anterograde events. In the face of bulk flow export, how incompletely folded proteins and ER residents might be prevented from being captured into vesicles is unclear and warrants further characterization. Retention signals and extensive interactions among ER resident chaperones might prevent some proteins from entering vesicles, but the nature and magnitude of these effects is largely unknown.
Related to the problem of selectivity is understanding how protein folding influences ER–Golgi traffic, in the context of both signal-mediated sorting and bulk flow. This is an important problem, because nascent cargoes that are prematurely exported lose access to the ER folding and degradation machinery. In principle, cargo adaptors and cargo receptors in both the ER and Golgi could play direct roles in determining the fidelity of transport. ER receptors would discriminate between fully folded proteins and those that need further chaperone action; Golgi receptors would bind misfolded cargo for retrieval back to the ER. In practice, this model may suffice for some misfolded proteins but is unlikely to apply to all proteins. First, some ER export signals are simple peptides that seem unlikely to change as a protein folds on the luminal side of the membrane. Perhaps more important and puzzling is the observation that many incompletely folded and resident proteins are actually transport competent (Hong et al., 1996; Jenness et al., 1997; Spear and Ng, 2003; Liu et al., 2006; Kincaid and Cooper, 2007; Ashok and Hegde, 2009; Wang and Ng, 2010). Thus, it is more accurate to say that functional ER export signals coexist with ERAD and retention signals and forward traffic is under dynamic control of multiple competing interactions. Furthermore, under severe ER stress, sorting signals can be used as an ER detoxification pathway (Kawaguchi et al., 2010; Satpute-Krishnan et al., 2014). Similar questions pertain as to how misfolded proteins are recognized in the Golgi, and the extent to which retrieval contributes to steady-state ER retention needs to be quantified. Understanding the interplay between these pathways lies at the heart of numerous misfolding diseases that stem from defects in quality control of protein sorting at the ER–Golgi interface.
Acknowledgments
The authors are supported by the Medical Research Council under award number MC_UP_1201/10.
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- AP
- adaptor–protein
- ERES
- ER exit site
- GPI
- glycosylphosphatidylinositol
References
- Anantharaman V., and Aravind L.. 2002. The GOLD domain, a novel protein module involved in Golgi function and secretion. Genome Biol. 3:0023.1–0023.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anelli T., Alessio M., Bachi A., Bergamelli L., Bertoli G., Camerini S., Mezghrani A., Ruffato E., Simmen T., and Sitia R.. 2003. Thiol-mediated protein retention in the endoplasmic reticulum: the role of ERp44. EMBO J. 22:5015–5022. 10.1093/emboj/cdg491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anelli T., Ceppi S., Bergamelli L., Cortini M., Masciarelli S., Valetti C., and Sitia R.. 2007. Sequential steps and checkpoints in the early exocytic compartment during secretory IgM biogenesis. EMBO J. 26:4177–4188. 10.1038/sj.emboj.7601844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appenzeller C., Andersson H., Kappeler F., and Hauri H.P.. 1999. The lectin ERGIC-53 is a cargo transport receptor for glycoproteins. Nat. Cell Biol. 1:330–334. 10.1038/14020 [DOI] [PubMed] [Google Scholar]
- Appenzeller-Herzog C., Roche A.C., Nufer O., and Hauri H.P.. 2004. pH-induced conversion of the transport lectin ERGIC-53 triggers glycoprotein release. J. Biol. Chem. 279:12943–12950. 10.1074/jbc.M313245200 [DOI] [PubMed] [Google Scholar]
- Ashok A., and Hegde R.S.. 2009. Selective processing and metabolism of disease-causing mutant prion proteins. PLoS Pathog. 5:e1000479 10.1371/journal.ppat.1000479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard F., Mazelin L., Péchoux-Longin C., Malhotra V., and Jurdic P.. 2003. Src regulates Golgi structure and KDEL receptor-dependent retrograde transport to the endoplasmic reticulum. J. Biol. Chem. 278:46601–46606. 10.1074/jbc.M302221200 [DOI] [PubMed] [Google Scholar]
- Barlowe C., and Helenius A.. 2016. Cargo capture and bulk flow in the early secretory pathway. Annu. Rev. Cell Dev. Biol. 32:197–222. 10.1146/annurev-cellbio-111315-125016 [DOI] [PubMed] [Google Scholar]
- Barlowe C., Orci L., Yeung T., Hosobuchi M., Hamamoto S., Salama N., Rexach M.F., Ravazzola M., Amherdt M., and Schekman R.. 1994. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 77:895–907. 10.1016/0092-8674(94)90138-4 [DOI] [PubMed] [Google Scholar]
- Belden W.J., and Barlowe C.. 1996. Erv25p, a component of COPII-coated vesicles, forms a complex with Emp24p that is required for efficient endoplasmic reticulum to Golgi transport. J. Biol. Chem. 271:26939–26946. 10.1074/jbc.271.43.26939 [DOI] [PubMed] [Google Scholar]
- Belden W.J., and Barlowe C.. 2001. Deletion of yeast p24 genes activates the unfolded protein response. Mol. Biol. Cell. 12:957–969. 10.1091/mbc.12.4.957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya N., O Donnell J., and Stagg S.M.. 2012. The structure of the Sec13/31 COPII cage bound to Sec23. J. Mol. Biol. 420:324–334. 10.1016/j.jmb.2012.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X., Mancias J.D., and Goldberg J.. 2007. Insights into COPII coat nucleation from the structure of Sec23.Sar1 complexed with the active fragment of Sec31. Dev. Cell. 13:635–645. 10.1016/j.devcel.2007.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum R., Feick P., Puype M., Vandekerckhove J., Klengel R., Nastainczyk W., and Schulz I.. 1996. Tmp21 and p24A, two type I proteins enriched in pancreatic microsomal membranes, are members of a protein family involved in vesicular trafficking. J. Biol. Chem. 271:17183–17189. 10.1074/jbc.271.29.17183 [DOI] [PubMed] [Google Scholar]
- Castillon G.A., Watanabe R., Taylor M., Schwabe T.M.E., and Riezman H.. 2009. Concentration of GPI-anchored proteins upon ER exit in yeast. Traffic. 10:186–200. 10.1111/j.1600-0854.2008.00857.x [DOI] [PubMed] [Google Scholar]
- Castillon G.A., Aguilera-Romero A., Manzano-Lopez J., Epstein S., Kajiwara K., Funato K., Watanabe R., Riezman H., and Muñiz M.. 2011. The yeast p24 complex regulates GPI-anchored protein transport and quality control by monitoring anchor remodeling. Mol. Biol. Cell. 22:2924–2936. 10.1091/mbc.E11-04-0294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copic A., Latham C.F., Horlbeck M.A., D’Arcangelo J.G., and Miller E.A.. 2012. ER cargo properties specify a requirement for COPII coat rigidity mediated by Sec13p. Science. 335:1359–1362. 10.1126/science.1215909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortini M., and Sitia R.. 2010. ERp44 and ERGIC-53 synergize in coupling efficiency and fidelity of IgM polymerization and secretion. Traffic. 11:651–659. 10.1111/j.1600-0854.2010.01043.x [DOI] [PubMed] [Google Scholar]
- Dancourt J., and Barlowe C.. 2010. Protein sorting receptors in the early secretory pathway. Annu. Rev. Biochem. 79:777–802. 10.1146/annurev-biochem-061608-091319 [DOI] [PubMed] [Google Scholar]
- Devos D., Dokudovskaya S., Alber F., Williams R., Chait B.T., Sali A., and Rout M.P.. 2004. Components of coated vesicles and nuclear pore complexes share a common molecular architecture. PLoS Biol. 2:e380 (published erratum appears in PLoS Biol. 2005. 3:e80) 10.1371/journal.pbio.0020380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodonova S.O., Diestelkoetter-Bachert P., von Appen A., Hagen W.J., Beck R., Beck M., Wieland F., and Briggs J.A.. 2015. VESICULAR TRANSPORT. A structure of the COPI coat and the role of coat proteins in membrane vesicle assembly. Science. 349:195–198. 10.1126/science.aab1121 [DOI] [PubMed] [Google Scholar]
- Emery G., Rojo M., and Gruenberg J.. 2000. Coupled transport of p24 family members. J. Cell Sci. 113:2507–2516. [DOI] [PubMed] [Google Scholar]
- Eugster A., Frigerio G., Dale M., and Duden R.. 2000. COP I domains required for coatomer integrity, and novel interactions with ARF and ARF-GAP. EMBO J. 19:3905–3917. 10.1093/emboj/19.15.3905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fath S., Mancias J.D., Bi X., and Goldberg J.. 2007. Structure and organization of coat proteins in the COPII cage. Cell. 129:1325–1336. 10.1016/j.cell.2007.05.036 [DOI] [PubMed] [Google Scholar]
- Fiedler K., Veit M., Stamnes M.A., and Rothman J.E.. 1996. Bimodal interaction of coatomer with the p24 family of putative cargo receptors. Science. 273:1396–1399. 10.1126/science.273.5280.1396 [DOI] [PubMed] [Google Scholar]
- Füllekrug J., Boehm J., Röttger S., Nilsson T., Mieskes G., and Schmitt H.D.. 1997. Human Rer1 is localized to the Golgi apparatus and complements the deletion of the homologous Rer1 protein of Saccharomyces cerevisiae. Eur. J. Cell Biol. 74:31–40. [PubMed] [Google Scholar]
- Geva Y., and Schuldiner M.. 2014. The back and forth of cargo exit from the endoplasmic reticulum. Curr. Biol. 24:R130–R136. 10.1016/j.cub.2013.12.008 [DOI] [PubMed] [Google Scholar]
- Goldberg J. 2000. Decoding of sorting signals by coatomer through a GTPase switch in the COPI coat complex. Cell. 100:671–679. 10.1016/S0092-8674(00)80703-5 [DOI] [PubMed] [Google Scholar]
- Gommel D.U., Memon A.R., Heiss A., Lottspeich F., Pfannstiel J., Lechner J., Reinhard C., Helms J.B., Nickel W., and Wieland F.T.. 2001. Recruitment to Golgi membranes of ADP-ribosylation factor 1 is mediated by the cytoplasmic domain of p23. EMBO J. 20:6751–6760. 10.1093/emboj/20.23.6751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Sirkis D.W., and Schekman R.. 2014. Protein sorting at the trans-Golgi network. Annu. Rev. Cell Dev. Biol. 30:169–206. 10.1146/annurev-cellbio-100913-013012 [DOI] [PubMed] [Google Scholar]
- Hara-Kuge S., Kuge O., Orci L., Amherdt M., Ravazzola M., Wieland F.T., and Rothman J.E.. 1994. En bloc incorporation of coatomer subunits during the assembly of COP-coated vesicles. J. Cell Biol. 124:883–892. (published erratum appears in J. Cell Biol. 1994. 126:589) 10.1083/jcb.124.6.883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig Y., Sharpe H.J., Elbaz Y., Munro S., and Schuldiner M.. 2012. A systematic approach to pair secretory cargo receptors with their cargo suggests a mechanism for cargo selection by Erv14. PLoS Biol. 10:e1001329 10.1371/journal.pbio.1001329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong E., Davidson A.R., and Kaiser C.A.. 1996. A pathway for targeting soluble misfolded proteins to the yeast vacuole. J. Cell Biol. 135:623–633. 10.1083/jcb.135.3.623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa Y., Ito S., Nagata K., Sakai L.Y., and Bächinger H.P.. 2016. Intracellular mechanisms of molecular recognition and sorting for transport of large extracellular matrix molecules. Proc. Natl. Acad. Sci. USA. 113:E6036–E6044. 10.1073/pnas.1609571113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson L.P., Kelly B.T., McCoy A.J., Gaffry T., James L.C., Collins B.M., Höning S., Evans P.R., and Owen D.J.. 2010. A large-scale conformational change couples membrane recruitment to cargo binding in the AP2 clathrin adaptor complex. Cell. 141:1220–1229. 10.1016/j.cell.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson L.P., Lewis M., Kent H.M., Edeling M.A., Evans P.R., Duden R., and Owen D.J.. 2012. Molecular basis for recognition of dilysine trafficking motifs by COPI. Dev. Cell. 23:1255–1262. 10.1016/j.devcel.2012.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenness D.D., Li Y., Tipper C., and Spatrick P.. 1997. Elimination of defective alpha-factor pheromone receptors. Mol. Cell. Biol. 17:6236–6245. 10.1128/MCB.17.11.6236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L., Pahuja K.B., Wickliffe K.E., Gorur A., Baumgärtel C., Schekman R., and Rape M.. 2012. Ubiquitin-dependent regulation of COPII coat size and function. Nature. 482:495–500. 10.1038/nature10822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaether C., Scheuermann J., Fassler M., Zilow S., Shirotani K., Valkova C., Novak B., Kacmar S., Steiner H., and Haass C.. 2007. Endoplasmic reticulum retention of the gamma-secretase complex component Pen2 by Rer1. EMBO Rep. 8:743–748. 10.1038/sj.embor.7401027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya Y., Kamiya D., Yamamoto K., Nyfeler B., Hauri H.-P., and Kato K.. 2008. Molecular basis of sugar recognition by the human L-type lectins ERGIC-53, VIPL, and VIP36. J. Biol. Chem. 283:1857–1861. 10.1074/jbc.M709384200 [DOI] [PubMed] [Google Scholar]
- Kawaguchi S., Hsu C.-L., and Ng D.T.W.. 2010. Interplay of substrate retention and export signals in endoplasmic reticulum quality control. PLoS One. 5:e15532 10.1371/journal.pone.0015532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly B.T., Graham S.C., Liska N., Dannhauser P.N., Höning S., Ungewickell E.J., and Owen D.J.. 2014. Clathrin adaptors. AP2 controls clathrin polymerization with a membrane-activated switch. Science. 345:459–463. 10.1126/science.1254836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid M.M., and Cooper A.A.. 2007. Misfolded proteins traffic from the endoplasmic reticulum (ER) due to ER export signals. Mol. Biol. Cell. 18:455–463. 10.1091/mbc.E06-08-0696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung L.F., Pagant S., Futai E., D’Arcangelo J.G., Buchanan R., Dittmar J.C., Reid R.J.D., Rothstein R., Hamamoto S., Snapp E.L., et al. 2012. Sec24p and Sec16p cooperate to regulate the GTP cycle of the COPII coat. EMBO J. 31:1014–1027. 10.1038/emboj.2011.444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara T., Hamamoto S., Gimeno R.E., Kaiser C.A., Schekman R., and Yoshihisa T.. 2000. Sec24p and Iss1p function interchangeably in transport vesicle formation from the endoplasmic reticulum in Saccharomyces cerevisiae. Mol. Biol. Cell. 11:983–998. 10.1091/mbc.11.3.983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhans M., Marcote M.J., Pimpl P., Virgili-López G., Robinson D.G., and Aniento F.. 2008. In vivo trafficking and localization of p24 proteins in plant cells. Traffic. 9:770–785. 10.1111/j.1600-0854.2008.00719.x [DOI] [PubMed] [Google Scholar]
- Lee C., and Goldberg J.. 2010. Structure of coatomer cage proteins and the relationship among COPI, COPII, and clathrin vesicle coats. Cell. 142:123–132. 10.1016/j.cell.2010.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.C.S., Miller E.A., Goldberg J., Orci L., and Schekman R.. 2004. Bi-directional protein transport between the ER and Golgi. Annu. Rev. Cell Dev. Biol. 20:87–123. 10.1146/annurev.cellbio.20.010403.105307 [DOI] [PubMed] [Google Scholar]
- Lewis M.J., and Pelham H.R.. 1992. Ligand-induced redistribution of a human KDEL receptor from the Golgi complex to the endoplasmic reticulum. Cell. 68:353–364. 10.1016/0092-8674(92)90476-S [DOI] [PubMed] [Google Scholar]
- Liu Y., Flanagan J.J., and Barlowe C.. 2004. Sec22p export from the endoplasmic reticulum is independent of SNARE pairing. J. Biol. Chem. 279:27225–27232. 10.1074/jbc.M312122200 [DOI] [PubMed] [Google Scholar]
- Liu Y., Sitaraman S., and Chang A.. 2006. Multiple degradation pathways for misfolded mutants of the yeast plasma membrane ATPase, Pma1. J. Biol. Chem. 281:31457–31466. 10.1074/jbc.M606643200 [DOI] [PubMed] [Google Scholar]
- Ma W., and Goldberg J.. 2016. TANGO1/cTAGE5 receptor as a polyvalent template for assembly of large COPII coats. Proc. Natl. Acad. Sci. USA. 113:10061–10066. 10.1073/pnas.1605916113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majoul I., Straub M., Hell S.W., Duden R., and Söling H.D.. 2001. KDEL-cargo regulates interactions between proteins involved in COPI vesicle traffic: measurements in living cells using FRET. Dev. Cell. 1:139–153. 10.1016/S1534-5807(01)00004-1 [DOI] [PubMed] [Google Scholar]
- Malhotra V., and Erlmann P.. 2011. Protein export at the ER: loading big collagens into COPII carriers. EMBO J. 30:3475–3480. 10.1038/emboj.2011.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra V., and Erlmann P.. 2015. The pathway of collagen secretion. Annu. Rev. Cell Dev. Biol. 31:109–124. 10.1146/annurev-cellbio-100913-013002 [DOI] [PubMed] [Google Scholar]
- Mancias J.D., and Goldberg J.. 2007. The transport signal on Sec22 for packaging into COPII-coated vesicles is a conformational epitope. Mol. Cell. 26:403–414. 10.1016/j.molcel.2007.03.017 [DOI] [PubMed] [Google Scholar]
- Mancias J.D., and Goldberg J.. 2008. Structural basis of cargo membrane protein discrimination by the human COPII coat machinery. EMBO J. 27:2918–2928. 10.1038/emboj.2008.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzano-Lopez J., Perez-Linero A.M., Aguilera-Romero A., Martin M.E., Okano T., Silva D.V., Seeberger P.H., Riezman H., Funato K., Goder V., et al. 2015. COPII coat composition is actively regulated by luminal cargo maturation. Curr. Biol. 25:152–162. 10.1016/j.cub.2014.11.039 [DOI] [PubMed] [Google Scholar]
- Mezzacasa A., and Helenius A.. 2002. The transitional ER defines a boundary for quality control in the secretion of tsO45 VSV glycoprotein. Traffic. 3:833–849. 10.1034/j.1600-0854.2002.31108.x [DOI] [PubMed] [Google Scholar]
- Michelsen K., Schmid V., Metz J., Heusser K., Liebel U., Schwede T., Spang A., and Schwappach B.. 2007. Novel cargo-binding site in the beta and delta subunits of coatomer. J. Cell Biol. 179:209–217. 10.1083/jcb.200704142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E., Antonny B., Hamamoto S., and Schekman R.. 2002. Cargo selection into COPII vesicles is driven by the Sec24p subunit. EMBO J. 21:6105–6113. 10.1093/emboj/cdf605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E.A., Beilharz T.H., Malkus P.N., Lee M.C.S., Hamamoto S., Orci L., and Schekman R.. 2003. Multiple cargo binding sites on the COPII subunit Sec24p ensure capture of diverse membrane proteins into transport vesicles. Cell. 114:497–509. 10.1016/S0092-8674(03)00609-3 [DOI] [PubMed] [Google Scholar]
- Mossessova E., Bickford L.C., and Goldberg J.. 2003. SNARE selectivity of the COPII coat. Cell. 114:483–495. 10.1016/S0092-8674(03)00608-1 [DOI] [PubMed] [Google Scholar]
- Nakanishi H., Suda Y., and Neiman A.M.. 2007. Erv14 family cargo receptors are necessary for ER exit during sporulation in Saccharomyces cerevisiae. J. Cell Sci. 120:908–916. 10.1242/jcs.03405 [DOI] [PubMed] [Google Scholar]
- Nichols W.C., Seligsohn U., Zivelin A., Terry V.H., Hertel C.E., Wheatley M.A., Moussalli M.J., Hauri H.P., Ciavarella N., Kaufman R.J., and Ginsburg D.. 1998. Mutations in the ER-Golgi intermediate compartment protein ERGIC-53 cause combined deficiency of coagulation factors V and VIII. Cell. 93:61–70. 10.1016/S0092-8674(00)81146-0 [DOI] [PubMed] [Google Scholar]
- Pagant S., Wu A., Edwards S., Diehl F., and Miller E.A.. 2015. Sec24 is a coincidence detector that simultaneously binds two signals to drive ER export. Curr. Biol. 25:403–412. 10.1016/j.cub.2014.11.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers J., and Barlowe C.. 1998. Transport of axl2p depends on erv14p, an ER-vesicle protein related to the Drosophila cornichon gene product. J. Cell Biol. 142:1209–1222. 10.1083/jcb.142.5.1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers J., and Barlowe C.. 2002. Erv14p directs a transmembrane secretory protein into COPII-coated transport vesicles. Mol. Biol. Cell. 13:880–891. 10.1091/mbc.01-10-0499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvirenti T., Giannotta M., Capestrano M., Capitani M., Pisanu A., Polishchuk R.S., San Pietro E., Beznoussenko G.V., Mironov A.A., Turacchio G., et al. 2008. A traffic-activated Golgi-based signalling circuit coordinates the secretory pathway. Nat. Cell Biol. 10:912–922. 10.1038/ncb1751 [DOI] [PubMed] [Google Scholar]
- Raykhel I., Alanen H., Salo K., Jurvansuu J., Nguyen V.D., Latva-Ranta M., and Ruddock L.. 2007. A molecular specificity code for the three mammalian KDEL receptors. J. Cell Biol. 179:1193–1204. (published erratum appears in J. Cell Biol. 2008. 180:645) 10.1083/jcb.200705180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberg K.J., Crotwell M., Espenshade P., Gimeno R., and Kaiser C.A.. 1999. LST1 is a SEC24 homologue used for selective export of the plasma membrane ATPase from the endoplasmic reticulum. J. Cell Biol. 145:659–672. 10.1083/jcb.145.4.659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K., Chen M., Bard F., Chen S., Zhou H., Woodley D., Polischuk R., Schekman R., and Malhotra V.. 2009. TANGO1 facilitates cargo loading at endoplasmic reticulum exit sites. Cell. 136:891–902. 10.1016/j.cell.2008.12.025 [DOI] [PubMed] [Google Scholar]
- Saito K., Yamashiro K., Ichikawa Y., Erlmann P., Kontani K., Malhotra V., and Katada T.. 2011. cTAGE5 mediates collagen secretion through interaction with TANGO1 at endoplasmic reticulum exit sites. Mol. Biol. Cell. 22:2301–2308. 10.1091/mbc.E11-02-0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmann T., Herrmann J.M., Dengjel J., Schwarz H., and Spang A.. 2003. Suppression of coatomer mutants by a new protein family with COPI and COPII binding motifs in Saccharomyces cerevisiae. Mol. Biol. Cell. 14:3097–3113. 10.1091/mbc.E02-11-0736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannino S., Anelli T., Cortini M., Masui S., Degano M., Fagioli C., Inaba K., and Sitia R.. 2014. Progressive quality control of secretory proteins in the early secretory compartment by ERp44. J. Cell Sci. 127:4260–4269. 10.1242/jcs.153239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., and Nakano A.. 2007. Mechanisms of COPII vesicle formation and protein sorting. FEBS Lett. 581:2076–2082. 10.1016/j.febslet.2007.01.091 [DOI] [PubMed] [Google Scholar]
- Sato K., Sato M., and Nakano A.. 1997. Rer1p as common machinery for the endoplasmic reticulum localization of membrane proteins. Proc. Natl. Acad. Sci. USA. 94:9693–9698. 10.1073/pnas.94.18.9693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Sato M., and Nakano A.. 2003. Rer1p, a retrieval receptor for ER membrane proteins, recognizes transmembrane domains in multiple modes. Mol. Biol. Cell. 14:3605–3616. 10.1091/mbc.E02-12-0777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Sato K., and Nakano A.. 2004. Endoplasmic reticulum quality control of unassembled iron transporter depends on Rer1p-mediated retrieval from the golgi. Mol. Biol. Cell. 15:1417–1424. 10.1091/mbc.E03-10-0765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpute-Krishnan P., Ajinkya M., Bhat S., Itakura E., Hegde R.S., and Lippincott-Schwartz J.. 2014. ER stress-induced clearance of misfolded GPI-anchored proteins via the secretory pathway. Cell. 158:522–533. 10.1016/j.cell.2014.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel A.A., and Pelham H.R.. 1996. Purification and characterization of the human KDEL receptor. Biochemistry. 35:10203–10209. 10.1021/bi960807x [DOI] [PubMed] [Google Scholar]
- Schindler R., Itin C., Zerial M., Lottspeich F., and Hauri H.P.. 1993. ERGIC-53, a membrane protein of the ER-Golgi intermediate compartment, carries an ER retention motif. Eur. J. Cell Biol. 61:1–9. [PubMed] [Google Scholar]
- Schweizer A., Fransen J.A., Bächi T., Ginsel L., and Hauri H.P.. 1988. Identification, by a monoclonal antibody, of a 53-kD protein associated with a tubulo-vesicular compartment at the cis-side of the Golgi apparatus. J. Cell Biol. 107:1643–1653. 10.1083/jcb.107.5.1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza J.C., Hardwick K.G., Dean N., and Pelham H.R.. 1990. ERD2, a yeast gene required for the receptor-mediated retrieval of luminal ER proteins from the secretory pathway. Cell. 61:1349–1357. 10.1016/0092-8674(90)90698-E [DOI] [PubMed] [Google Scholar]
- Shibuya A., Margulis N., Christiano R., Walther T.C., and Barlowe C.. 2015. The Erv41-Erv46 complex serves as a retrograde receptor to retrieve escaped ER proteins. J. Cell Biol. 208:197–209. 10.1083/jcb.201408024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoni Y., Kurihara T., Ravazzola M., Amherdt M., Orci L., and Schekman R.. 2000. Lst1p and Sec24p cooperate in sorting of the plasma membrane ATPase into COPII vesicles in Saccharomyces cerevisiae. J. Cell Biol. 151:973–984. 10.1083/jcb.151.5.973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear E.D., and Ng D.T.W.. 2003. Stress tolerance of misfolded carboxypeptidase Y requires maintenance of protein trafficking and degradative pathways. Mol. Biol. Cell. 14:2756–2767. 10.1091/mbc.E02-11-0717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg S.M., Gürkan C., Fowler D.M., LaPointe P., Foss T.R., Potter C.S., Carragher B., and Balch W.E.. 2006. Structure of the Sec13/31 COPII coat cage. Nature. 439:234–238. 10.1038/nature04339 [DOI] [PubMed] [Google Scholar]
- Stamnes M.A., Craighead M.W., Hoe M.H., Lampen N., Geromanos S., Tempst P., and Rothman J.E.. 1995. An integral membrane component of coatomer-coated transport vesicles defines a family of proteins involved in budding. Proc. Natl. Acad. Sci. USA. 92:8011–8015. 10.1073/pnas.92.17.8011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strating J.R.P.M., Hafmans T.G.M., and Martens G.J.M.. 2009. COP-binding sites in p24δ2 are necessary for proper secretory cargo biosynthesis. Int. J. Biochem. Cell Biol. 41:1619–1627. 10.1016/j.biocel.2009.02.010 [DOI] [PubMed] [Google Scholar]
- Suckling R.J., Poon P.P., Travis S.M., Majoul I.V., Hughson F.M., Evans P.R., Duden R., and Owen D.J.. 2015. Structural basis for the binding of tryptophan-based motifs by δ-COP. Proc. Natl. Acad. Sci. USA. 112:14242–14247. 10.1073/pnas.1506186112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor F., Gautschi M., Geiger R., and Helenius A.. 2009. Bulk flow revisited: transport of a soluble protein in the secretory pathway. Traffic. 10:1819–1830. 10.1111/j.1600-0854.2009.00989.x [DOI] [PubMed] [Google Scholar]
- Tu L., Tai W.C.S., Chen L., and Banfield D.K.. 2008. Signal-mediated dynamic retention of glycosyltransferases in the Golgi. Science. 321:404–407. 10.1126/science.1159411 [DOI] [PubMed] [Google Scholar]
- Tu L., Chen L., and Banfield D.K.. 2012. A conserved N-terminal arginine-motif in GOLPH3-family proteins mediates binding to coatomer. Traffic. 13:1496–1507. 10.1111/j.1600-0854.2012.01403.x [DOI] [PubMed] [Google Scholar]
- Valkova C., Albrizio M., Röder I.V., Schwake M., Betto R., Rudolf R., and Kaether C.. 2011. Sorting receptor Rer1 controls surface expression of muscle acetylcholine receptors by ER retention of unassembled alpha-subunits. Proc. Natl. Acad. Sci. USA. 108:621–625. 10.1073/pnas.1001624108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavassori S., Cortini M., Masui S., Sannino S., Anelli T., Caserta I.R., Fagioli C., Mossuto M.F., Fornili A., van Anken E., et al. 2013. A pH-regulated quality control cycle for surveillance of secretory protein assembly. Mol. Cell. 50:783–792. 10.1016/j.molcel.2013.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venditti R., Scanu T., Santoro M., Di Tullio G., Spaar A., Gaibisso R., Beznoussenko G.V., Mironov A.A., Mironov A. Jr., Zelante L., et al. 2012. Sedlin controls the ER export of procollagen by regulating the Sar1 cycle. Science. 337:1668–1672. 10.1126/science.1224947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada I., Rindress D., Cameron P.H., Ou W.J., Doherty J.J. II, Louvard D., Bell A.W., Dignard D., Thomas D.Y., and Bergeron J.J.. 1991. SSR alpha and associated calnexin are major calcium binding proteins of the endoplasmic reticulum membrane. J. Biol. Chem. 266:19599–19610. [PubMed] [Google Scholar]
- Wang S., and Ng D.T.W.. 2010. Evasion of endoplasmic reticulum surveillance makes Wsc1p an obligate substrate of Golgi quality control. Mol. Biol. Cell. 21:1153–1165. 10.1091/mbc.E09-10-0910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters M.G., Serafini T., and Rothman J.E.. 1991. ‘Coatomer’: a cytosolic protein complex containing subunits of non-clathrin-coated Golgi transport vesicles. Nature. 349:248–251. 10.1038/349248a0 [DOI] [PubMed] [Google Scholar]
- Wieland F.T., Gleason M.L., Serafini T.A., and Rothman J.E.. 1987. The rate of bulk flow from the endoplasmic reticulum to the cell surface. Cell. 50:289–300. 10.1016/0092-8674(87)90224-8 [DOI] [PubMed] [Google Scholar]
- Yorimitsu T., and Sato K.. 2012. Insights into structural and regulatory roles of Sec16 in COPII vesicle formation at ER exit sites. Mol. Biol. Cell. 23:2930–2942. 10.1091/mbc.E12-05-0356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti G., Prinz S., Daum S., Meister A., Schekman R., Bacia K., and Briggs J.A.G.. 2013. The structure of the COPII transport-vesicle coat assembled on membranes. eLife. 2:e00951 10.7554/eLife.00951 [DOI] [PMC free article] [PubMed] [Google Scholar]